Abstract
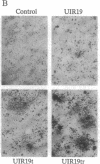
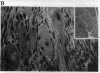
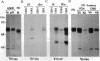
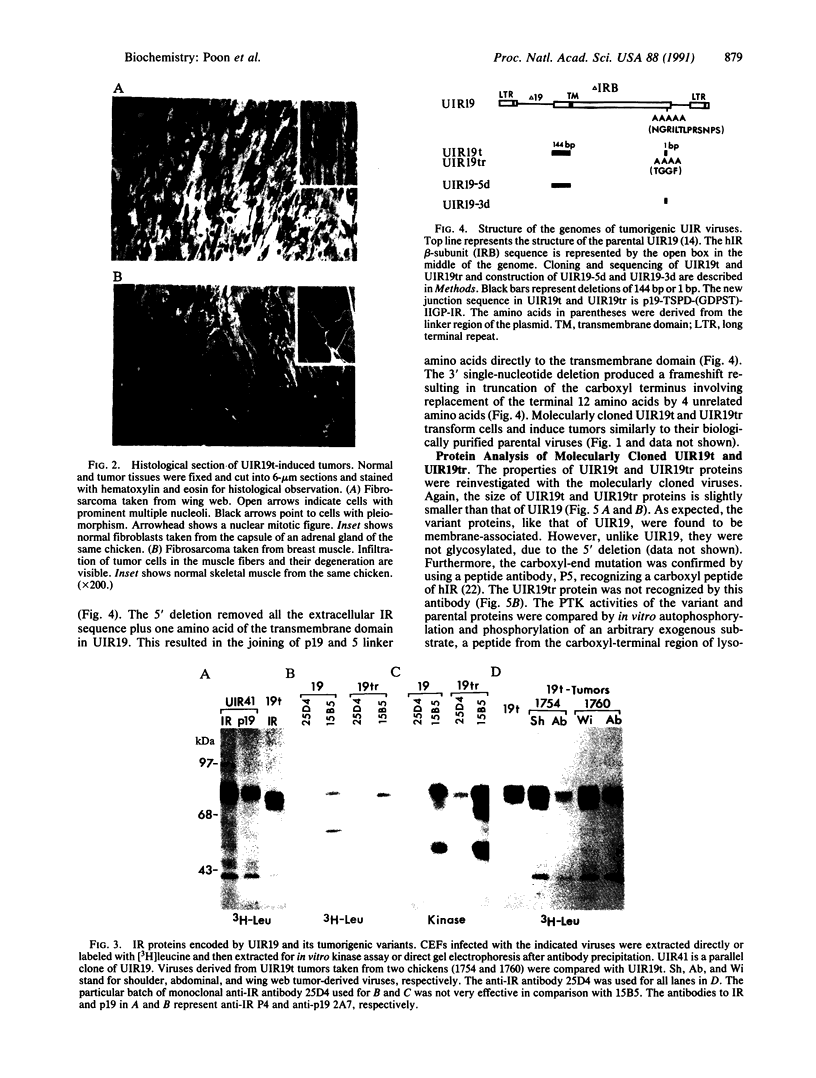
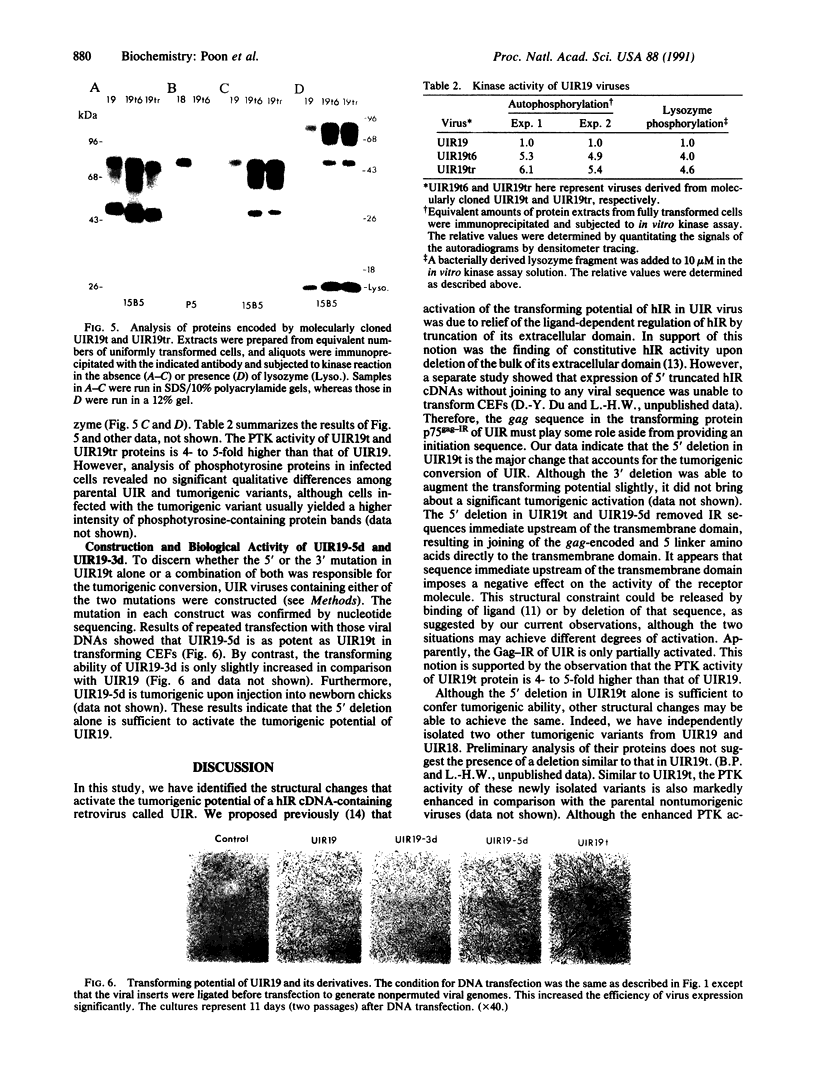
A previous study showed that the human insulin receptor (IR) could be activated by insertion of a 3' portion of the cDNA encoding the beta subunit into a retrovirus genome to form a Gag-IR fusion protein. While capable of transforming cells in culture, this IR cDNA-containing virus, called UIR, was not able to induce tumors in animals. Subsequently, we isolated a spontaneous sarcomagenic variant called UIR19t from the parental UIR. UIR19t was molecularly cloned, sequenced, and found to harbor two mutations. A 44-amino acid deletion immediately upstream from the transmembrane domain of the Gag-IR fusion protein removes all the extracellular sequence of the IR remaining in the original UIR construct. In addition, a single nucleotide deletion at the 3' end results in truncation and replacement of the carboxyl-terminal 12 amino acids by 4 new amino acids. The specific kinase activity of UIR19t is 4- to 5-fold higher than that of the parental UIR. However, no new cellular substrates were detected in UIR19t-transformed cells as compared to UIR cells. Viruses containing either the 5' or the 3' deletion mutation were constructed and assessed for their biological function. Our data indicate that the 5' deletion alone is sufficient to confer tumorigenic ability. We conclude that sequence immediately upstream from the transmembrane domain imposes a negative effect on the transforming and tumorigenic potential of the Gag-IR fusion protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Hafen E. Control of photoreceptor cell fate by the sevenless protein requires a functional tyrosine kinase domain. Cell. 1988 Jul 29;54(3):299–311. doi: 10.1016/0092-8674(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Bowtell D. D., Simon M. A., Rubin G. M. Nucleotide sequence and structure of the sevenless gene of Drosophila melanogaster. Genes Dev. 1988 Jun;2(6):620–634. doi: 10.1101/gad.2.6.620. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Yee D., Sly W. S., Perdue J., Hampton B., Lippman M. E., Rosen N. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 1990 Jan 1;50(1):48–53. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Edery M., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Mechanisms of receptor-mediated transmembrane communication. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):773–784. doi: 10.1101/sqb.1986.051.01.090. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Roth R. A., Rutter W. J. A membrane-anchored cytoplasmic domain of the human insulin receptor mediates a constitutively elevated insulin-independent uptake of 2-deoxyglucose. Mol Endocrinol. 1987 Jan;1(1):15–24. doi: 10.1210/mend-1-1-15. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Heterologous transmembrane signaling by a human insulin receptor-v-ros hybrid in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5101–5105. doi: 10.1073/pnas.84.15.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Petruzzelli L., Thomas N., Bramson H. N., Kaiser E. T., Rosen O. M. An antipeptide antibody that specifically inhibits insulin receptor autophosphorylation and protein kinase activity. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7899–7903. doi: 10.1073/pnas.82.23.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong S. M., Wang L. H. The transforming protein P68gag-ros of avian sarcoma virus UR2 is a transmembrane protein with the gag portion protruding extracellularly. Oncogene Res. 1987 Jun;1(1):7–21. [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Molecular cloning and characterization of avian sarcoma virus UR2 and comparison of its transforming sequence with those of other avian sarcoma viruses. J Virol. 1984 Jun;50(3):914–921. doi: 10.1128/jvi.50.3.914-921.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms R. W., Bose H. R., Jr Viral oncogenes and signal transduction. Virus Res. 1989 Mar;12(3):251–282. doi: 10.1016/0168-1702(89)90043-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Lin B., Jong S. M., Dixon D., Ellis L., Roth R. A., Rutter W. J. Activation of transforming potential of the human insulin receptor gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5725–5729. doi: 10.1073/pnas.84.16.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]