Abstract
Micelles have been successfully used for the delivery of anticancer drugs. Amphiphilic polymers form core-shell structured micelles in an aqueous environment through self-assembly. The hydrophobic core of micelles functions as a drug reservoir and encapsulates hydrophobic drugs. The hydrophilic shell prevents the aggregation of micelles and also prolongs their systemic circulation in vivo. In this protocol, we describe a method to synthesize a doxorubicin lipophilic pro-drug, doxorubicin-palmitic acid (DOX-PA), which will enhance drug loading into micelles. A pH-sensitive hydrazone linker was used to conjugate doxorubicin with the lipid, which facilitates the release of free doxorubicin inside cancer cells. Synthesized DOX-PA was purified with a silica gel column using dichloromethane/methanol as the eluent. Purified DOX-PA was analyzed with thin layer chromatography (TLC) and 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR). A film dispersion method was used to prepare DOX-PA loaded DSPE-PEG micelles. In addition, several methods for characterizing micelle formulations are described, including determination of DOX-PA concentration and encapsulation efficiency, measurement of particle size and distribution, and assessment of in vitro anticancer activities. This protocol provides useful information regarding the preparation and characterization of drug-loaded micelles and thus will facilitate the research and development of novel micelle-based cancer nanomedicines.
Keywords: Bioengineering, Issue 114, Doxorubicin, Pro-drug, Nanomedicine, Cancer, Micelles, Cell Viability, Drug Delivery
Introduction
Chemotherapy is commonly used to treat various forms of cancers. Most, if not all, chemotherapy drugs have toxic side effects which may vary from manageable minor conditions, such as nausea and diarrhea, to more life threatening conditions. Because most anticancer drugs are toxic, non-selective exposure of these drugs to normal tissue inevitably causes toxicity. Therefore, there is a great need for a therapeutic approach that can selectively deliver drugs into cancer cells. Another challenge with the administration of anticancer drugs is their poor water solubility. Usually, solubilizing agents are needed to formulate these poorly soluble drugs. However, most solubilizing agents, such as dimethyl sulfoxide (DMSO), Cremophor EL, and Polysorbate 80 (Tween 80) may cause liver and kidney toxicity, hemolysis, acute hypersensitivity reactions and peripheral neuropathies.1 Therefore, safe and biocompatible formulations are needed for the clinical use of poorly soluble anticancer drugs. Nanocarriers are promising drug delivery systems for addressing the above challenges. These nanocarriers include liposomes,2 nanoparticles,3 micelles,4-7 polymer-drug conjugates,8 and inorganic materials.9 Several nanomedicine products (e.g., Doxil, Abraxane, and Genexol) have been approved by the regulatory agencies to treat cancer patients.10
Polymeric micelles are promising nano-scale drug delivery carriers, which have been successfully used for the delivery of anticancer drugs.4-7,11,12 Typical polymeric micelles are prepared from amphiphilic polymers through a self-assembly process. The core-shell structured polymeric micelles include a hydrophilic shell and a hydrophobic core. The hydrophilic shell can sterically stabilize micelles and prolong their circulation in blood stream. The hydrophobic core can effectively encapsulate hydrophobic drugs. Because of the small size of micelles (typically less than 200 nm) and long-circulation properties, polymeric micelles are believed to achieve tumor targeting through enhanced permeability and retention (EPR) effects (passive tumor targeting).
Drug loading stability is critical for the tumor targeting ability of micelles. To achieve optimal tumor targeting, micelles should have minimal drug leakage before reaching the tumor site, yet quickly release the drug after entering cancer cells. In addition, formulation stability is also an essential requirement for product development, because formulation stability determines the feasibility of product development, as well as the shelf-life of developed products. Recently, much effort has been made to improve the loading of drugs into delivery carriers. The lipophilic pro-drug approach is a strategy which has been explored to improve drug loading into lipid nanoparticles and emulsions.13,14 The conjugation of lipids with drugs can significantly improve their lipophilicity and enhance loading and retention in the lipophilic components of nanocarriers.
Here, we describe a protocol for preparing lipophilic doxorubicin pro-drug loaded micelles. First, the synthesis procedure for doxorubicin lipophilic pro-drug is described. Then, a protocol for generating micelles with a film-dispersion method is introduced. This method has been successfully used in our previous studies.5 DSPE-PEG was selected as the carrier material for preparing micelles because it has been successfully used for micelle drug delivery.15,16 Finally, we describe several in vitro assays used to characterize micelle formulations and to evaluate anticancer activity.
Protocol
1. Synthesis of DOX-PA
Weigh 390 mg of doxorubicin and 243 mg of palmitic acid hydrazide, and transfer to a round bottom flask.
Add 150 ml of anhydrous methanol to the flask with a glass syringe. Add 39 µl of trifluoroacetic acid (TFA) with a pipette. Using a magnetic stirrer, stir the reaction mixture for 18 hr at RT in the dark. NOTE: The quantities of reaction materials can be scaled up or down to obtain different amounts of DOX-PA. The ratio of reactants should be maintained in the same proportions. Reactions using DOX quantities in the range of 78 mg to 1,170 mg can be performed in a regular chemistry laboratory.
- Purification of DOX-PA using a silica gel column.17
- Remove the solvent in the reaction mixture with a rotary evaporator. Add 3 g of silica gel after the volume of the mixture is reduced to around 20 ml. Continue rotary evaporation to yield dry powders and to allow the adsorption of products onto the silica gel. Keep the sample under a vacuum for an additional 30 min after the dry powders are formed.
- Pack 50 g of silica gel into a column using dichloromethane as a solvent. Carefully add the silica gel sample containing adsorbed product to the column.
- Elute the column with a mixture of dichloromethane and methanol, while gradually increasing the percentage of methanol, thereby increasing solvent polarity (Table 1).
- Collect fractions of eluent in test tubes (25 ml/tube) and monitor the progress by thin layer chromatography (TLC).
- Combine all fractions containing pure DOX-PA and remove the solvent using a rotary evaporator until dry powder is formed. Further dry the product under a vacuum O/N.
- Analysis of DOX-PA by TLC.
- Cut a 4 cm x 8 cm section of TLC plate. Spot sample solutions 0.5 cm from the bottom of the plate with TLC spotting capillaries using methanol as the solvent.
- Place the TLC plate into a developing chamber containing a mixture of dichloromethane and methanol (3/1, v/v). The depth of solvent should be just less than 0.5 cm.
- Remove the plate from the developing chamber when the solvent front reaches the top of the plate. Mark the location of the solvent front with a pencil and allow the plate to dry. Place the TLC plate into a staining chamber containing saturated iodine vapor in order to visualize samples.
- Analysis of DOX-PA with 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR).18
- Dissolve 15 mg of DOX-PA in 1 ml of methyl sulfoxide-d6 (DMSO) and transfer the sample into an NMR tube.
- Insert the NMR tube into the magnet of the NMR instrument. Measure the proton spectrum, selecting DMSO as a solvent. Remove the NMR tube from the magnet. Analyze the NMR result18.
2. Preparation of DOX-PA Micelles by Film-dispersion Method
Dissolve DSPE-PEG (40 mg) and DOX-PA (4 mg) with 2 ml of methanol in a 10 ml glass vial.
Remove the organic solvent under a vacuum using a rotary evaporator until a thin film forms in the vial. NOTE: Alternatively, evaporate the organic solvents under inert gas (e.g., argon or nitrogen gas) to form a film and keep the vial in a vacuum desiccator to further remove residual solvent.
Transfer 2 ml of Dulbecco's phosphate buffered saline (pH 7.4, DPBS) to the glass vial.
Place the vial in an ultrasonic bath for 3 min at RT to generate micelles. NOTE: Ultrasonic power varies among different models of ultrasonic baths. Select a unit which can generate enough ultrasonic power to disperse the thin polymer/drug film. The output power of ultrasonic bath used in this protocol is 110 W.
Keep micelles at 4 °C for short-term storage and -20 °C for long-term storage. NOTE: Alternatively, micelles can also be freeze-dried and reconstituted with water before use. Usually, no cryoprotectant or lyoprotectant is needed for this formulation.
3. Characterization of DOX-PA Micelles
- Determination of DOX-PA concentration in micelles and drug encapsulation efficiency
- Dissolve DOX-PA synthesized in previous steps in DMSO to prepare DOX-PA solutions of five different concentrations: 1 µg/ml, 5 µg/ml, 20 µg/ml, 50 µg/ml, and 100 µg/ml. Measure the absorption of DOX-PA solutions with a UV-VIS spectrometer at 490 nm. Generate a standard curve based on the DOX-PA drug concentrations and their corresponding absorption at 490 nm.
- Dilute 25 µl of drug-loaded micelle with 500 µl of DMSO. Measure the absorption at 490 nm with a UV-VIS spectrometer. Calculate drug concentrations with the standard curve generated in 3.1.1.
- Calculate encapsulation efficiency using the following equation: Drug Encapsulation Efficiency (%) = (amount of drugs in micelles)/(amount of added drug) × 100%
- Characterization of particle size with dynamic light scattering (DLS)
- Dilute micelles with DPBS (pH 7.4) to a final DSPE-PEG concentration of 1 mg/ml. Analyze a 2 ml sample with a particle size analyzer to obtain the Z-average size and polydispersity index (PDI).
- Evaluation of in vitro anticancer activity NOTE: Use appropriate sterile technique and operate inside a biosafety cabinet.
- Remove the cell culture medium from a cell culture flask (e.g., T25) containing DU-145 human prostate cancer cells and rinse the cells with 2 ml of DPBS (pH 7.4).
- Aspirate DPBS, add 1 ml of trypsin solution (0.25%), and incubate for 2 min at 37 °C to detach the cells.
- Add 10 ml of cell culture medium (RPMI 1640 + 10% Fetal Bovine Serum + 1% Antibiotic-Antimycotic), when most of cells are detached from the flask. Transfer cells to a 15-ml centrifuge tube and centrifuge the cells at 1,000 x g for 5 min.
- Re-suspend the cell pellet with 5 ml of cell culture medium and remove a sample for counting cell numbers with a hemocytometer. Dilute the cells with cell culture medium to a density of 50,000 cells/ml. Add the diluted-cell suspensions into a 96-well cell culture plate (100 µl/well). Incubate the cells in a cell culture incubator (37 °C, 5% CO2) for 18 hr to allow cell attachment.
- Dilute DOX dimethyl sulfoxide (DMSO) solution and DOX-PA DMSO solution with cell culture medium to obtain final drug concentrations of 0.1 µM, 0.5 µM, 2 µM, 5 µM, and 10 µM, respectively. Keep final DMSO concentration in all above samples to 0.5%. Dilute DOX-PA micelle with cell culture medium to obtain final drug concentrations of 0.1 µM, 0.5 µM, 2 µM, 5 µM, and 10 µM, respectively. Use the blank cell culture medium a control.
- Remove the 96-well cell culture plate from the incubator and replace the cell culture medium with 100 µl of medium containing different treatment agents prepared in step 3.3.5 (n = 4 for each group). Incubate the cells in the cell culture incubator (37 °C, 5% CO2) for an additional 72 hr.
- Aspirate the medium and add 100 µl of medium containing 0.5 mg/ml of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT).
- Incubate the cells in the cell culture incubator for additional 2 hr. Carefully remove the medium and add 100 µl of DMSO to dissolve formazan crystals.
- Measure absorbance with the microplate spectrophotometer at a wavelength of 570 nm and a reference wavelength of 670 nm.
- Calculate the cell viability using the following equation: Cell Viability (%) = (ATest ⁄ Acontrol) × 100% NOTE: Compare the cell viability between different groups using a one-way analysis of variance (ANOVA) statistical test. Calculate the IC50 based on cell viability vs. drug concentration data.
Representative Results
Figure 1 shows the synthesis scheme of DOX-PA. DOX-PA was synthesized by conjugation of palmitic acid with doxorubicin through a pH-sensitive hydrazone bond. A slight excess of palmitic acid hydrazide was used to facilitate the completion of the reaction. This reaction method has a very high efficiency and only a small amount of doxorubicin remained after an 18 hr reaction (Figure 2). The yield was approximately 88%. At the end of the reaction, DOX-PA was purified using a silica gel column and dried to obtain a pure red solid product. DOX-PA was analyzed with TLC, which showed a single spot for purified DOX-PA (Figure 2). Figure 3 shows the 1H-NMR spectrum of DOX-PA. Characteristic NMR peaks from both doxorubicin and palmitic acid were observed, which further confirmed success of the conjugation reaction.
The appearance of micelles prepared in this protocol is shown in Figure 4. Blank DSPE-PEG micelles without drug appear as a transparent liquid. DOX-PA DSPE-PEG micelles appear as a red liquid; the red color is due to the DOX-PA loaded in the micelles. Representative results of particle size analysis are shown in Figure 5. The Z-average mean particle size for blank DSPE-PEG micelles was 17.0 ± 0.5 nm (PDI = 0.034 ± 0.019). The loading of DOX-PA increased micelle particle size slightly; the Z-average mean particle size for DOX-PA DSPE-PEG micelles was 25.7 ± 1.6 nm (PDI = 0.407 ± 0.035).
The DOX-PA concentration in the micelle formulation was determined based on the absorption at 490 nm. DSPE-PEG has negligible absorption at this wavelength, thus does not interfere in the analysis of DOX-PA concentration. The DOX-PA concentration in the micelle formulation was 1.99 ± 0.11 mg/ml and the drug loading efficiency was 99.3 ± 5.7%. The high encapsulation efficiency is due to the lipophilic feature of DOX-PA, which enhances its retention in the hydrophobic core of micelles. The formulation showed excellent stability. There was no visible precipitation when stored at - 4 °C for 3 weeks. No significant change in drug concentration was observed during storage.
DU-145 human prostate cancer cells were treated with different concentrations of free doxorubicin, free DOX-PA, and DOX-PA DSPE-PEG micelles. Cell viability was determined at the end of a 72 hr treatment using a MTT assay (Figure 6). A concentration-dependent reduction in cell viability was achieved by treating DU145 cells with free doxorubicin (IC50 = 0.10 µM), free DOX-PA (IC50 = 0.33 µM), or DOX-PA micelles (IC50 = 0.25 µM). Although free doxorubicin is more effective than free DOX-PA or DOX-PA micelles at lower concentrations (0.1 µM and 0.5 µM), DOX-PA groups showed greater reduction in cell viability than free doxorubicin at higher concentrations (2-10 µM). Also, there appeared to be no significant difference between DOX-PA and DOX-PA micelles at both higher and lower concentrations. Blank DSPE-PEG micelles showed no toxicity (data not shown), indicating good biocompatibility and safety of DSPE-PEG as a drug delivery carrier material.
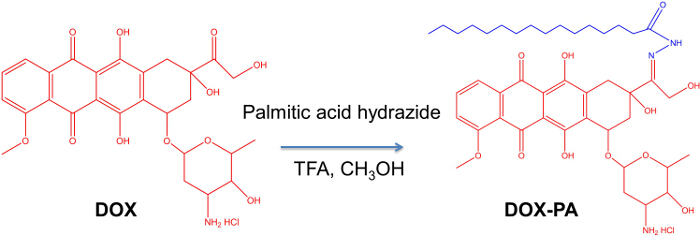 Figure 1. Synthesis of lipophilic pro-drug of doxorubicin (DOX-PA). Reagents and conditions: doxorubicin (DOX), palmitic acid hydrazide (PA) and trifluoroacetic acid (TFA) dissolved in methanol were stirred for 18 hr at RT in the dark. Please click here to view a larger version of this figure.
Figure 1. Synthesis of lipophilic pro-drug of doxorubicin (DOX-PA). Reagents and conditions: doxorubicin (DOX), palmitic acid hydrazide (PA) and trifluoroacetic acid (TFA) dissolved in methanol were stirred for 18 hr at RT in the dark. Please click here to view a larger version of this figure.
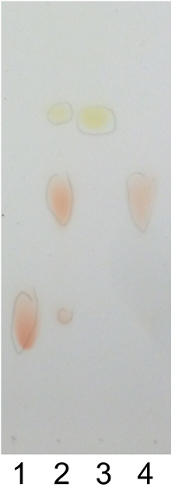 Figure 2. Thin layer chromatography (TLC). TLC of (1) doxorubicin, (2) raw reaction mixture, (3) palmitic acid hydrazide, and (4) DOX-PA conjugate. Samples were developed with a mixture of dichloromethane and methanol (3/1, v/v) and stained with iodine. Please click here to view a larger version of this figure.
Figure 2. Thin layer chromatography (TLC). TLC of (1) doxorubicin, (2) raw reaction mixture, (3) palmitic acid hydrazide, and (4) DOX-PA conjugate. Samples were developed with a mixture of dichloromethane and methanol (3/1, v/v) and stained with iodine. Please click here to view a larger version of this figure.
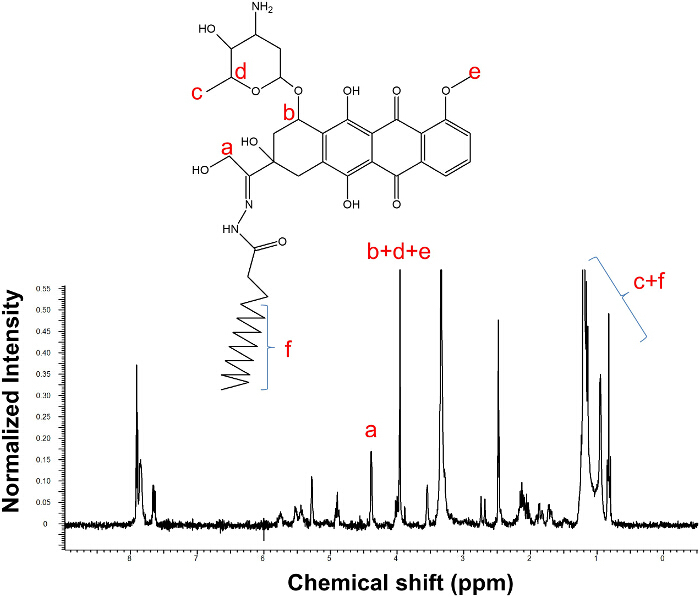 Figure 3.1H-NMR of DOX-PA in deuterated DMSO. The presence of characteristic peaks from both DOX and PA demonstrates the success of the conjugation reaction. Please click here to view a larger version of this figure.
Figure 3.1H-NMR of DOX-PA in deuterated DMSO. The presence of characteristic peaks from both DOX and PA demonstrates the success of the conjugation reaction. Please click here to view a larger version of this figure.
 Figure 4.Appearance of micelles. (A) DSPE-PEG micelles and (B) DOX-PA DSPE-PEG micelles. Representative figures are presented to demonstrate the appearance of blank DSPE-PEG micelles and DOX-PA DSPE-PEG micelles. Please click here to view a larger version of this figure.
Figure 4.Appearance of micelles. (A) DSPE-PEG micelles and (B) DOX-PA DSPE-PEG micelles. Representative figures are presented to demonstrate the appearance of blank DSPE-PEG micelles and DOX-PA DSPE-PEG micelles. Please click here to view a larger version of this figure.
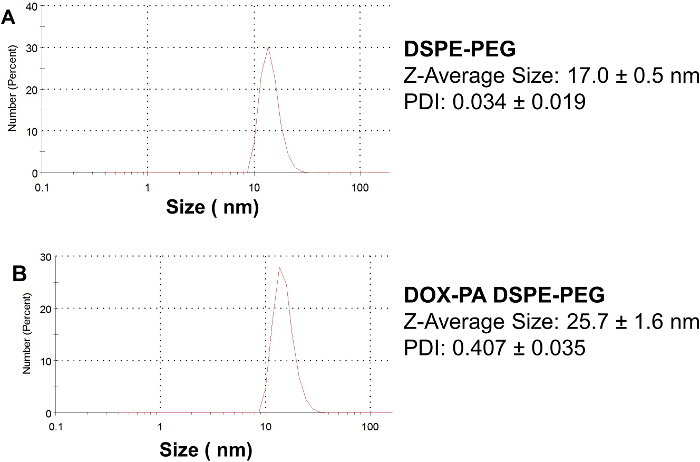 Figure 5.Particle size and size distribution of micelles. (A) DSPE-PEG micelles and (B) DOX-PA DSPE-PEG micelles. The micelle size is determined by dynamic light scattering. Representative figures are presented to demonstrate particle size and size distribution. Data presented are Mean ± SD (n = 3). Please click here to view a larger version of this figure.
Figure 5.Particle size and size distribution of micelles. (A) DSPE-PEG micelles and (B) DOX-PA DSPE-PEG micelles. The micelle size is determined by dynamic light scattering. Representative figures are presented to demonstrate particle size and size distribution. Data presented are Mean ± SD (n = 3). Please click here to view a larger version of this figure.
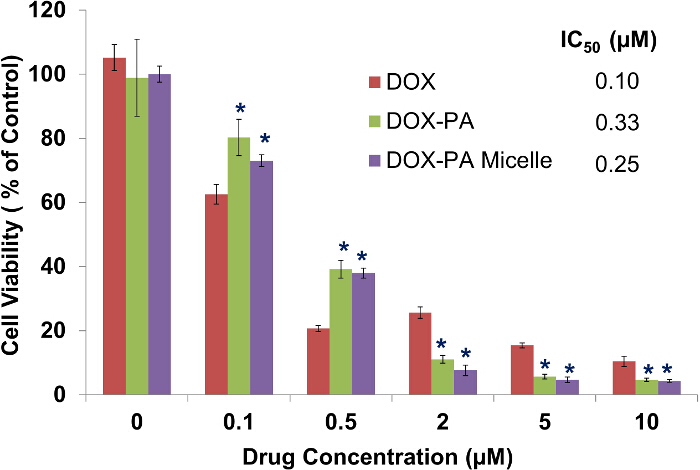 Figure 6.Anticancer activity of micelle formulations. DU145 cells were treated for 72 hr and cell viability was measured using the MTT assay. Data presented in the figure are Mean ± SD (n =4). *, P < 0.01, compared to DOX treated groups. There is no statistical difference between DOX-PA and DOX-PA micelles treated groups. IC50 was calculated based on cell viability vs. drug concentration data. Please click here to view a larger version of this figure.
Figure 6.Anticancer activity of micelle formulations. DU145 cells were treated for 72 hr and cell viability was measured using the MTT assay. Data presented in the figure are Mean ± SD (n =4). *, P < 0.01, compared to DOX treated groups. There is no statistical difference between DOX-PA and DOX-PA micelles treated groups. IC50 was calculated based on cell viability vs. drug concentration data. Please click here to view a larger version of this figure.
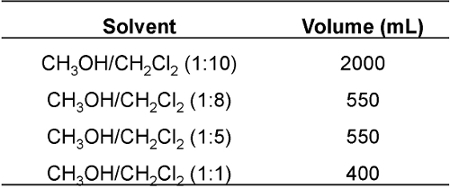
Table 1. CH 2 Cl 2 /CH 3 OH eluting solvents used in the purification of DOX-PA.
Discussion
In this work, we describe an uncomplicated, rapid film-dispersion method for the preparation of micelles. This method utilizes the self-assembly properties of an amphiphilic polymer (e.g., DSPE-PEG) to form core-shell structured micelles in an aqueous environment. This micelle preparation method has several advantages. 1. It involves a simple formulation process, which avoids the use of complicated size-reduction steps (such as extrusion or homogenization) commonly used in the preparation of liposomes, nanoparticles, and nanoemulsions.19 2. It has a good reproducibility. Excellent batch-to-batch consistency can be achieved once the formulation has been optimized and established. The protocol is tolerant to variations in preparation process variables, such as sonication strength and time. Although other methods (such as the dialysis approach or the emulsion-solvent evaporation approach) are available for preparing micelles, the film dispersion method is more convenient and efficient. Therefore, it has a great potential to be adapted for use in large-scale manufacturing of micelles in the pharmaceutical industry. The limitation of this method is that it depends on the self-assembly properties of carrier materials and thus is restricted to materials with such properties. The inappropriate selection of polymer materials may lead to failure in generating satisfactory micelle formulations. In addition, the preparation method relies on ultrasonication to disperse the polymer/drug film and to facilitate the formation of micelles. Most commercially available ultrasonic baths are suitable because only a weak ultrasonic power is needed to form micelles. However, it might be a problem if an ultrasonic bath with a very weak power is used.
Poor drug loading and premature drug release are major concerns for micelle drug delivery. In this protocol, we developed a lipophilic pro-drug strategy to enhance the loading of doxorubicin into DSPE-PEG micelles. Conjugation of doxorubicin with lipids significantly improved compatibility and interaction of the drug with the lipid core of DSPE-PEG micelles. The pro-drug, DOX-PA, was synthesized by conjugating doxorubicin with lipid through an acidic pH-responsive hydrazone linker. This linker is stable at neutral pH and cleavable at acidic pH.20,21 Thus, DOX is stably loaded in DSPE-PEG micelles as a pro-drug at neutral pH and has minimal drug leakage during storage and during circulation in the blood. Once DOX-PA micelles enter tumor cells through endocytosis, DOX-PA pro-drug will be cleaved and converted into free DOX in response to the acidic endosome environment (pH 5-6). Since DOX is more hydrophilic than DOX-PA, this conversion will disrupt the interactions between DOX and the hydrophobic core of micelles, and thus facilitate the quick release of DOX from micelles. This innovative design feature will avoid incomplete drug release, which is a major problem associated with drug conjugates using non-cleavable or slow-cleaving linkers. 22 This approach may also be applied to the delivery of other drugs. The selection of an appropriate linker is critical for the success of this approach. The linkers should be stable in a physiological environment in order to maximize the stability of the formulation. The linkers should also be efficiently cleaved in response to triggers in the tumor microenvironment (e.g., pH, enzymes, etc.) to release the parent drug.
In this protocol, DOX-PA was efficiently loaded into micelles with high encapsulation efficiency (~ 100%). The micelle formulation also demonstrated superior stability and remained intact for at least three weeks without drug precipitation or reduction in drug concentration. This is due to the enhanced interaction between lipid moieties in the pro-drug and the lipid core of DSPE-PEG micelles. Engineering the compatibility of carrier molecules with drugs to enhance their interaction is a promising strategy for improving the performance of micelles and other nanocarriers. This approach can enhance drug loading and minimize pre-mature drug release for these nanocarriers.23 The selection of an appropriate drug/polymer pair is a critical step in designing the micelle formulation. The structure of drug molecules can be modified (pro-drug approach) or the design of carrier materials can be optimized in order to improve compatibility and to enhance drug/carrier interactions.2324 In addition, computational modeling can be a useful tool to assist in the optimization of nanocarriers and make it possible to prepare a tailor-designed nanocarrier for specific a specific drug.
In summary, we describe here a method to synthesize a lipophilic pro-drug of doxorubicin. Protocols for the preparation and characterization of drug loaded micelles are also described. The film-dispersion method is a simple and promising method for preparing a variety of nanoscale self-assembly delivery systems. The characterization methods described here can be used as standard in vitro assays to determine the properties of nanomedicines, thus can facilitate the optimization of formulation and product development processes.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the following grants: NIH-SC3 grant, NSF-PREM grant, Hampton University Faculty Research Grant. We would like to thank Mrs. Michele A. Cochran at Virginia Institute of Marine Science (VIMS) for the use of the particle size analyzer. We would also like to thank Mrs. Corinne R. Ramaley for reviewing the manuscript.
References
- Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? ESMO. 2006;17(5):735–749. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- Paliwal SR, Paliwal R, Agrawal GP, Vyas SP. Liposomal nanomedicine for breast cancer therapy. Nanomedicine. 2011;6(6):1085–1100. doi: 10.2217/nnm.11.72. [DOI] [PubMed] [Google Scholar]
- Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9(55) doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danquah M, Li F, Duke CB, Miller 3rd , D D, Mahato RI. Micellar delivery of bicalutamide and embelin for treating prostate cancer. Pharm Res. 2009;26(9):2081–2092. doi: 10.1007/s11095-009-9903-5. [DOI] [PubMed] [Google Scholar]
- Li F, Danquah M, Mahato RI. Synthesis and characterization of amphiphilic lipopolymers for micellar drug delivery. Biomacromolecules. 2010;11(10):2610–2620. doi: 10.1021/bm100561v. [DOI] [PubMed] [Google Scholar]
- Li F, Danquah M, Singh S, Hao W, Mahato R. Paclitaxel- and lapatinib-loaded lipopolymer micelles overcome multidrug resistance in prostate cancer. Drug Deliv. and Transl. Res. 2011;1(6):9. doi: 10.1007/s13346-011-0042-2. [DOI] [PubMed] [Google Scholar]
- Li F, Lu Y, Li W, Miller DD, Mahato RI. Synthesis, formulation and in vitro evaluation of a novel microtubule destabilizer, SMART-100. J Control Release. 2010;143(1):151–158. doi: 10.1016/j.jconrel.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Minko T, Kopeckova P, Pozharov V, Kopecek J. HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J Control Release. 1998;54(2):223–233. doi: 10.1016/s0168-3659(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Rosenholm JM, Mamaeva V, Sahlgren C, Linden M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine. 2012;7(1):111–120. doi: 10.2217/nnm.11.166. [DOI] [PubMed] [Google Scholar]
- Kaur IP, et al. Issues and concerns in nanotech product development and its commercialization. J Control Release. 2014;193:51–62. doi: 10.1016/j.jconrel.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Jones M, Leroux J. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Li F, Du C, Mahato RI, Huang Y. Doxorubicin and lapatinib combination nanomedicine for treating resistant breast cancer. Mol Pharm. 2014;11(8):2600–2611. doi: 10.1021/mp400687w. [DOI] [PubMed] [Google Scholar]
- Ma P, Rahima Benhabbour S, Feng L, Mumper RJ. 2'-Behenoyl-paclitaxel conjugate containing lipid nanoparticles for the treatment of metastatic breast cancer. Cancer Lett. 2013;334(2):253–262. doi: 10.1016/j.canlet.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg BB, Risovic V, Ramaswamy M, Wasan KM. A lipophilic paclitaxel derivative incorporated in a lipid emulsion for parenteral administration. J Control Release. 2003;86(1):93–100. doi: 10.1016/s0168-3659(02)00323-1. [DOI] [PubMed] [Google Scholar]
- Perche F, Patel NR, Torchilin VP. Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG-PE micelles in ovarian cancer cell spheroid model. J Control Release. 2012;164(1):95–102. doi: 10.1016/j.jconrel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG(2000)-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur J Pharm Sci. 2012;46(1-2):67–71. doi: 10.1016/j.ejps.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Still WC, Kahn M, Mitra A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978;43(14):2923–2925. [Google Scholar]
- New Mexico State University. NMR Protocols and Specifications. 2015. Available from: http://web.nmsu.edu/~kburke/Instrumentation/NMSU_NMR_300.html.
- Morton LA, Saludes JP, Yin H. Constant pressure-controlled extrusion method for the preparation of Nano-sized lipid vesicles. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Ulbrich K, Etrych T, Chytil P, Jelinkova M, Rihova B. HPMA copolymers with pH-controlled release of doxorubicin: in vitro cytotoxicity and in vivo antitumor activity. J Controlled Release. 2003;87(1-3):33–47. doi: 10.1016/s0168-3659(02)00348-6. [DOI] [PubMed] [Google Scholar]
- Patil R, et al. Cellular Delivery of Doxorubicin via pH-Controlled Hydrazone Linkage Using Multifunctional Nano Vehicle Based on Poly(beta-L-Malic Acid) Int J Mol Sci. 2012;13(9):11681–11693. doi: 10.3390/ijms130911681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Liu S, Huang Y, Chen X, Jing X. Biodegradable block copolymer-doxorubicin conjugates via different linkages: preparation, characterization, and in vitro evaluation. Biomacromolecules. 2010;11(8):2094–2102. doi: 10.1021/bm100458n. [DOI] [PubMed] [Google Scholar]
- Huynh L, Neale C, Pomes R, Allen C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomedicine. 2012;8(1):20–36. doi: 10.1016/j.nano.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Shi C, et al. A drug-specific nanocarrier design for efficient anticancer therapy. Nat Commun. 2015;6:7449. doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]


