Abstract
The measurement of sediment-water exchange of gases and solutes in aquatic sediments provides data valuable for understanding the role of sediments in nutrient and gas cycles. After cores with intact sediment-water interfaces are collected, they are submerged in incubation tanks and kept under aerobic conditions at in situ temperatures. To initiate a time course of overlying water chemistry, cores are sealed without bubbles using a top cap with a suspended stirrer. Time courses of 4-7 sample points are used to determine the rate of sediment water exchange. Artificial illumination simulates day-time conditions for shallow photosynthetic sediments, and in conjunction with dark incubations can provide net exchanges on a daily basis. The net measurement of N2 is made possible by sampling a time course of dissolved gas concentrations, with high precision mass spectrometric analysis of N2:Ar ratios providing a means to measure N2 concentrations. We have successfully applied this approach to lakes, reservoirs, estuaries, wetlands and storm water ponds, and with care, this approach provides valuable information on biogeochemical balances in aquatic ecosystems.
Keywords: Environmental Sciences, Issue 114, Sediment-water exchange, sediment biogeochemistry, denitrification, nitrogen cycling, sediment oxygen demand, benthic-pelagic coupling
Introduction
Sediments are critical biogeochemical components of aquatic ecosystems and often are important sinks of nutrients and contaminants. Pioneering studies of nutrient, gas and transition metal biogeochemistry in lacustrine sediments revealed sediment exchange of solutes and gases with overlying water that had varying redox conditions1,2. For nutrient elements, sediments can be a source of phosphorus and fixed nitrogen after remineralization of organic matter, and a sink for oxygen in non-photosynthetic environments3,4. Photosynthesis of submerged macrophytes, macroalgae and benthic microalgae can have profound influences on the exchange of dissolved substances across the sediment-water interface5,6.
Measurements of the exchange of solutes and gases across the sediment-water interface are carried out for both basic science and applied science purposes, including calibration of engineering and scientific water quality models7,8. The goal of these methods, to the greatest extent possible, is to provide reliable and accurate sediment-water exchange rates. A wide variety of approaches have been used to assess chemical exchange at the sediment-water interface. Bottom water accumulation of gases and solutes in stratified systems can be useful9, but is not valid for sediment-water exchange above thermoclines or pycnoclines. Eddy correlation requires high frequency measurements of gases, generally oxygen, combined with high frequency measurement of vertical water velocities; this technique has enormous promise but currently cannot provide data for nutrient exchange studies. In situ domes or chambers are a highly preferred method, with the advantage of covering a larger surface area of sediment and maintaining in situ temperatures, deep-water pressures and light levels10. In practice, these are very expensive measurements requiring extensive time on larger research vessels; most applications are deeper coastal zone or oceanic sediments. Core incubation techniques using flow through chambers that reach steady state are excellent for maintaining relatively constant overlying water chemistry, including oxygen, during incubations11. Because the rate is determined at steady state by concentration differences between inflowing and outflowing water, and by water exchange rates, these incubations can take a considerable amount of time.
The time-course core incubation approach used by our laboratory was adapted from approaches used by a number of different laboratories in North America and Europe, and there is a considerable amount of literature based on this general approach. We adapted this approach to the measurement of N2-N fluxes12, often referred to as denitrification, and have applied it to photosynthetic and non-photosynthetic sediment environments, including estuaries13, lakes, reservoirs, and wetlands14. Through these studies we have found many environments in which our overall approach works well, and some in which it does not. The measurement of denitrification has been carried out in many different terrestrial and aquatic environments because this process represents a key loss of nitrogen to ecosystems. Numerous approaches have been used to make denitrification measurements, some direct and some indirect15. Direct N2 flux measurements are very difficult because of the high atmospheric content N2, and subsequent high concentrations dissolved in water16. Two approaches have emerged as having the best representation of environmentally relevant rates: isotope pairing using N isotopes17 and the N2:Ar ratio used in our laboratory. The isotope pairing method has been used successfully in many environments and has very high sensitivity at low rates. We employ the N2:Ar ratio approach because of its simplicity, and because it is sufficiently sensitive in the impacted environments we often study.
In this paper, we describe the technical approach we have used over the last two decades to make measurement of the sediment-water exchange of gases and solutes. Any measurements of sediment-water exchange need to take into consideration field conditions and a number of experimental parameters. These factors include temperature, light/dark conditions18, mixing/physical flow at the sediment-water interface19, dissolved oxygen concentrations20, and other factors that are key elements of making good measurements. For example, if cores are collected from areas which receive sufficient illumination for the growth of benthic microalgae, it is necessary to devise experiments that include both dark and light conditions21. Similarly, adding oxygenated overlying water to anoxic cores does not replicate field conditions. Experimental enclosure of any part of aquatic ecosystems may lead to unavoidable artifacts22; it is critical that the approaches used in a sediment-water exchange measurement program 1) recognize the factors controlling sediment-water exchange in each ecosystem and 2) minimize artifacts derived from experimental manipulation.
Protocol
Note: The collection of cores with undisturbed sediment-water interfaces is essential to making good experimental measurements of exchange; highly-disturbed cores are likely to exchange pore water solutes with overlying water and have enhanced uptake of oxygen via the oxidation of Fe(II) and reduced sulfur compounds. In this paper, we emphasize sediment incubation procedures of sediments with only a cursory inclusion of sediment sampling techniques and chemical analyses of solutes and gases. Prior to sampling, or based on initial results, determine the degree of replication by the overall project needs, statistical design or expected amount of small scale spatial variability. Duplicate cores are the minimum used by many studies and triplicates are useful for allowing a better statistical analysis.
1. Sediment Collection and Handling
Note: The collection of sediment for exchange experiments is carried out using 1) manual insertion of cores using divers or in shallow water or wetland, by wading, 2) pole coring using an aluminum pole with a manually closed valve to retain sediments, or 3) box coring.
- At each site, record the site location using GPS, determine bottom water oxygen, temperature, and salinity using a water quality sonde, and determine the photosynthetically active radiation (PAR) at the surface and bottom using a PAR sensor/meter.
- Lower the water quality sonde to ~1 m above the sediment and record bottom water characteristics (depth, temperature, dissolved oxygen, temperature and salinity/conductivity).
- Lower a PAR sensor with an underwater probe to the sediment-water interface using a lowering frame. Compare PAR readings near the sediment surface to PAR readings immediately below the air-water interface to estimate the light attenuation under the ambient light conditions.
- Deploy a box corer over the side of the boat/ship, lowering it slowly to minimize disturbances upon penetrating the sediment. Examine the core box for visible disturbance or excessive resuspension.
- For a box corer, insert core tubes into the sediment, and use a butyl stopper to cover the top of the core. For flux experiments, while the ideal sediment/water balance within the core is 15 cm of water and 15 cm of sediment, in coarse or highly compacted sediments collecting less sediment depth is an acceptable outcome. If rates of oxygen depletion are excessive, shift the balance towards more water column height.
- Typically use 6.35 cm inner diameter cores for deep water studies and for sediments with benthic microalgae or large animal populations, use 10 cm ID cores. The main limit to core size is the ability to cap the bottom of the core.
- Cap the bottom with an acrylic bottom plate that has an embedded O-ring. Repeat this process until sufficient replicates are collected. With the pole corer, first place the acrylic bottom plate in the core liner, remove the core from the corer, and add the stopper.
Place cores in a tall insulated water cooler that is flooded with ambient water from the site; this helps maintain in situ temperatures. Ensure that the cooler remains upright. Discard cores that are disturbed during transport.
- Pump bottom water taken near the sediment surface into 20 L carboys for use in the experiments. Use a diaphragm pump with 10-20 L/min capacity or a high speed peristaltic pump.
- In shallow unstratified water, fill the carboy by "dunking" it in the water. Filtration of the bottom water using a high capacity inline cartridge filter may be useful at sites with high rates of water column oxygen uptake or photosynthesis (in the light), minimizing the correction from the water column only control cores.
Transport the cores as quickly as possible to the incubation facility. In the case of extended transport, aerobic cores can become anoxic and artificial bubbling or circulation are necessary.
2. Initial Setup
At the incubation facility, place cores in an incubation tub either in an environmental room with controlled temperature, or in a double walled incubator with temperature control via a heating/cooling circulator. Set the temperature to bottom water temperatures measured in 1.1.1.
Add bottom water to the incubator, completely submerging the sediment cores. Also add water to 5 L carboys with spigots that will be used to dispense replacement water.
Add a water-only core (without sediment) to the incubator. The use of water column blanks is important in most environments to compensate for any water column processes that affect gases and solutes. For the measurement of denitrification, these blanks can reflect not only water column processes but the exchange of gases with the acrylic walls of the core.
- Bubble cores with air for a minimum of 2 hr to ensure thermal equilibrium and full oxygenation of overlying water. They may be kept overnight and time courses initiated the next morning. Longer pre-incubation periods have not been evaluated for efficacy.
- For aeration, use a small "T" bubbler consisting of ½" PVC pipe with a three way coupler; a 1/8' tube inserted to the bottom of the T results in entrainment of water upward during bubbling and ensures not only oxygenation, but circulation of the water in the core with water in the incubation tub.
3. Sediment-water Incubation Procedures
After checking the temperature to ensure it matches the field conditions, attach spinning tops to the tops of the cores. At this point, seal the core from the tank water. Leave the sampling valve on the core open during this process. Manually sweep any air bubbles gently from the bottom side of the spinning top.
Elevate the replacement water carboy ~30-40 cm higher than the tops of the incubation cores and drain the lines downward to eliminate any air in the line. While still flowing, attach the lines to the core tops and close the valves.
Turn on the central stirring turntable and adjust the rotation speed so that cores rotate ~40 times per minute, or at a rate that is sufficient to mix the water column but not resuspend the sediment.
- Approximately 5 min after all cores are sealed, open the replacement water valve and the sample valve, and then attach a short piece of tubing to the sample valve using a Luer fitting. Place this sampling tube in the bottom of a 7 ml glass tube, which is filled to overflowing. Prior to capping the tube, add 10 µl of 30 g L-1 HgCl2 as a preservative.
- Store these samples underwater at temperatures close to the incubation temperature. Other laboratories have successfully used 12 ml "Exetainers" for sample storage.
For solute sampling, attach a 20 ml syringe barrel to the sample valve and open the replacement water valve. The syringe barrel fills until full using only gravity. Attach a plunger and a filter disk, and then filter the samples into vials. These samples for nutrient analysis are frozen at -20°C until analysis. Note: The time course of sampling in the dark typically involves 4 sampling periods with the intervals between sampling ranging from 0.5 to > 2 hr, depending on the rate of oxygen uptake. With low rates of oxygen uptake, the time intervals are long; in sediments with high rates of respiration, intervals need to be short. Excessively high volumes of sample taken at each sample point may result in sampling too big a proportion of the whole sample volume; in our work these sample volumes result in a negligible correction. If greater volume of sample is needed, larger diameter cores or an increased water column height may be necessary.
- Do not proceed with a time course of sampling below a threshold of 50% oxygen depletion, with oxygen depletions of 25% usually providing sufficient signal in nutrient concentrations. Here, use calibrated optodes for direct analysis of oxygen concentrations and oxygen saturation.
- If the sediments are from shallow, illuminated environments, at the 4th sampling, turn on the lights and take 3 subsequent samples. Note that in highly photosynthetic sediments, supersaturation of O2 and bubble formation limits the measurement of gas fluxes in some cases. The continual monitoring of oxygen is an increasingly viable and valuable alternative, with fiber optic measurement technology having relatively small probes that are highly reliable and precise.
At the conclusion of the sediment incubations, either measure the height of the water column or siphon the water column in to a graduated cylinder to directly determine the water volume, and take photos of each core.
4. Sample Analysis
- Pump samples for the analysis of N2, O2 and Ar into a membrane inlet mass spectrometer, and determine the ratios of N2:Ar and O2:Ar to < 0.03% precision12,23.
- Couple a quadrupole mass spectrometer to a membrane inlet. Push the sample into the membrane tubing using a peristaltic pump. Collect the sample waste in a plastic carboy and treat as chemical waste due to the Hg preservative. Calibrate with deionized water equilibrated with air at the temperature of incubation.
- Perform nutrient analyses manually on ≤ 5 ml samples or on smaller volumes using automated analyzers. Upon sample thawing, start analyses immediately. The choice of nutrient analysis procedure must yield a precision sufficient to observe changes in nutrient concentration during incubation. Typical detection limits are < 0.05 µmol L-1 and time course trends can be difficult to observe under both extremely low and extremely high nutrient concentrations.
- For colorimetric analyses of soluble reactive phosphorus, use the ascorbic acid phosphomolybdate technique. For the ammonium analyses, utilize an overnight color development using a phenol hypochlorite reagent24. Automated colorimetric analyses, either using segmented flow or a discrete analyzer, are a great alternative and utilize lower sample volume.
- For analyses of nitrate plus nitrite, utilize overnight color development using vanadium chloride as a reductant25, or use an automated analyzer
- Compare absorbances determined on a UV/VIS spectrophotometer to standard curves and determine concentrations from a regression of standards concentrations and absorbances.
5. Calculation of Sediment-water Exchange Rates
Regress the concentrations of gas or nutrient versus time independently for both dark and illuminated incubations, with the slope expressed as µmol L-1 hr-1. Correct the slopes of the incubation cores for the slope of the water column-only cores. Only use significant regressions (P < 0.05) for calculations; identify non-significant data in the final data spreadsheets.
Calculate sediment-water exchange rates from the slope of the change of chemical constituent concentrations in the overlying water:
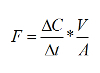 Where F is the flux (µmol m-2 hr-1), ΔC/Δt is the slope of the concentration change in overlying water (µmol L-1 hr-1), V is the volume of the overlying water (L) and A is the area of the incubated core (m-2).To estimate the daily flux, multiply the illuminated rate by the hours of light and add to the dark rate multiplied by the hours of darkness.
Where F is the flux (µmol m-2 hr-1), ΔC/Δt is the slope of the concentration change in overlying water (µmol L-1 hr-1), V is the volume of the overlying water (L) and A is the area of the incubated core (m-2).To estimate the daily flux, multiply the illuminated rate by the hours of light and add to the dark rate multiplied by the hours of darkness.
6. Reporting
When reporting results from sediment-water exchange measurements, provide sufficient information for other scientists to understand the environment that has been sampled. Essential information includes: 1) site location and water depth, 2) physical characteristics such as field and incubation temperature, and PAR, 3) bottom water characteristics such as oxygen, nutrient concentrations and salinity, and 4) sediment characteristics such as grain size, organic matter concentrations, and the presence of benthic animals.
Representative Results
Results from sediment flux measurements near an aquaculture facility on the Choptank River (Chesapeake Bay, MD) are shown in Figure 1 and the interpretation of these results in an ecosystem context are presented elsewhere26. The incubations were carried out over 7 hours, with dark incubations followed by illuminated incubations data. Data from two cores are shown as well as the water column only control. The rapid decrease in oxygen in the dark was attenuated somewhat by illumination; the photosynthesis rate of microalgal production was not as high as respiration, with the main effect of illumination being a decreased rate of change of oxygen. The control core experienced small decreases in oxygen concentration in the dark and small increases in the light.
The N2 concentrations were determined by the N2:Ar ratio and the calculated Ar saturation literature values for the observed temperature and salinity27. At a typical precision of 0.02% for the N2:Ar ratio, these data are precise to ~0.1 µmol L-1 N2. The sediment cores and the water column blank cores had increases in N2 over time, with much higher rates of increase for the cores. Under illumination, slopes were generally similar to the dark rate of N2 change.
Fluxes of dissolved NH4+ were quite high at this site, with dark increase of > 20 µmol L-1 for one core. Illuminated NH4+ fluxes were much lower. Both cores and the water column blank had decreasing NOx- concentration over time, leveling out during illumination. For all of the fluxes, the concentration data and data on the core volume and other relevant parameters are shown in Tables 1 and 2.
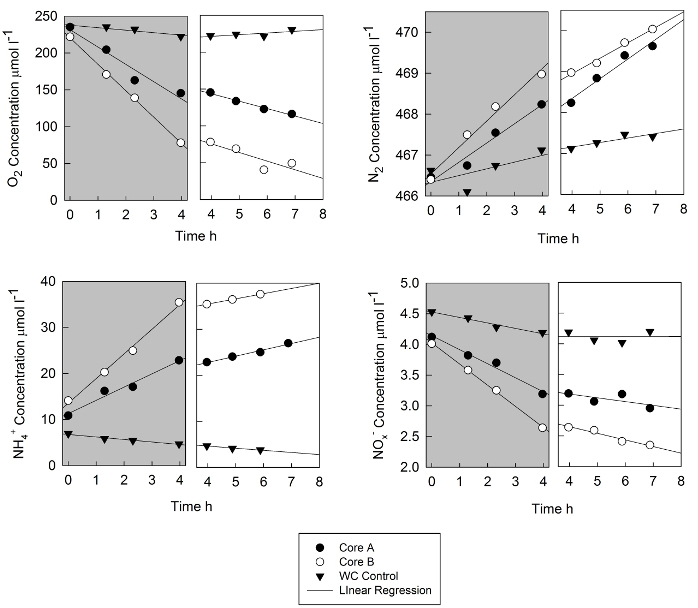 Figure 1. Time course data from a shallow water site in the Choptank River that was covered with floats containing cultured oysters. The data are from replicate cores (A and B) and the data from a water column blank are shown. Concentrations of oxygen N2, NH4+ and NOx- (the sum of NO3- and NO2- are presented for both the dark part of the incubation (shaded area) and for the illuminated part of the incubation. The fourth time point of the dark incubation is also the first time point of the illuminated time series; lights were turned on at the time of sampling. The lines are linear regressions and slopes are presented in Table 1. Please click here to view a larger version of this figure.
Figure 1. Time course data from a shallow water site in the Choptank River that was covered with floats containing cultured oysters. The data are from replicate cores (A and B) and the data from a water column blank are shown. Concentrations of oxygen N2, NH4+ and NOx- (the sum of NO3- and NO2- are presented for both the dark part of the incubation (shaded area) and for the illuminated part of the incubation. The fourth time point of the dark incubation is also the first time point of the illuminated time series; lights were turned on at the time of sampling. The lines are linear regressions and slopes are presented in Table 1. Please click here to view a larger version of this figure.
| Oxygen - Dark | Time (hr) | Core A | Core B | Control |
| 0 | 235.1 | 221.7 | 235.2 | |
| 1.3 | 204.3 | 170.6 | 235.3 | |
| 2.32 | 162.7 | 138.9 | 232 | |
| 3.97 | 145.3 | 77.9 | 222.2 | |
| R2 | 0.943 | 0.999 | 0.836 | |
| Slope (µmol L-1 hr-1) | -23.5 | -35.9 | -3.4 | |
| Corrected Slope (µmol L-1 hr-1) | -20.1 | -32.5 | ||
| Rate (µmol m-2 hr-1) | -3,095 | -4,875 | ||
| Oxygen - Light | Time (hr) | Core A | Core B | Control |
| 3.97 | 145.3 | 77.9 | 222.2 | |
| 4.88 | 133.5 | 68.8 | 224.3 | |
| 5.88 | 122.8 | 40.3 | 221.6 | |
| 6.88 | 116 | 49.2 | 230.5 | |
| R2 | 0.981 | 0.999 | 0.994 | |
| Slope (µmol L-1 hr-1) | -10.1 | -9.8 | 2.9 | |
| Corrected Slope (µmol L-1 hr-1) | -13 | -12.7 | ||
| Rate (µmol m-2 hr-1) | -2,000 | -1,905 | ||
| N2 - Dark | Time (hr) | Core A | Core B | Control |
| 0 | 466.46 | 466.40 | 466.62 | |
| 1.3 | 466.74 | 467.49 | 466.11 | |
| 2.32 | 467.55 | 468.18 | 466.74 | |
| 3.97 | 468.24 | 468.98 | 467.12 | |
| R2 | 0.963 | 0.98 | 0.854 | |
| Slope N2 (µmol L-1 hr-1) | 0.471 | 0.645 | 0.12 | |
| Corrected Slope N2 (µmol L-1 hr-1) | 0.351 | 0.525 | ||
| Rate N2-N (µmol m-2 hr-1) | 108.1 | 157.5 | ||
| N2 - Light | Time (hr) | Core A | Core B | Control |
| 3.97 | 468.24 | 468.98 | 467.12 | |
| 4.88 | 468.84 | 469.21 | 467.26 | |
| 5.88 | 469.39 | 469.71 | 467.47 | |
| 6.88 | 469.62 | 470.04 | 467.41 | |
| R2 | 0.96 | 0.987 | 0.967 | |
| Slope N2 (µmol L-1 hr-1) | 0.481 | 0.378 | 0.096 | |
| Corrected Slope N2 (µmol L-1 hr-1) | 0.386 | 0.282 | ||
| Rate N2-N (µmol m-2 hr-1) | 118.9 | 84.6 | ||
| Core Surface Area (m2) | 0.003165 | 0.003165 | ||
| Core Volume (L) | 0.4874 | 0.4747 |
Table 1. Time course data for O2 and N2 from sediments underneath oyster aquaculture floats in the Choptank River, a subestuary of the Chesapeake Bay. The gas concentrations are derived from O2:Ar and N2:Ar ratios determined via membrane inlet mass spectrometry. The time course regression R2 values are significant for values > 0.9025 (P < 0.05). Slopes are determined by linear regression and corrected slopes are determined by subtracting the rate of change of the water column only blank. Positive rates are net fluxes out of the sediment, negative rates indicate flux into the sediment. The N2 flux data are expressed as N2-N, making comparison to NH4+ and NOx- fluxes easier. This site had sediments primarily consisting of silt and clay with fully aerobic water column conditions. The area of the cores was 31.65 cm-2 and the water column depths were 15.4 cm for core A and 15.0 for core B. All concentrations for N2 and O2 are µmol L-1. The final rate for N2 flux is expressed at N2-N.
| NH4+ - Dark | Time (hr) | Core A | Core B | Control |
| 0 | 10.84 | 14.09 | 6.91 | |
| 1.3 | 16.19 | 20.26 | 5.83 | |
| 2.32 | 17.07 | 24.93 | 5.42 | |
| 3.97 | 22.83 | 35.43 | 4.67 | |
| R2 | 0.968 | 0.993 | 0.853 | |
| Slope (µmol L-1 hr-1) | 2.88 | 5.36 | -0.53 | |
| Corrected Slope (µmol L-1 hr-1) | 3.41 | 5.89 | ||
| Rate (µmol m-2 hr-1) | 525 | 884 | ||
| NH4+ - Light | Time (hr) | Core A | Core B | Control |
| 3.97 | 22.83 | 35.43 | 4.67 | |
| 4.88 | 24.05 | 36.45 | 4.13 | |
| 5.88 | 25.00 | 37.60 | 3.79 | |
| 6.88 | 26.96 | |||
| R2 | 0.978 | 1 | 0.966 | |
| Slope (µmol L-1 hr-1) | 1.37 | 1.13 | -0.55 | |
| Corrected Slope (µmol L-1 hr-1) | 1.92 | 1.68 | ||
| Rate (µmol m-2 hr-1) | 296 | 252 | ||
| NOx- - Dark | Time (hr) | Core A | Core B | Control |
| 0 | 4.12 | 4.01 | 4.53 | |
| 1.3 | 3.82 | 3.58 | 4.43 | |
| 2.32 | 3.70 | 3.25 | 4.28 | |
| 3.97 | 3.19 | 2.64 | 4.19 | |
| R2 | 0.976 | 0.992 | 0.967 | |
| Slope (µmol L-1 hr-1) | -0.229 | -0.345 | -0.089 | |
| Corrected Slope (µmol L-1 hr-1) | -0.14 | -0.256 | ||
| Rate (µmol m-2 hr-1) | -21.6 | -38.4 | ||
| NOx- - Light | Time (hr) | Core A | Core B | Control |
| 3.97 | 3.19 | 2.64 | 4.19 | |
| 4.88 | 3.06 | 2.59 | 4.06 | |
| 5.88 | 3.18 | 2.41 | 4.02 | |
| 6.88 | 2.95 | 2.35 | 4.2 | |
| R2 | 0.934 | 0.909 | 0.9 | |
| Slope (µmol L-1 hr-1) | -0.078 | -0.103 | 0 | |
| Corrected Slope (µmol L-1 hr-1) | -0.078 | -0.103 | ||
| Rate (µmol m-2 hr-1) | -12 | -15.5 | ||
| Core Surface Area (m2) | 0.003165 | 0.003165 | ||
| Core Volume (L) | 0.4874 | 0.4747 |
Table 2. Time course data for NH4+ and NOx- from the same sediment cores used for Table 1. The time course regression R2 values are significant for values > 0.9025 (P < 0.05). Slopes are determined by linear regression and corrected slopes are determined by subtracting the rate of change of the water column only blank. Positive rates are net fluxes out of the sediment, negative rates indicate flux into the sediment. All concentrations for NH4+ and NO2- are µmol L-1.
Discussion
The technique described here has been applied to numerous kinds of aquatic systems, both shallow and deep, and we have found it to work well in most circumstances. This approach was adapted from approaches used by colleagues and presented in the literature; it is optimized for the measurement of denitrification via membrane inlet mass spectrometry. One of the strengths of this approach is the ability to handle a large number of cores simultaneously. Replicating each site with duplicate or triplicate cores increases the confidence in measurements, though an alternative approach is to maximize sites with less replication, under those circumstances the average value for an environmental segment may be more representative of the variability in nature. For elucidating seasonal differences, a measurement time series at a fewer number of sites may be a useful strategy.
In this protocol, there are several critical steps. Paramount to making successful measurements is the collection of cores with an intact sediment-water interface. Although rejecting cores that do not meet this criterion in the field can be tiring, poor cores will lead to poor accuracy and precision. Keeping aerobic cores aerated and close to the original collection temperature will minimize artifacts and maintain healthy, intact microbial and metazoan populations. Finally, for O2 and N2 samples, the addition of mercuric chloride preservative is critical. We have observed that improper preservation of gas samples, including excessive heating and cooling of the vials, can compromise these flux measurements. Other laboratories have successfully employed 7.0 M ZnCl2 as a less toxic preservative that has lower waste disposal costs; for a 7 ml sample a 30 μl addition is appropriate.
The precise and accurate analysis of the ratio of N2 and Ar is key to the determination of the N2 fluxes. Observed N2:Ar ratios change as a function of the oxygen concentration leading some investigators to advocate oxygen removal prior to analysis, generally using heated copper28. The instrumentation used in our laboratory was used to determine the effect of oxygen on N2:Ar ratios23 and the effect was found to be very small, < 0.03% for modest oxygen depletion. Differences in the approach to assessing the oxygen "effect" appear to lead to different conclusions by different investigators23,28,29. A large oxygen effect on N2:Ar ratios would lead to erroneously high rates of N2-N efflux; in our experience, we have many observations of negligible N2-N efflux under high rate of oxygen depletion. In labs in which the oxygen effect on N2:Ar ratios appear large, a useful alternative is the independent measurement of oxygen concentrations using electrodes or optodes and oxygen removal from the mass spectrometric analysis using inline heated Cu.
Troubleshooting this technique is possible only upon examination of the sediment flux data. Key factors to consider when regressions are poor are whether stirring was continuous, samples were collected and preserved correctly, and whether time courses were too short to allow estimation of low rates. The length of experiments generally is set by the oxygen time course, with low rates of metabolism requiring longer incubations to increase the signal to noise ratio embedded in the time course regressions. High rates of oxygen production that yield O2 bubbles make gas fluxes difficult, but solute fluxes may be unaffected.
It is necessary to understand the limitations of this approach. The small cores cover 0.3% of a square meter and the larger cores cover 0.6%. In sites with substantial heterogeneity at the meter scale, heterogeneous distributions of animals or plants may suggest that one or two cores may not be a sufficient representation. There are also some environments that present measurement difficulties. For the measurement of denitrification, the presence of methane or oxygen bubbles may invalidate the technique, with N2:Ar ratios influenced by differential incorporation of gases into the bubbles. In sediment colonized by benthic microalgae, the formation of oxygen bubbles results in a preferential stripping of N2 relative to Ar, and decrease in the N2:Ar ratio. In general, we cannot measure denitrification at the point where bubbles form. Anaerobic environments pose different challenges, and aeration of cores changes the redox dynamics at the sediment-water interface. We seal up cores with stirring tops immediately after collection and start the fluxes without replacing the water column completely30. Our experiments with illuminated sediments typically have saturating or near-saturating levels of illumination31, and thus maximize the effect of benthic microalgae.
Sediment-water exchange measurements are a measurement of the net flux of materials across the sediment-water interface. However, these measurements alone often cannot identify the mechanisms controlling these interfacial exchanges. If the research question involves understanding mechanisms, other information on organic matter reactivity, terminal electron acceptor zonation, bioirrigation and bioturbation, and photosynthetic organisms may be necessary. Modeling efforts7 may require determination of pore water chemistry, direct measures of organic matter reactivity32, enumeration of animal populations, sediment bio-irrigation, sediment accretion, or experimental manipulations of redox or overlying water chemistry13. In our studies, good sediment-water exchange data is a key component of understanding the chemistry of aquatic sediments, and in conjunction with other measurements, identifies the role of sediment recycling processes in aquatic biogeochemical cycles.
With care regarding sediment handling, temperature control, and water column mixing, core incubations are a useful approach to the estimation of the exchange of solutes and gases at the sediment-water interface. However, the techniques used here may need modification for some environments and for difficult logistics, such as extended time periods before incubation. Thus far, we have successfully applied this incubation approach to estuarine, coastal, wetland, lake, reservoir, river and retention pond environments with minimal modification.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors developed this approach using our observations of work carried out by Walter Boynton and Pete Sampou and cooperative work on denitrification with Todd Kana at the University of Maryland Center for Environmental Science. Development of our denitrification approaches would not have been possible without the support of the Maryland Sea Grant Program and the National Science Foundation. The representative data used here were collected with funding from Maryland Sea Grant (R/AQ-5c) and writing efforts were supported by Maryland Sea Grant (R/SV-2), the NOAA Chesapeake Bay Office (NA13NMF4570210), the Oyster Recovery Partnership, the National Science Foundation (OCE1427019), Exelon Corporation, and the Maryland Environmental Service/Maryland Port Administration.
References
- Einsele W. Ueber die Beziehungen des Eisenkreislaufes zum Phosphatkreislauf im Eutrophen See. Arch.Hydrobiol. 1936;29:664–686. [Google Scholar]
- Mortimer CH. The exchange of dissolved substances between mud and lake water. J Ecol. 1941;29:280–329. [Google Scholar]
- Cowan JLW, Boynton WR. Sediment-water oxygen and nutrient exchanges along the longitudinal axis of Chesapeake Bay: Seasonal patterns, controlling factors and ecological significance. Estuaries. 1996;19:562–580. [Google Scholar]
- Fisher TR, Carlson PR, Barber RT. Sediment nutrient regeneration in three North Carolina estuaries. Estuar. Coast. Shelf S.e. 1982;14:101–116. [Google Scholar]
- McGlathery KJ, Sundback K, Anderson IC. Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Mar. Ecol-Prog Ser. 2007;348:1–18. [Google Scholar]
- Eyre BD, Ferguson AJP. Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae- and macroalgae-dominated warm-temperate Australian lagoons. Mar. Ecol-Prog Ser. 2002;229:43–59. [Google Scholar]
- DiToro DM. Sediment Flux Modeling. Wiley-Interscience; 2001. [Google Scholar]
- Testa JM, et al. Sediment flux modeling: Simulating nitrogen, phosphorus, and silica cycles. Estuar. Coast. Shelf S. 2013;131:245–263. [Google Scholar]
- Kana TM, Cornwell JC, Zhong LJ. Determination of denitrification in the Chesapeake Bay from measurements of N-2 accumulation in bottom water. Estuar. Coasts. 2006;29:222–231. [Google Scholar]
- Hammond DE, Cummins KM, McManus J, Berelson WM, Smith G, Spagnoli F. Methods for measuring benthic nutrient flux on the California Margin: Comparing shipboard core incubations to in situ lander results. Limnol. Oceanog Methods. 2004;2:146–159. [Google Scholar]
- Miller-Way T, Boland GS, Rowe GT, Twilley RR. Sediment oxygen consumption and benthic nutrient fluxes on the Louisiana continental shelf: a methodological comparison. Estuaries. 1994;17:809–815. [Google Scholar]
- Kana TM, et al. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal. Chem. 1994;66:4166–4170. [Google Scholar]
- Gao Y, Cornwell JC, Stoecker DK, Owens MS. Influence of cyanobacteria blooms on sediment biogeochemistry and nutrient fluxes. Limnol. Oceanogr. 2014;59:959–971. [Google Scholar]
- Hopfensperger KN, Kaushal SS, Findlay SEG, Cornwell JC. Influence of Plant Communities on Denitrification in a Tidal Freshwater Marsh of the Potomac River, United States. J. Environ. Qual. 2009;38:618–626. doi: 10.2134/jeq2008.0220. [DOI] [PubMed] [Google Scholar]
- Cornwell JC, Kemp WM, Kana TM. Denitrification in coastal ecosystems: environmental controls and aspects of spatial and temporal scale. Aquat. Ecol. 1999;33:41–54. [Google Scholar]
- LaMontagne MG, Valiela I. Denitrification measured by a direct N2 flux method in sediments of Waquoit Bay, MA. Biogeochemistry. 1995;31:63–83. [Google Scholar]
- Nielsen LP. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Ecol. 1992;86:357–362. [Google Scholar]
- Ferguson AJP, Eyre BD, Gay JM. Organic matter and benthic metabolism in euphotic sediments along shallow sub-tropical estuaries, northern New South Wales, Australia. Aq. Microb. Ecol. 2003;33:137–154. [Google Scholar]
- Coley TL. The effect of flow on the fluxes of oxygen, dinitrogen gas, nitrate and ammonium in diffusively controlled sediments using stirred experimental chambers. MEES Program, University of Maryland; 2003. M.S. Thesis. [Google Scholar]
- Owens MS. Nitrogen cycling and controls on denitrification in mesoahaline sediment of Chesapeake Bay. MEES Program, University of Maryland; 2009. M.S. Thesis. [Google Scholar]
- Sundback K, Jonsson B. Microphytobenthic productivity and biomass in sublittoral sediments of a stratified bay, southeastern Kattegat. J. Exp. Mar. Biol. Ecol. 1988;122:63–81. [Google Scholar]
- Petersen JE, Cornwell JC, Kemp WM. Implicit scaling in experimental enclosed aquatic ecosystems. Oikos. 1999;85:3–18. [Google Scholar]
- Kana TM, Weiss DL. Comment on "Comparison of isotope pairing and N-2 : Ar methods for measuring sediment denitrification" by B. D. Eyre, S. Rysgaard, T. Daisgaard, and P. Bondo Christensen. 2002. Estuaries 25: 1077-1087. Estuaries. 2004;27:173–176. [Google Scholar]
- Parsons TR, Maita Y, Lalli CM. A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press; 1984. [Google Scholar]
- Doane TA, Horwath WR. Spectrophotometric determination of nitrate with a single reagent. Analytical Letters. 2003;36:2713–2722. [Google Scholar]
- Testa JM, et al. Modeling the impact of floating oyster (Crassostrea virginica) aquaculture on sediment-water nutrient and oxygen fluxes. Aquac. Environ. Interact. 2015;7:205–222. [Google Scholar]
- Hamme RC, Emerson SR. The solubility of neon, nitrogen and argon in distilled water and seawater. Deep-Sea Res. Part I-Oceanogr. Res. Papers. 2004;51:1517–1528. [Google Scholar]
- Chong LS, Prokopenko MG, Berelson WM, Townsend-Small A, McManus J. Nitrogen cycling within suboxic and anoxic sediments from the continental margin of Western North America. Marine Chemistry. 2012;128:13–25. [Google Scholar]
- Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB. Comparison of isotope pairing and N-2 : Ar methods for measuring sediment-denitrification-assumptions, modifications, and implications. Estuaries. 2002;25:1077–1087. [Google Scholar]
- Lee DY, et al. The Effects of Oxygen Transition on Community Respiration and Potential Chemoautotrophic Production in a Seasonally Stratified Anoxic Estuary. Estuar.Coasts. 2015;38:104–117. [Google Scholar]
- MacIntyre HL, Geider RJ, Miller DC. Microphytobenthos: The ecological role of the "secret garden" of unvegetated, shallow-water marine habitats .1. Distribution, abundance and primary production. Estuaries. 1996;19:186–201. [Google Scholar]
- Aller RC, Mackin JE. Open-incubation, diffusion methods for measuring solute reaction rates in sediments. J. Mar. Res. 1989;47:411–440. [Google Scholar]


