Abstract
Backgrounds and Aims In cultivated rice, phosphorus (P) in grains originates from two possible sources, namely exogenous (post-flowering root P uptake from soil) or endogenous (P remobilization from vegetative parts) sources. This study investigates P partitioning and remobilization in rice plants throughout grain filling to resolve contributions of P sources to grain P levels in rice.
Methods Rice plants (Oryza sativa ‘IR64’) were grown under P-sufficient or P-deficient conditions in the field and in hydroponics. Post-flowering uptake, partitioning and re-partitioning of P was investigated by quantifying tissue P levels over the grain filling period in the field conditions, and by employing 33P isotope as a tracer in the hydroponic study.
Key Results Post-flowering P uptake represented 40–70 % of the aerial plant P accumulation at maturity. The panicle was the main P sink in all studies, and the amount of P potentially remobilized from vegetative tissues to the panicle during grain filling was around 20 % of the total aerial P measured at flowering. In hydroponics, less than 20 % of the P tracer taken up at 9 d after flowering (DAF) was found in the above-ground tissues at 14 DAF and half of it was partitioned to the panicle in both P treatments.
Conclusions The results demonstrate that P uptake from the soil during grain filling is a critical contributor to the P content in grains in irrigated rice. The P tracer study suggests that the mechanism of P loading into grains involves little direct transfer of post-flowering P uptake to the grain but rather substantial mobilization of P that was previously taken up and stored in vegetative tissues.
Keywords: P loading into grains, post-flowering P uptake, P partitioning, 33P tracer, P fluxes, P remobilization
INTRODUCTION
In annual plants, the senescence period involves the intensive transfer of carbon and other nutrients including phosphorus (P) from vegetative organs to developing seeds. In natural ecosystems, the storage of large quantities of P in seeds offers the emerging seedlings a competitive advantage because many soils are inherently low in bioavailable P (White and Veneklaas, 2012). In agricultural systems soil P levels are typically higher than in native ecosystems, and in high-input farming systems soil P levels may be at or above the optimum levels required for productive crop growth so responses to P fertiliser may be limited (Scanlan et al., 2015). There is growing evidence that maintaining high seed P content in agricultural crops may not be as critical as earlier reports suggested, particularly in soils with high P fertility or soils supplemented with P fertiliser (Rose et al., 2012; Pariasca-Tanaka et al., 2015). However, the process of active loading of P into grains appears to have been conserved in most major crop species throughout domestication and subsequent breeding programmes, because the P harvest index (the proportion of above-ground P located in grain tissue; PHI) of staple cereal and oilseed crops is generally well above the biomass (carbon) harvest index (HI; Batten and Khan, 1987; Rose et al., 2007; Bi et al., 2013). The consequence of this in an agricultural sense is that an estimated equivalent of 80 % of the P applied as fertiliser across the globe is removed from fields at harvest each year, mostly in the grains of staple cereal crops (Lott et al., 2000).
Reducing the amount of P accumulated into the grains would minimize the amount of P that is unnecessarily exported from the field at harvest, which would consequently improve the P use efficiency of cropping systems (Richardson et al., 2011; Rose et al., 2013; Vandamme et al., 2016a). At maturity, P is principally found in cereal grains in the form of phytic acid, an anti-nutrient that cannot be digested by humans and other monogastric animals, which results in high loads of P in animal waste (Lott et al., 2000; Raboy, 2009). High P loads in animal manures are a major contributor to the eutrophication of rivers and lakes across the globe (Raboy, 2007). Thus, a reduction in P content in cereal grains may also have beneficial consequences for water quality, and reduce the impact of agriculture on the environment.
Rice (Oryza sativa) is a major staple cereal crop that provides calories for more than a third of the world’s population, and estimates suggest that the global rice harvest removes around $11 billion worth of P from fields each year (Rose et al., 2010). Phosphorus is rapidly loaded into grains of both indica and japonica rice genotypes between 6 and 15 d after flowering (Ogawa et al., 1979; Wang et al., 2016), although the physiological and genetic mechanisms governing P transfer into the grain remain unclear (Wang et al., 2016). Ultimately, P residing in grains at maturity comes from two possible sources: exogenous (post-flowering root P uptake from soil) and/or endogenous (P remobilization from vegetative parts of the plant) sources.
Previous studies under high P conditions have shown extensive post-flowering P uptake in aerobically grown rice amounting to around 30 % of total plant P at maturity (Cheng et al., 2003; Chen et al., 2008; Rose et al., 2010); however, the fate of this P is not known. In theory, P taken up by rice during grain filling could be directly loaded into nucellar epidermis and aleurone cells of the grain via the xylem, because unlike in other cereals such as wheat, the xylem is not discontinuous at the base of the grain (Krishnan and Dayanandan, 2003). However, a degree of perenniality exists in many rice cultivars and the late tillers that continue to emerge and grow during grain filling may be a strong sink for P. A reduction in leaf and stem P concentrations between flowering and maturity suggests that remobilization of P from vegetative tissues to the developing grain is also an important process in rice (Rose et al., 2010), but the exact contributions of exogenous versus endogenous P sources to P loading in rice grains, and the influence of soil P fertility on these contributions, remains unclear.
The overall objectives of the study were therefore to determine the extent to which post-flowering P uptake occurs in lowland rice, to establish its contribution to grain P, and to improve our knowledge on the possible mechanisms responsible for the P loading into the grains. Isotopic tracers allow tracking of the uptake and movement of nutrients by plant roots. For example, Jiang et al. (2008) reported continued uptake of 65Zn by roots of anaerobic (upland) rice cultivars beyond 15 d after flowering (DAF) in hydroponic culture and concluded that post-flowering uptake of Zn was substantial and an important contributor to grain Zn content. Using 70Zn in hydroponic rice cultures, Stomph et al. (2014) were able to propose a model describing the allocation and re-translocation of Zn throughout the cereal cycle. However, results from such hydroponic studies should be interpreted with a degree of caution. In the case of Brassica napus, we observed negligible uptake of P in the field during the later stages of pod filling (Rose et al., 2009), but in sand culture where soluble P was supplied as nutrient solution, P uptake continued until maturity in the same cultivar (Rose et al., 2008). It is therefore critical to ascertain whether, and to what extent, nutrient uptake occurs during grain filling under field conditions before making conclusions based on hydroponic experiments.
Using a 33P isotope tracer, we studied the uptake, distribution and redistribution of P during grain filling in rice plants grown in optimum and low P hydroponic environments. We also measured the evolution of total P partitioning within the plants by measuring P content of various organs at different times during grain filling. To test the hypothesis that the results obtained in hydroponic studies are consistent with the observations made in the field and are therefore relevant, we investigated the post-flowering P uptake and partitioning in field-grown rice under P-deficient and P-fertilized conditions in the tropics (Los Baños, the Philippines).
MATERIALS AND METHODS
Experiment 1: Accumulation and partitioning of P under P-deficient and P-sufficient conditions in the field
To determine whether hydroponic experiments involving P uptake and distribution during grain filling have any relevance to rice cultivated in practice, we investigated the post-flowering uptake and partitioning of P in field-grown rice.
Field site.
The experiment was undertaken at the P demonstration plots at the International Rice Research Institute (IRRI), Los Baños, The Philippines, from January to April 2013. The experimental design consisted of three P-fertilized and three P-deficient replicate plots of 5 ×10 m each and containing soil in bunkers transported from a P-deficient site at Pangil, Laguna, The Philippines. Briefly, soil in the P-deficient plots had a pH (1:1 soil–water) of 7·7, % carbon (C) 2·34, % nitrogen (N) 0·175, Bray P 1·6 mg kg−1, exchangeable potassium (K) (meq 100 g−1) 0·44 and available zinc (Zn) 1·9 mg kg−1 while the P-fertilized soil had a pH (1:1 soil–water) of 7·3, % C 2·33, % N 0·192, Bray P 8·5 mg kg−1, exchangeable K (meq 100 g−1) 0·54 and available Zn 0·9 mg kg−1. On 4 January 2013, 3 d prior to transplanting, basal fertiliser was broadcast onto the plots at the following rates (kg ha−1): 90 N, 26 P (P-fertilized plots only), 33 K and 2 Zn.
Plant cultivation.
Rice (Oryza sativa L. ‘IR64’) seeds were sown into trays containing commercial nursery seedling mix in a glasshouse at IRRI. At 21 d after sowing (DAS) – 7 January 2013 – seedlings were transplanted by hand into the field plots in rows 0·2 m apart and a hill spacing of 0·2 m within rows (i.e. 25 hills m−2), with one plant per hill. A further 90 kg ha−1 N fertiliser as urea was applied (broadcast) in three splits of 30 kg ha−1 N at 20, 40 and 60 d after transplanting. Plants were cultivated under standard, fully flooded conditions, with weeds controlled by hand and snails controlled by applications of appropriate chemical treatments when required.
Measurements.
Flowering occurred on 18 March and 28 March for P-fertilized and P-deficient plots, respectively, and plants reached physiological maturity at 28 DAF regardless of P treatment. At sampling, three plants per replicate plot were randomly harvested (excluding border plants) at 0, 5, 8–10, 12–14, 17–19, 22–23 and 28 DAF by severing plant shoots about 10 mm above the soil surface. The three plants were pooled before undertaking any measurement, so each measurement reported represents the average value of three plants. The range of days (e.g. 8–10 DAF) reflects the range of maturity dates among plants of a same plot (differing by up to 2 d). For simplicity, the days of plant harvest are herein referred to as 0, 5, 9, 13, 18, 23 and 28 DAF.
Plant material was dried in an oven at 60 °C for 5 d and then separated into grain (husk plus caryopsis), stem (stem plus leaf sheath), flag leaf (blade), second and third leaves (blade), older leaves (blade) and late tillers (tillers that had not yet reached flowering). Any grain from tillers that may not have been physiologically mature at the time of harvest was recorded in the grain yield, as late tillers were defined as those that had not yet flowered. A 0·2-g subsample of finely ground tissue was digested with nitric acid in a MARS microwave oven (CEM Corp., Matthews, NC, USA) and concentration of P in the digest solutions was quantified using inductively coupled plasma optical emission spectroscopy (ICP-OES 4300D, Perkin Elmer, Waltham, MA, USA).
Experiment 2: 33P tracer hydroponic experiment
To investigate the partitioning and remobilization of P taken up by roots during grain filling, a hydroponic experiment was conducted in a naturally lit, temperature-controlled glasshouse at Southern Cross University (Lismore, NSW, Australia) between August and December 2014. During the growth period, maximum daily temperatures ranged from 28 to 32 °C and minimum temperatures from 22 to 24 °C.
Plant growth conditions and experimental design.
Seeds of rice (‘IR64’) were rinsed with 1 % bleach solution and distilled water before being germinated in Petri dishes in an incubator at 30 °C. After 3 d, germinated seeds were transferred to the glasshouse onto floating mesh above a solution containing 0·1 mm calcium (Ca) and 36 μm iron (Fe). After 2 weeks, healthy seedlings were transferred to 5-litre pots filled with full strength (minus P) Yoshida nutrient solution (Yoshida et al., 1976), with two seedlings per pot supported by foam material. A preliminary experiment presented in Vandamme et al. (2016b) allowed us to identify precisely the minimum amount of P to be imposed to the optimum P plants (Opt-P) to reach maximum yield (that is 1·2 mg P per pot d−1). The amount of P to be applied to low P plants (Low-P) nutrient solution to obtain deficient plants (consistent reduction in biomass and yield compared to Opt-P plants), was calculated from the same previous study and set to 0·3mg P per pot day−1. Phosphorus was applied twice per week on the same days and times by pipetting the appropriate volume of stock P solution (150 mg P L−1 as K2HPO4) into each 5-litre pot. Nutrient solutions were changed every week and pH was monitored on a daily basis and adjusted to 5·5 when necessary.
The experiment was designed to allow three and four replicate pots (RP) in Low-P and Opt-P, respectively, to be harvested at five time points (TP) during the plant cycle to undertake measurements. Consequently, a total of 30 plants (2 plants × 3 RP × 5 TP) were grown under Low-P and 40 plants (2 plants × 4 RP × 5 TP) were grown under Opt-P conditions.
TP were 0 DAF (Flowering), 9 DAF, 10 DAF, 14 DAF and 30 DAF (Maturity). Biomass and P content were measured at each TP in both P treatments. As 33P tracer was spiked at 9 DAF in both P treatments, 33P tracer content was also measured at TP subsequent to spiking (i.e. 9 DAF-3 h after spiking, 10 DAF-24 h after spiking, 14 DAF-120 h after spiking and also at 30 DAF-15 d after spiking in Opt-P plants only). Details on the 33P tracer measurements are described in the following section.
33P tracer study.
Previous studies have indicated that the bulk of P loading into grains of rice cultivar ‘IR64’ on an individual tiller occurs between 6 and 15 DAF (Wang et al., 2016). Thus, at 9 DAF 33P supplied as orthophosphoric acid (Perkin Elmer) was spiked into the nutrient solution of nine Low-P pots (3 RP × 3 STP) and 16 Opt-P pots (4 RP × 4 TP), at a rate of 500 MBq per pot for Low-P pots and 1500 MBq per pot for Opt-P. Pots were selected for uniformity and appropriate time of flowering. After the 33P spike was applied at 9 DAF, plants were retained in the spiked solution for 3 h. After 3 h, we harvested three and four RP of the Low-P and Opt-P treatment, respectively, while plants from the remaining RP were removed from the 33P-containing nutrient solution and the roots systems were rinsed twice with deionized water to remove any nutrient solution adhering to roots. The 33P-containing nutrient solution and deionized water used for rinsing were sampled in triplicate to quantify 33P uptake per pot. The plants of the six remaining replicate pots per P treatment were then returned to their initial pots containing full-strength Yoshida nutrient solution with the appropriate P treatment (0·3 mg P per pot d−1 or 1·2 mg P per pot d−1) for later harvest at 24 h and 120 h after 33P spiking (i.e. 10 and 14 DAF).
At each sampling, and for each replicate pot, plants were separated into six organs including panicles, flag leaves (blade), leaves (blade), dead leaves (blade and sheath), stem (stem plus leaf sheath) and roots. At maturity, unproductive or late tillers (LT) were separated and counted. Organs were dried in an air-forced oven at 70 °C for a minimum or 4 d before panicles were manually separated into rachis and grains before grains were manually de-husked. Tissues were then ground to a fine powder for subsequent analyses.
Between 400 mg and 1 g of ground sample for each organ was acid digested in a 7-mL mix of nitric and sulphuric acid (3 : 1) using a VELP DK10 series block digester. The concentration of (unlabelled) P in the digest solutions was quantified as per samples from the field study.
The amount of 33P in the digest solutions were quantified using a 1450 MicroBeta TriLux counter (Perkin Elmer) with scintillation cocktail Ultima Gold AB and a 500 μL/3·5 mL acid sample/scintillation fluid ratio. Each 33P count was run in triplicate with 33P standards made up in acid digest blanks, which allow correlation of sample counts and activities (R2 > 0·99).
From the activity measured in each digest, the total activity per organ was calculated as:
where Aorgan is the total activity in the organ (in Bq), Adigest the activity in the organ digest (in Bq g−1) and DWorgan is the organ dry weight per pot (in g).
For each replicate, the total 33P activity (At) in Bq per pot was calculated by summing the total activities of each individual organ (Aorgan).
The percentage of 33P accumulated in each organ was calculated as:
The fraction of 33P tracer translocated from roots to aerial parts (FR→S) was defined as the ratio of the 33P activity measured above ground by the total 33P tracer in the plant (At) and calculated as:
Statistical analyses
Statistical analyses were undertaken with R version 3.2.0 (R Core Team, 2012) using the ‘agricolae’ library. Two-way analyses of variance were done fitting in rice tissue biomass or P content and time point, but also fitting in the percentage of 33P tracer translocated above ground and P treatment. Differences between time point mean values for each tissue and between treatments for the percentage of 33P translocated above ground were tested using Duncan’s multiple range tests with a probability level of 0·05.
RESULTS
Experiment 1 – field experiment investigating uptake and partitioning of P during grain filling
Plant growth and grain yields at maturity.
Plants grown in the absence of P fertiliser showed typical symptoms of P deficiency throughout vegetative growth including bronzing of older leaves, reduced tillering (data not recorded) and biomass production (Table 1). Flowering was delayed by approx. 10 d when no P fertiliser was applied, but the grain ripening period was the same (28 d) as for P-fertilized plots. At flowering, plants in the low P plots had less than half the biomass of plants grown in the P-fertilized plots (around 13 vs. 33 g per plant) (Fig. 1A, B) although at maturity P-deficient plants suffered only around a 30 % biomass reduction compared to P-fertilized plants (Table 1). This was due to the fact that P-deficient plants accumulated around 70 % of their final biomass yields during the grain filling period, compared to around 50 % accumulated during this period in P-fertilized plants (Fig. 1A, B). In P-fertilized plants, vegetative biomass remained relatively constant during grain filling and biomass increase was mainly due to the increase in grain biomass. In P-deficient plants, the vegetative biomass – particularly in leaves and flag leaves – continued increasing in the first 10 d of grain filling as leaves were progressively emerging from later tillers (Fig. 1A, B). Ultimately, grain yields of P-fertilized plants were almost double those of P-deficient plants (42 vs. 24 g per plant; Table 1).
Table 1.
Summary of the four experiments run in the field and in the glasshouse: P treatments and yield components at maturity
| Growing conditions | Name | Applied P | Above-ground P content (mg per plant) | Grain DW (g per plant) | Shoot DW (g per plant) | HI | PHI | Productive tillers (n) | Late tillers (n) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Field | P-fertilized | 26 kg ha−1 | 122·2 ± 10·3 | 41·8 ± 4·8 | 25·7 ± 0·7 | 0·62 | 0·90 | NA | NA | NA |
| P-deficient | No application | 42·6 ± 14·9 | 23·9 ± 5·9 | 18·2 ± 2·9 | 0·56 | 0·85 | NA | NA | NA | |
| Hydroponic | Opt-P | 89·7 mg per plant d−1 | 54·6 ± 7·3 | 28·4 ± 3·1 | 38·9 ± 3·7 | 0·42 | 0·65 | 16·3 ± 2·4 | 2·4 ± 1·5 | <3 % |
| Low-P | 16·4 mg per plant d−1 | 13·6 ± 0·4 | 7·9 ± 0·85 | 10·1 ± 0·6 | 0·44 | 0·65 | 5·5 ± 0·5 | 1·3 ± 0·3 | <3 % | |
Mean values of three to four replicates are given. Standard deviations are presented in italic. NA, not applicable.
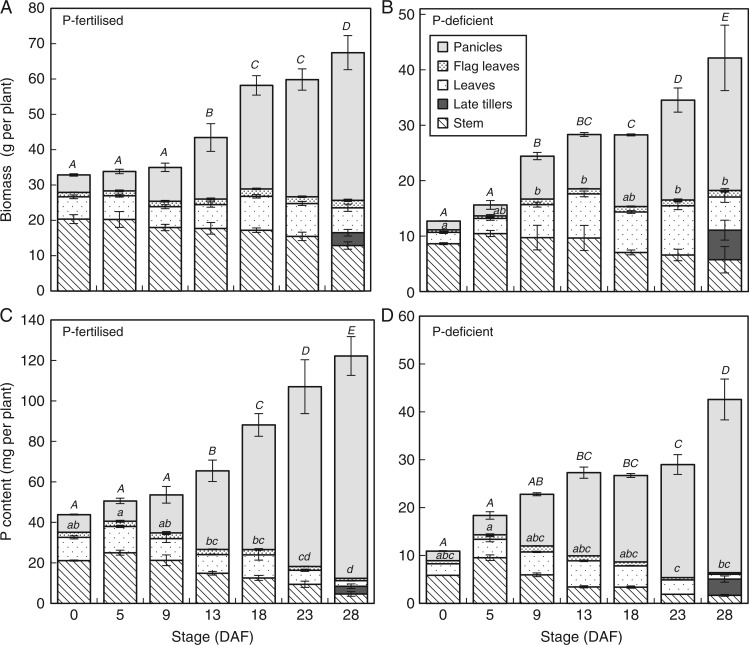
Fig. 1.
Above-ground biomass (A, B) and P content (C, D) in five different rice tissues of P-fertilized (A, C) and P-deficient (B, D) plants during grain filling of the field study. Error bars depict standard deviation of the mean of three replicates plants per time point. The letters present the results of Duncan’s multiple range tests (α = 0·05, n = 3) done for ‘Panicles’ (capital letters) and for the vegetative above-ground compartment (lowercase letters) using the stage (DAF) as factor. No letter indicates no significant difference between time points.
Tissue P concentrations, P accumulation and P partitioning during grain filling.
At flowering, P-deficient plants had accumulated around a quarter of the P in aerial plant tissue of plants in the P-fertilized plots (around 10 vs. 40 mg per plant) (Fig. 1C, D). Accumulation of P continued until maturity under both P treatments, with approx. 70 % of total plant P accumulated during the grain filling phase in both P-fertilized and P-deficient plants (Fig. 1C, D). The proportion of plant P located in vegetative tissues (especially in leaves) declined throughout grain filling in both P treatments while the proportion of total plant P located in grains continued to increase until maturity (Fig. 1C, D). Ultimately, 85 and 90 % of above-ground P was located in the grains at maturity in P-deficient and P-fertilized plants, respectively.
The concentrations of P in above-ground vegetative tissues (flag leaves, leaves 2 and 3, older leaves and stems) of P-deficient plants were lower than those of P-fertilized plants over the course of grain filling, but followed a similar pattern (Fig. 2). Phosphorus concentrations in above-ground vegetative tissues were in the order of flag leaves > 2nd and 3rd leaves > older leaves > stems, and this trend was maintained as P concentrations declined over the course of grain filling in both fertilized and non-fertilized treatments (Fig. 2B, C). In P-fertilized plants, P concentrations in flag leaves and 2nd and 3rd leaves declined gradually from flowering to 13 DAF, but then declined sharply until maturity. Phosphorus concentrations in older leaves and stems of P-fertilized plants were relatively constant until 9 DAF but declined progressively from 9 DAF until maturity (Fig. 2B). At maturity, flag leaves still had P concentrations above 0·5 mg g−1 in P-fertilized plants while P concentrations of other leaves and stem tissues declined to 0·4 mg g−1 or below (Fig. 2B).
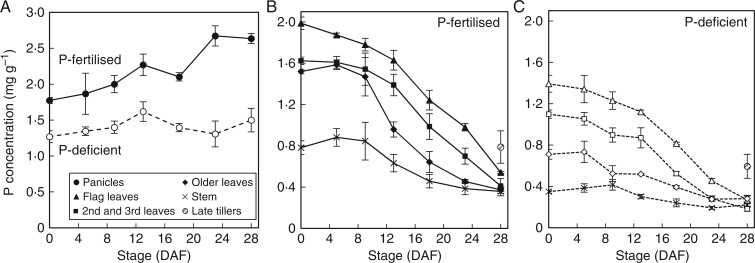
Fig. 2.
Average P concentration of rice panicles (A) and vegetative tissues (B, C) during grain filling in P-fertilized plants (closed symbols and solid lines) and P-deficient plants (open symbols and dotted lines) of the field experiment. Error bars depict standard error of the mean of three replicates plants per time point.
While the P concentrations in leaf and stem tissues were lower in P-deficient plants than in P-fertilized plants at flowering and maturity, similar trends in the declines in P concentrations throughout the course of grain filling were observed. At maturity, P concentrations in stem and leaf tissues were similar (<0·25 mg g−1). Phosphorus concentration in late tillers was also higher in P-fertilized plants (0·79 mg g−1) than in P-deficient plants (0·59 mg g−1) at maturity.
A net flux out of the above-ground vegetative compartment of 28·0 and 5·6 mg P per plant was observed between flowering and maturity in P-fertilized and P-deficient plants, respectively.
Experiment 2: Hydroponic experiment investigating 33P uptake and distribution during grain filling
P accumulation, biomass production and grain yields at maturity.
Plants that received optimum P (Opt-P) supply had around four-fold the P accumulation and the biomass production of deficient (Low-P) plants (Fig. 3). Grain yields were also nearly four-fold higher in Opt-P plants (Table 1), as was the percentage of filled grains (data not presented). Opt-P plants had 16·3 tillers per plant compared to 5·5 tillers per plant in P-deficient plants, and also produced almost twice the number of late-forming tillers as the P-deficient plants (Table 1).
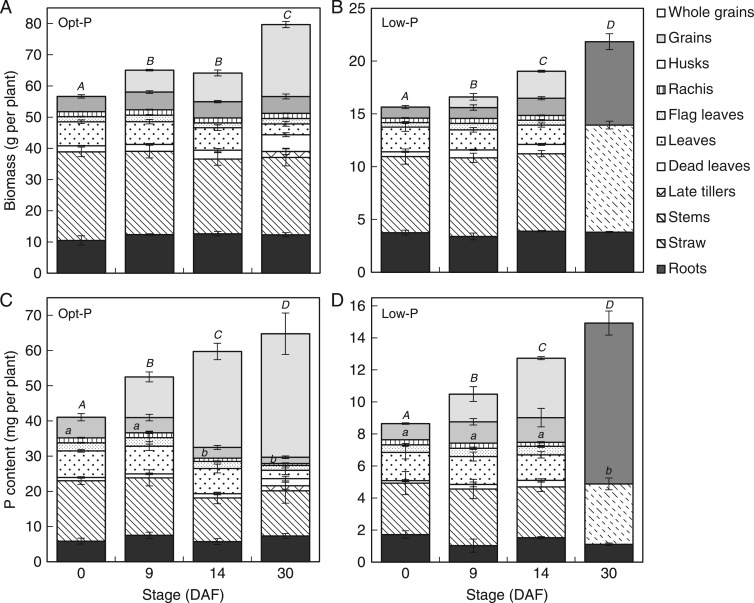
Fig. 3.
Average biomass (A, B) and P content (B, D) of rice tissues during grain filling in Opt-P (A, C) and Low-P (B, D) treatments of the hydroponic experiment. Error bars depict standard deviation of the mean of three and four replicates pots in Low-P and Opt-P, respectively. The letters present results of Duncan’s multiple range tests (α = 0·05, n = 3 for Low-P, n = 4 for Opt-P) done for ‘Whole grains’ (capital letters) and for the rest of the plant* (lowercase letters). No letter indicates no significant difference between time points. *Roots + all leaves + rachis.
Biomass and P accumulation during grain filling.
Biomass continued to accumulate from flowering to maturity in both Opt-P plants and Low-P plants, with over 25 % of total plant biomass accumulated in the post-flowering period under both P treatments (Fig. 3A, B). Aerial vegetative biomass (stem and leaves) did not vary significantly during grain filling and remained about 45–50 % of plant biomass at maturity under both P treatments. Root biomass also remained relatively stable throughout grain filling and comprised around 15 % of plant biomass at maturity in both P treatments (Fig. 3A, B). As expected during grain filling, the most significant biomass increase was observed in the grains under both treatments (Fig. 3A, B), with grain biomass accounting for around 35 % of total plant biomass and nearly 45 % of the aerial biomass at maturity under both P treatments (Table 1).
Plant P content also continued to accumulate during the grain filling period with around 35 and 40 % of the total plant P content accumulated during grain filling in Opt-P plants and P-deficient plants, respectively (Fig. 4C, D). While the P content of Opt-P plants plateaued from 14 DAF, the P content of deficient plants increased up to maturity.
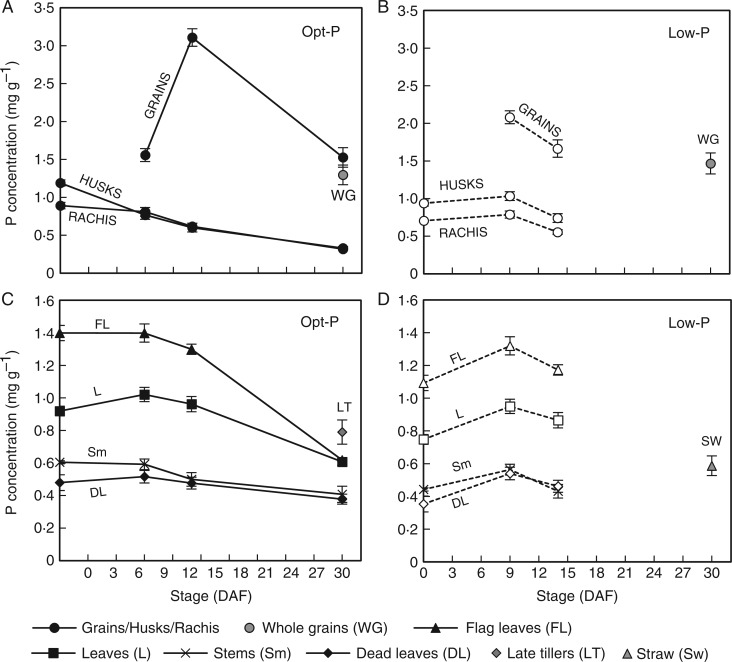
Fig. 4.
Average P concentrations of panicle tissues (A, B) and vegetative tissues (C, D) during grain filling in Opt-P (closed symbols and solid lines) and Low-P (open symbols and dotted lines) treatments of the hydroponic glasshouse experiment. Error bars depict standard deviation of the mean of three and four replicates pots in Low-P and Opt-P, respectively.
Tissue P concentrations and P partitioning during grain filling.
As expected, the amount of P and proportion of total plant P in the grains increased throughout grain filling such that nearly 70 % of the above-ground P was located in grains at maturity under both P treatments (Fig 3C, D). As was observed in the field experiment at flowering, leaf P concentrations were in the order flag leaf > other leaves > stems > dead leaves in both P treatments (Fig. 4B–D). The order was maintained throughout grain filling, but unlike in the field, a sharp decline in the P concentration only occurred after 14 DAF (Fig. 4C).
A significant decline in husk P concentration was observed from 0 DAF in the Opt-P treatment while the decline only started after 9 DAF in the Low-P treatment (Fig. 4A). In Opt-P, P concentration in grains greatly increased from 1·55 to 3·1 mg g−1 between 9 and 14 DAF and declined to 1·5 mg g−1 between 14 DAF and maturity, a consequence of the large increase in grain biomass between 14 DAF and maturity (dilution effect) (Fig. 4A). In Low-P, grain P concentration decreased between 9 and 14 DAF (small dilution effect) and probably plateaued afterwards until maturity (only whole grain P was measured but grain P concentration is generally only slightly higher than whole grain P). Ultimately, whole grain P concentration was slightly higher in Low-P plants (1·45 mg g−1) than in Opt-P plants (1·3 mg g−1) (Fig. 4A, B).
Hydroponic cultivation allowed us to harvest clean roots to measure P content. A small decrease in root P concentration was observed in Low-P plants throughout grain filling (from 0·46 to 0·29 mg P g−1), while no significant trend was observed in Opt-P plants (mean 0·55 mg P g−1). As a result, root P content did not follow any clear trend over grain filling in both P treatments (Fig 4C, D).
The variation of P concentrations in the above-ground vegetative tissues (stem + all leaves) throughout grain filling resulted in a net flux out of the above-ground vegetative compartment of 8·8 mg P per plant in Opt-P and 2·5 mg P per plant in Low-P plants (Fig. 3C, D). Interestingly, most of this P flux occurred after 9 DAF in Opt P and later after 14 DAF in Low-P plants.
33P partitioning and redistribution during grain filling.
Uptake of 33P tracer by plants deduced from the 33P readings of the nutrient solutions spiked after 3 h, in agreement with the total 33P activity measured in the plants at sampling with an acceptable error of 5–10 % (data not presented). According to the total activity measured in the plants at sampling, more than 50 % of the 33P tracer was absorbed by the plants 3 h following the spiking in Low-P and Opt-P treatments. Consequently, the amount of 33P tracer in each set of two plants per pot represented a minimum average of 5–15 Bq mg−1 and was therefore well above the detection limit.
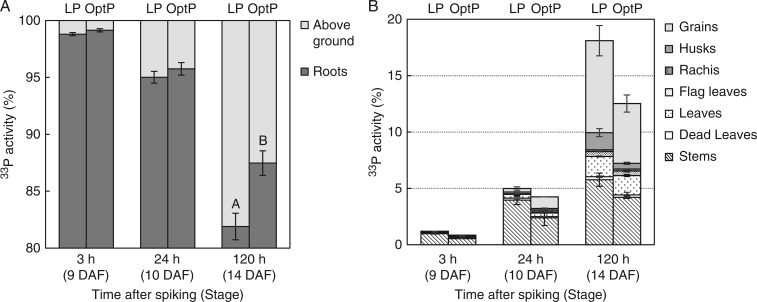
In both P treatments, nearly 100 % of the P tracer absorbed at 9 DAF was found in the roots 3 h after spiking 33P into the nutrient solution (Fig. 5A). At 24 h, Opt-P and Low-P plants had translocated almost 5 % of the P tracer to the aerial parts. At 120 h, a clear and significant difference was observed between P treatments as P-deficient plants had translocated 5·6 % more P tracer to the above-ground tissues (18·1 %) than Opt-P plants (12·5 %) (Fig. 5A). At maturity, nearly 35 % of 33P tracer absorbed at 9 DAF was found in the aerial tissue in Opt-P plants (Fig. 6A).
Fig. 5.
33Phosphorus tracer accumulation in rice plant tissues 3, 24 and 120 h after spiking in Opt-P and Low-P (LP) treatments of the hydroponic glasshouse experiment. General partitioning of 33P between roots and above-ground compartment (A), and 33P accumulation in different above-ground tissues (B). Error bars depict standard error of the mean of three or four replicates plants per time point. The letters present the results of Duncan’s multiple range tests (α = 0·05, n = 3 for Low-P, n = 4 for Opt-P) done for the vegetative above-ground compartment using the treatment as factor. No letter indicates no significant difference between treatments.
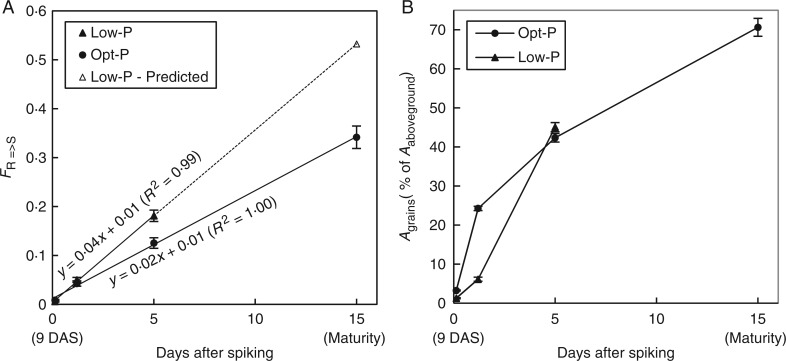
Fig. 6.
Fraction of 33P tracer translocated to the above-ground tissues of rice plants, FR→S (A), and percentage of the total-above ground 33P allocated to the grains (B) after spiking 33P in the hydroponic nutrient solution at 9 DAS in Low-P (triangle) and Opt-P (circle) plants of the glasshouse experiment. Error bars depict standard deviation of the mean of three and four replicates pots in Low-P and Opt-P, respectively.
In Opt-P plants, the 33P relative activities per above-ground organ at 24 h were in the order stem > grains > leaves > husk > late tillers > dead leaves > rachis > flag leaves, with more than 50 % of the above-ground 33P tracer found in the stem and nearly 30 % already found in the whole grains (husks/grains) (Fig. 5B). Between 24 and 120 h, the proportion of 33P tracer partitioned to the panicle (whole grain and rachis), leaves and flag leaves increased significantly, particularly in grains. At 120 h, the whole grains contained more than 45 % of the above-ground 33P tracer while another 45 % was found in the stem and leaves. Nearly 70 % of above-ground 33P tracer was found in the panicle at maturity (Fig. 6B).
In Low-P plants, 80 % of the above-ground P tracer was located in the stem at 24 h (Fig. 5A) while less than 10 % of the aerial 33P tracer was found in the grains, and another 10 % went to the other aerial parts in particular leaves (Fig. 5B). Low-P plants were therefore slower in transferring tracer-P to the grains than Opt-P plants, possibly because P-deficient leaves and stems were stronger sinks than Opt-P leaves and stems. Interestingly, between 24 and 120 h, the P tracer moved to the leaves and to the panicle in higher proportion than in Opt-P plants. At 120 h, nearly 55 % of the aerial 33P tracer was found in the whole grains, a higher proportion than in Opt-P plants, demonstrating that ultimately the transfer of 33P tracer to husk and grains was greater in P-deficient plants than in Opt-P plants (Fig. 5B). The proportion of 33P tracer contained in the husk at 120 h was greater in Low-P than in Opt-P (Fig. 5B).
In both treatments, we found a small proportion of 33P tracer absorbed at 9 DAF present in dead leaves at 24 and 120 h. In Opt-P, only 0·63 % was found in the late tillers at maturity.
DISCUSSION
Yield components and HIs in the field experiment
Plants accumulated 50 % of total biomass during the post-flowering period, which is typical of field-grown flooded rice (Dunn et al., 2014). Average grain yields with modern varieties in irrigated rice fields in the Philippines are typically around 6 t ha−1 per crop (Brennan and Malabayabas, 2011). The high grain yields observed in this study in the field (around 10 t ha−1) probably reflect the adequate nutrition, water and weed control to a large degree, but are also due in part to the fact that grains from late-forming tillers that may not have been completely physiologically mature at harvest, but were heavy enough to be threshed, were included in the grain yield measurements. As a result the HI was high (0·62) and the PHIs of 0·85 and 0·9 in P-deficient and P-fertilized plants, respectively, are higher than the typical range of 0·6–0·8 for modern rice varieties (Rose et al., 2010; Bi et al., 2013). These high values for HI and PHI in the field study therefore reflect patterns of carbon and nutrient partitioning to grains observed in annual crops that fully senesce at maturity, in contrast to the PHI values of 0·72 for Low-P and 0·65 for Opt-P in the hydroponic study, which reflect the typical degree of perenniality observed in many rice cultivars. In addition the drying of the soil that occurred in the field during grain filling in the field probably enhanced carbon partitioning to the grains (Yang and Zhang, 2010) compared to the hydroponic conditions, which would further exacerbate the observed differences in HI and PHI between the field and hydroponics study. Nonetheless, the P concentrations of 1·5 and 3·0 mg g−1 in grains of the P-deficient and P-fertilized plants, respectively, are within the range of typical concentrations observed in farmers’ fields in South East Asia (Dobermann and Fairhurst, 2000; Pariasca-Tanaka et al., 2015), and suggest that the crops were representative of P-sufficient and P-deficient crops grown in the region.
Late tillers at maturity represented less than 3 % of the aerial biomass and of the total P in both treatments, which indicates that eventually the majority of the tillers had developed sufficiently to contribute to grain yield at maturity.
Importance of post-flowering P uptake
Several studies have shown that post-flowering P accumulation is important in upland or aerobically grown rice (Cheng et al., 2003; Chen et al., 2008; Rose et al., 2010), amounting to around 30 % of total above-ground plant P accumulation at maturity. A recent study (Ye et al., 2014) indicated that post-flowering P uptake may be even more critical in field-grown flooded rice, representing nearly 70 % of above-ground plant P accumulation. We obtained similar results in the present study, with around 70 % of above-ground plant P accumulated during grain filling in both P-fertilized and P-deficient plants in the field (Fig. 1C, D). In hydroponic conditions, post-flowering P uptake was also significant, representing 40 and 50 % of the aerial P accumulation in Opt-P and Low-P plants, respectively (Fig. 3C, D). In all studies, post-flowering P uptake contributed substantially to plant P accumulation.
The two sources of grain P: major contribution of post-flowering P uptake and proportion of P remobilized from the vegetative tissues
The decline in the P content of vegetative organs throughout grain filling in both P-sufficient and P-deficient plants in the field and in hydroponics indicated that P was remobilized to developing grains. However, calculations of net fluxes out of vegetative tissue suggest that remobilized P represented only 20 % of the P found in the panicle at maturity in both P treatments in the field (Fig. 1C, D), and about 24 % in both treatments of the hydroponic study (Fig. 4C, D). This suggests that P uptake from the soil during grain filling is a critical contributor to the grains’ P content in rice, but also that the amount of P potentially remobilized from vegetative tissues to the panicle is around 20 % of the total P accumulated in the aerial plant compartment at flowering. Jiang et al. (2008) similarly reported that remobilization of Zn from aerial tissues was not as important for grain Zn content as uptake by roots during grain filling in flooded rice. In this regard, rice appears to be different to other small-grain cereal crops such as wheat and barley, which tend to rely heavily on P remobilized from vegetative tissues during grain filling, as post-flowering P uptake from the soil is typically low (Römer and Schilling, 1986; Hocking, 1994; Rose et al., 2007). This uptake pattern may be specific for rice grown in flooded soils where water and P availability are adequate through grain filling: in upland rice where the topsoil may dry out during grain filling, the contribution of post-flowering P uptake to the P accumulation in the plants is lower than in lowland rice. Thus, the proportion of P remobilized from vegetative tissues to grains is likely to be higher.
Timing of P loading into the grains
Earlier studies focusing on individual tillers have shown that peak loading of P into grains occurs from 6 to 15 DAF (Ogawa et al., 1979; Wang et al., 2016). In our studies, plants continued partitioning a consistent amount of P to the grains after 14 DAF and up to maturity, particularly in the field study (Figs 1 and 3). These differences are probably due to the fact that in our studies, plants and tillers of different physiological ages were pooled. That is particularly true with field studies because plants are generally less synchronized than in controlled environments. When P partitioning is assessed at the whole plant level, the presence of physiologically younger tillers is likely to shift the partitioning of P to grains to later stages of growth. As such it is possible that the mild declines in leaf P concentrations observed from 0 to 9 DAF or 14 DAF may actually have been more rapid in tillers that flowered first.
Remobilization of P from the vegetative tissues to the grains
Interestingly, in the field, a significant decline in P concentrations in vegetative tissues (stem and all leaves) only occurred beyond 9 and 13 DAF in P-fertilized and P-deficient P treatments, respectively (Fig. 2). In hydroponic conditions the remobilization of P from leaves and stems generally also occurred towards the later stages of grain filling (beyond 9 and 14 DAF in Opt-P and Low-P treatments, respectively) (Fig. 4).
Given that the remobilization of minerals from leaves during senescence is typically linked to a decline in photosynthesis (Kong et al., 2013), the delay in remobilization of P from leaves until the later stages of senescence may be a key strategy to enable the continuation of photosynthesis and dry matter accumulation throughout grain filling. Logically, some coordination of P depletion from vegetative organs would make sense to enable key photosynthetic organs (e.g. the flag leaf) to continue to function optimally for as long as possible – a strategy that appears to be employed by wheat plants in relation to nitrogen allocations to photosynthetic tissues during grain filling (Bertheloot et al., 2008). Subsequent experiments in our lab suggest that photosynthesis in flag leaves begins to decline when leaf P concentrations drop below 1 mg g−1, and these concentrations were evident by 15, 18, 21 and 23 DAF in the P-deficient, Low-P, Opt-P and P-fertilized experiments, respectively. The fact that P concentrations in vegetative tissues in the field and hydroponic study were in the order flag leaves > lower leaves > stems in both sufficient P and deficient P treatments at flowering is consistent with some degree of coordination; however, P concentrations (and content) of both flag leaves and lower leaves began to decline beyond 8–10 DAF, with no clear evidence of delayed P remobilization in flag leaves in order to maintain leaf function (Figs 2B, C and 4D, E). In addition, in the hydroponic study, rachis and husk P concentrations actually began to decline significantly between 0 and 9 DAF in the Opt-P, prior to significant declines in P concentrations in other vegetative organs, indicating that the transfer of P to the grain might also follow a gradient orientated downward from the grain so the closer the organ is to the grain, the earlier the P depletion starts (Fig. 4A, B). However, our study did not determine if such signalling orientated towards the grain during grain filling is indeed involved.
33P tracer movement within rice plants
If at least 20 % of P contained in grains at maturity potentially comes from P remobilization from the vegetative parts of the plant, up to 80 % of grain P was the result of post-flowering P uptake. Repartition of the 33P tracer indicated that grain is indeed by far the main P sink during grain filling as more than 70 % of P tracer absorbed at 9 DAF that was translocated above ground was found in the grains at maturity in Opt-P plants (Fig. 6B). Opt-P plants partitioned P to the grains earlier than Low-P plants (Fig. 6B – 3 and 24 h), although low-P plants had caught up and partitioned even more P to the grains than Opt-P plants at 120 h. The partitioning of the 33P was therefore in good agreement with the total P partitioning observed in both treatments over grain filling (Fig. 3C, D).
The fraction of 33P tracer translocated from roots to aerial parts (FR→S) followed a strong linear trend over time after spiking (Fig. 6A). Low-P plants translocated 33P to the above-ground compartment more quickly than plants grown under Opt-P and the estimated difference at maturity was nearly 20 % (Fig. 6A). As it seems logical that P-deficient plants rely more on external P uptake to meet grain P demands, the fact that 45 % of total 33P tracer absorbed at 9 DAF was still in the roots at maturity (Fig. 6A) provides us with a better understanding of P loading into the grain.
In theory, it is possible for P to move from roots to grains in the xylem flow because the xylem is not discontinuous at the base of the seed (Krishnan and Dayanandan, 2003). The results of our 33P tracer study demonstrate that some P absorbed at 9 DAS was found in the grains within 3 h, which suggests that there is indeed a direct pathway to the grains, although only a small fraction of P was mobilized in this way. That the great majority of 33P tracer was still located in roots at maturity indicates that the P coming from post-flowering P uptake is not directly loaded to the grain.
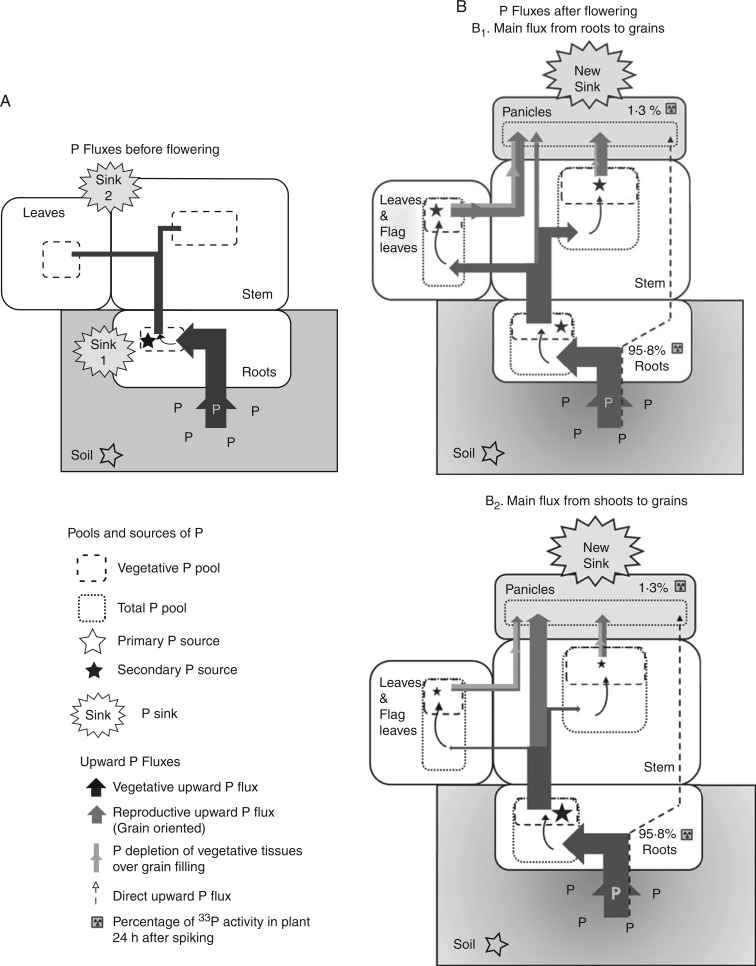
Proposed model for P loading into grains
Our results suggest that the mechanism of P loading into rice grains involves indirect transfer of P from the vegetative tissues to the panicles.
At the early stage of plant development, the seedling establishes a vegetative upward P flux to transport P from the soil (primary P source) and to provide it to the developing organs (P sinks), namely roots and then shoots. This vegetative upward P flux remains during the whole growth cycle (Fig. 7A – dark grey). After flowering, the development of the new P sink that is the panicle triggers a new P flux that is grain-orientated and plugged into the vegetative flux to enable P to move to the developing grains (Fig. 7B – Reproductive upward P flux – lighter grey). While a quick and direct transport of P from roots to grains seems to be possible (Fig. 7B – Direct upward P flux) it only involves a small proportion of P (Fig. 7B – Percentage of 33P measured in grains 24 h after spiking) and P that was taken up before grain filling is mobilized first (Fig. 7B – Vegetative pool). From flowering to maturity, the amount of P mobilized to the grains through the reproductive upward P flux is equal to the post-flowering P uptake plus the P remobilization from vegetative tissues. In our studies, the proportion of P depleted from the vegetative tissues to fill the grains accounted for around 20 % of the reproductive P flux (Fig. 7B – pale grey). There are two possible models of reproductive P flux that would account for the P loading into grains: (1) a model with roots as the main secondary source of P for grains (Fig. 7B1), and (2) a model with shoots as main secondary sources of P for grains (Fig. 7B2). One thing we did not determine was whether the P that was distributed to vegetative tissues prior to moving to the grain was stored as inorganic P or whether it cycled into the organic P pool in vegetative tissues. The likelihood of each flux system depends on the form in which P is preferentially transported within the plant, that is to say as inorganic P (Fig. 7B2) or bound to assimilates (Fig. 7B2) or both. Ye et al. (2014) did report a similar pattern of C and P partitioning over the growth cycle, particularly between grain filling and maturity, which could indicate that P would be transported bound to assimilates, but this would require further study.
Fig. 7.
Proposed model of upward P fluxes in rice plants before (A) and after (B) flowering. Model variations B1 and B2 represent the two possible reproductive fluxes enabling P to be loaded into grains, that is either using roots (B1) or vegetative tissues (B2) as the main secondary P source. Size of arrows is proportional to the amount of P mobilized by fluxes from sowing to flowering (A) and during grain filling (B) measured in Opt-P treatment of the hydroponic study. Values in italic depict the percentage of 33P activity in plants 24 h after spiking in Opt-P.
Proposed model relevance
Our results show that the mechanism of P loading into grains cannot be explained by a basic model representing the stem as a simple ‘pipe’ leading post-flowering P uptake to grains. Such a model would leave grains strongly dependent on post-flowering P supply and vulnerable in the case of sudden P supply interruption. By contrast, a model using a buffer stock of P taken up previously as source of P would allow a constant and regular flow of P to the grains.
From an evolutionary point of view, such models based on indirect P fluxes and limited remobilization are relevant because the ancestor of cultivated rice (O. rufipogon) is perennial. In addition, the breeding pressure that was imposed to increase grain yield took place recently at the scale of species evolution (i.e. during rice domestication). It would therefore make sense that the vegetative P flux hypothesized in Fig. 7A would indeed remain without fundamentally being changed or suppressed by the sudden reproductive P flux (Fig. 7B) orientated towards the grains during grain filling.
CONCLUSION
Post-flowering P uptake was important and contributed substantially to plant P accumulation in both hydroponic and field experiments. Plants partitioned the majority of above-ground P to the grains. The potential contribution of P re-mobilization from vegetative tissues to grain P was around 20 % of grain P in all cases. The majority of 33P tracer taken up by roots during grain filling remained in the roots up to maturity. This suggests that post-flowering P uptake does not move directly to the grains but that grain filling involves indirect P fluxes originating from P previously taken up and located in the vegetative tissues. Any attempts to breed rice cultivars with lower grain P concentrations to reduce the amount of P exported from fields at harvest could feasibly focus on reducing the re-partitioning of P from vegetative tissues, originating from both pre-anthesis and post-anthesis uptake, to developing grains rather than limiting post-anthesis P uptake from the soil.
REFERENCES
- Batten GD, Khan MA. 1987. Uptake and utilisation of phosphorus and nitrogen by bread wheats grown under natural rainfall. Australian Journal of Experimental Agriculture 27: 405–410. [Google Scholar]
- Bertheloot J, Martre P, Andrieu B. 2008. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiology 148: 1707–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Liu Z, Lin Z, et al. 2013. Phosphorus accumulation in grains of japonica rice as affected by nitrogen fertilizer. Plant Soil 369: 231–240. [Google Scholar]
- Brennan J.P, Malabayabas A. 2011. International Rice Research Institute’s contribution to rice varietal yield improvement in South-East Asia . ACIAR Impact Assessment Series Report No. 74. Canberra: Australian Centre for International Agricultural Research. [Google Scholar]
- Chen S, Xia Z, Zhang G. 2008. Nutrient accumulation, remobilization and partitioning in rice on no-tillage soil. Journal of Plant Nutrition 31: 2044–2058. [Google Scholar]
- Cheng W, Zhang G, Yao H, Zhao G, Wu W, Wang R. 2003. Nutrient accumulation and utilization in rice under film-mulched and flooded cultivation. Journal of Plant Nutrition 26: 2489–2501. [Google Scholar]
- Dobermann A, Fairhurst TH. 2000. Nutrient disorders and nutrient management. Singapore: Potash and Phosphate Institute, Potash and Phosphate Institute of Canada and International Rice Research Institute. [Google Scholar]
- Dunn BW, Dunn TS, Beecher HG. 2014. Nitrogen timing and rate effects on growth and grain yield of delayed permanent-water rice in south-eastern Australia. Crop and Pasture Science 65: 878–887. [Google Scholar]
- Hocking PJ. 1994. Dry-matter production, mineral nutrient concentrations, and nutrient distribution and redistribution in irrigated wheat. Journal of Plant Nutrition 17: 1289–1308. [Google Scholar]
- Jiang W, Struik PC, Van KH, Zhao M, Jin LN, Stomph TJ. 2008. Does increased Zn uptake enhance grain Zn mass concentration in rice? Annals of Applied Biology 153: 135–147. [Google Scholar]
- Kong X, Luo Z, Dong H, Eneji AE, Li W, Lu H. 2013. Gene expression profiles deciphering leaf senescence variation between early- and late-senescence cotton lines. PLoS One 8: e69847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Dayanandan P. 2003. Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.). Journal of Biosciences 28: 455–469. [DOI] [PubMed] [Google Scholar]
- Lott JNA, Ockenden I, Raboy V, Batten GD. 2000. Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Science Research 10: 11–33. [Google Scholar]
- Ogawa M, Tanaka K, Kasai Z. 1979. Accumulation of phosphorus, magnesium and potassium in developing rice grains: followed by electron microprobe X-ray analysis focusing on the aleurone layer. Plant and Cell Physiology 20: 19–27. [Google Scholar]
- Pariasca-Tanaka J, Vandamme E, Mori A, et al. 2015. Does reducing seed-P concentrations affect seedling vigor and grain yield of rice? Plant and Soil 392: 253–266. [Google Scholar]
- R Core Team 2012. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raboy V. 2007. The ABCs of low-phytate crops. Nature Biotechnology 25: 874–875. [DOI] [PubMed] [Google Scholar]
- Raboy V. 2009. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Science 177: 281–296. [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349: 121–156. [Google Scholar]
- Römer W, Schilling G. 1986. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 91: 221–229. [Google Scholar]
- Rose TJ, Rengel Z, Ma Q, Bowden JW. 2007. Differential accumulation patterns of phosphorus and potassium by canola cultivars compared to wheat. Journal of Plant Nutrition and Soil Science 170: 404–411. [Google Scholar]
- Rose TJ, Rengel Z, Ma Q, Bowden JW. 2008. Post-flowering supply of P, but not K, is required for maximum canola seed yields. European Journal of Agronomy 28: 371–379. [Google Scholar]
- Rose TJ, Rengel Z, Ma Q, Bowden JW. 2009. Phosphorus accumulation by field-grown canola crops and the potential for deep phosphorus placement in a Mediterranean-type climate. Crop and Pasture Science 60: 987–994. [Google Scholar]
- Rose TJ, Pariasca-Tanaka J, Rose MT, Fukuta Y, Wissuwa M. 2010. Genotypic variation in grain phosphorus concentration, and opportunities to improve P-use efficiency in rice. Field Crops Research 119: 154–160. [Google Scholar]
- Rose TJ, Pariasca‐Tanaka J, Rose MT, Mori A, Wissuwa M. 2012. Seeds of doubt: re‐assessing the impact of grain P concentrations on seedling vigor. Journal of Plant Nutrition and Soil Science 175: 799–804. [Google Scholar]
- Rose TJ, Liu L, Wissuwa M. 2013. Improving phosphorus efficiency in cereal crops: is breeding for reduced grain phosphorus concentration part of the solution? Frontiers in Plant Science 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan C, Brennan R, Sarre GA. 2015. Effect of soil pH and crop sequence on the response of wheat (Triticum aestivum) to phosphorus fertiliser. Crop and Pasture Science 66: 23–31. [Google Scholar]
- Stomph T, Jiang W, Van Der Putten P, Struik P. 2014. Zinc allocation and re-allocation in rice. Frontiers in Plant Science 5: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme E, Wissuwa M, Rose T, Ahouanton K, Saito K. 2016a. Strategic phosphorus (P) application to the nursery bed increases seedling growth and yield of transplanted rice at low P supply. Field Crop Research 186: 10–17. [Google Scholar]
- Vandamme E, Rose T, Saito K, Jeong K, Wissuwa M. 2016b. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutrient Cycling in Agroecosystems 104: 413–427. [Google Scholar]
- Wang F, Rose T, Jeong K, Kretzschmar T, Wissuwa M. 2016. The knowns and unknowns of phosphorus loading into grains, and implications for phosphorus efficiency in cropping systems. Journal of Experimental Botany 67: 1221–1229. [DOI] [PubMed] [Google Scholar]
- White PJ, Veneklaas EJ. 2012. Nature and nurture: the importance of seed phosphorus content. Plant and Soil 357: 1–8. [Google Scholar]
- Yang J, Zhang J. 2010. Grain-filling problem in ‘super’ rice. Journal of Experimental Botany 61: 1–5. [DOI] [PubMed] [Google Scholar]
- Ye Y, Liang X, Chen Y, Li L, Ji Y, Zhu C. 2014. Carbon, nitrogen and phosphorus accumulation and partitioning, and C:N:P stoichiometry in late-season rice under different water and nitrogen managements. PLoS One 9: e101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cock J, Gomez KA. 1976. Laboratory manual for physiological studies of rice. Los Baños (Philippines: ): International Rice Research Institute. [Google Scholar]