Abstract
Background and Aims Cytological parameters such as chromosome numbers and genome sizes of plants are used routinely for studying evolutionary aspects of polyploid plants. Members of Zingiberaceae show a wide range of inter- and intrageneric variation in their reproductive habits and ploidy levels. Conventional cytological study in this group of plants is severely hampered by the presence of diverse secondary metabolites, which also affect their genome size estimation using flow cytometry. None of the several nuclei isolation buffers used in flow cytometry could be used very successfully for members of Zingiberaceae to isolate good quality nuclei from both shoot and root tissues.
Methods The competency of eight nuclei isolation buffers was compared with a newly formulated buffer, MB01, in six different genera of Zingiberaceae based on the fluorescence intensity of propidium iodide-stained nuclei using flow cytometric parameters, namely coefficient of variation of the G0/G1 peak, debris factor and nuclei yield factor. Isolated nuclei were studied using fluorescence microscopy and bio-scanning electron microscopy to analyse stain–nuclei interaction and nuclei topology, respectively. Genome contents of 21 species belonging to these six genera were determined using MB01.
Key Results Flow cytometric parameters showed significant differences among the analysed buffers. MB01 exhibited the best combination of analysed parameters; photomicrographs obtained from fluorescence and electron microscopy supported the superiority of MB01 buffer over other buffers. Among the 21 species studied, nuclear DNA contents of 14 species are reported for the first time.
Conclusions Results of the present study substantiate the enhanced efficacy of MB01, compared to other buffers tested, in the generation of acceptable cytograms from all species of Zingiberaceae studied. Our study facilitates new ways of sample preparation for further flow cytometric analysis of genome size of other members belonging to this highly complex polyploid family.
Keywords: bio-SEM, chromosome number, flow cytometry, fluorescence microscopy, genome size, nuclei isolation buffer, UPGMA-dendrogram, Zingiberaceae
INTRODUCTION
Since the inception of flow cytometry (FCM) and its use in plant science in 1973 by Friedrich Otto Heller (Heller, 1973), assessment of nuclear DNA content, determination of DNA ploidy level and cell cycle analysis have emerged as its most popular applications (Galbraith, 2004; Bennett and Leitch, 2005). However, this technique has been plagued by a myriad of problems, such as the presence of cell walls and thick tissues, which compromised its utility, until the pioneering work of David W. Galbraith, whose innovative homogenization technique facilitated rapid nuclei isolation (Galbraith et al., 1983). Galbraith’s nuclei isolation method was fast, easy and accurate, with a higher degree of resolution, and had distinct advantages over the enzymatic method of Heller (1973) and the hypotonic lysis of protoplast method (Ulrich and Ulrich, 1986; Bergounioux et al., 1988, 1992; Ulrich et al., 1988).
A distinct disadvantage of FCM in plants is that the diversity of plant tissues and their chemical composition necessitates the use of specific buffers for each type of plant tissue under study. Although the quest for the most effective nuclei isolation buffer began nearly three decades ago, even today, successful preparation of nuclei suspension remains problematic in many plant species. Terpenoids, essential oils, alkaloids, phenolics, flavonoids, tannins, glycosides and saponins are the most commonly present secondary compounds in plants (Kabera et al., 2014). These compounds aggravate the problem of nuclei isolation by mixing with the isolated nuclei, thus affecting sample quality and causing stoichiometric errors in DNA staining (Noirot et al., 2000, 2003; Pinto et al., 2004; Loureiro et al., 2006a Walker et al., 2006). This contention was emphasized when Loureiro et al. (2006a) showed that the light scattering property of nuclei changed in Pisum sativum and Zea mays in the presence of a common phenolic compound, tannic acid.
Zingiberaceae, the pantropical family of aromatic rhizomatous perennial herbs, comprises 53 genera and over 1200 species worldwide (Kress et al., 2002). Many taxonomic problems are associated with this huge group of plants. Many authors argue that the characters governing the classification and definition of the four subfamilies and six tribes are often variable and conflicting (Kress et al., 2002). As the members are mostly rhizomatous with a fleshy inflorescence, it is extremely difficult to preserve them and the lack of a type specimen frequently leads to ambiguous assignment of names and usage. The very short flowering period and the predominance of vegetative propagation have added to the confusion in the taxonomic circumscription of Zingiberaceae.
C-values and genome size (= size of the monoploid chromosome set) are the most important markers of biodiversity, which can help in infrageneric classification, species delimitation and identification of hybrids (Keller et al., 1996; Buitendijk et al., 1997; Bare et al., 1998; Morgan et al., 1998; Ohri, 1998; Thibault, 1998; Thalmann et al., 2000; Zonneveld, 2001; Šiško et al., 2003). Genome size also reveals plant phenology (Grime and Mowforth, 1982; Baranyi and Greilhuber, 1999) and life history (Bennett, 1972; Price and Bachmann, 1975; Nandini et al., 1997) and can be correlated with environmental factors, climatic variation and geographical plant distribution (Bennett, 1976; Levin and Funderburg, 1979; Ohri and Khoshoo, 1986; Poggio et al., 1989, 1998; Wakamiya et al., 1993; MacGillivray and Grime, 1995; Bottini et al., 2000; Hall et al., 2000; Knight and Ackerly, 2002). Therefore, genome size estimation of the members of Zingiberaceae may be used as a vital parameter in resolving its taxonomic problems. It is pertinent to mention here that members of the family show huge variability in somatic chromosome numbers and variable ploidy ranges. Natural hybridization and subsequent neutralization of these crosses have also been reported in this family (Škorničková and Sabu, 2005; Škorničková et al., 2007), indicating a potential role of chromosomal events in the evolution of Zingiberaceae.
Zingiberaceae is well known for the presence of different types of bioactive metabolites, namely flavonoids, phenolic acids, essential oils, oleoresin, etc. (Conell, 1970; Chan et al., 2008; Santos et al., 2012; Nag et al., 2013; Yusuf et al., 2013; Taheri et al., 2014). This perhaps explains why DNA content estimates of only five genera, Alpinia, Curcuma, Hitchenia, Kaempferia and Stahlianthus (Bharathan et al., 1994; Leong-Škorničková et al., 2007), could be reported using FCM (FC:PI) while the DNA content of only three genera, Amomum, Curcuma and Zingiber, were reported using feulgen microdensitometry (FE) (Rai et al., 1997; Das et al., 1999). A review of all available literature revealed the lack of uniformity in buffer compositions in the protocols reported for nuclei extraction from different members of Zingiberaceae and that no single reported buffer could successfully isolate nuclei from both shoot and root tissues of the same species.
According to the FLOWer database (http://flower.web.ua.pt/; Loureiro et al., 2007a) only eight of the 28 lysis buffers listed are commonly used. The buffers devised by different authors share certain common characteristics that facilitate efficient nuclei release from intact cells, ensure nuclei stability throughout the experiment, provide protection to the DNA from degradation and promote stoichiometric staining. Although the chemical constitution of the buffers varies, each nuclei isolation buffer primarily includes organic pH-stabilizing buffers (e.g. MOPS, Tris, HEPES), chromatin stabilizers (e.g. MgCl2, MgSO4, Spermine); divalent cation binding metal chelators (e.g. EDTA, sodium citrate) to bind divalent cations that serve as a nuclease cofactor; inorganic salts (e.g. KCl, NaCl) to achieve proper ionic strength; and non-ionic detergents (e.g. Triton X-100, Tween 20) to release nuclei, disrupt chloroplasts and remove debris from the surface of nuclei (Coba de la Peňa and Brown, 2001; Doležel and Bartoš, 2005).
In the present study, we propose a novel nuclei isolation buffer, Modified Buffer 01 (MB01) that is capable of isolating good quality nuclei in various species of Zingiberaceae that are rich in phenolic compounds and essential oils. The efficiency of MB01 was assessed against eight widely used nuclei isolation buffers: Arumuganathan and Earle (A&E), Galbraith’s buffer (GB), general purpose buffer (GPB), LB01, Marie’s nuclear isolation buffer (MNB), Otto buffers (OB), Tris-MgCl2 buffer (TMB) and Tris-MgCl2 buffer with 1 % PVP (TMP). An array of FCM parameters, namely relative fluorescence intensity of propidium iodide (PI)-stained nuclei (FL), half peak coefficient of variation (CV) of the G0/G1 peak, debris factor (DF, i.e. background debris) and nuclei yield factor (YF, i.e. amount of nuclei in the suspension independently of the amount of tissue used), were analysed in six species belonging to Alpinia, Curcuma, Globba, Hedychium, Kaempferia and Zingiber to evaluate the efficacy of MB01. Furthermore, MB01 was used to isolate nuclei from 21 Zingiberaceae species that were analysed using FCM for genome size estimation. Genome sizes of 14 such species are reported here for the first time. Our results affirm the proficiency of the novel buffer over conventionally used buffers in terms of the above critical FCM determinants in members of Zingiberaceae.
MATERIAL AND METHODS
Plant material
Plant rhizomes collected from different areas of India (Table 1) were cultivated for at least one year under homogeneous conditions in the Experimental Garden of the Department of Botany, University of Calcutta, West Bengal, India. Plants were identified and herbarium sheets were submitted to the University of North Bengal, West Bengal, India. For FCM estimation of nuclear DNA, Raphanus sativus ‘Saxa’, Lycopersicon esculentum ‘Stupicke polni tyckove rane’ and Glycine max ‘Polanka’ were used as internal standards, kindly supplied by Dr Jaroslav Doležel (Laboratory of Molecular Cytogenetics and Cytometry, Institute of Experimental Botany, Czech Republic). The 2C DNA contents of these plants are 1·11, 1·96 and 2·5 pg, respectively (Doležel et al., 1992, 1994, 1998).
Table 1.
Place of collection and herbarium accession numbers of the plants analysed
| Sl. no. | Plant | Place of collection | Accession no. |
|---|---|---|---|
| 1 | Alpinia calcarata (Haw.) Roscoe | North Bengal University, Siliguri, West Bengal | MB-LAB-A14* |
| 2 | Alpinia galanga (L.) Willd. | North Bengal University, Siliguri, West Bengal | NBU- 09697 |
| 3 | Alpinia malaccensis (Burm.f.) Roscoe | Botanical Survey of India, Eastern Regional Center, Umiam, Shillong, Meghalaya | MB-LAB-A13* |
| 4 | Alpinia zerumbet (Pers.) B.L.Burtt & R.M.Sm. | Botanical Survey of India, Eastern Regional Center, Umiam, Shillong, Meghalaya | NBU-09713 |
| 5 | Curcuma amada Roxb. | Kolkata, West Bengal | NBU-09701 |
| 6 | Curcuma aurantiaca Zijp | Calicut University, Kozhikode, Kerala | NBU-09705 |
| 7 | Curcuma caesia Roxb. | Experimental Garden, Dept of Botany, CU, Kolkata, West Bengal | NBU-09709 |
| 8 | Curcuma inodora Blatt. | Calicut University, Kozhikode, Kerala | NBU-09704 |
| 9 | Curcuma longa L. | Ram Krishna Mission, Medicinal Plant Garden, Narendrapur, Kolkata, West Bengal | NBU-09703 |
| 10 | Curcuma rubescens Roxb. | Kalimpong, West Bengal | NBU-09699 |
| 11 | Globba marantina L. | Experimental Garden, Dept of Botany, CU, Kolkata, West Bengal | NBU-09706 |
| 12 | Globba sessiliflora Sims | Calicut University, Kozhikode, Kerala | NBU-09707 |
| 13 | Globba schomburgkii Hook.f. | Calicut University, Kozhikode, Kerala | NBU-09708 |
| 14 | Hedychium spicatum Sm. | National Bureau of Plant Genomic Resources, Bhowali Research Station, Uttarakhand | NBU-09696 |
| 15 | Hedychium coronarium J.Koenig | Agrihorticultural Garden, Kolkata, West Bengal | MB-LAB-He6* |
| 16 | Kaempferia angustifolia Roscoe | North Bengal University, Siliguri, West Bengal | MB-LAB-Ka4* |
| 17 | Kaempferia elegans (Wall.) Baker | Kolkata, West Bengal | NBU-09711 |
| 18 | Kaempferia galanga L. | North Bengal University, Siliguri, West Bengal | MB-LAB-Ka2a* |
| 19 | Kaempferia rotunda L. | National Bureau of Plant Genomic Resources, Umiam, Shillong, Meghalaya | NBU-09700 |
| 20 | Zingiber officinale Roscoe | Ram Krishna Mission, Medicinal Plant Garden, Narendrapur, Kolkata, West Bengal | NBU-09702 |
| 21 | Zingiber zerumbet (L.) Roscoe ex Sm. | National Bureau of Plant Genomic Resources, Bhowali Research Station, Uttarakhand | NBU-09716 |
*Live collection, Department of Botany, University of Calcutta.
Chromosomal study
Mitotic chromosome numbers of these plants were studied in young root tips according to the protocol of Bhadra and Bandyopadhyay (2015, 2016). Somatic chromosome numbers of each species were confirmed by examination of at least 50 independent plates from at least five different root tips.
Sample preparation for flow cytometry
Approximately, 50 mg of young leaves ∼5–10 mm long were selected from each plant. Nuclei suspensions were prepared by mechanically chopping the tissue using a sharp razor blade according to Galbraith et al. (1983) in a Petri dish with chilled nuclei isolation buffers. Eight widely used nuclei isolation buffers were tested along with the prepared buffer (Table 2). In total, 500 μL of each nuclear suspension was taken in a 2-mL centrifuge tube and, to prevent staining of double-stranded RNA, 50 μg mL−1 RNase A was added. The suspension was then filtered through a 50-μm nylon mesh to remove cell fragments and large debris.
Table 2.
Nuclei isolation buffers and their compositions
| Buffer | Composition |
|---|---|
| Arumuganathan and Earle (Arumuganathan and Earle, 1991) | 9·53 mm MgSO4.7H2O; 47·67 mm KCl; 4·77 mm HEPES; 6·48 mm DTT; 0·25 % (v/v) Triton X-100; pH 8·0 |
| Galbraith’s buffer (Galbraith et al., 1983) | 45 mm MgCl2; 30 mm sodium citrate; 20 mm MOPS; 0·1 % (v/v) Triton X-100; pH 7·0 |
| GPB (Loureiro et al., 2007b) | 0·5 mm spermine.4HCl, 30 mm sodium citrate, 20 mm MOPS, 80 mm KCl, 20 mm NaCl, 0·5 % (v/v) Triton X-100; pH 7·0 |
| LB01 (Doležel et al., 1989) | 15 mm Tris; 2 mm Na2EDTA; 0·5 mm spermine.4HCl; 80 mm KCl; 20 mm NaCl; 15 mm β-mercaptoethanol; 0·1 % (v/v) Triton X-100; pH 7·5 |
| Marie’s nuclear isolation buffer (Marie and Brown, 1993) | 50 mm glucose; 15 mm KCl; 15 mm NaCl; 5 mm Na2EDTA; 50 mm sodium citrate; 0·5 % (v/v) Tween 20; 50 mm HEPES; 0·5 % (v/v) β-mercaptoethanol; pH 7·2 |
| MB01 | 20 mm MOPS; 2·5 mm Na2EDTA; 0·7 mm spermine.4HCl; 80 mm KCl; 20 mm NaCl; 1 % (w/v) PVP; 0·5 % (v/v) β-mercaptoethanol; 0·2 % (v/v) Triton X-100; pH 7·4 |
| Otto buffers* |
|
| Tris-MgCl2 buffer (Pfosser et al., 1995) | 200 mm Tris; 4 mm MgCl2.6H2O; 0·5 % (v/v) Triton X-100; pH 7·5 |
| Tris-MgCl2 buffer with 1 % PVP (Doležel et al., 1989) | 200 mm Tris; 4 mm MgCl2.6H2O; 0·5 % (v/v) Triton X-100; 1 % (w/v) PVP; pH 7·5 |
*The pH of these buffers was not adjusted. The nuclei isolation was done in Otto I buffer; fluorochrome was added in a mixture of Otto I and Otto II buffers (1 : 2)
To minimize the pipetting error and ensure uniform dye distribution, PI was added to each buffer and the PI-containing buffer solutions were added to the filtered nuclear suspension making the final volume up to 1 mL. The final concentration of PI in the suspension was 50 μg mL−1. After addition of PI, the samples were incubated on ice in the dark and analysed within 10 min.
The FCM parameters were analysed in shoots of all six genera using all nine lysis buffers and three best working buffers were chosen for analysis in roots of the two most abundantly available plants, C. amada and G. sessiliflora.
To estimate the genome size, shoots of the reference standards were co-chopped with sample shoots of unknown genome size using MB01 buffer, following the same protocol. External standard nuclei suspensions were also prepared separately using the same isolation method.
Flow cytometric analysis
A BD FACSVerse (Becton Dickinson, Franklin Lakes, NJ, USA) laser flow cytometer was used to analyse PI-stained nuclei. A blue laser operating at 488 nm of 20 mW power was used for excitation. Fluorescence emission of PI (>615 nm) reaches the photomultiplier through a 560-nm long pass dichroic mirror (560 LP) and a 586/42 band pass filter. Prior to sample run, the instrument was set up with BD DNA QC Particles (Becton Dickinson) for DNA analysis and linearity checking. Sample analysis was performed according to the method of Doležel et al. (2007). A medium flow rate (60 μL min−1) was used and at least 5000 nuclei were analysed from each sample. The flow rate and amplification settings were kept constant throughout the experiment to compare the buffers.
A PI fluorescence area (PI-A) vs. PI fluorescence width (PI-W) dot plot was drawn on a linear scale to eliminate clumps and aggregates using qualitative gating. A PI-A histogram was drawn to view nuclear DNA content. The data were recorded and analysed using BD FACSuite software version 1·0·5·3841 (Becton Dickinson).
The relative fluorescence intensity of PI-stained nuclei (FL), half peak coefficient of variation (CV) of the G0/G1 peak (to evaluate nuclei integrity and staining variation), debris factor (DF, i.e. background debris) and nuclei yield factor (YF, i.e. amount of nuclei in the suspension independently of the amount of tissue used) were assessed for each sample (Loureiro et al., 2006b).
The PMT voltages for forward light scatter (FS), side light scatter (SS) and fluorescence intensity of PI-stained nuclei (FL) were adjusted. A threshold was given to cut low-channel signals of cell debris or autofluorescent compounds. The number of events in G0/G1 and G2 peaks as verified by the median PI fluorescence intensity value was added to obtain the nuclei yield. All experiments were repeated three times, and each sample was analysed five times in each buffer. For genome size estimation five replicates from different individuals were analysed and the 2C values of the unknown samples were calculated using the following formula:
The mass values were converted to base-pair numbers by the factor 1 pg = 978 Mbp (Doležel et al., 2003).
Physical quality of isolated nuclei
To visualize the quality of isolated nuclei, the parameters shape, agglomeration property, stain–nuclei interaction and nuclear topology were analysed using fluorescence microscopy and bio-scanning electron microscopy (bio-SEM).
Fluorescence microscopy of the nuclei
Nuclei from A. zerumbet shoot tissue were scanned under a Leica DM IL LED (Leica, Wetzlar, Germany) fluorescence microscope to check the quality of nuclei. Nuclei were isolated from 50 mg of tissue according to the method described above using each of the nine buffers stained by PI (50 μg mL−1). After incubation for 10 min, nuclei suspensions were passed through a 50-μm nylon mesh to remove cellular debris. To remove excess PI, nuclei were centrifuged at 3000 r.p.m. for 5 min and resuspended in 500 μL of the respective buffers. Nuclei were analysed in 35-mm Petri dishes and photomicrographs were taken using a Leica DFC 450C camera (Leica) using Leica Application Suite V.4.7.1 software (Leica).
bio-SEM of the nuclei
As chemical constituents of the buffers can alter the nuclei structure, nuclei isolated from a representative species, A. zerumbet, with each of the nine buffers were investigated by SEM. To evaluate the nuclei topology, 10 μL of each nuclei suspension isolated by the method described above was drop-casted on grease-free glass coverslips and analysed in a Zeiss EVO-MA 18 special edition (Zeiss, Oberkochen, Germany), under variable pressure (75 Pa; for biological samples) using VPSE G3 detectors and 20 kV EHT voltage.
Statistical analysis
Statistical analyses of the mean values were carried out using SigmaPlot 12.1 software. One-way analysis of variance (ANOVA) was performed to detect significant statistical differences among the buffers. The means were compared using the Holm–Sidak multiple comparison test for pairwise comparison at a 5 % probability level. Hierarchical cluster analysis was done using FL, G0/G1 CV, DF and YF parameters of the isolated nuclei as multistate data. A simple matching coefficient (Sneath and Sokal, 1973) was calculated using the program SIMQUAL of NTSYSpc 2·02 (Rohlf, 1999). Utilizing this data matrix, a UPGMA (unweighted pair group method with arithmetic mean) dendrogram was generated using the SAHN (sequential, agglomerative, hierarchical and nested clustering methods) module of NTSYSpc. All data are expressed as mean ± SD.
RESULTS
Comparative analysis of flow cytometric parameters
The efficiency of the buffers was evaluated depending on the best combination of high YF and FL values and low DF and G0/G1 peak CV values. In this study, species belonging to six different genera of Zingiberaceae were chosen to compare the nuclei isolation ability of the buffers given that each genus exhibits unique phytochemical background. With the exception of MB01, which was able to isolate a reasonable number of nuclei (4·26–18·43 nuclei s−1 mg−1 of tissue) and secure well-defined histograms with DNA peaks demonstrating acceptable CV values (<5 %; Galbraith et al., 2002; Table 3), no other buffer was able to isolate a good quality of nuclei from all the samples studied.
Table 3.
Flow cytometric parameters (FL, CV, DF, YF) analysed in each species from shoot tissue
| Species | Buffer | FL (channel unit) | CV (%) | DF (%) | YF (nuclei s−1 mg−1) |
|---|---|---|---|---|---|
| Alpinia zerumbet | Arumugnathan and Earle | 63·57a ± 3·732 | 4 ·36 ± 0·044 | 77 ·79 ± 0·286 | 0 ·61a ± 0·062 |
| Galbraith’s buffer | 61·50a ± 2·436 | 8·20 ± 0·315 | 79·83 ± 0·490 | 0·62a ± 0·046 | |
| GPB | – | – | – | – | |
| LB01 | 96·24b ± 1·138 | 4·64 ± 0·128 | 52·84 ± 0·715 | 1·61b ± 0·208 | |
| Marie’s nuclear isolation buffer | – | – | – | – | |
| MB01 | 103·10 ± 2·443 | 3·87 ± 0·157 | 22·00 ± 0·509 | 6·63 ± 0·897 | |
| Otto buffers | 90·41 ± 0·584 | 3·06 ± 0·077 | 33·04 ± 2·650 | 2·68 ± 0·266 | |
| Tris-MgCl2 buffer | – | – | – | – | |
| Tris-MgCl2 buffer with 1 % PVP | 94·34b ± 1·456 | 3·41 ± 0·073 | 48·20 ± 1·978 | 1·15a,b ± 0·075 | |
| Curcuma amada | Arumugnathan and Earle | – | – | – | – |
| Galbraith’s buffer | 81·77a ± 1·159 | 5·35a ± 0·098 | 64·47a ± 1·034 | 1·01a ± 0·180 | |
| GPB | 69·70 ± 1·091 | 6·36 ± 0·054 | 80·06 ± 0·356 | 0·54 ± 0·020 | |
| LB01 | – | – | – | – | |
| Marie’s nuclear isolation buffer | 81·38a ± 1·760 | 7·43 ± 0·071 | 60·62b ± 0·792 | 1·31a ± 0·080 | |
| MB01 | 96·95 ± 1·517 | 3·91b ± 0·114 | 20·23 ± 2·336 | 18·43 ± 5·400 | |
| Otto buffers | 92·56 ± 1·228 | 4·07b ± 0·384 | 27·65 ± 0·744 | 11·99 ± 2·810 | |
| Tris-MgCl2 buffer | 77·80 ± 1·128 | 5·48a ± 0·131 | 62·65a,b ± 0·937 | 6·69 ± 1·580 | |
| Tris-MgCl2 buffer with 1 % PVP | 74·09 ± 2·808 | 6·71 ± 0·170 | 51·67 ± 2·357 | 0·61a ± 0·100 | |
| Globba sessiliflora | Arumugnathan and Earle | 146·49 ± 2·756 | 3·71a ± 0·024 | 71·54 ± 1·357 | 0·97a ± 0·040 |
| Galbraith’s buffer | – | – | – | – | |
| GPB | – | – | – | – | |
| LB01 | 125·80 ± 0·346 | 3·67a ± 0·032 | 42·89 ± 2·530 | 1·74 ± 0·105 | |
| Marie’s nuclear isolation buffer | – | – | – | – | |
| MB01 | 168·45 ± 1·645 | 3·78a ± 0·182 | 28·70 ± 0·301 | 7·21 ± 0·282 | |
| Otto buffers | 96·25 ± 2·294 | 3·89a ± 0·157 | 75·50 ± 0·915 | 1·51 ± 0·094 | |
| Tris-MgCl2 buffer | 139·29a ± 4·419 | 4·85 ± 0·084 | 92·22 ± 0·381 | 0·79a ± 0·041 | |
| Tris-MgCl2 buffer with 1 % PVP | 138·16a ± 1·995 | 4·15 ± 0·171 | 89·85 ± 0·455 | 0·85a ± 0·055 | |
| Hedychium spicatum | Arumugnathan and Earle | 58·64 ± 0·444 | 3·41a ± 0·092 | 19·10 ± 0·503 | 6·74 ± 0·042 |
| Galbraith’s buffer | – | – | – | – | |
| GPB | 49·57b ± 0·697 | 5·64 ± 0·254 | 37·65 ± 0·654 | 3·74 ± 0·354 | |
| LB01 | 52·31a ± 0·673 | 3·28a ± 0·087 | 17·57 ± 0·572 | 4·34 ± 0·195 | |
| Marie’s nuclear isolation buffer | 43·04 ± 0·351 | 4·11b ± 0·048 | 40·56 ± 0·870 | 2·89 ± 0·069 | |
| MB01 | 51·78a, b ± 0·382 | 2·97 ± 0·115 | 11·35 ± 0·284 | 7·52 ± 0·172 | |
| Otto buffers | 53·03 ± 0·410 | 4·13b ± 0·076 | 16·05 ± 0·464 | 7·00 ± 0·039 | |
| Tris-MgCl2 buffer | – | – | – | – | |
| Tris-MgCl2 buffer with 1 % PVP | 4·02 ± 0·464 | 5·43b ± 0·375 | 99·41 ± 0·215 | 0·04 ± 0·011 | |
| Kaempferia elegans | Arumugnathan and Earle | 41·14 ± 0·698 | 3·19 ± 0·287 | 54·50a ± 10·109 | 1·60 ± 0·107 |
| Galbraith’s buffer | 22·49a ± 11·444 | 4·14a ± 0·105 | 86·68b ± 1·083 | 0·35a ± 0·225 | |
| GPB | 20·54a ± 3·578 | 5·89b ± 0·457 | 88·38 ± 3·677 | 0·31a ± 0·379 | |
| LB01 | 19·21a ± 0·893 | 3·80 ± 0·429 | 86·14b ± 10·371 | 0·77a ± 0·114 | |
| Marie’s nuclear isolation buffer | 27·14a ± 0·273 | 5·13b ± 0·705 | 52·92a ± 14·074 | 3·41 ± 0·071 | |
| MB01 | 70·52 ± 0·809 | 2·90 ± 0·400 | 8·62 ± 14·193 | 4·26 ± 0·914 | |
| Otto buffers | 55·87 ± 8·121 | 4·18a ± 0·031 | 54·87a ± 3·374 | 0·17a ± 0·139 | |
| Tris-MgCl2 buffer | – | – | – | – | |
| Tris-MgCl2 buffer with 1 % PVP | – | – | – | – | |
| Zingiber zerumbet | Arumugnathan and Earle | – | – | – | – |
| Galbraith’s buffer | – | – | – | – | |
| GPB | – | – | – | – | |
| LB01 | – | – | – | – | |
| Marie’s nuclear isolation buffer | – | – | – | – | |
| MB01 | 83·08 ± 1·903 | 3·40 ± 0·181 | 21·00 ± 0·750 | 8·43 ± 0·517 | |
| Otto buffers | – | – | – | – | |
| Tris-MgCl2 buffer | – | – | – | – | |
| Tris-MgCl2 buffer with 1 % PVP | – | – | – | – |
Values are given as mean and standard deviation of the mean (SD) of fluorescence (FL, channel units); coefficient of variation of G0/G1 DNA peak (CV, %); debris factor (DF, %) and nuclear yield factor (YF, nuclei s−1 mg−1). Means followed by the same letter (a or b) are not statistically different according to the multiple comparison Holm–Sidak test at P < 0·05. –, buffer failed to generate acceptable results.
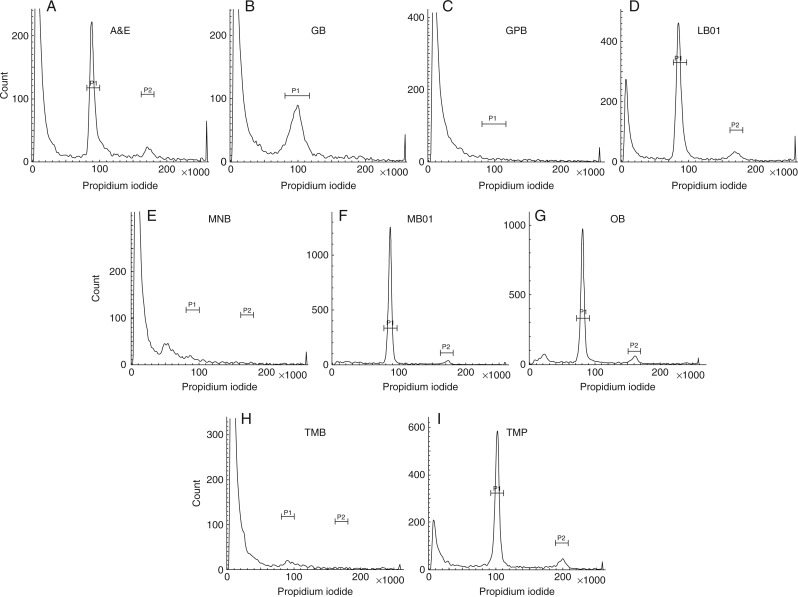
Alpinia zerumbet
GPB, MNB and TMB failed to generate acceptable results for all parameters studied (Fig. 1C, E, H). The highest FL value was observed in nuclei isolated by MB01 (Table 3), while GB yielded nuclei with the lowest FL value, which was statistically similar to A&E nuclei. The lowest G0/G1 CV was obtained from nuclei extracted using OB, which was approx. 20 % less than MB01. It was evident that MB01 significantly reduced the background factors (Table 3) and increased YF compared with other buffers (Table 3; Fig. 1F).
Fig. 1.
FCM histograms of nuclei suspensions prepared from shoot tissues of A. zerumbet in different buffers. (A) Arumuganathan and Earle buffer, (B) Galbraith’s buffer, (C) General purpose buffer, (D) LB01 buffer, (E) Marie’s nuclear isolation buffer, (F) MB01 buffer, (G) Otto buffers, (H) Tris-MgCl2 buffer, (I) Tris-MgCl2 buffer with 1 % PVP.
Curcuma amada
Although Leong-Škorničková et al. (2007) indicated the efficiency of OB in yielding an acceptable amount of nuclei from this species, use of MB01 gave a higher FL value and YF as well as lower G0/G1 CV and DF, when compared to OB, as well as all the other buffers (Table 3). Neither A&E- nor LB01-extracted nuclei generated an evaluable histogram, indicating failure of these buffers in providing the requisite amount of nuclei. GB and MNB gave statistically similar results for the FL value, while the CV of G0/G1 MB01-extracted nuclei was not significantly different from that of OB. MB01 showed the highest nuclei YF in all the species among the nine buffers analysed and hence indicated its efficiency.
Globba sessiliflora
Acceptable results for this species were obtained from all the buffers except GB, GPB and MNB. The lowest FL value was demonstrated by OB and the highest by MB01 (Table 3). The nuclei suspension prepared using MB01 showed significantly low DF, i.e. 28·70 %, while LB01, OB and TMB showed DF values of 42·89, 75·50 and 92·22 %, respectively. The YF value was also higher in samples prepared with MB01 (>5 nuclei s−1 mg−1) compared with the other buffers used.
Hedychium spicatum
All buffers used showed mixed results except GB and TMB. Nuclei isolated with A&E showed the highest FL value, although LB01 and MB01 nuclei showed significantly homogenous results. Nuclei isolated using MB01 showed the lowest CV of G0/G1 (Table 3), with lowest background DF, and thus YF value was higher in MB01 (>7 nuclei s−1 mg−1 of tissue) compared with the other buffers.
Kaempferia elegans
Nuclei extracted using MB01 showed the best combination of parameters analysed here, whereas nuclei extracted with TMB and TMP failed to generate any estimable histogram. With regard to FL and YF values, MB01 showed acceptable results that were higher than for the other buffers, along with the lowest G0/G1 CV and DF values among all the species and buffers analysed. In terms of DF value, A&E, MNB, GB and LB01 buffers showed similar results, while values for GB, LB01 and OB were similar.
Zingiber zerumbet
Only MB01 was able to isolate nuclei from this species. None of the other buffers could generate any evaluable histogram when scanned in the flow cytometer. FL and YF values were fairly high with low CV and DF values (Table 3).
Three of the best-performing buffers, i.e. MB01, OB and LB01, were also analysed in root tissues of C. amada and G. sessiliflora (Supplementary Data Fig. S1). In C. amada, the FL and YF values were significantly higher for MB01 than for OB and LB01, while G0/G1 CV and DF values were lower for MB01 than for OB and LB01. Although in G. sessiliflora the FL value was higher in OB-isolated nuclei than MB01 nuclei, the other parameters investigated supported the use of MB01 with a higher YF value, and lower G0/G1 CV and DF values than for OB buffer, while LB01 did not generate any evaluable cytogram (Fig. S1).
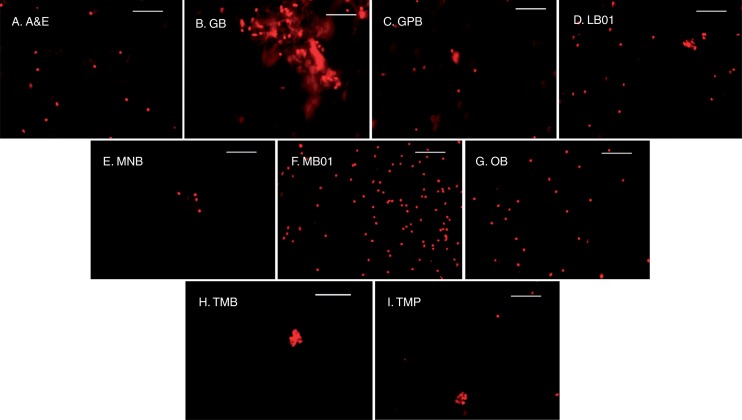
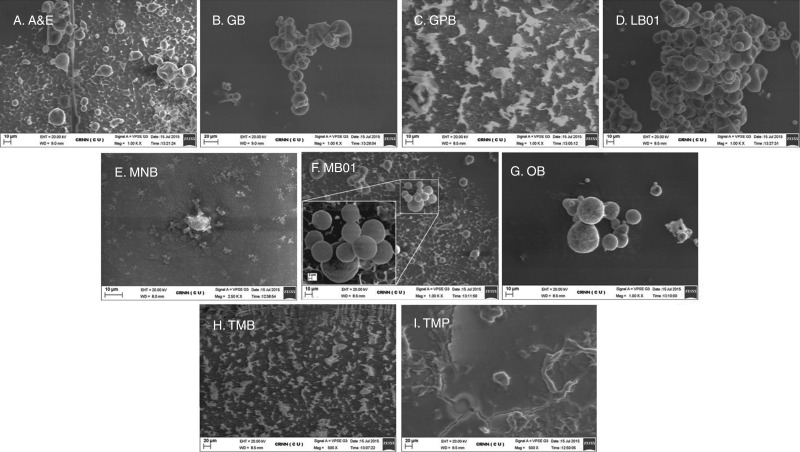
Quality of isolated nuclei
Both FM and bio-SEM analysis showed similar observations for each of the buffers in A. zerumbet, congruent with the FCM data. As GPB, MNB and TMB failed to yield a legitimate number of nuclei for generation of histograms in FCM (Fig. 1C, E, H), nothing other than cellular debris was found under the FM (Fig. 2C, E, H). SEM photomicrographs showed only debris along with salt depositions for the above buffers (Fig. 3C, E and H). Large clumps were observed in GB-isolated nuclei suspension (Fig. 2B), whereas small clumps were formed in LB01-isolated nuclei (Fig. 2D), which resulted in poor-quality FCM histograms (Fig. 1B, D). Nuclear deformities, due to inadequate ionic strength of the buffer and lack of stabilizing agents, were confirmed by SEM in GB-extracted nuclei (Fig. 3B), which impaired stain–nuclei interactions, leading to a high DF and higher G0/G1 CV of nuclei. A&E, MB01, OB and TMP nuclei were clearly visible under the FM. As YF velues were lower and not significantly different in A&E and TMP suspensions with higher DF (Table 3), fewer nuclei were seen under FM (Fig. 2A, I). SEM images revealed deposition of salt crystals along with a few nuclei in some buffers (Fig. 3A, C and I). Although OB was a potent competitor to MB01, the number of nuclei was higher in MB01 (Table 3; Figs 1F, G and 2F, G). Nuclei isolated by MB01 also lacked deformities (Fig. 3F) and thus proper stain–nuclei interaction helped in the estimation of genome size.
Fig. 2.
Alpinia zerumbet shoot nuclear suspension under fluorescence microscopy in different buffers. (A) Arumuganathan and Earle buffer, (B) Galbraith’s buffer, (C) General purpose buffer, (D) LB01 buffer, (E) Marie’s nuclear isolation buffer, (F) MB01 buffer, (G) Otto buffers, (H) Tris-MgCl2 buffer, (I) Tris-MgCl2 buffer with 1 % PVP. Scale bars = 50 μm.
Fig. 3.
Photomicrographs obtained from bio-SEM analyses of A. zerumbet shoot nuclear suspensions using different isolation buffers. (A) Arumuganathan and Earle buffer, (B) Galbraith’s buffer, (C) General purpose buffer, (D) LB01 buffer, (E) Marie’s nuclear isolation buffer, (F) MB01 buffer, (G) Otto buffers, (H) Tris-MgCl2 buffer, (I) Tris-MgCl2 buffer with 1 % PVP.
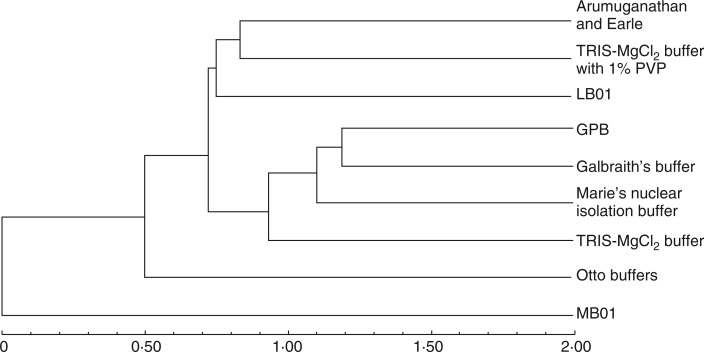
Hierarchical cluster analysis
Analysis of FCM data clustered the buffers according to their effectiveness and chemical composition. The UPGMA dendrogram, generated using hierarchical cluster analysis of the buffers based on FCM parameters and nuclei isolation ability in all six species, separated MB01 buffer from the other buffer in the dendrogram, again confirming its effectiveness among the buffers analysed (Fig. 4). OB segregated next to MB01, away from the remaining buffers, which formed two sub-clusters. In the first sub-cluster, LB01 was separate from the A&E and TMP clade, indicating its difference from these buffers and its nuclei isolation ability. In the next sub-cluster, GB, GPB and MNB yielding nearly identical results, separate from TMB.
Fig. 4.
Dendrogram obtained after hierarchical cluster analysis of FCM parameters in all species for all buffers studied.
2C nuclear DNA contents
Table 4 shows the DNA values (2C) of the species investigated, reconfirming the efficiency of MB01 buffer. Relative 2C nuclear DNA contents were estimated by comparing them with plant DNA standards (Fig. 5). In Kaempferia the genome content ranged from 3·43 to 8·61 pg with highest intrageneric variation of 86·04 % among the species studied. No statistical differences were found among nuclear DNA contents in different individuals of the same species. 2C nuclear DNA content varied from 1·59 pg (C. amada) to 8·61 pg (K. galanga). Plants from different genera showed variable somatic chromosome numbers, according to which the 1Cx value varied from 0·265 pg (C. amada, 2n = 42, x = 6) to 1·945 pg (Z. zerumbet, 2n = 22, x = 2).
Table 4.
Somatic chromosome number (2n), ploidy level (x), 2C nuclear DNA content with standard deviation, monoploid genome size (Cx-value, determined as 2C DNA amount/ploidy level) expressed in DNA picograms and megabase pairs, for 21 Indian plants belonging to six genera of Zingiberaceae and previous reports; using flow cytometry (FC:PI) and Feulgen microdensitometry (FE)
| Sl. no. | Taxon | Present report | Previous reports | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2n | Ploidy level (x) | 2C DNA content (pg) ± SD | 1Cx-value (pg) | 1Cx-value (Mbp) | 2n | Ploidy level (x) | 2C DNA content (pg) | Method | Reference | ||
| 1 | Alpinia calcarata | 48 | 4 | 4·43 ± 0·015 | 1·108 | 1083·62 | – | – | – | – | – |
| 2 | Alpinia galanga | 48 | 4 | 3·86 ± 0·018 | 0·965 | 943·77 | – | – | – | – | |
| 3 | Alpinia malaccensis | 48 | 4 | 4·38 ± 0·012 | 1·095 | 1070·91 | – | – | – | – | – |
| 4 | Alpinia zerumbet | 52 | 4 | 3·95 ± 0·014 | 0·988 | 966·26 | – | – | – | – | – |
| 5 | Curcuma amada | 42 | 6 | 1·59 ± 0·042 | 0·265 | 259·17 | 42 | 6 | 1·86 | FC:PI | Leong-Skornickova et al. (2007) |
| 40 | – | 1·56 | FE | Das et al. (1999) | |||||||
| 6 | Curcuma aurantiaca | 42 | 6 | 2·84 ± 0·083 | 0·473 | 462·59 | 42 | 6 | 2·2 | FC:PI | Leong-Skornickova et al. (2007) |
| 7 | Curcuma caesia | 63 | 9 | 2·52 ± 0·013 | 0·280 | 273·84 | 63 | 9 | 2·82 | FC:PI | Leong-Skornickova et al. (2007) |
| 8 | Curcuma inodora | 42 | 6 | 2·03 ± 0·009 | 0·338 | 330·56 | 42 | 6 | 2·29 | FC:PI | Leong-Skornickova et al. (2007) |
| 9 | Curcuma longa | 63 | 9 | 2·40 ± 0·010 | 0·267 | 261·13 | 63 | 9 | 2·71 | FC:PI | Leong-Skornickova et al. (2007) |
| 10 | Curcuma rubescens | 63 | 9 | 2·71 ± 0·027 | 0·301 | 294·38 | 42 | 6 | 1·87 | FC:PI | Leong-Skornickova et al. (2007) |
| 11 | Globba marantina | 52 | 4 | 3·63 ± 0·139 | 0·908 | 888·02 | – | – | – | – | – |
| 12 | Globba sessiliflora | 52 | 4 | 3·05 ± 0·134 | 0·763 | 746·21 | – | – | – | – | – |
| 13 | Globba schomburgkii | 48 | 4 | 3·00 ± 0·053 | 0·750 | 733·50 | – | – | – | – | – |
| 14 | Hedychium spicatum | 68 | 4 | 2·71 ± 0·048 | 0·678 | 663·08 | – | – | – | – | – |
| 15 | Hedychium coronarium | 34 | 2 | 2·14 ± 0·011 | 1·070 | 1046·46 | – | – | – | – | – |
| 16 | Kaempferia angustifolia | 36 | 3 | 5·21 ± 0·040 | 1·370 | 1339·86 | – | – | – | – | – |
| 17 | Kaempferia elegans | 22 | 2 | 3·43 ± 0·004 | 1·715 | 1677·27 | – | – | – | – | – |
| 18 | Kaempferia galanga | 54 | 5 | 8·61 ± 0·055 | 1·722 | 1684·12 | – | – | – | – | – |
| 19 | Kaempferia rotunda | 44 | 4 | 7·45 ± 0·163 | 1·863 | 1822·01 | – | – | – | – | – |
| 20 | Zingiber officinale | 22 | 2 | 3·60 ± 0·045 | 1·800 | 1760·40 | 22 | – | 12·05 | FE | Rai et al. (1997) |
| 21 | Zingiber zerumbet | 22 | 2 | 3·89 ± 0·036 | 1·945 | 1902·21 | – | – | – | – | – |
–, no previous genome size report.
Fig. 5.
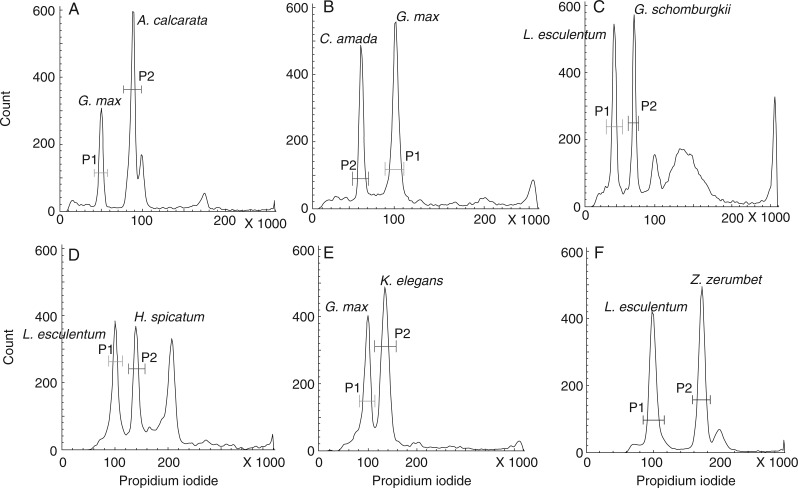
Representative FCM histograms documenting genome size determination in six genera evaluated. (A) The P1 peak representing the G0/G1 peak of internal standard G. max and P2 peak representing the G0/G1 peak of unknown sample A. calcarata. (B) The P1 peak representing the G0/G1 peak of internal standard G. max and P2 peak representing the G0/G1 peak of unknown sample C. amada. (C) The P1 peak representing the G0/G1 peak of internal standard L. esculentum and P2 peak representing the G0/G1 peak of unknown sample G. schomburgkii. (D) The P1 peak representing the G0/G1 peak of internal standard L. esculentum and P2 peak representing the G0/G1 peak of unknown sample H. spicatum. (E) The P1 peak representing the G0/G1 peak of internal standard G. max and P2 peak representing the G0/G1 peak of unknown sample K. elegans. (F) The P1 peak representing the G0/G1 peak of internal standard L. esculentum and P2 peak representing the G0/G1 peak of unknown sample Z. zerumbet.
DISCUSSION
The estimation of absolute DNA content depends on the preparation of a suspension of intact nuclei, while the quality of nuclei depends on the chemical constituents of nuclei isolation buffers. The aim of this study was to standardize a single nuclei isolation buffer that would be capable of isolating nuclei from all members of Zingiberaceae in comparison with eight widely used nuclei isolation buffers.
Members of Zingiberaceae contain a wide variety of secondary metabolites, which can potentially interfere with the isolated nuclei in the cell-free cytoplasmic lysate. Among them, different types of essential oils, viscous hydrophobic compounds and phenols are predominant. Moreover, nuclei suspensions are prepared by mechanical chopping, thus containing cell-wall remnants (cellulose, hemicellulose) as well as Ca2+-pectate gel residues, a major component of the sticky middle lamella (Buren, 1991; Vincken et al., 2003). Pectin is made up of galacturonans (homogalacturonan, rhamnogalacturonan, xylogalacturonan) mainly consisting of glucuronic acid, galacturonic acid and rhamnose (Ovodov, 1998). These components promote isolated nuclei to aggregate, and such clumping is enhanced in the presence of the sticky essential oils, making the samples unstable for FCM estimation. This agglomeration is enhanced by the hydrophobic layer that forms around nuclei and induces them to stick together.
In this study, OB and LB01 buffers performed best as they showed higher FL and YF values and lower G0/G1 CV and DF values than the other buffers investigated. However, their failure in Z. zerumbet and C. amada led to the formulation of MB01. Although, citric acid (the chief constituent of OB) is known to be a polymer stabilizer forming ester or amide bonds (Wing, 1996; Gaffar, 2002), also acting as a reducing agent (Robertson et al., 1940; Hale, 1944), it causes increasing nuclei agglomeration in suspension with time (data is not shown) perhaps due to its pH, which is also supported by the findings of Nath et al. (2014). By contrast, MB01 showed better results than OB and LB01. This buffer provided better stain–nuclei interactions than other buffers, which was reflected by a higher FL value. Only in H. spicatum was the FL value highest in A&E nuclei. Although G0/G1 CV was lower in OB-isolated nuclei of A. zerumbet and in LB01-isolated nuclei of G. sessiliflora (not statistically significant) than in MB01-isolated nuclei, the MB01 values were acceptable according to the recommendadtions of Galbraith et al. (2002). A perfect nuclei suspension should not contain any cellular debris and autofluorescent compounds, as they increase background noise. High background noise is usually the cause of high CV values (Emshwiller, 2002) and lower yield of nuclei. In all genera, the chemical makeup of MB01 helped to minimize background noise, i.e. DF, and consequently increased the overall nuclei count, i.e. YF.
Because the original composition of the buffers did not work well, the formulation of MB01 was carried out based on previous reports (Doležel et al., 1989; Nath et al., 2014) taking into account the properties of the constituent chemicals and the effect of pH. The pH of MB01 buffer was fixed based on Shen et al.’s (2013) observation that while many cellular organelles possess high pH, nuclei remain viable at pH 7·2. As MOPS has a better buffering capacity (pKa of 7·2) than Tris (pKa of 8·1) (Loureiro et al., 2006b), the former was favoured over the latter during the formulation of MB01. To increase nucleic acid stability, magnesium ions in magnesium chloride buffers (Galbraith et al., 1983; Pfosser et al., 1995), magnesium sulfate buffers (Arumuganathan and Earle, 1991) and glucose in MNB (Marie and Brown, 1993) was replaced with 0·7 mm spermine in MB01 at a concentration higher than in LB01 buffer (Doležel et al., 1989). KCl and NaCl concentrations were kept unchanged. To protect nucleic acids from nuclease activity, EDTA was used (Doležel et al., 1989; Marie and Brown, 1993) as a chelating agent for divalent cations, which acted as a nuclease cofactor (Doležel et al., 1989). Previous reports had shown that PVP decreased the effect of polyphenols by changing their conformational structure, forming hydrogen bonds, and maintaining them in a reduced state (Doyle and Doyle, 1987; Greilhuber et al., 2007; Loureiro et al., 2007b). A higher concentration of the non-ionic detergent Triton X-100 in MB01 buffer facilitated the higher chloroplast lysis, and resulted in a decreased number of fluorescent debris particles (Coba de la Peña and Brown, 2001). β-ME, being a strong reducing agent, breaks the hydrophobic interactions and checks the activity of phenolic compounds in the presence of another competitor (e.g. PVP) (Greilhuber et al., 2007). Some buffers are supplemented with sodium metabisulfite (Loureiro et al., 2007b), which acts as a reducing agent. However, sodium metabisulfite is also known to have genotoxic properties (Rencüzoğullari, 2001), which can result in erroneous genome evaluation and thus, this compound and buffers containing it were avoided in this study. Therefore, the addition of PVP with increased concentrations of β-ME and Triton X-100 in MB01 buffer was perhaps instrumental in yielding higher YF and lower DF than the other reported buffers.
The photomicrographs (Figs 2 and 3) also depicted buffer-specific nuclei quality. While MB01 showed the highest number of well-dispersed nuclei (Fig. 2F) of proper shape and size (Fig. 3F), GB showed clumped nuclei (Fig. 2B) of deformed shape (Fig. 3B). Thus, MB01 buffer provided improved nuclei quality for FCM analysis, with higher fluorescence level and yield, lower G0/G1 peak CV values and background debris factors. These favourable properties allowed the estimation of the genome size of the Zingiberaceae species studied here. The UPGMA dendrogram provided insight into the efficacy of the buffers by comparing them on the basis of the above parameters, which can be correlated with the respective buffer compositions. In the dendrogram, MB01 buffer separated from the remaining buffers as it performed best with its unique chemical constitution (Fig. 4).
Therefore, the formulation of MB01 buffer improved sample quality for FCM analysis of the chosen plants, and facilitated the estimation of DNA contents of 14 new species among the investigated taxa. Although there are previous reports of the genome sizes of six species of Curcuma, using FC:PI (Leong-Škorničková et al., 2007) and FE (Rai et al., 1997), the results obtained by MB01 are comparable. Moreover, the effects of OB in other genera were largely unknown. 2C content of Zingiber officinale (2n = 22) by Rai et al. (1997) was reported as 12·05 pg/2C, which is higher than the putative value as inferred from doubling the genome size of the diploid species studied here (2n = 22; 3·60 ± 0·045 pg). Analysis of the non-replicated monoploid genome (1Cx-value) reaffirmed the reduction in genome size as a common trend among polyploids (Leitch and Bennett, 2004). The mean 2C value of the family Zingiberaceae as given in the Kew online database (Bennett and Leitch, 2012) for C-value of angiosperms based on 39 species (one each of Alpinia, Hitchenia, Kaempferia, Paracautleya, Stahlianthus and Zingiber, and 33 Curcuma), is 2·79 ± 1·7 pg/2C. Our estimation of novel 2C nuclear genome sizes in this study has increased the average genome size of the family to 3·18 ± 1·8 pg/2C, an increase of 13·06 %.
Thus, this is the first study in which eight widely used nuclei isolation buffers were compared with a newly formulated buffer, MB01, for Zingiberaceae plant flow cytometry. The results revealed that MB01 yielded superior quality of nuclei from plants of different genera of the family from both shoot and root tissues. This buffer might be used to improve nuclei quality in other plant families rich in secondary metabolites. Nevertheless, further studies are required to gain thorough knowledge of the interaction between buffers and cellular components. Thus, using this study as a platform, it will be possible to unlock new ways of FCM sample preparation in plants to decrypt the correlation among chromosome number, ploidy and genome size.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Figure S1: FCM histograms, of nuclei suspensions prepared from root tissues of G. sessiliflora in different buffers. (A) LB01 buffer, (B) MB01 buffer, (C) Otto buffers.
ACKNOWLEDGMENTS
We thank Dr. Jarošlav Doležel; Laboratory of Molecular Cytogenetics and Cytometry, Institute of Experimental Botany, Czech Republic, for providing us with the seeds of flow cytometry standard plants; Professor Mamiyil Sabu, Department of Botany, Calicut University, Calicut, India; Prof. Abhaya Pada Das, Department of Botany, North Bengal University, West Bengal, India; Botanical survey of India, Government of India, and National Bureau of Plant Genomic Resources, Government of India, for providing the plant materials. The Director, Centre for Research in Nanoscience & Nanotechnology (CRNN), is acknowledged for providing the instrumentation facility used in this study.
LITERATURE CITED
- Arumuganathan K, Earle ED. 1991. Estimation of nuclear DNA content of plants by flow cytometry. Plant Molecular Biology Reporter 9: 229–241. [Google Scholar]
- Baranyi M, Greilhuber J. 1999. Genome size in Allium: in quest of reproducible data. Annals of Botany 83: 687–695. [Google Scholar]
- Bare P, Layssac M, D’Hont A, et al. 1998. Relationship between parental chromosomic contribution and nuclear DNA content in the coffee interspecific hybrid C. pseudozanguebariae x C. liberica var. ‘dewevrei’. Theoretical and Applied Genetics 96: 301–305. [Google Scholar]
- Bennett MD. 1972. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London. Series B, Biological Sciences 181: 109–135. [DOI] [PubMed] [Google Scholar]
- Bennett MD. 1976. DNA amount, latitude and crop plant distribution. Environmental and Experimental Botany 16: 93–108. [Google Scholar]
- Bennett MD, Leitch I. 2005. Genome size evolution in plants In: T Gregory, ed. The evolution of the genome. London: Elsevier Academic Press, 89–162. [Google Scholar]
- Bennett MD, Leitch IJ. 2012. Plant DNA C-values Database. http://data.kew.org/cvalues/. 4 April 2016.
- Bergounioux C, Perennes C, Brown SC, Gadal P. 1988. Cytometric analysis of growth regulator-dependent transcription and cell cycle progression in Petunia protoplast cultures. Planta 175: 500–505. [DOI] [PubMed] [Google Scholar]
- Bergounioux C, Brown SC, Petit PX. 1992. Flow cytometry and plant protoplast cell biology. Physiologia Plantarum 85: 374–386. [Google Scholar]
- Bhadra S, Bandyopadhyay M. 2015. Karyomorphological investigations on some economically important members of Zingiberaceae from Eastern India. Caryologia 68: 184–192. [Google Scholar]
- Bhadra S, Bandyopadhyay M. 2016. New chromosome number counts and karyotype analyses in three genera of Zingiberaceae. The Nucleus 59: 35–40. [Google Scholar]
- Bharathan G, Lambert G, Galbraith DW. 1994. Nuclear DNA content of monocotyledons and related taxa. American Journal of Botany 81: 381-386. [Google Scholar]
- Bottini MCJ, Greizerstein EJ, Aulicino MB, Poggio L. 2000. Relationships among genome size, environmental conditions and geographical distribution in natural populations of NW Patagonian species of Berberis L. (Berberidaceae). Annals of Botany 86: 565–573. [Google Scholar]
- Buitendijk JH, Boon EJ, Ramanna MS. 1997. Nuclear DNA content in twelve species of Alstroemeria L. and some of their hybrids. Annals of Botany 79: 343–353. [Google Scholar]
- Buren JPV. 1991. Function of pectin in plant tissue structure and firmness In: RH Walter, ed. The chemistry and technology of pectin. San Diego: Academic Press Inc, 1–22. [Google Scholar]
- Chan EWC, Lim YY, Wong LF, et al. 2008. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chemistry 109: 477–483. [Google Scholar]
- Coba de la Peña T, Brown SC. 2001. Cytometry and fluorimetry In: C Hawes, B Satiat-Jeunemaître, eds. Plant cell biology: a practical approach, 2nd edn Oxford: IRL Press, 85–106. [Google Scholar]
- Conell DW. 1970. The chemistry of the essential oil and oleoresin of ginger (Zingiber officinale Roscoe). Flavour Industry 1: 677–693. [Google Scholar]
- Das AB, Rai S, Das P. 1999. Karyotype analysis and cytophotometric estimation of nuclear DNA content in some members of the Zingiberaceae. Cytobios 97: 23–33. [Google Scholar]
- Doležel J, Bartoš J. 2005. Plant DNA flow cytometry and estimation of nuclear genome size. Annals of Botany 95: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doležel J, Göhde W. 1995. Sex determination in dioecious plants Melandrium album and M. rubrum using high-resolution flow cytometry. Cytometry 19: 103–106. [DOI] [PubMed] [Google Scholar]
- Doležel J, Binarová P, Lucretti S. 1989. Analysis of nuclear DNA content in plant cells by flow cytometry. Biologia Plantarum 31: 113–120. [Google Scholar]
- Doležel J, Sgorbati S, Lucretti S. 1992. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiologia Plantarum 85: 625–631. [Google Scholar]
- Doležel J, Doleželova M, Novák FJ. 1994. Flow cytometric estimation of nuclear DNA amount in diploid bananas (Musa acuminata and M. balbisiana). Biologia Plantarum 36: 351–357. [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, et al. 1998. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany 82: 17–26. [Google Scholar]
- Doležel J, Bartoš J, Voglmayr H, Greilhuber J. 2003. Nuclear DNA content and genome size of trout and human. Cytometry 51: 127–128. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233–2244. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. Genomic plant DNA preparation from fresh tissue-CTAB method. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Emshwiller E. 2002. Ploidy levels among species in the ‘Oxalis tuberosa Alliance’ as inferred by flow cytometry. Annals of Botany 89: 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffar MA. 2002. Preparation and utilization of new carboxyl group containing cation exchangers based on starch using a dry reaction method. Starch 54: 185–192. [Google Scholar]
- Galbraith DW. 2004. Cytometry and plant sciences: a personal retrospective. Cytometry 58: 37–44. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. 1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Lambert GM, Macas J, Doležel J. 2002. Analysis of nuclear DNA content and ploidy in higher plants In: JP Robinson, Z Darzynkiewicz, PN Dean, et al. , eds. Current protocols in cytometry. New York: John Wiley & Sons, 7.6.1–7.6.22. [DOI] [PubMed] [Google Scholar]
- Greilhuber J, Temsch E, Loureiro J. 2007. Nuclear DNA content measurement In: J Doležel, J Greilhuber, J Suda, eds. Flow cytometry with plant cells. Weinheim: Wiley-VCH, 67–101. [Google Scholar]
- Grime JP, Mowforth MA. 1982. Variation in genome size – An ecological interpretation. Nature 299: 151–153. [Google Scholar]
- Hale CW. 1944. Studies on diffusing factors: 4. The action of reducing agents on hyaluronic acid and other polysaccharides. Biochemical Journal 38: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SE, Dvorak WS, Johnston JS, Price HJ, Williams CG. 2000. Flow cytometric analysis of DNA content for tropical and temperate new world pines. Annals of Botany 86: 1081–1086. [Google Scholar]
- Heller FO. 1973. DNS-Bestimmung an Keimwurzeln von Vicia faba L. mit Hilfe der Impulscytophotometrie. Bericht der Deutschen Botanischen Gesellschaft 86, 437–441. [Google Scholar]
- Kabera JN, Semana E, Mussa AR, He X. 2014. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. Journal of Pharmacy and Pharmacology 2: 377–392. [Google Scholar]
- Keller ERJ, Schubert I, Fuchs J, Meister A. 1996. Interspecific crosses of onion with distant Allium species and characterization of the presumed hybrids by means of flow cytometry, karyotype analysis and genomic in situ hybridization. Theoretical and Applied Genetics 92: 417–424. [DOI] [PubMed] [Google Scholar]
- Knight CA, Ackerly DD. 2002. Variation in nuclear DNA content across environmental gradients: a quantile regression analysis. Ecology Letters 5: 66–76. [Google Scholar]
- Kress WJ, Prince LM, Williams KJ. 2002. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. American Journal of Botany 89: 1682–1696. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Bennett MD. 2004. Genome size downsizing in polyploid plants. Biological Journal of the Linnean Society 82: 651–663. [Google Scholar]
- Leong-Škorničková J, Šída O, Jarolímová V, et al. 2007. Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae). Annals of Botany 100, 505–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA, Funderburg SW. 1979. Genome size in angiosperms: temperate versus tropical species. American Naturalist 114: 784–795. [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. 2006a. Flow cytometric and microscopic analysis of the effect of tannic acid on plant nuclei and estimation of DNA content. Annals of Botany 98: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. 2006b. Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Annals of Botany 98: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Suda J, Doležel J, Santos C. 2007a. FLOWer: a plant DNA flow cytometry database In: J Doležel, J Greilhuber, J Suda, eds. Flow cytometry with plant cells. Weinheim: Wiley-VCH, 423–438. [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. 2007b. Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Annals of Botany 100: 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray CW, Grime JP. 1995. Genome size predicts frost resistance in British herbaceous plants: Implications for rates of vegetation response to global warming. Functional Ecology 9: 320–325. [Google Scholar]
- Marie D, Brown SC. 1993. A cytometric exercise in plant DNA histograms, with 2C values for 70 species. Biology of the Cell 78: 41–51. [DOI] [PubMed] [Google Scholar]
- Morgan ER, Burge GK, Seelye JF, Hopping ME, Grant JE. 1998. Production of inter-specific hybrids Limonium perezii (Stapf) Hubb. and Limonium sinuatum (L.) Mill. Euphytica 102: 109–115. [Google Scholar]
- Nag A, Bandyopadhyay M, Mukherjee A. 2013. Antioxidant activities and cytotoxicity of Zingiber zerumbet (L.) Smith rhizome. Journal of Pharmacognosy and Photochemistry 2: 102–108. [Google Scholar]
- Nandini AV, Murray BG, O’Brien IEW, Hammett KRW. 1997. Intra- and interspecific variation in genome size in Lathyrus (Leguminosae). Botanical Journal of the Linnean Society 125: 359–366. [Google Scholar]
- Nath S, Mallick SK, Jha S. 2014. An improved method of genome size estimation by flow cytometry in five mucilaginous species of Hyacinthaceae. Cytometry A 85: 833-840. [DOI] [PubMed] [Google Scholar]
- Noirot M, Barre P, Louarn J, Duperray C, Hamon S. 2000. Nucleus-cytosol interactions – A source of stoichiometric error in flow cytometric estimation of nuclear DNA content in plants. Annals of Botany 86: 309–316. [Google Scholar]
- Noirot M, Barre P, Duperray C, Louarn J, Hamon S. 2003. Effects of caffeine and chlorogenic acid on propidium iodide accessibility to DNA: Consequences on genome size evaluation in coffee tree. Annals of Botany 92: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohri D. 1998. Genome size variation and plant systemetics. Annals of Botany 82: 75–83. [Google Scholar]
- Ohri D, Khoshoo TN. 1986. Genome size in gymnosperms. Plant Systematics and Evolution 153: 119–132. [Google Scholar]
- Otto FJ. 1990. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA In: Z Darzynkiewicz, HA Crissman, eds. Methods in cell biology, Vol. 33 San Diego: Academic Press, 105–110. [DOI] [PubMed] [Google Scholar]
- Ovodov I. 1998. Polysaccharides of flower plants: structure and physiological activity. Russian Journal of Bioorganic Chemistry 24: 483–501. [PubMed] [Google Scholar]
- Pfosser M, Amon A, Lelley T, Heberle-Bors E. 1995. Evaluation of sensitivity of flow cytometry in detecting aneuploidy in wheat using disomic and ditelosomic wheat-rye addition lines. Cytometry 21: 387–393. [DOI] [PubMed] [Google Scholar]
- Pinto G, Loureiro J, Lopes T, Santos C. 2004. Analysis of the genetic stability of Eucalyptus globulus Labill. somatic embryos by flow cytometry. Theoretical and Applied Genetics 109: 580–587. [DOI] [PubMed] [Google Scholar]
- Poggio L, Burghardt AD, Hunziker JH. 1989. Nuclear DNA variation in diploid and polyploid taxa of Larrea (Zygophyllaceae). Heredity 63: 321–328. [Google Scholar]
- Poggio L, Rosato M, Chiavarino AM, Narajano CA. 1998. Genome size and environmental correlations in maize (Zea mays ssp. mays, Poaceae). Annals of Botany 82: 107–115. [Google Scholar]
- Price HJ, Bachmann K. 1975. DNA content evolution in the Microseridinae. American Journal of Botany 62: 262–267. [Google Scholar]
- Rai S, Das AB, Das P. 1997. Estimation of 4C DNA and karyotype analysis in ginger (Zingiber officinale Rosc.). Cytologia 62: 133–141. [Google Scholar]
- Rencüzoğullari E. 2001. The cytogenetic effects of sodium metabisulfite, a food preservative in root tip cells of Allium cepa L. Turkish Journal of Biology 25: 361–370. [Google Scholar]
- Robertson WVMW, Ropes MW, Bauer W. 1940. Mucinase: A bacterial enzyme which hydrolyzes synovial fluid mucin and other mucins. The Journal of Biological Chemistry 133: 261–276. [Google Scholar]
- Rohlf FJ. 1999. NTSYSpc numerical taxonomy and multivariate analysis system. Version 2.02i. Exeter Software, Setauket, New York, US.
- Santos GKN, Dutra KA, Barros RA, et al. 2012. Essential oils from Alpinia purpurata (Zingiberaceae): Chemical composition, oviposition deterrence, larvicidal and antibacterial activity. Industrial Crops and Products 40: 254–260. [Google Scholar]
- Shen J, Zeng Y, Zhuang X, et al. 2013. Organelle pH in the Arabidopsis endomembrane system. Molecular Plant 6, 1419–1437. [DOI] [PubMed] [Google Scholar]
- Šiško M, Ivanič A, Bohanec B. 2003. Genome size analysis in the genus Cucurbita and its use for determination of interspecific hybrids obtained using the embryo-rescue technique. Plant Science 165: 663–669. [Google Scholar]
- Škorničková J, Sabu M. 2005. Curcuma roscoeana Wall. (Zingiberaceae) in India. Gardens’ Bulletin Singapore 57: 187–198. [Google Scholar]
- Škorničková J, Rehse T, Sabu M. 2007. Other economically important Curcuma species In: PN Ravindran, KN Babu, K Sivaraman, eds. Turmeric: the genus Curcuma. Boca Raton, FL: CRC Press, 451–467. [Google Scholar]
- Sneath PHA, Sokal RR. 1973. The study of phylogeny In: D Kennedy, RB Park, eds. Numerical taxonomy: the principles and practice of numerical classification. San Francisco: WH Freeman and company, 309–361. [Google Scholar]
- Taheri S, Abdullah TL, Karimi E, Oskoueian E, Ebrahimi M. 2014. Antioxidant capacities and total phenolic contents enhancement with acute gamma irradiation in Curcuma alismatifolia (Zingiberaceae) leaves. International Journal of Molecular Sciences 15: 13077–13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann C, Guadagnuolo R, Felber F. 2000. Search for spontaneous hybridization between oilseed rape (Brassica napus L.) and wild radish (Raphanus raphanistrum L.) in agricultural zones and evaluation of the genetic diversity of the wild species. Botanica Helvetica 111: 107–119. [Google Scholar]
- Thibault J. 1998. Nuclear DNA amount in pure species and hybrid willows (Salix): a flow cytometric investigation. Canadian Journal of Botany 76: 157–165. [Google Scholar]
- Ulrich I, Ulrich W. 1986. Flow cytometric DNA analysis of plant protoplasts with DAPI . Zeitschrift für Naturforschung 41: 1052–1056. [Google Scholar]
- Ulrich I, Fritz B, Ulrich W. 1988. Application of DNA fluorochromes for flow cytometric DNA analysis of plant protoplasts. Plant Science 55: 151–158. [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, et al. 2003. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiology 132: 1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamiya I, Newton RJ, Johnston JS, Price HJ. 1993. Genome size and environmental factors in the genus Pinus. American Journal of Botany 80: 1235–1241. [Google Scholar]
- Walker D, Moñino I, Correal E. 2006. Genome size in Bituminaria bituminosa (L.) C.H. Stirton (Fabaceae) populations: separation of ‘true’ differences from environmental effects on DNA determination. Environmental and Experimental Botany 55: 258–265. [Google Scholar]
- Wing RE. 1996. Starch citrate: preparation and ion exchange properties. Starch 48: 275–279. [Google Scholar]
- Yusuf NA, Suffian M, Annuar M, Khalid N. 2013. Existence of bioactive flavonoids in rhizomes and plant cell cultures of Boesenbergia rotunda (L.) Mansf. Kulturpfl. Australian Journal of Crop Science 7: 730–734. [Google Scholar]
- Zonneveld BJM. 2001. Nuclear DNA content of all species of Helleborus (Ranunculaceae) discriminate between species and sectional divisions. Plant Systematics and Evolution 229: 125–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.