Abstract
Background and Aims Bromeliads are able to occupy some of the most nutrient-poor environments especially because they possess absorptive leaf trichomes, leaves organized in rosettes, distinct photosynthetic pathways [C3, Crassulacean acid metabolism (CAM) or facultative C3–CAM], and may present an epiphytic habit. The more derived features related to these traits are described for the Tillandsioideae subfamily. In this context, the aims of this study were to evaluate how terrestrial predators contribute to the nutrition and performance of bromeliad species, subfamilies and ecophysiological types, whether these species differ in their ecophysiological traits and whether the physiological outcomes are consistent among subfamilies and types (e.g. presence/absence of tank, soil/tank/atmosphere source of nutrients, trichomes/roots access to nutrients).
Methods Isotopic (15N-enriched predator faeces) and physiological methods (analyses of plant protein, amino acids, growth, leaf mass per area and total N incorporated) in greenhouse experiments were used to investigate the ecophysiological contrasts between Tillandsioideae and Bromelioideae, and among ecophysiological types when a predatory anuran contributes to their nutrition.
Key Results It was observed that Bromelioideae had higher concentrations of soluble protein and only one species grew more (Ananas bracteatus), while Tillandsioideae showed higher concentrations of total amino acids, asparagine and did not grow. The ecophysiological types that showed similar protein contents also had similar growth. Additionally, an ordination analysis showed that the subfamilies and ecophysiological types were discrepant considering the results of the total nitrogen incorporated from predators, soluble protein and asparagine concentrations, relative growth rate and leaf mass per area.
Conclusions Bromeliad subfamilies showed a trade-off between two strategies: Tillandsioideae stored nitrogen into amino acids possibly for transamination reactions during nutritional stress and did not grow, whereas Bromelioideae used nitrogen for soluble protein production for immediate utilization, possibly for fast growth. These results highlight that Bromeliaceae evolution may be directly associated with the ability to stock nutrients.
Keywords: Bromelioideae, Tillandsioideae, ecophysiological types, trichomes, phytotelmata, amino acids, asparagine, nitrogen flux, stable isotopes
INTRODUCTION
Bromeliaceae is considered a monophyletic family and comprises 58 genera and 3346 Neotropical species within eight subfamilies (Crayn et al., 2004; Bremer et al., 2009; Givnish et al., 2011; Escobedo-Sarti et al., 2013). Among flowering plants, Bromeliaceae are remarkable considering their vegetative features that allow them to occur in many habitats, such as tropical forests, dry savannas, rocky fields and semi-arid regions, from sea level to mountainous areas (Benzing, 1986, 2000; Crayn et al., 2004; Gitaí et al., 2005). Bromeliads are capable of occurring in resource-poor environments because they possess a number of traits that allow efficient uptake and use of water and nutrients, including foliar trichomes (i.e. epidermal cells that absorb water and nutrients) and the improvement of their absorptive capacity, the presence of phytotelmata (i.e. tanks with water) and the diversification of carbon metabolism (Benzing and Burt, 1970; Benzing, 2000; Givnish et al., 2014).
Bromeliad subfamilies (i.e. Brocchinioideae, Lindmanioideae, Hechtioideae, Navioideae, Pitcairnioideae, Puyoideae, Bromelioideae and Tillandsioideae) have contrasting growth forms, morphological and physiological features, and distinct flower morphology, features that may explain their different habitat preferences (Benzing and Burt, 1970; Givnish et al., 2011). Benzing (2000) divided the family into five ecophysiological types according to their sources of nutrients (i.e. soil, tank or atmosphere) and their traits to access them (i.e. foliar trichomes and/or roots). Bromelioideae is a monophyletic subfamily with some exclusively terrestrial genera included in the ecophysiological type II (e.g. Ananas and Bromelia); they do not form phytotelmata, have non-specialized trichomes in absorbing complex nitrogen compounds (e.g. amino acids) and bear well-developed roots responsible for water and nutrient acquisition from soil (Benzing and Burt, 1970; Benzing, 2000; Endres and Mercier, 2003). Moreover, other Bromelioideae genera are type III (e.g. Aechmea, Neoregelia and Quesnelia), are terrestrial or epiphytic, and have phytotelmata, specialized trichomes and roots responsible for fixing the plant into the substrate (Benzing and Burt, 1970; Benzing, 2000). Furthermore, the Tillandsioideae subfamily is monophyletic and shares the most derived morphological features of the family (Benzing et al., 1985; Benzing, 2000). All species of this subfamily are epiphytic and dependent on their leaves to absorb nutrients; some of them are type IV and have phytotelmata (e.g. Vriesea) while others are known as atmospheric epiphytes, included in the ecophysiological type V (e.g. some Tillandsia), which absorb nutrients from the atmosphere, representing the most derived response of Bromeliaceae to multiple environmental stresses (Benzing, 1986, 2000; Martin, 1994). Benzing (2000) described the ecophysiological types III and IV as a tank-absorbing trichome group with anchorage and conditionally absorptive roots; however, type II is predominantly CAM (i.e. performs Crassulacean acid metabolism) while type IV is C3.
Epiphytic bromeliads obtain nutrients mostly from the deposition of canopy leaves, the atmosphere and interaction with animals. Since these nutrient sources are intermittent, epiphytic bromeliads may store them to use during nutritional stress conditions instead of using them for growth (Laube and Zotz, 2003). On the other hand, terrestrial bromeliads may utilize nutrients to grow as their roots are in constant contact with soil (Laube and Zotz, 2003). Indeed, Benzing (1983) reported that atmospheric bromeliads of the Tillandsioideae family did not grow with added fertilizer, suggesting that these plants have a slow-growing strategy, an adaptive response to oligotrophic environments (Aerts and Chapin, 2000). In more recent experiments, Endres and Mercier (2001a, b, 2003) showed that the epiphytic Vriesea gigantea (Tillandsioideae) presented higher levels of total free amino acids on leaves and accumulated asparagine after the addition of ammonium compared with the terrestrial tankless Ananas comosus (Bromelioideae). In contrast, the addition of this nutrient had little effect on the amino acid profile of A. comosus. The authors commented that the production of amino acids, especially asparagine, might be associated with temporary nutrient storage. Furthermore, these studies suggest differences in the allocation of nutrients into amino acids or growth between epiphytes and terrestrial bromeliads, but we still do not know whether this mechanism is consistent among other species from the two subfamilies or among bromeliad ecophysiological types (see Benzing, 2000).
Bromeliad leaves are organized in rosettes that allow the development of terrestrial and aquatic ecosystems (e.g. in tank bromeliads) where many organisms can live and drive bromeliad functioning (Benzing, 2000; Ngai and Srivastava, 2006; Romero et al., 2006, 2010; Gonçalves et al., 2014). These organisms are usually divided into functional groups, such as micro-organisms that mineralize organic compounds retained by bromeliad rosettes, detritivores (e.g. Chironomidae and Scirtidae) that are responsible for debris decomposition, and aquatic (e.g. Zygoptera and Tabanidae) and terrestrial predators (e.g. spiders and anurans) that can control bromeliad food chains (Romero et al., 2006, 2010; Romero and Srivastava, 2010). Additionally, aquatic and terrestrial predators can directly improve bromeliad nutrition through their faeces, prey carcasses and other detritus from their activities (Benzing, 2000; Ngai and Srivastava, 2006; Romero et al., 2006, 2010; Gonçalves et al., 2011, 2014, 2016; Leroy et al., 2016). However, preliminary evidence suggests that the contribution of terrestrial predators to the mineral nutrition of bromeliads can vary among different subfamilies and plant life forms. For example, bromeliads with developed trichomes and with poorly developed root systems are more dependent on leaves to obtain water and nutrients, and those with epiphytic habit and/or with a tank may derive more nutrients from terrestrial predators (Benzing, 2000; Gonçalves et al., 2011; Leroy et al., 2016).
Many species of predatory anurans can use bromeliads temporarily or may depend on these plants for their survival and reproduction in tropical forests (Silva et al., 1989; Endres and Mercier, 2001a, b; Romero et al., 2010). Faeces of anurans and other predators are rich in nitrogen (i.e. urea and guanina; Lehninger et al., 1993; Duellman and Trueb, 1994; Romero et al., 2006) and can be an important nutrient source for bromeliad species in nutrient-poor habitats. Distinct morphological and physiological features among bromeliads can also affect the relative contribution of animal-derived nutrient sources to bromeliad nutrition. Although all Tillandsioideae species were described to have the more derived traits within bromeliads (i.e. developed trichomes, dependence on leaves to obtain water and nutrients, a poorly developed root system, epiphytism and amino acid storage), to the best of our knowledge there are no controlled experiments showing that these traits benefit Tillandsioideae more than other bromeliad species. In this study, we conducted an experiment using isotopic and physiological methods to evaluate (1) how predators (i.e. the anuran Dendropsophus nanus) contribute to the nutrition and performance of several Bromelioideae and Tillandsioideae species of distinct ecophysiological types; (2) whether these subfamilies and types differ in ecophysiological traits; and (3) whether the physiological outcomes are consistent among species within each subfamily and type.
MATERIALS AND METHODS
Organisms and experiment design
The Bromelioideae species Ananas bracteatus, Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta (all CAM), and the Tillandsioideae species Vriesea gigantea, Vriesea bituminosa and Tillandsia cyanea (all C3) were chosen to evaluate how terrestrial predators contribute to the nutrition and physiological attributes of bromeliads with distinct eco-physiological traits. Ananas bracteatus, an ecophysiological type II, is a terrestrial tankless species that occurs in Colombia, Brazil, Paraguay and Argentina (Benzing, 2000). Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta are type III, are terrestrial or epiphytic, have phytotelmata and are found in restingas and outcrops of Brazil (Benzing, 2000; Romero and Vasconcellos-Neto, 2004; Romero, 2006). Vriesea gigantea and V. bituminosa are type IV, are epiphytic, have phytotelmata and occur in Brazil, but V. bituminosa also occurs in Venezuela (Benzing, 2000; Endres and Mercier, 2001a, b; Romero, 2006). Tillandsia cyanea is type V, and an epiphytic tankless species that occur in coastal rainforests of Ecuador (Gilmartin, 1977; Benzing, 2000).
All bromeliad species were bought at Veiga Arquitetura e Paisagismo® (CEASA, Campinas, São Paulo state, Brazil) and were similar in number of leaves and size to minimize the effects of N dilution [number of leaves between 11 and 36 units, mean ± s.e.: 22·4 ± 0·7 leaves among species, one-way analysis of variance (ANOVA) of number of leaves among species: P = 0·098; the longest leaf length between 10 and 15 cm, mean ± s.e.: 12·6 ± 0·1 cm of leaf length, one-way ANOVA of leaf length among species: P = 0·155]. Bromeliads were grown in a homogeneous soil mixture of a bark of Pinus sp., vermiculite and peat. To test the contribution of predatory anurans to the nutrition and performance of bromeliad subfamilies and types, we performed a greenhouse experiment from February to April 2009, lasting 70 d, at the campus of UNESP University in São José do Rio Preto (São Paulo state, Brazil). All plants were kept in a net greenhouse (1 mm mesh diameter) and were watered with a limited amount of water with an automatic irrigation system by a fine spray using three sprinklers, each with a release of 8 L h−1 that was activated for 15 min every 2 h, just enough to avoid excessive desiccation. The greenhouse net impeded the contact of bromeliads with any metazoans as we did not observe any organism in bromeliads throughout the experiment.
The experiment had two treatments that were applied to each species of bromeliad: (1) 15N-labelled anuran faeces (n = 6 bromeliads) and (2) no faeces (control; n = 6). Control and treatment bromeliads were kept in the same greenhouse, but placed in separate stands 2 m apart. The anuran Dendropsophus nanus (Hylidae) is a small predator and was used as a model organism. Fifty D. nanus males were collected in the field (Nova Itapirema, São Paulo state) and kept in glass flasks of approx. 7 cm diameter and 10 cm in height in the laboratory at UNESP. Each anuran was fed every 2 d with 20 15N-enriched fruit flies (Drosophila melanogaster), an interval sufficient for the anuran to capture all the flies and produce faeces (mean ± s.e. of dry weight of faeces: 97 ± 15 mg). At 2 d intervals, anuran faeces were stored in polypropylene tubes for subsequent application to experimental bromeliads every 2 d (35 applications). The 15N-enriched anuran faeces were carefully applied with tweezers to the central part of the rosettes at the base of the leaves. The flies were cultured from eggs in a medium of 15N-labelled yeast. Details of the laboratory procedure for yeast and fly enrichment can be found in Romero et al. (2006).
Isotopic analyses
Two of the youngest leaves of each bromeliad rosette were randomly collected at the end of the experiment for isotopic analyses. The leaves were homogenized together and were dried at 60 °C for 48 h, crushed to obtain a fine powder and stored dry in polypropylene tubes until analysis. The total N concentration (μg mg−1 dry leaf tissue) of bromeliad leaves and the δ15N of flies, faeces and bromeliads were determined with an isotope ratio mass spectrometer (20–20 mass spectrometer; PDZ Europa, Sandbach, UK) after sample combustion to N2 at 1000 °C by an on-line elemental analyser (PDZ Europa ANCA-GSL) in the Stable Isotope Facility at the University of California, Davis. The nitrogen fraction in bromeliads that received faeces (fA) was calculated using mixing model equations with two sources of nitrogen (i.e. soil and faeces) and one single isotopic signature (e.g. δ15N; see Phillips and Gregg, 2001). The 15N fractioning during its assimilation and the metabolic process of plants was calculated according to the equation of McCutchan et al. (2003): fA = (δM − δB − Δδ15N)/(δA − δB). The fA is the proportionate contribution of labelled faeces absorbed by bromeliads (%), δM is the isotope ratio of bromeliads that received faeces, δA and δB are the isotope ratios of potential nitrogen sources (faeces and soil, respectively) and Δδ15N is the trophic shift for nitrogen between diet (e.g. faeces or soil) and consumer (e.g. bromeliads). The values of Δδ15N used were +3·3 ± 0·26‰ [(mean ± s.e.); Caut et al., 2009].
Analyses of plant protein, total amino acids and asparagine
To determine if the nitrogen derived from predators had some influence on soluble protein concentration, two leaves from the intermediate part of each bromeliad rosette were randomly collected, were cut together into small pieces (1 cm2), and 1 g of these leaves was frozen in liquid nitrogen and homogenized with 3 mL of ultra-pure water. The homogenate was centrifuged at 12 000 rpm (g) for 10 min, and the supernatant (15 μL) was used to measure the protein concentration (Bradford, 1976). The absorbance was measured using a spectrophotometer at 595 nm, and a standard curve was obtained with bovine serum albumin. Total amino acid and asparagine concentrations were analysed by reverse phase HPLC with o-phthaldialdehyde (OPA) derivates as described previously in Puiatti and Sodek (1999).
Bromeliad growth, leaf mass per area and total N incorporated
To determine if the nitrogen derived from predators affected bromeliad growth, two leaves from the intermediate part of each bromeliad rosette but different from those used in physiological analysis were randomly labelled and their lengths were measured individually at the beginning and end of the experiment. The bromeliad leaf length was directly related to the leaf biomass (linear regressions: A. bracteatus, r2 = 0·69, P < 0·001; N. cruenta, r2 = 0·68, P < 0·001; Q. arvensis, r2 = 0·73, P < 0·001; A. blanchetiana, r2 = 0·59, P = 0·006; V. bituminosa, r2 = 0·75, P < 0·001; V. gigantea, r2 = 0·68, P < 0·001; T. cyanea, r2 = 0·69, P = 0·003). Bromeliad leaves showed continuous growth during the experiment and their relative growth rate [RGR; ln(cm) d−1] was calculated using the following equation: RGR = [ln(Lfinal) – ln(Linitial)]/(t2 − t1). The ln(Lfinal) and ln(Linitial) are, respectively, the natural logarithm of the foliar final length and the natural logarithm of the foliar initial length, with t2 – t1 being the number on days between the initial and final measurements.
To estimate the bromeliad leaf dry mass per area (LMA, g m−2), three leaves of the fourth inner node from each bromeliad rosette of the control treatment (different from leaves used for physiological analyses) had their central portion cut into 5 cm2 pieces, dried at 60 °C for 48 h and the weight of each piece was obtained individually. LMA was taken only from control bromeliads to avoid the measurement of an enhancement of leaf mass when bromeliads received 15N-enriched anuran faeces. For each experimental bromeliad, the total N incorporated during the experiment was calculated by multiplying the total nitrogen concentration of bromeliad leaves with their RGR and their LMA. This measure shows the allocation of N in the construction of leaf structures, either to growth or to increasing the weight per unit of leaf area.
Statistical analyses
Comparisons of total N concentration, total N incorporated, concentrations of soluble protein, amino acids and asparagine, RGR and LMA between subfamilies and among ecophysiological types were performed using linear mixed models [LME; subfamilies or ecophysiological types and treatments (faeces addition) were fixed effects and bromeliad species were a random effect]. We checked the normality and heteroscedasticity of residuals with standard diagnostic plots and metrics. Comparisons among bromeliad species were performed using nested ANOVA (species nested within subfamilies or ecophysiological types, Supplementary Data Tables S1 and S2) and Tukey HSD post-hoc tests were used for pair-wise comparisons. Statistical differences between treatments within each bromeliad species were performed using the t-test. The percentage of nitrogen in leaves of bromeliad species derived from predator faeces was compared using ANOVA and Tukey HSD post-hoc tests for pair-wise comparisons. We performed a t-test to compare the percentage of nitrogen derived from faeces between the two subfamilies and ANOVA to compare the percentage of nitrogen derived from faeces among ecophysiological types. Non-metric multidimensional scaling (NMDS) plot ordination was constructed considering the results of the total nitrogen incorporated, soluble protein and asparagine concentrations, RGR and LMA of leaves of each bromeliad species after receiving faeces in order to show the discrepancy between subfamilies and ecophysiological types. All statistical analyses were conducted with the statistical platform R (R Core Team, 2014).
RESULTS
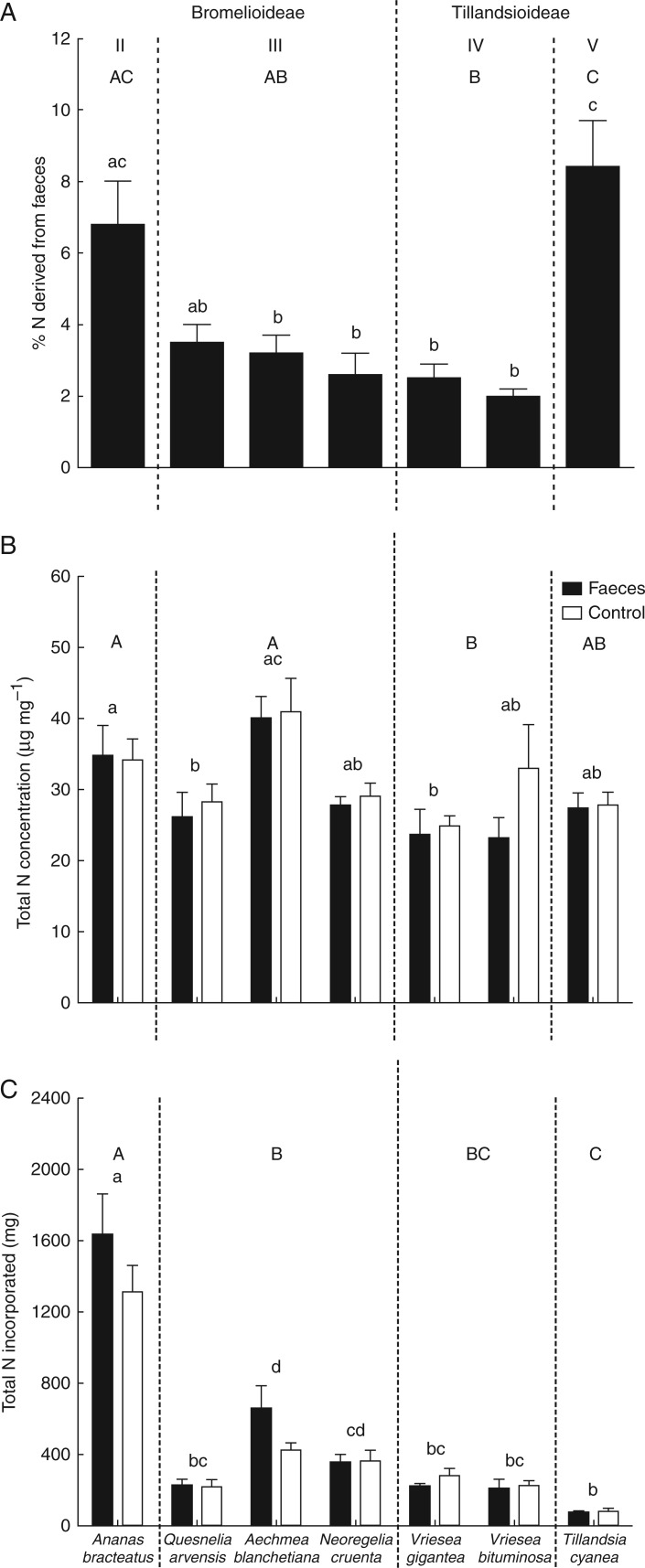
The amount of nitrogen derived from anuran faeces did not differ between subfamilies (d.f. = 2, t = 0·22, P = 0·845), but was variable among bromeliad species (F6,41 = 10·07, P < 0·001; Fig. 1A; Table S1) and bromeliad ecophysiological types (F3,23 = 8·64, P < 0·001; Fig. 1A; Table S2). Tillandsia cyanea (type V, mean ± s.e., 8·4 ± 1·3 %) and Ananas bracteatus (type II, 6·8 ± 1·2 %) derived a similar amount of nitrogen from predators and were the species that derived more nitrogen (Table 1; Fig. 1A). Quesnelia arvensis (type III, 3·5 ± 0·5 %), Aechmea blanchetiana (type III, 3·2 ± 0·5 %), Neoregelia cruenta (type III, 2·6 ± 0·6 %), Vriesea gigantea (type IV, 2·5 ± 0·4 %) and Vriesea bituminosa (type IV, 2 ± 0·2 %) derived a similar amount of nitrogen from predators, but those from the ecophysiological type IV differed from those from type V, while type III was similar to type II (Table 1; Fig. 1A). Despite predators having contributed to bromeliad nutrition, total nitrogen concentration and total nitrogen incorporated by bromeliad leaves did not differ between the subfamilies (total N concentration, F1,75 = 3·44, P = 0·122; N incorporated, F1,61 = 2·53, P = 0·172; Table 2; Fig. 1B, C). The ecophysiological types showed a similar total nitrogen concentration, but differed in the total nitrogen incorporated (total N concentration, F3,73 = 1·34, P = 0·407; N incorporated, F3,59 = 31·40, P = 0·009; Table 2, Fig. 1B, C). Nevertheless, comparisons among species and ecophysiological types revealed that A. bracteatus (type II) incorporated the greatest amount of nitrogen, while T. cyanea (type V) incorporated the lowest amount (Fig. 1C; Tables S1 and S2). The addition of faeces did not alter the total N concentration or the N incorporated within each species (Fig. 1B, C; Table S1).
Fig. 1.
(A) The percentage of nitrogen in the Bromelioideae species Ananas bracteatus (ecophysiological type II), Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta (type III), and in the Tillandsioideae species Vriesea gigantea and Vriesea bituminosa (type IV) and Tillandsia cyanea (type V) derived from Dendropsophus nanus faeces. Values were obtained from the two-source mixing models equations (see the Materials and Methods for details). (B) Values of total nitrogen concentration of each species after treatments with predator faeces and controls. (C) Total nitrogen incorporated by leaves of each bromeliad species after the two treatments. Bars indicate the s.e.m. Different lower case letters indicate statistical differences among species, and upper case letters indicate statistical differences among ecophysiological types (ANOVA/Tukey HSD post-hoc test, α = 0·05).
Table 1.
Average δ15N values of Drosophila melanogaster flies, Dendropsophus nanus anurans and their enriched faeces, and values of leaves of Ananas bracteatus, Quesnelia arvensis, Aechmea blanchetiana, Neoregelia cruenta, Vriesea gigantea, Vriesea bituminosa and Tillandsia cyanea that received anuran faeces and controls
| Treatment | δ15N (s.e.m.) | n |
|---|---|---|
| Drosophila melanogaster | ||
| Natural abundance | 3·07 (0·19) | 2 |
| Enriched | 1530·51 (76·95) | 5 |
| Anuran (adult male) | ||
| Natural abundance | 12·64 (0·37) | 2 |
| Enriched | 542·04 (82·58) | 5 |
| Anuran faeces | ||
| Natural abundance | 8·97 (0·21) | 3 |
| Enriched | 1046·47 (50·10) | 5 |
| A. bracteatus | ||
| Faeces | 70·43 (11·79) | 6 |
| Control | 10·68 (0·31) | 6 |
| Q. arvensis | ||
| Faeces | 52·64 (4·62) | 6 |
| Control | 8·33 (0·31) | 6 |
| A. blanchetiana | ||
| Faeces | 46·42 (4·63) | 6 |
| Control | 11·56 (0·26) | 6 |
| N. cruenta | ||
| Faeces | 46·59 (5·72) | 6 |
| Control | 9·21 (0·31) | 6 |
| V. gigantea | ||
| Faeces | 38·86 (3·85) | 6 |
| Control | 9·38 (0·44) | 6 |
| V. bituminosa | ||
| Faeces | 30·33 (1·39) | 6 |
| Control | 8·75 (0·38) | 6 |
| T. cyanea | ||
| Faeces | 84·57 (11·98) | 6 |
| Control | 9·02 (0·34) | 6 |
n, number of replicates.
Table 2.
Linear mixed models (LME) summarizing the effects of treatments (Dendropsophus nanus faeces and control) on the total nitrogen concentration, total nitrogen incorporated, soluble protein, total amino acids and asparagine concentrations, relative growth rate of leaves and leaf mass per area (LMA) of Bromeliaceae subfamilies and ecophysiological types (Bromelioideae, type II, Ananas bracteatus; type III, Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta; Tillandsioideae, type IV, Vriesea gigantea and Vriesea bituminosa; type V, Tillandsia cyanea)
| Source of variation | Treatments | Subfamilies | Treatments vs. subfamilies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | d.f. | F | P | d.f. | F | P | |
| Total N concentration | 75 | 1·42 | 0·235 | 5 | 3·44 | 0·122 | 75 | 0·71 | 0·399 |
| Total N incorporated | 61 | 0·27 | 0·603 | 5 | 2·51 | 0·173 | 61 | 4·14 | 0·046 |
| Soluble protein | 74 | 10·08 | 0·002 | 5 | 0·11 | 0·748 | 74 | 12·13 | <0·001 |
| Total amino acids | 75 | 151·16 | <0·001 | 5 | 41·71 | 0·001 | 75 | 189·16 | <0·001 |
| Asparagine | 75 | 65·01 | <0·001 | 5 | 96·70 | <0·001 | 75 | 87·05 | <0·001 |
| Relative growth rate | 75 | 6·88 | 0·010 | 5 | 2·74 | 0·158 | 75 | 2·62 | 0·109 |
| LMA | – | – | – | 5 | 1·37 | 0·293 | – | – | – |
| Treatments | Types | Treatments vs. types | |||||||
| d.f. | F | P | d.f. | F | P | d.f. | F | P | |
| Total N concentration | 73 | 1·06 | 0·305 | 3 | 1·34 | 0·407 | 73 | 0·45 | 0·715 |
| Total N incorporated | 59 | 0·88 | 0·350 | 3 | 31·40 | 0·009 | 59 | 1·47 | 0·229 |
| Soluble protein | 73 | 9·16 | 0·003 | 3 | 5·31 | 0·101 | 73 | 22·7 | <0·001 |
| Total amino acids | 73 | 186·7 | <0·001 | 3 | 37·5 | 0·007 | 73 | 84·8 | <0·001 |
| Asparagine | 73 | 63·4 | <0·001 | 3 | 49·2 | 0·004 | 73 | 28·4 | <0·001 |
| Relative growth rate | 73 | 17·4 | <0·001 | 3 | 23·9 | 0·013 | 73 | 1·4 | 0·229 |
| LMA | – | – | – | 3 | 16·9 | 0·021 | – | – | – |
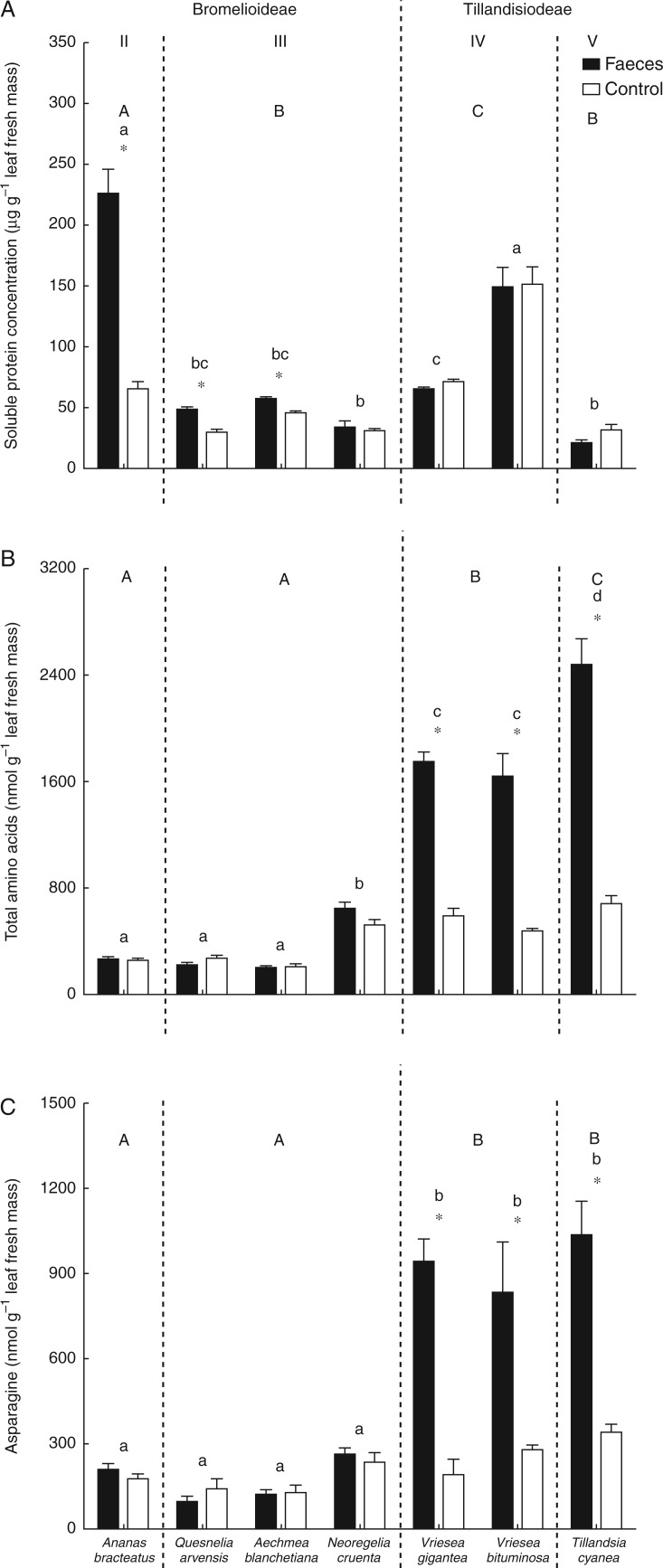
Soluble protein concentration on leaves did not differ between subfamilies or ecophysiological types (subfamilies, F1,74 = 0·11, P = 0·748; types, F3,73 = 5·31, P = 0·101) especially because types III and V showed similar soluble protein concentration (Table 2; Fig. 2A). Ananas bracteatus, Q. arvensis and A. blanchetiana (Bromelioideae) had more soluble protein after receiving predator faeces than Tillandsioideae species (treatments, F1,74 = 10·08, P = 0·002; subfamilies vs. treatments, F1,74 = 12·13, P < 0·001; Table 2; Fig. 2A; Table S1). Species also differed in their soluble protein content as A. bracteatus and V. bituminosa had the highest concentrations while N. cruenta and T. cyanea had the lowest concentrations (Fig. 2A; Table S1). On the other hand, total amino acid and asparagine concentrations were higher in Tillandsioideae if compared with Bromelioideae (amino acids, F1,75 = 41·71, P = 0·001; asparagine, F1,75 = 96·70, P < 0·001), thus they were high in the ecophysiological types IV and V (Table 2; Fig. 2B, C). Despite the fact that types IV and V had a similar amount of asparagine, type V showed more amino acid content than type IV (Fig. 2B, C). Additionally, only Tillandsioideae species had higher contents of amino acids and asparagine after receiving predator faeces (Fig. 2B, C; Table S1).
Fig. 2.
(A) Soluble protein concentration for the Bromelioideae species Ananas bracteatus (ecophysiological type II), Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta (type III), and in the Tillandsioideae species Vriesea gigantea and Vriesea bituminosa (type IV) and Tillandsia cyanea (type V) after treatments with Dendropsophus nanus faeces and controls. (B) Total amino acid concentration for each species of bromeliads after the two treatments. (C) Total asparagine concentration for each species of bromeliads after the two treatments. Bars indicate the s.e.m. Asterisks (*) indicate statistical differences between treatments within each bromeliad species (t-test, α = 0·05), different lower case letters indicate statistical differences among species, and upper case letters indicate statistical differences among ecophysiological types (ANOVA/Tukey HSD post-hoc test, α = 0·05).
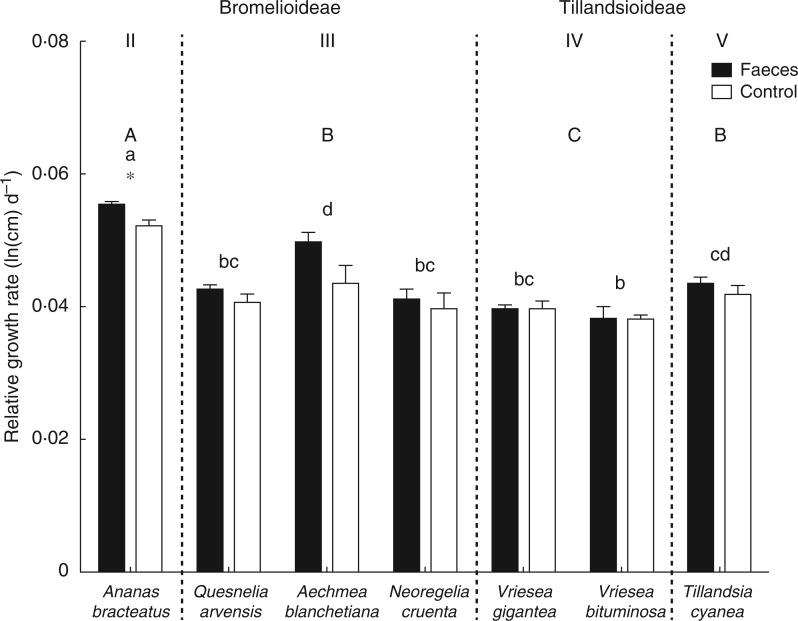
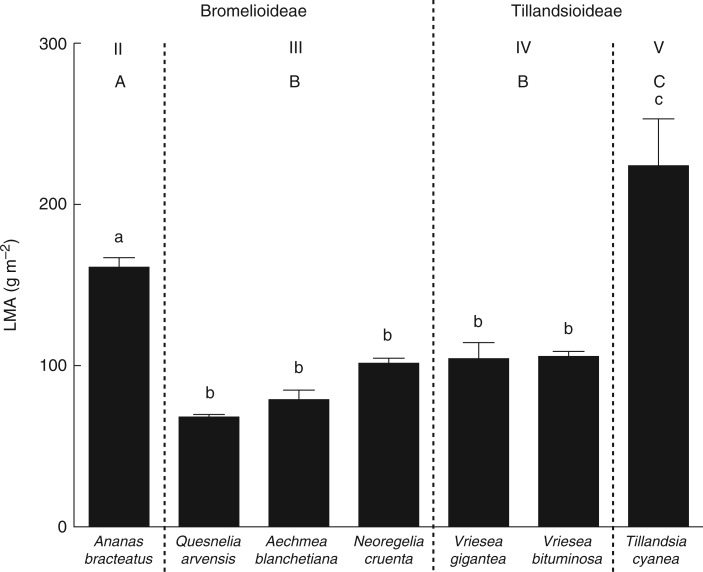
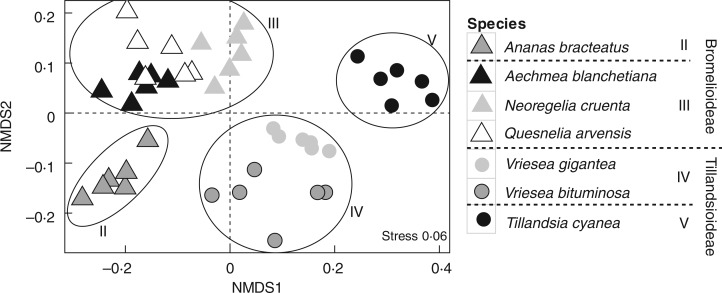
The RGR did not differ between subfamilies, but differed among ecophysiological types (subfamilies, F1,75 = 2·74, P = 0·158; types, F3,73 = 23·9, P = 0·013; Table 2; Fig. 3). However, types III and V showed a similar RGR (Fig. 3). Ananas bracteatus grew more than other species and was the only species that had a higher growth rate after receiving predator faeces (treatment, F1,75 = 6·88, P = 0·010; Fig. 3; Table S1). The LMA did not differ between subfamilies, but differed among ecophysiological types (subfamilies, F1,28 = 1·37, P = 0·293; types, F3,28 = 16·9, P = 0·021; Table 2; Fig. 4). The tankless species T. cyanea (type V) and A. bracteatus (type II) had the highest LMA, while tank species from types III and IV had the lowest leaf mass per area (Fig. 4; Table S1). The NMDS ordination plot reinforces that Bromelioideae and Tillandsioideae subfamilies and the ecophysiological types are discrepant in the use and allocation of nitrogen (Fig. 5).
Fig. 3.
Relative growth rate of the leaves of the Bromelioideae species Ananas bracteatus (ecophysiological type II), Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta (type III), and in the Tillandsioideae species Vriesea gigantea and Vriesea bituminosa (type IV) and Tillandsia cyanea (type V) after treatments with Dendropsophus nanus faeces and controls. Asterisks (*) indicate statistical differences between treatments within each bromeliad species (t-test, α = 0·05), different lower case letters indicate statistical differences among species, and upper case letters indicate statistical differences among ecophysiological types (ANOVA/Tukey HSD post-hoc test, α = 0·05).
Fig. 4.
Leaf mass per area (LMA) of the leaves of the Bromelioideae species Ananas bracteatus (ecophysiological type II), Quesnelia arvensis, Aechmea blanchetiana and Neoregelia cruenta (type III), and in the Tillandsioideae species Vriesea gigantea and Vriesea bituminosa (type IV) and Tillandsia cyanea (type V). Different lower case letters indicate statistical differences among species, and upper case letters indicate statistical differences among ecophysiological types (ANOVA/Tukey HSD post-hoc test, α = 0·05).
Fig. 5.
Non-metric multidimensional scaling (NMDS) plot derived from the total nitrogen incorporated, soluble protein and asparagine concentrations, relative growth rate and leaf mass per area of leaves of each bromeliad species after receiving Dendropsophus nanus faeces. Triangles indicate Bromelioideae and circles indicate the Tillandsioideae subfamily. Roman letters indicate the ecophysiological types of each bromeliad species (see the Materials and Methods for details).
DISCUSSION
Our results indicate a trade-off between soluble protein production and nutritional storage in Bromeliaceae, as the bromeliad subfamilies and ecophysiological types showed contrasting strategies in nitrogen use. Apparently, Tillandsioideae species have the strategy to store nutrients, and this may be an evolutionary trait selected to live in oligotrophic environments. Furthermore, Bromelioideae species seem to produce proteins and may use them for growth since their developed roots may access nutrients more easily. We observed that the ecophysiological types with similar soluble protein production showed similar growth. In addition, one of the features that stood out in bromeliads was the presence or absence of the tank, as species without a tank derived more nitrogen from predators and showed the highest leaf biomass.
Bromelioideae had increased the concentration of soluble proteins after receiving predator faeces, and only one Bromelioideae species grew more (i.e. Ananas bracteatus). In contrast, Tillandsioideae showed high concentrations of amino acids and asparagine but did not grow after receiving faeces. Apparently, a trade-off in protein production or accumulating nitrogen may exist among Bromeliaceae subfamilies: while Bromelioideae species allocate nitrogen to soluble proteins that may be converted into growth but have a lower ability to store nitrogen, Tillandsioideae species accumulate nitrogen in amino acids, probably keeping the nitrogen stored under conditions of nutritional stress. Soluble proteins are a source of nitrogen circulating on the leaves of bromeliads that can be used at any time, while asparagine appears to be a sink of nitrogen that can be converted into other amino acids by transamination reactions (Endres and Mercier, 2001a, b, 2003). Additionally, we observed that the ecophysiological types that showed similar soluble protein content also had similar growth (i.e. types III and V). Other studies showed a similar pattern between these two subfamilies, as a Bromelioideae species (i.e. Ananas comosus) had a lower concentration of asparagine in leaves while a Tillandsioideae species (i.e. Vriesea gigantea) accumulated this amino acid (Endres and Mercier, 2003). In addition, Romero et al. (2010) showed that V. bituminosa (Tillandsioideae) may have accumulated amino acids after receiving frog faeces since its soluble protein concentration decreased by a half. Nitrogen storage appears to be an adaptive response of Tillandsioideae to survive in oligotrophic environments and should be even more intense in atmospheric epiphytes since these plants depend on nutrients that are in low concentrations in the atmosphere (Benzing, 2000).
Bromeliad leaves are considered the most essential vegetative organs due to their ability to absorb nutrients through trichomes (Benzing, 2000). This ability was particularly important to T. cyanea and A. bracteatus, since they derived more nitrogen from predators through their leaves if compared with other species. These species have no phytotelmata and, therefore, have no external reservoir to accumulate nutrients and water. Thus, since nutrients come into contact with trichomes of tankless species, the mechanism of efficient nutrient acquisition by leaves could have been selected during Bromeliaceae evolution to avoid nutrient losses. On the other hand, regardless of the subfamily and ecophysiological types, bromeliads with a tank obtained less nitrogen from predators than species without phytotelmata, possibly because of the dilution of nutrients by the water and the occurrence of a diversity of micro-organisms on phytotelmata. The tank water may dilute nutrients that should be concentrated at the base of leaves, where there is a larger amount of trichomes (Takahashi et al., 2007). Thus, water reduces the contact of nutrients with the base of leaves, increasing nutrient contact with apical parts where the number of trichomes is gradually replaced by stomata. Additionally, bromeliads with phytotelmata might possess a more favourable environment for the occurrence of a greater diversity of micro-organisms, algae and metazoans compared with bromeliads without a tank. In fact, numerous micro-organisms can live in the bromeliad phyllosphere, where mineralization provides nutrients that can be absorbed easily by trichomes (Inselsbacher et al., 2007; Gonçalves et al., 2014). These micro-organisms and algae can immobilize amino acids and/or ammonium and compete for nutrients with bromeliads (Benzing, 2000; Inselsbacher et al., 2007). Additionally, some insects can immobilize nutrients from the tank in their bodies and take them out of the bromeliad system when they emerge as adults (Ngai and Srivastava, 2006). Therefore, bromeliads with phytotelmata may be competing more with micro-organisms, algae and some metazoans compared with T. cyanea and A. bracteatus.
Tillandsia cyanea derived 20 % more nitrogen from predators than A. bracteatus, possibly because Tillandsioideae species, especially that from ecophysiological type V, possess more developed trichomes and in a greater abundance with numerous mitochondria that assist them during the absorption of nutrients (Sakai and Sanford, 1980; Benzing, 1986, 2000). Leaf nutrient uptake is significant for T. cyanea because it occupies habitats with scarce rainfall periods where water and nutrients must be absorbed quickly and in a greater amount when available (Benzing et al., 1976). In contrast, A. bracteatus is in contact with soil nutrients and incorporated the largest amount of nitrogen among the species tested, which possibly allowed its greatest growth. These results may be related to the greater amount of nitrogen that A. bracteatus derived from predators, the allocation of this nutrient in the production of soluble proteins, and its use in vegetative development. On the other hand, T. cyanea have obtained as much nitrogen from predators as A. bracteatus; however, T. cyanea showed lower growth and accumulated asparagine as did other species of Tillandsioideae.
The two tankless bromeliads, T. cyanea and A. bracteatus, also showed the highest values of LMA. This result may be linked to the allocation of nutrients to the construction of leaf tissues since building leaves with high LMA requires a greater investment (Wright et al., 2004). However, it is possible that these species have shown a greater LMA for different reasons, i.e. while T. cyanea must have more sclerenchyma tissues to increase its leaf life span, A. bracteatus should have more chlorenchyma, allowing a greater photosynthetic activity and growth (d’Eeckenbrugge and Leal, 2002). Future studies may further investigate the differences between photosynthetic and mechanical tissues in bromeliads with similar LMA but with distinct ecophysiological strategies. High values of LMA allow continuous operation of the functions performed by leaves and are associated with long leaf life span, extent and abundance of air spaces and water-storing tissues (i.e. hydrenchyma) (Rascio et al., 1990; Witkowski and Lamont, 1991; Wright et al., 2002, 2004; Freschi et al., 2010). Since the species with high LMA did not have phytotelmata and species with low LMA had a tank with water, high LMA may be related to water storage. Thus, we can observe two distinct strategies concerning water storage that are clearly unrelated to the bromeliad subfamilies or ecophysiological types: those bromeliads that may store water in the hydrenchyma with high LMA, and those that store water outside the leaves (i.e. in phytotelmata) with low LMA.
In this study, we demonstrate that predators contribute to the nutrition of bromeliads, as already reported by Romero et al. (2006, 2010) and Gonçalves et al. (2011, 2014, 2016). Since many species of bromeliads live in oligotrophic environments such as rocky outcrops, sandy soils and canopy forests, these plants need to obtain nutrients from sources other than soil. Here, we showed that species of distinct subfamilies and types possess contrasting resource-use strategies, as they showed distinct ecophysiological plasticity. So far, CAM metabolism and the trichomes have dominated discussions about the evolution of Bromeliaceae, in which CAM allowed the evolution of epiphytism, for example, and epiphytism allowed speciation (Givnish et al., 2014; Donoghue and Sanderson, 2015). In turn, this study highlights the exceptional influence of storage of amino acids for bromeliad occurrence in nutrient-poor environments, which may have favoured its radiation into a wide range of environments. The metabolic pathways associated with amino acid production and transamination, and the presence of a tank may be the important directions for future studies on Bromeliaceae evolution.
SUPPLEMENTARY DATA
Supplementary data are available online at ww.aob.oxfordjournals.org and consist of the following. Table S1: ANOVA of the percentage of nitrogen in bromeliad leaves derived from Dendropsophus nanus faeces, and nested ANOVA (bromeliad species nested within subfamilies) summarizing the effects of treatments (faeces and control) on the total nitrogen concentration, total nitrogen incorporated, soluble protein, total amino acids and asparagine concentrations, relative growth rate of leaves and leaf mass per area of Ananas bracteatus, Quesnelia arvensis, Aechmea blanchetiana, Neoregelia cruenta (Bromelioideae) and Vriesea gigantea, Vriesea bituminosa, Tillandsia cyanea (Tillandsioideae). Table S2: ANOVA of the percentage of nitrogen in bromeliad leaves derived from Dendropsophus nanus faeces, and nested ANOVA (bromeliad species nested within ecophysiological types) summarizing the effects of treatments (faeces and control) on the total nitrogen concentration, total nitrogen incorporated, soluble protein, total amino acids and asparagine concentrations, relative growth rate of leaves and leaf mass per area of Ananas bracteatus (ecological type II), Quesnelia arvensis (type III), Aechmea blanchetiana (type III), Neoregelia cruenta (type III), Vriesea gigantea (type IV), Vriesea bituminosa (type IV) and Tillandsia cyanea (type V).
ACKNOWLEDGEMENTS
We thank Hilario Azol for help with sampling of anurans in the field, Dr Ladaslav Sodek, Dr Camila Aguetoni Cambuí, Cassia Ayumi Takahashi and Patrícia Britto helped with the physiological analyses. This study was supported by a FAPESP scholarship to A.Z.G. [2011/10137-8], and by FAPESP and CNPq research grants to G.Q.R.
LITERATURE CITED
- Aerts R, Chapin FS., III 2000. The mineral nutrition of wild plants revisited: a reevaluation of processes and patterns. Advances in Ecological Research 30: 1–67. [Google Scholar]
- Benzing DH. 1983. Vascular epiphytes: a survey with special reference to their interactions with other organisms In: Sutton SL, Whitmor TC, Chadwick AC, eds. Tropical rain forest: ecology and management. Oxford: Blackwell Scientific Publications, 11–24. [Google Scholar]
- Benzing DH. 1986. Foliar specializations for animal-assisted nutrition in Bromeliaceae In: Juniper B, Southwood R, eds. Insects and the plant surface. London: Edward Arnold, 235–256. [Google Scholar]
- Benzing DH. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge: Cambridge University Press. [Google Scholar]
- Benzing DH, Burt KM. 1970. Foliar permeability among twenty species of the Bromeliaceae. Bulletin of the Torrey Botanical Club 5: 269–279. [Google Scholar]
- Benzing DH, Givinish TJ, Bermudes D. 1985. Absorptive trichomes in Brocchinia reducta (Bromeliaceae) and their evolutionary and systematic significance. Systematic Botany 10: 81–91. [Google Scholar]
- Benzing DH, Henderson K, Kessel B, Sulak J. 1976. The absorptive capacities of bromeliad trichomes. American Journal of Botany 63: 1009–1014. [Google Scholar]
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bremer K, Chase M, Fay M, Reveal J, Soltis P, Stevens P. 2009. An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. [Google Scholar]
- Caut S, Angulo E, Courchamp F. 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453. [Google Scholar]
- Crayn DM, Winter K, Smith JAC. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the neotropical family Bromeliaceae. Proceedings of the National Academy of Sciences, USA 101: 3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Eeckenbrugge GC, Leal F. 2002. Morphology, anatomy and taxonomy In: Bartholomew DP, Paull RE, Rohrbach KG, eds. The pineapple: botany, production and uses. Honolulu: CABI Publishing, 13–32. [Google Scholar]
- Donoghue MJ, Sanderson MJ. 2015. Confluence, synnovation, and depauperons in plant diversification. New Phytologist 207: 260–274. [DOI] [PubMed] [Google Scholar]
- Duellman WE, Trueb L. 1994. Biology of amphibians. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Endres L, Mercier H. 2001a. Influence of nitrogen forms on the growth and nitrogen metabolism of bromeliads. Journal of Plant Nutrition 24: 29–42. [Google Scholar]
- Endres L, Mercier H. 2001b. Ammonium and urea as nitrogen sources for bromeliads. Journal of Plant Pysiology 158: 205–212. [Google Scholar]
- Endres L, Mercier H. 2003. Amino acid uptake and profile in bromeliads with different habitats cultivated in vitro. Plant Physiology and Biochemistry 41: 181–187. [Google Scholar]
- Escobedo-Sarti J, Ramírez I, Leopardi C, et al. 2013. A phylogeny of Bromeliaceae (Poales, Monocotyledoneae) derived from an evaluation of nine supertree methods. Journal of Systematics and Evolution 51: 743–757. [Google Scholar]
- Freschi L, Takahashi CA, Cambui CA, et al. 2010. Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water storage. Journal of Plant Physiology 167: 526–533. [DOI] [PubMed] [Google Scholar]
- Gilmartin AJ. 1977. Variation and distribution: a new bromeliad taxon from Ecuador. Taxon 26: 223–226. [Google Scholar]
- Gitaí J, Horres R, Benko-Iseppon AM. 2005. Chromosomal features and evolution of Bromeliaceae. Plant Systematics and Evolution 253: 65–80. [Google Scholar]
- Givnish TJ, Barfus MHJ, Van Ee B, et al. 2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. American Journal of Botany 98: 872–895. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Barfuss MHJ, Van Ee BV, et al. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78. [DOI] [PubMed] [Google Scholar]
- Gonçalves AZ, Mercier H, Mazzafera P, Romero GQ. 2011. Spider-fed bromeliads: seasonal and interspecific variation in plant performance. Annals of Botany 107: 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves AZ, Hoffmann FL, Mercier H, Mazzafera P, Romero GQ. 2014. Phyllosphere bacteria improve animal contribution to plant nutrition. Biotropica 46: 170–174. [Google Scholar]
- Gonçalves AZ, Oliveira RS, Oliveira PS, Romero GQ. 2016. Species-specific effects of ant inhabitants on bromeliad nutrition. PLoS One 11: e0152113. doi:10.1371/journal.pone.0152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W. 2007. Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea. New Phytologist 175: 311–320. [DOI] [PubMed] [Google Scholar]
- Laube S, Zotz G. 2003. Which abiotic factors limit vegetative growth in a vascular epiphyte? Functional Ecology 17: 598–604. [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. 1993. Amino acid oxidation and the production of urea In: AL Lehninger, DL Nelson, MM Cox, eds. Principles of biochemistry. New York: Worth Publisher, 506–537. [Google Scholar]
- Leroy C, Carrias JF, Céréghino R, Corbara B. 2016. The contribution of microorganisms and metazoans to mineral nutrition in bromeliads. Journal of Plant Ecology 9: 241–255. [Google Scholar]
- Martin CE. 1994. Physiological ecology of the Bromeliaceae. Botanical Review 1: 1–82. [Google Scholar]
- McCutchan JHJ, Lewis WMJ, Kendall C, McGrath C. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulfur. Oikos 102: 378–390. [Google Scholar]
- Ngai JT, Srivastava DS. 2006. Predators accelerate nutrient cycling in a bromeliad ecosystem. Science 314: 963. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Gregg JW. 2001. Uncertainty in source partitioning using stable isotopes. Oecologia 127: 171–179. [DOI] [PubMed] [Google Scholar]
- Puiatti M, Sodek L. 1999. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiology and Biochemistry 37: 767–773. [Google Scholar]
- R Core Team. 2014. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria: URL http://www.R-project.org/ (last accessed 1 May 2016). [Google Scholar]
- Rascio A, Cedola MC, Toponi M, Flagella Z, Wittmer G. 1990. Leaf morphology and water status change in Triticum durum under water stress. Physiologia Plantarum 78: 462–467. [Google Scholar]
- Romero GQ. 2006. Geographic range, habitats and host plants of bromeliad-living jumping spiders (Salticidae). Biotropica 38: 522–530. [Google Scholar]
- Romero GQ, Srivastava DS. 2010. Food-web composition affects cross-ecosystem interactions and subsidies. Journal of Animal Ecology 79: 1122–1131. [DOI] [PubMed] [Google Scholar]
- Romero GQ, Vasconcellos-Neto J. 2004. Spatial distribution patterns of jumping spiders associated with terrestrial bromeliads. Biotropica 36: 596–601. [Google Scholar]
- Romero GQ, Mazzafera P, Vasconcellos-Neto J, Trivelin PCO. 2006. Bromeliad-living spiders improve host plant nutrition and growth. Ecology 87: 803–808. [DOI] [PubMed] [Google Scholar]
- Romero GQ, Nomura F, Gonçalves AZ, et al. 2010. Nitrogen fluxes from treefrogs to tank epiphytic bromeliads: an isotopic and physiological approach. Oecologia 162: 941–949. [DOI] [PubMed] [Google Scholar]
- Sakai WS, Sanford WG. 1980. Ultrastructure of the water-absorbing trichomes of pineapple (Ananas comosus, Bromeliaceae). Annals of Botany 46: 7–11. [Google Scholar]
- Silva HR, Britto-Pereira MC, Caramaschi U. 1989. Frugivory and seed dispersal by Hyla truncata, a neotropical treefrog. Copeia 3: 781–783. [Google Scholar]
- Takahashi CA, Ceccantini GCT, Mercier H. 2007. Differential capacity of nitrogen assimilation between apical and basal leaf portions of a tank epiphytic bromeliad. Brazilian Journal of Plant Physiology 19: 119–126. [Google Scholar]
- Witkowski ETF, Lamont B. 1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88: 486–493. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M, Reich PB. 2002. Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology 90: 534–543. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.