Abstract
Objective
To study metabolic/inflammatory biomarker risk profiles in women with PCOS and PCOS offspring.
Design
Cross-sectional comparison of serum biomarkers.
Setting
University Medical Center Utrecht.
Patients
Hyperandrogenic PCOS women (HA-PCOS, n = 34), normoandrogenic PCOS women (NA-PCOS, n = 34), non-PCOS reference population (n = 32), PCOS offspring (n = 14, age 6–8 years), and a paedriatic reference population (n = 30).
Main Outcome Measure(s)
Clustering profile of adipocytokines (IL-1b, IL-6, IL-13, IL-17, IL-18, TNF-α, adiponectin, adipsin, leptin, chemerin, resistin, RBP4, DPP-IV/sCD26, CCL2/MCP-1), growth factors (PIGF, VEGF, sVEGF-R1), soluble cell adhesion molecules (sICAM-1/sCD54, sVCAM-1/sCD106), and other inflammatory related proteases (MMP-9, S100A8, Cathepsin S). Differences in median biomarker concentrations between groups, and associations with the free androgen index (FAI; Testosterone/SHBG x100).
Results
The cluster analysis identified leptin, RBP-4, DPP-IV and adiponectin as potential discriminative markers for HA-PCOS with a specifically strong correlation in cases with increased BMI. Leptin (R2 = 0.219) and adiponectin (R2 = 0.182) showed the strongest correlation with the FAI. When comparing median protein concentrations adult PCOS women with or without hyperandrogenemia, the most profound differences were observed for leptin (P < 0.001), DPP-IV (P = 0.005), and adiponectin (P < 0.001). Adjusting for age, BMI and multiple testing attenuated all differences. In PCOS offspring, MMP-9 (P = 0.001) and S100A8 (P < 0.001) concentrations were significantly higher compared to a healthy matched reference population, even after correcting for age and BMI and adjustment for multiple testing.
Conclusion
In this preliminary investigation we observed significant differences in adipocytokines between women with or without hyperandrogenic PCOS and non-PCOS controls, mostly influenced by BMI. Leptin and adiponectin showed the strongest correlation with the FAI in adult women with PCOS. In PCOS offspring other inflammatory biomarkers (MMP-9, S100A8) were increased, suggesting that these children may exhibit increased chronic low-grade inflammation. Additional research is required to confirm results of the current exploratory investigation.
Introduction
The polycystic ovary syndrome (PCOS) is the most common endocrinopathy amongst women of reproductive age, with a prevalence up to 15%.[1] PCOS is a heterogeneous condition of unknown origin, and is characterized by the presence of at least two of the following features: ovulatory dysfunction, hyperandrogenism, and polycystic ovarian morphology.[2] PCOS is associated with various cardiometabolic risk factors including obesity, hyperinsulinemia and dyslipidemia, which may result in type 2 diabetes mellitus, atherosclerosis and cardiovascular disease (CVD) later in life.[3–6] Moreover, PCOS is known to be associated with chronic low-grade inflammation as demonstrated by increased circulating inflammatory markers such as CRP, TNF-α, Il-18.[7–9]
Insulin resistance along with hyperandrogenism appear to play pivotal roles in the pathophysiology of PCOS.[10,11] Hyperandrogenism and obesity induce cardiometabolic dysfunction and chronic low-grade inflammation in women with PCOS.[12] The extent of increased cardiovascular events during later life in women with PCOS remains to be established. Children born to PCOS mothers may even exhibit increased cardiometabolic risk since early endocrine abnormalities (e.g. hyperandrogenism, hyperinsulinemia) and endothelial dysfunction have previously been reported in these children.[13,14]
Current research is therefore increasingly focusing on the discovery of novel biomarker profiles to further elucidate the complex pathophysiology of PCOS.[15,16] In the future, such biomarkers may serve to identify women with PCOS who are at particular risk for later life metabolic and cardiovascular disease, and who may benefit from secondary prevention strategies.
The current explorative study was designed to compare metabolic and inflammatory biomarker risk profiles between women with different PCOS phenotypes, offspring of PCOS mothers and reference populations. We hypothesized that metabolic and inflammatory biomarkers may be increased in women with PCOS and PCOS offspring at a young age. In doing so, we focussed on a wide range of tailored biomarkers based on the recent literature also including various closely-related novel biomarkers.
Materials and Methods
Conduction of the current study was approved by the official Medical Ethical Committee Board of the University Medical Center Utrecht, and conducted according to the principles expressed in the Declaration of Helsinki. All included adult participants provided written informed consent, and written informed parental consent was obtained of all included children. Clinical trials were registered at www.clinicaltrials.gov, trial registration number NCT02309047 (adult PCOS), and NCT01767051 (PCOS offspring).
Study population
PCOS
We included women with PCOS from a large prospective cohort study on menstrual cycle disturbances within the University Medical Center Utrecht. All women were evaluated through a standardized screening protocol which has been previously described in detail elsewhere.[5] PCOS was diagnosed according to the Rotterdam criteria in the presence of two or more of the following criteria: oligo‐ and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovarian morphology as assessed by transvaginal ultrasound.[2]
We included n = 34 hyperandrogenic women with PCOS (HA-PCOS), who exhibited ovulatory dysfunction, polycystic ovarian morphology and a free androgen index (FAI) > 4.5 [FAI: (Testosterone(nmol/L)/ SHBG(nmol/L)) x100)].[17] Subsequently we included n = 34 normoandrogenic women with PCOS (NA-PCOS) who exhibited ovulatory dysfunction, polycystic ovarian morphology, and a normal free androgen index (FAI) < 4.5. In this group only women with a normal body mass index (BMI) ≤25 kg/m2 were included, hence reflecting a truly mild PCOS reference group.
Non-PCOS reference population
We included n = 32 women without PCOS with regular menstrual cycles (21–35 days) from a cohort study regarding characteristics of women undergoing IVF/ICSI treatment within the University Medical Center Utrecht.All women included in the reference population were clinically evaluated and definitely classified as non-PCOS, hence composing a non-PCOS reference population. Serum samples were collected prior to the start of fertility treatment.
PCOS offspring
We included n = 14 children (6–8 years of age) who were born to PCOS mothers, from a cohort study regarding child health of children born to PCOS mothers within the University Medical Center Utrecht. Included children underwent a standardized screening with study procedures identical to those performed in the reference population (see below).[18,19]
Pediatric reference population. We included n = 30 children (7–8 years of age) from a population-based birth cohort study regarding determinants of wheezing illnesses and cardiovascular disease.[19] Included children underwent a standardized screening which has been described in detail elsewhere.[18,19]
Fasting serum samples were collected from all participants included in the current study. Serum samples were stored within 4 hours after withdrawal in -80 degrees Celsius.
Multiplex immunoassay
Serum samples were used to measure the concentrations of 22 proteins, being: IL-1b, IL-6, IL-13, IL-17, IL-18, TNF-α, adiponectin, adipsin, leptin, chemerin, resistin, retinol-binding protein 4 (RBP4), dipeptidyl peptidase IV (DPP-IV/sCD26), monocyte chemotactic protein 1 (CCL2/MCP-1), placental growth factor (PIGF), vascular endothelial growth factor (VEGF), soluble VEGF receptor-1 (sVEGF-R1), soluble intercellular adhesion molecule 1 (sICAM-1/sCD54), soluble vascular cell adhesion molecule 1 (sVCAM-1/sCD106), matrix metallopeptidase 9 (MMP-9), S100A8, Cathepsin S.
Laboratory measurements were performed using an in-house developed and validated multiplex immunoassay based on Luminex technology (xMAP, Luminex Austin TX USA). The assay was performed using previously described methods.[20–22] In short, a-specific heterophilic immunoglobulins were preabsorbed from all samples with heteroblock (Omega Biologicals, Bozeman MT, USA). Then samples were incubated with antibody-conjugated MagPlex microspheres for one hour at room temperature with continuous shaking, followed by one hour incubation with biotinylated antibodies, and 10 min incubation with phycoerythrin-conjugated streptavidin diluted in high performance ELISA buffer (HPE, Sanquin the Netherlands). Data acquisition was performed with the Biorad FlexMAP3D (Biorad laboratories, Hercules USA) in combination with xPONENT software version 4.2 (Luminex). Laboratory data were analyzed by 5-parametric curve fitting using Bio-Plex Manager software, version 6.1.1 (Biorad).
Statistical analyses
Study sample size was determined according to availability of serum samples. Basic descriptive statistics were used to describe the patient population.
Kruskal Wallis tests were performed to compare patient characteristics and one-way ANOVA were performed to compare log-transformed biomarker values between HA-PCOS women, NA-PCOS women and non-PCOS women. When this resulted in a P-value < 0.05, pairwise Student’s T-test were used to calculate P-values between specific groups. Next, P-values were adjusted for age and BMI using general linear models, and false discovery rates (FDR) were calculated to correct for multiple testing. In the pediatric populations, Mann-Whitney U tests and Chi-square tests were used to compare baseline characteristics. Student’s t-tests were used to assess differences in log-transformed biomarker concentrations between both groups, including specific sub-analyses for gender. P-values were adjusted for age and BMI using general linear models and FDR was calculated to correct for multiple testing.
Before log-transformation, biomarker values that were below the lower limit of detection were imputed at 35% of the lower detection limit concentration. Values which were above the highest limit of detection were imputed as the highest value of detection concentration plus one pg/ml.
Finally, as described previously, an unsupervised hierarchal clustering analysis, with min-max normalization per protein, was performed to investigate the discriminative potential of a single or a combination of proteins [23].
All statistical analyses were performed with SPSS Statistics 21.0. Hierarchical cluster analyses were performed using Omniviz 6.1.2 (Instem Scientific).
Results
First, we assessed whether protein concentrations differ between HA-PCOS, NA-PCOS and the non-PCOS reference group. Clinical baseline characteristics of involved study groups are shown in Table 1.
Table 1. Baseline characteristics of women with PCOS and non PCOS reference group.
| HA-PCOS (n = 34) | NA-PCOS (n = 34) | non-PCOS (n = 32) | P-value | ||
|---|---|---|---|---|---|
| Ethnicity | 0.54 | ||||
| Caucasian | 31 (91) | 33 (97) | 28 (90) | ||
| Mediterranean | 1 (3) | - | 2 (7) | ||
| Indian | 2 (6) | - | - | ||
| Black | - | 1 (3) | - | ||
| Asian | - | - | 1 (3) | ||
| Age (years) | 28.5 [23.5–32.5] | 28.8 [25.8–31.2] | 34.5 [30.7–37.7] | < 0.001 a,c | |
| FAI | 6.7 [5.0–10.2] | 2.0 [1.4–2.6] | - | ||
| BMI (kg/m2) | 29.5 [23.3–35.6] | 21.8 [19.8–22.2] | 22.5 [21.2–24.5] | < 0.001a,b,c | |
| ART indication | - | ||||
| Idiopathic infertility | - | - | 13 (41) | ||
| Male factor | - | - | 15 (47) | ||
| Tubal factor | - | - | 2 (6) | ||
| Low ovarian reserve | - | - | 1 (3) | ||
| Donor semen | - | - | 1 (3) |
PCOS: polycystic ovary syndrome, NA: normoandrogenic, HA: hyperandrogenic, FAI: free androgen index (Testosterone/SHBG)x100), BMI: body mass index, ART: assisted reproduction technology. Values are depicted as medians [interquartile ranges], or absolute numbers (percentages). Depicted variables contained a maximum of 3% missing values. P-values were calculated using Kruskal Wallis ANOVA. When a P-value < 0.05 was detected pairwise Mann Whitney U tests were used to assess differences between specific groups.
a P < 0.05 between HA PCOS and non PCOS.
b P < 0.05 between HA PCOS and NA PCOS.
c P < 0.05 between NA PCOS and non PCOS
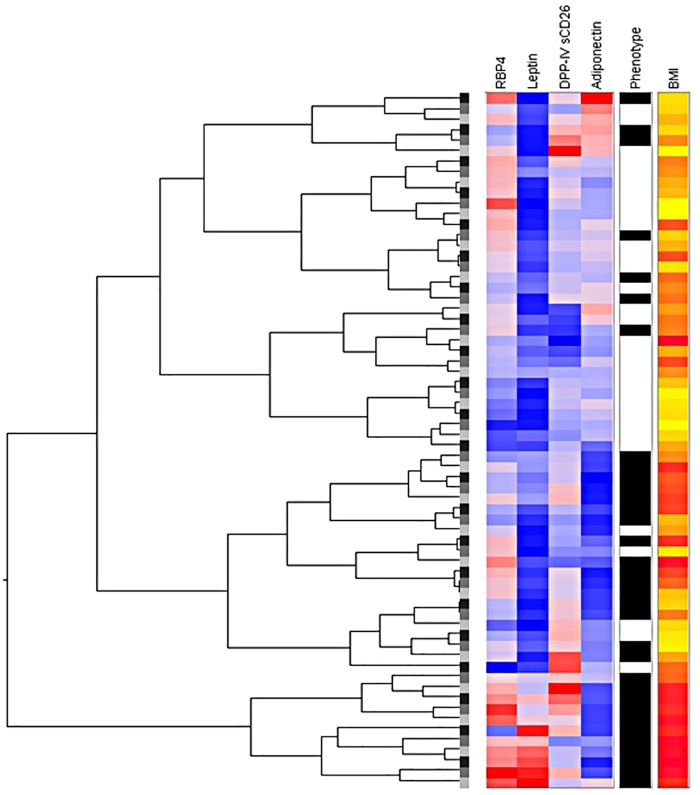
In order to assess the discriminative potential of a single or a combination of any marker(s) in HA-PCOS, NA-PCOS and the non-PCOS group, we performed a hierarchical cluster analysis as described by van den Ham et al.[23] This analysis showed clustering of leptin, RBP-4, DPP-IV and adiponectin specific for HA-PCOS with a strong correlation of cases with increased BMI, as is depicted in Fig 1. The complete clustering of all proteins is shown in S1 Fig.
Fig 1. Hierarchical cluster analysis in women with hyperandrogenic PCOS and non-PCOS women.
PCOS: polycystic ovary syndrome, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV. Phenotype: black represents hyperandrogenic PCOS; White represents non-PCOS reference population. BMI: yellow represents low BMI, red represents high BMI.
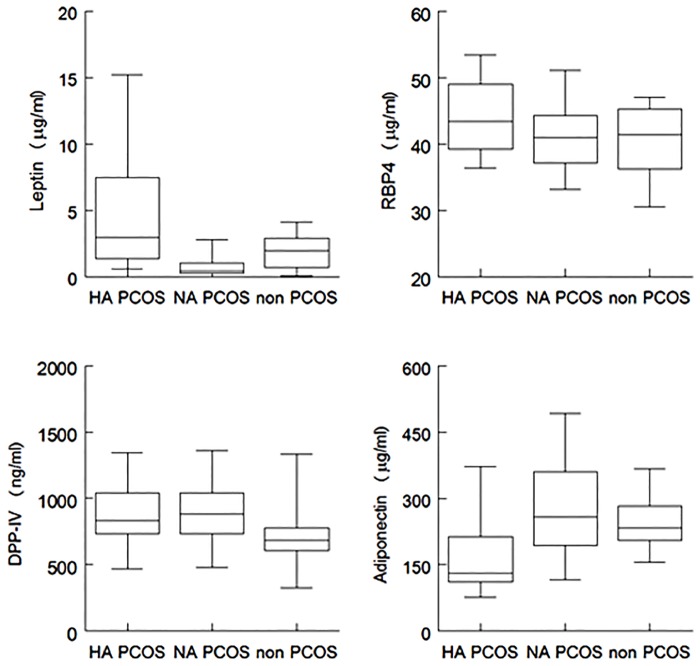
Fig 2 shows the discriminative potential of these four markers in both PCOS groups and the non-PCOS group.
Fig 2. Biomarker concentrations of most discriminative markers in women with PCOS and non-PCOS women.
RBP4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV. PCOS: polycystic ovary syndrome, HA: hyperandrogenic, NA: normoandrogenic, IL: interleukin,, DPP-IV/sCD26: dipeptidyl peptidase IV.
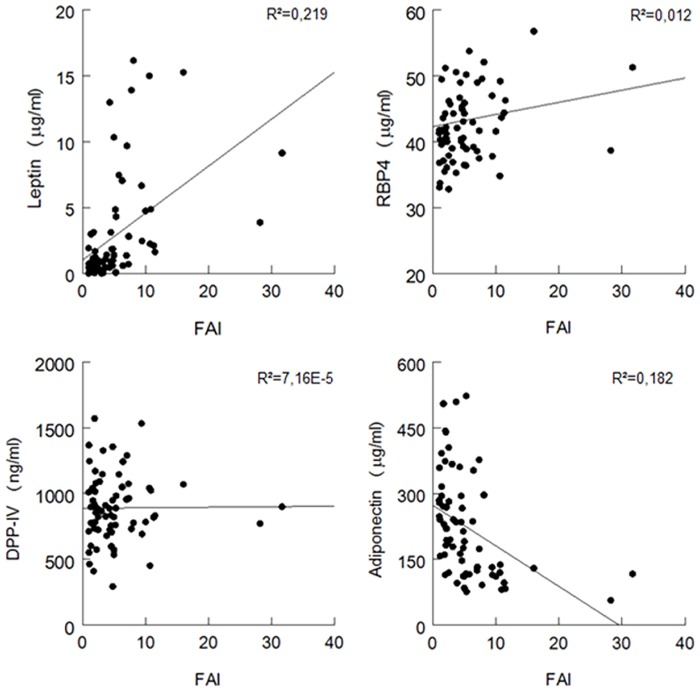
Subsequently, we assessed the correlation between these four potential biomarkers and the FAI, being one of the clinical features of PCOS. The strongest correlations were found for leptin (R2 = 0.219) and adiponectin (R2 = 0.182) as shown in, Fig 3.
Fig 3. Correlation between biomarker concentrations and the free androgen index (FAI) in women with PCOS.
RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV.
Next, we compared differences in median protein concentrations between adult PCOS groups (see Tables 2 and 3). The most profound differences were found for leptin (P <0.001), DPP-IV (P = 0.005), and adiponectin (P < 0.001). Differences in leptin and adiponectin concentrations between groups were no longer significant after adjustment for BMI and age. DPP-IV remained significantly increased in HA-PCOS versus non-PCOS women after adjustment for BMI and age (P = 0.049), however significance was last after correction for multiple testing (P = 0.29).
Table 2. Biomarker concentrations in PCOS women and non-PCOS reference group.
| HA-PCOS (n = 34) | NA-PCOS (n = 34) | non-PCOS (n = 32) | P-value | |
|---|---|---|---|---|
| IL-13 (pg/ml) | 4.5 [0.9–19.5] | 5.4 [1.5–29.2] | 0.9 [0.9–6.6] | 0.043 |
| IL-18 (pg/ml) | 95 [52–135] | 75 [47–103] | 87 [61–116] | 0.53 |
| CCL2/MCP-1 (pg/ml) | 101 [83–139] | 111 [82–136] | 112 [80–152] | 0.25 |
| PIGF (pg/ml) | 35 [18–46] | 28 [14–42] | 28 [15–44] | 0.33 |
| VEGF (ng/ml) | 0.4 [0.3–0.5] | 0.3 [0.1–0.5] | 0.4 [0.2–0.7] | 0.34 |
| MMP-9 (μg/ml) | 1.8 [1.2–3.1] | 1.3 [0.8–2.2] | 1.7 [0.8–2.4] | 0.16 |
| sVEGF-R1 (ng/ml) | 1.4 [1.2–1.7] | 1.5 [1.4–1.9] | 1.4 [1.1–1.6] | 0.053 |
| S100A8 (ng/ml) | 2.5 [1.2–4.5] | 0.9 [0.3–4.6] | 2.6 [0.7–5.3] | 0.19 |
| Adipsin (ng/ml) | 0.4 [0.2–0.4] | 0.3 [0.2–0.4] | 0.3 [0.2–0.4] | 0.31 |
| Leptin (ng/ml) | 3.0 [1.4–7.9] | 0.4 [0.3–1.1] | 1.9 [0.7–2.9] | <0.001 |
| Resistin (ng/ml) | 38 [27–54] | 31 [24–39] | 35 [30–45] | 0.017 |
| RBP4 (μg/ml) | 43 [39–49] | 41 [37–44] | 41 [36–45] | 0.10 |
| DPP-IV/ sCD26 (μg/ml) | 0.8 [0.7–1.0] | 0.9 [0.7–1.0] | 0.7 [0.6–0.8] | 0.005 |
| sICAM1/ sCD54(μg/ml) | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.97 |
| sVCAM (μg/ml) | 3.7 [3.2–4.5] | 3.8 [3.2–4.5] | 4.0 [3.2–5.1] | 0.42 |
| Cathepsin S (ng/ml) | 13 [10–15] | 11 [9–12] | 11 [10–14] | 0.009 |
| Adiponectin (μg/ml) | 131 [111–219] | 258 [192–362] | 233 [204–285] | <0.001 |
Values represent median concentrations [interquartile ranges]. P-values were calculated with ANOVA on logtransformed values for difference between all groups. PCOS: polycystic ovary syndrome, HA: hyperandrogenic, NA: normoandrogenic, IL: interleukin, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-1b, IL-6, IL-17, TNF-α and chemerin are not shown as the majority of samples (>57%) were undetectable measurements evenly distributed amongst the study population.
Table 3. P-values, after adjustment for BMI and Age, and correction for multiple testing (FDR).
| ANOVA all groups | HA-PCOS vs non-PCOS | HA-PCOS vs NA-PCOS | NA-PCOS vs non-PCOS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-value | P-value | adjustedP-value | FDR | P-value | Adjusted P-value | FDR | P-value | Adjusted P-value | FDR | |
| IL-13 (pg/ml) | 0.043 | 0.10 | 0.30 | 0.45 | 0.39 | 0.83 | 0.91 | 0.013 | 0.46 | 0.92 |
| Leptin (ng/ml) | <0.001 | 0.006 | 0.29 | 0.45 | <0.001 | 0.08 | 0.24 | 0.013 | 0.08 | 0.48 |
| Resistin (ng/ml) | 0.017 | 0.72 | 0.42 | 0.50 | 0.010 | 0.40 | 0.60 | 0.021 | 0.92 | 0.92 |
| DPP-IV/sCD26 (μg/ml) | 0.005 | 0.014 | 0.049 | 0.29 | 0.70 | 0.91 | 0.91 | 0.005 | 0.48 | 0.92 |
| Cathepsin S (ng/ml) | 0.009 | 0.85 | 0.67 | 0.67 | 0.005 | 0.20 | 0.40 | 0.012 | 0.65 | 0.92 |
| Adiponectin (μg/ml) | <0.001 | <0.001 | 0.15 | 0.45 | <0.001 | 0.07 | 0.24 | 0.38 | 0.85 | 0.92 |
When a P-value < 0.05 was detected in ANOVA, pairwise T-test on logtransformed biomarkers were used to calculate P-values between specific groups. Furthermore P-values were adjusted for age and BMI, and false discovery rates (FDR) were calculated to correct for multiple testing. PCOS: polycystic ovary syndrome, HA: hyperandrogenic, NA: normoandrogenic, IL: interleukin,, DPP-IV/sCD26: dipeptidyl peptidase IV.
Since such shifted protein profiles as well as certain clinical characteristics (e.g. BMI) are known to be associated with a predisposition for cardiovascular disease, we assessed the identical protein panel in a cross sectional cohort of PCOS offspring and paediatric reference cohort of comparable age and sex, based on the assumption that PCOS offspring may already exhibit an increased cardiometabolic risk at young age. The baseline characteristics of these two groups are shown in Table 4. Results of the hierarchical cluster analysis in these groups are shown in S2 Fig When comparing protein concentrations between PCOS offspring and the reference population, we found higher MMP-9 (P = 0.001) and S100A8 (P < 0.001) concentrations in the PCOS offspring (see Table 5). Correction for BMI and age yielded similar results. and differences remained significant after correction for multiple testingSubanalyses regarding the potential influence of gender, comparing both sexes separately, showed that the significant difference in MMP-9 concentrations was mainly driven by the difference in girls (girls 1.0 μg/ml [0.7–2.0] vs. 0.2 μg/ml [0.1–0.5], boys 0.8 μg/ml [0.4–2.2] vs. 0.3 μg/ml [0.2–0.5]). Differences in S100A8 concentrations were significant in both sexes (girls 2.7 ng/ml [0.8–2.8] vs. 0.0 ng/ml [0.0–0.8], boys 2.2 ng/ml [0.2–3.7] vs. 0.0 ng/ml [0.0–0.0]).
Table 4. Baseline characteristics of PCOS offspring and reference group.
| PCOS offspring (n = 14) | Reference group (n = 30) | P-value | ||
|---|---|---|---|---|
| Complications during pregnancy | 0.08 | |||
| None | 8 (57) | 25 (86) | ||
| Hypertensive complications | 5 (36) | 2 (7) | ||
| Gestational diabetes | 1 (7) | - | ||
| Infection | 1 (7) | 2 (7) | ||
| Mode of delivery | 0.05 | |||
| Vaginal spontaneously | 7 (50) | 23 (79) | ||
| Caesarean section | 5 (36) | 6 (21) | ||
| Assisted vaginal delivery | 2 (14) | - | ||
| Gestational age at delivery (weeks) | 40.1 [37.5–40.4] | 40.4 [39.4–41.2] | 0.19 | |
| Preterm delivery | 0.15 | |||
| Yes | 1 (7) | - | ||
| No | 13 (93) | 29 (100) | ||
| Birth Weight (grams) | 3295 [3061–3651] | 3600 [3200–3940] | 0.27 | |
| Neonatal complications | ||||
| Small for gestational age | 1 (7) | 1 (3) | 0.15 | |
| Large for gestational age | 2 (14) | 2 (7) | 0.43 | |
| Sex | 0.98 | |||
| Male | 6 (43) | 13 (43) | ||
| Female | 8 (57) | 17 (57) | ||
| Age at screening (years) | 7.0 [6.6–8.1] | 7.8 [7.6–7.9] | 0.09 | |
| BMI (kg/m2) | 15.7 [14.5–16.8] | 14.9 [14.5–16.0] | 0.12 |
Values represent median values [interquartile range] or absolute numbers (percentages) Mann-Whitney U tests or Chi-square tests were used to calculate P-values. Variables contained a maximum of 3% missing values.
Table 5. Median biomarker concentrations in PCOS offspring and reference group.
| PCOS offspring (n = 14) | Reference group (n = 30) | P-value | Adjusted P-value | FDR | |
|---|---|---|---|---|---|
| IL-13 (pg/ml) | 8.5 [0.9–37.7] | 16.5 [5.0–77.6] | 0.14 | 0.06 | 0.26 |
| IL-18 (pg/ml) | 134 [85–196] | 139 [107–188] | 0.64 | 0.73 | 0.78 |
| CCL2/MCP-1 (pg/ml) | 113 [88–138.] | 134 [100–159] | 0.18 | 0.22 | 0.47 |
| PIGF (pg/ml) | 37 [32–59] | 31 [16–69] | 0.17 | 0.29 | 0.53 |
| VEGF (ng/ml) | 0.3 [0.1–0.6] | 0.2 [0.1–0.5] | 0.59 | 0.65 | 0.74 |
| MMP-9 (μg/ml) | 1.0 [0.6–2.2] | 0.2 [0.2–0.5] | 0.001 | 0.003 | 0.02 |
| sVEGF-R1 (ng/ml) | 1.4 [1.1–1.7] | 1.6 [1.1–2.0] | 0.47 | 0.46 | 0.71 |
| S100A8 (ng/ml) | 2.7 [0.7–3.1] | 0.0 [0.0–0.1] | <0.001 | <0.001 | <0.001 |
| Adipsin (ng/ml) | 0.3 [0.1–0.3] | 0.3 [0.2–0.3] | 0.71 | 0.96 | 0.96 |
| Leptin (ng/ml) | 0.1 [0.0–0.5] | 0.0 [0.0–0.3] | 0.31 | 0.63 | 0.74 |
| Resistin (ng/ml) | 26 [21–32] | 22 [19–36] | 0.53 | 0.18 | 0.44 |
| RBP4 (μg/ml) | 35 [32–37] | 35 [32–40] | 0.20 | 0.05 | 0.26 |
| DPP-IV/sCD26 (μg/ml) | 2.2 [1.8–2.7] | 2.5 [2.0–3.1] | 0.19 | 0.08 | 0.27 |
| sICAM1/sCD54(μg/ml) | 0.5[0.4–0.6] | 0.5 [0.4–0.6] | 0.50 | 0.50 | 0.71 |
| sVCAM (μg/ml) | 7.7 [6.5–10.3] | 6.9 [5.7–9.4] | 0.18 | 0.16 | 0.44 |
| Cathepsin S (ng/ml) | 12 [10–13] | 11 [11–13] | 0.42 | 0.31 | 0.53 |
| Adiponectin (μg/ml) | 273 [215–312] | 258 [239–325] | 0.70 | 0.61 | 0.74 |
Values represent median concentrations [interquartile ranges]. P-values were calculated using T-tests on logtransformed biomarkers. Furthermore P-values were adjusted for age and BMI (general linear models), and false discovery rates (FDR) were calculated to correct for multiple testing. PCOS: polycystic ovary syndrome, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM1/sCD54: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-1b, IL-6, IL-17, TNF-α and chemerin are not shown as the majority of samples (>57%) were undetectable measurements evenly distributed amongst the study population.
Discussion
The primary aim of this study was to compare metabolic/inflammatory biomarker risk profiles between women with different PCOS phenotypes, PCOS offspring and reference populations. We selected specific adipocytokines, growth factors, soluble cell adhesion molecules, and other proteins, which have previously been associated with chronic low-grade inflammation and cardiometabolic dysfunction.
Adipocytokines are known to affect vascular endothelium by stimulating the migration of monocytes into the vessel wall, and inducing the conversion of monocytes into macrophages.[24] These macrophages phagocytize LDL cholesterol and form fatty streaks within the vessel wall, ultimately resulting in atherosclerotic plaque formation.[25] Adipocytokines, such as IL-6 and TNF-alpha, may be valuable cardiovascular risk markers in women with PCOS, since they reflect chronic low-grade inflammation and have been associated with several insulin-resistant states.[24] Furthermore, vascular endothelial growth factors may stimulate the development of atherosclerosis and/or plaque instability through the formation of microvessels.[26] Increased expression of cell adhesion molecules on the vascular endothelium may play a significant role in atherosclerosis as well, by inducing solid adhesion of inflammatory cells to the vascular surface.[27] Hence these biomarkers were specifically selected to differentiate risk potential within PCOS, in view of the future appliance of secondary prevention measures.
When comparing women with PCOS to a non-PCOS reference population, we found significant differences in several adipocytokines, which were mostly influenced by BMI. Leptin and adiponectin appeared the most discriminative markers in women with PCOS, both showing the strongest correlation with the FAI. This finding corroborates with previous studies in which these adipocytokines were correlated with metabolic complications in women with PCOS.[28,29] Leptin is secreted by white adipose tissue and regulates food intake and energy expenditure through actions on the central nervous system.[30] Serum concentrations rise with increasing body weight and impair insulin activity, and may therefore attribute to the development of hyperandrogenism and infertility in PCOS.[31] High concentrations may also directly impair ovarian function by decreasing ovarian sensitivity for gonadotrophins.[32] Previous reports concerning leptin concentrations in PCOS and non-PCOS women have not been entirely consistent, although the majority of studies report no difference in leptin concentrations when comparing PCOS women to weight-matched non-PCOS controls, which is in line with our findings in the current study.[33–35]
Adiponectin is secreted in adipose tissue and exerts anti-atherogenic, anti-inflammatory and insulin-sensitizing effects.[36] It has been shown that serum concentrations of this protein are inversely correlated to obesity, insulin resistance and diabetes mellitus type 2.[36,37] Circulating concentrations appear to be lower in women with PCOS compared to non-PCOS women, even after adjustment for BMI-related effects.[38]
We observed no significant differences in other inflammatory biomarkers between women with and without PCOS (e.g. interleukins, TNF-α, resistin, MMP-2, MMP-9, MCP-1), as opposed to what has previously been reported by others.[7,8,39–41] This may be due to the relatively low BMI of women with PCOS included in the current study, even in the overweight HA-PCOS group in which the average BMI did not exert 30 kg/m2. [24]
Contrary to adult PCOS, we did observe significantly higher concentrations of MMP-9 and S100A8 in PCOS offspring, compared to the reference population. Increased circulating MMP-9 concentrations have been previously reported in adult women with PCOS.[40,42] MMP-9 is a zinc-binding proteolytic enzyme which is involved in remodelling of the extracellular matrix.[40] It originates from various inflammatory cells, however predominantly from neutrophils.[43] Within the ovary, MMPs are involved in follicular development and the process of ovulation.[42] It has been hypothesized that increased MMP-9 concentrations may be associated with the pathophysiology of PCOS, since altered extracellular matrix remodeling may be related to inappropriate follicular atresia and increased ovarian stroma tissue.[40,44]. Increased MMP activity has been associated with various other disease processes including asthma, cystic fibrosis, ulcerative colitis, atherosclerosis and cardiovascular disease. [45–49]
To our knowledge, the possible role of circulating S100A8 in women with PCOS or PCOS offspring has not been previously addressed. S100A8 is known as a damage-associated molecular pattern (DAMP) molecule because of its pro-inflammatory actions, and is secreted in response to cell damage, death, and stress.[50] Moreover, this chemotaxin is involved in various processes including calcium homeostasis, cellular migration, and energy metabolism.[51] Within the ovary, S100A8 might also play a role in primordial follicle formation, as was recently demonstrated in a fetal mice model.[52]
In PCOS offspring we did not observe a difference in adipocytokines compared to the reference group. This might be due to an absence of overweight and insulin resistance in these young prepuberal children. As children enter puberty, insulin resistance may develop in response to growth hormone secretion which induces accelerated growth during puberty.[53] It is known that insulin acts as a co-gonadotropin in ovarian steroidogenesis and as such may contribute to the development of PCOS.[11]
To our knowledge, this is the first study in which metabolic/inflammatory biomarkers were simultaneously assessed both in adult women with PCOS as well as PCOS offspring. Further strengths of this study are the large tailored series of potentially relevant biomarkers which were assessed in a well-phenotyped patient population, and the use of a validated multiplex immunoassay with standardized technology which has been repeatedly described before.[20–22]
Limitations of the current study are the relatively small sample size, and the characteristics of the reference populations. The current study was performed with a relatively limited sample size, especially concerning PCOS offspring. Therefore, reported results in these children may be regarded as preliminary and require validation in a larger cohort. The adult reference population consisted of women who were planned to undergo IVF/ICSI treatment, and therefore might not be considered as entirely healthy controls despite the fact that these women had regular menstrual cycles. However, post-hoc analyses including only women with infertility due to a male factor did not significantly alter results (data not shown). Moreover, all women included in the adult reference population were clinically evaluated and definitely classified as non-PCOS. Although the paediatric reference population was clinically well defined, there was no detailed reproductive history data available of their mothers and therefore PCOS could not be excluded. Hence, it is possible that observed differences in protein concentrations between PCOS offspring and the reference group in the current study may be underestimated.
In summary, we observed differences in adipocytokines between women with normoandrogenic and hyperandrogenic PCOS and women without PCOS, which were influenced by BMI. Leptin and adiponectin showed the strongest correlation with the FAI. Since these biomarkers are directly correlated with more easy assessable classical risk factors such as obesity and insulin resistance, routine assessment of these markers at this point may contribute little to the conventional risk assessment in women with PCOS.
In PCOS offspring other inflammatory biomarkers were higher, suggesting that these young children may exhibit increased risk of chronic low-grade inflammation. Although these results require validation in a larger cohort study, these findings may be of importance for future appliance of primary prevention measures. Foremost,longitudinal follow-up studies with repeated measurements are needed in order to assess the potential association between biomarker profiles, the development of PCOS and actual cardiovascular disease in later life.
Supporting Information
PCOS: polycystic ovary syndrome, IL: interleukin, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-1b, IL-6, IL-17, TNF-α and chemerin are not shown as the majority of samples (>57%) were undetectable measurements evenly distributed amongst the study population. Phenotype: black represents hyperandrogenic PCOS; White represents non-PCOS reference population. BMI: yellow represents low BMI, red represents high BMI.
(TIF)
PCOS: polycystic ovary syndrome, IL: interleukin, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-6, and chemerin are not shown as > 95% were undetectable measurements evenly distributed amongst the study population. Black represents PCOS offspring, white represents reference population.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
N.M.P. Daan has received funding from the Dutch Heart Foundation, grant number 2013T083. W. de Jager is supported by the Dutch Technology Foundation STW (project 11679), which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. During the recent 5 year period B.C.J.M. Fauser has received fees and grant support from the following companies (in alphabetic order); Actavis, COGI, Euroscreen, Ferring, Finox, Genovum, Gedeon-Richter, Merck-Serono, OvaScience, Pantharei Bioscience, PregLem, Roche, Uteron, and Watson laboratories. This funding is unrelated to the current investigation. The Children follow-up study from women with PCOS (CHOPS) was funded in part by the Child Health research programme of the University Medical Center Utrecht. A.M.V. Evelein being part of the Wheezing Illnesses Study Leidsche Rijn (WHISTLER) Study group received funding from The Netherlands Organisation for Health Research and Development, grant number 2100.0095 (ZonMw), the Dutch Asthma Foundation and from Glaxo Smith Kline (no personal grant). None of the funders stated above of any of the authors had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010. February;25(2):544–551. 10.1093/humrep/dep399 [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004. January;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 3.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010. Jul-Aug;16(4):347–363. 10.1093/humupd/dmq001 [DOI] [PubMed] [Google Scholar]
- 4.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012. January;97(1):28–38.e25. 10.1016/j.fertnstert.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 5.Daan NM, Louwers YV, Koster MP, Eijkemans MJ, de Rijke YB, Lentjes EW, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril 2014. September 16. [DOI] [PubMed] [Google Scholar]
- 6.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. J Clin Endocrinol Metab 2015. March;100(3):911–919. 10.1210/jc.2014-3886 [DOI] [PubMed] [Google Scholar]
- 7.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JL. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab 2004. February;89(2):806–811. 10.1210/jc.2003-031365 [DOI] [PubMed] [Google Scholar]
- 8.Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update 2011. Nov-Dec;17(6):741–760. 10.1093/humupd/dmr025 [DOI] [PubMed] [Google Scholar]
- 9.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 2011. March 1;95(3):1048–58.e1-2. 10.1016/j.fertnstert.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005. March 24;352(12):1223–1236. 10.1056/NEJMra041536 [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012. December;33(6):981–1030. 10.1210/er.2011-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012. Nov-Dec;18(6):618–637. 10.1093/humupd/dms030 [DOI] [PubMed] [Google Scholar]
- 13.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 2008. May;93(5):1662–1669. 10.1210/jc.2007-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia C, Mancini F, Cianciosi A, Busacchi P, Persico N, Paradisi R, et al. Cardiovascular risk in normal weight, eumenorrheic, nonhirsute daughters of patients with polycystic ovary syndrome: a pilot study. Fertil Steril 2009. July;92(1):240–249. 10.1016/j.fertnstert.2008.05.018 [DOI] [PubMed] [Google Scholar]
- 15.Atiomo W, Khalid S, Parameshweran S, Houda M, Layfield R. Proteomic biomarkers for the diagnosis and risk stratification of polycystic ovary syndrome: a systematic review. BJOG 2009. January;116(2):137–143. 10.1111/j.1471-0528.2008.02041.x [DOI] [PubMed] [Google Scholar]
- 16.Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update 2011. Nov-Dec;17(6):741–760. 10.1093/humupd/dmr025 [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999. October;84(10):3666–3672. 10.1210/jcem.84.10.6079 [DOI] [PubMed] [Google Scholar]
- 18.Geerts CC, Evelein AM, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CS. Body fat distribution and early arterial changes in healthy 5-year-old children. Ann Med 2012. June;44(4):350–359. 10.3109/07853890.2011.558520 [DOI] [PubMed] [Google Scholar]
- 19.Eikendal AL, den Ruijter HM, Uiterwaal CS, Pasterkamp G, Hoefer IE, de Kleijn DP, et al. Extracellular vesicle protein CD14 relates to common carotid intima-media thickness in eight-year-old children. Atherosclerosis 2014. October;236(2):270–276. 10.1016/j.atherosclerosis.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 20.de Jager W, Prakken BJ, Bijlsma JW, Kuis W, Rijkers GT. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods 2005. May;300(1–2):124–135. 10.1016/j.jim.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 21.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis 2007. May;66(5):589–598. 10.1136/ard.2006.061853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schipper HS, de Jager W, van Dijk ME, Meerding J, Zelissen PM, Adan RA, et al. A multiplex immunoassay for human adipokine profiling. Clin Chem 2010. August;56(8):1320–1328. 10.1373/clinchem.2010.146118 [DOI] [PubMed] [Google Scholar]
- 23.van den Ham HJ, de Jager W, Bijlsma JW, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology (Oxford) 2009. August;48(8):899–905. [DOI] [PubMed] [Google Scholar]
- 24.Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers 2009;26(4):163–170. 10.3233/DMA-2009-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology 2003. June;144(6):2195–2200. 10.1210/en.2003-0285 [DOI] [PubMed] [Google Scholar]
- 26.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 2005. September 20;112(12):1813–1824. 10.1161/CIRCULATIONAHA.105.535294 [DOI] [PubMed] [Google Scholar]
- 27.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med 1999. July;107(1):85–97. [DOI] [PubMed] [Google Scholar]
- 28.Chen CI, Hsu MI, Lin SH, Chang YC, Hsu CS, Tzeng CR. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol Endocrinol 2015. April;31(4):264–268. 10.3109/09513590.2014.984676 [DOI] [PubMed] [Google Scholar]
- 29.Sarray S, Madan S, Saleh LR, Mahmoud N, Almawi WY. Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome. Fertil Steril 2015. June 6. [DOI] [PubMed] [Google Scholar]
- 30.Chen CI, Hsu MI, Lin SH, Chang YC, Hsu CS, Tzeng CR. Adiponectin and leptin in overweight/obese and lean women with polycystic ovary syndrome. Gynecol Endocrinol 2015. April;31(4):264–268. 10.3109/09513590.2014.984676 [DOI] [PubMed] [Google Scholar]
- 31.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science 1996. November 15;274(5290):1185–1188. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs HS, Conway GS. Leptin, polycystic ovaries and polycystic ovary syndrome. Hum Reprod Update 1999. Mar-Apr;5(2):166–171. [DOI] [PubMed] [Google Scholar]
- 33.Plati E, Kouskouni E, Malamitsi-Puchner A, Boutsikou M, Kaparos G, Baka S. Visfatin and leptin levels in women with polycystic ovaries undergoing ovarian stimulation. Fertil Steril 2010. September;94(4):1451–1456. 10.1016/j.fertnstert.2009.04.055 [DOI] [PubMed] [Google Scholar]
- 34.Svendsen PF, Christiansen M, Hedley PL, Nilas L, Pedersen SB, Madsbad S. Adipose expression of adipocytokines in women with polycystic ovary syndrome. Fertil Steril 2012. July;98(1):235–241. 10.1016/j.fertnstert.2012.03.056 [DOI] [PubMed] [Google Scholar]
- 35.Cassar S, Teede HJ, Harrison CL, Joham AE, Moran LJ, Stepto NK. Biomarkers and insulin sensitivity in women with Polycystic Ovary Syndrome: Characteristics and predictive capacity. Clin Endocrinol (Oxf) 2015. July;83(1):50–58. [DOI] [PubMed] [Google Scholar]
- 36.Ardawi MS, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril 2005. June;83(6):1708–1716. 10.1016/j.fertnstert.2004.11.077 [DOI] [PubMed] [Google Scholar]
- 37.Spranger J, Mohlig M, Wegewitz U, Ristow M, Pfeiffer AF, Schill T, et al. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004. December;61(6):738–746. [DOI] [PubMed] [Google Scholar]
- 38.Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update 2009. May-Jun;15(3):297–307. 10.1093/humupd/dmp006 [DOI] [PubMed] [Google Scholar]
- 39.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchon R, Luque-Ramirez M, et al. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Hum Reprod 2006. September;21(9):2257–2265. 10.1093/humrep/del146 [DOI] [PubMed] [Google Scholar]
- 40.Lewandowski KC, Komorowski J, O'Callaghan CJ, Tan BK, Chen J, Prelevic GM, et al. Increased circulating levels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J Clin Endocrinol Metab 2006. March;91(3):1173–1177. 10.1210/jc.2005-0648 [DOI] [PubMed] [Google Scholar]
- 41.Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf) 2009. November;71(5):652–658. [DOI] [PubMed] [Google Scholar]
- 42.Goldman S, Shalev E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front Biosci 2004. September 1;9:2474–2483. [DOI] [PubMed] [Google Scholar]
- 43.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000. October 15;96(8):2673–2681. [PubMed] [Google Scholar]
- 44.Huet C, Monget P, Pisselet C, Monniaux D. Changes in extracellular matrix components and steroidogenic enzymes during growth and atresia of antral ovarian follicles in the sheep. Biol Reprod 1997. April;56(4):1025–1034. [DOI] [PubMed] [Google Scholar]
- 45.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994. December;94(6):2493–2503. 10.1172/JCI117619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke 2001. December 1;32(12):2762–2767. [DOI] [PubMed] [Google Scholar]
- 47.Kofla-Dlubacz A, Matusiewicz M, Krzesiek E, Noga L, Iwanczak B. Metalloproteinase-3 and -9 as novel markers in the evaluation of ulcerative colitis activity in children. Adv Clin Exp Med 2014. Jan-Feb;23(1):103–110. [DOI] [PubMed] [Google Scholar]
- 48.Rath T, Zwaschka L, Hage L, Kugler M, Menendez K, Naehrlich L, et al. Identification of neutrophil activation markers as novel surrogate markers of CF lung disease. PLoS One 2014. December 29;9(12):e115847 10.1371/journal.pone.0115847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turkeli A, Yilmaz O, Taneli F, Horasan GD, Kanik ET, Kizilkaya M, et al. IL-5, IL-8 and MMP -9 levels in exhaled breath condensate of atopic and nonatopic asthmatic children. Respir Med 2015. June;109(6):680–688. 10.1016/j.rmed.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 50.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007. January;81(1):28–37. 10.1189/jlb.0306170 [DOI] [PubMed] [Google Scholar]
- 51.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med 2013. January;13(1):24–57. [PMC free article] [PubMed] [Google Scholar]
- 52.Teng Z, Wang C, Wang Y, Huang K, Xiang X, Niu W, et al. S100A8, An Oocyte-Specific Chemokine, Directs the Migration of Ovarian Somatic Cells During Mouse Primordial Follicle Assembly. J Cell Physiol 2015. December;230(12):2998–3008. 10.1002/jcp.25032 [DOI] [PubMed] [Google Scholar]
- 53.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991. February;72(2):277–282. 10.1210/jcem-72-2-277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCOS: polycystic ovary syndrome, IL: interleukin, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-1b, IL-6, IL-17, TNF-α and chemerin are not shown as the majority of samples (>57%) were undetectable measurements evenly distributed amongst the study population. Phenotype: black represents hyperandrogenic PCOS; White represents non-PCOS reference population. BMI: yellow represents low BMI, red represents high BMI.
(TIF)
PCOS: polycystic ovary syndrome, IL: interleukin, CCL2/MCP-1: monocyte chemoattractant protein-1, PIGF: placental growth factor, VEGF: vascular endothelial growth factor, MMP-9: matrix metallopeptidase 9, RBP-4: retinol-binding protein 4, DPP-IV/sCD26: dipeptidyl peptidase IV, sICAM: soluble intercellular adhesion molecule 1, sVCAM: soluble vascular cell adhesion molecule 1. IL-6, and chemerin are not shown as > 95% were undetectable measurements evenly distributed amongst the study population. Black represents PCOS offspring, white represents reference population.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.