Abstract
Plants not only provide food, feed and raw materials for humans, but have also been developed as an economical production system for biopharmaceutical proteins, such as antibodies, vaccine candidates and enzymes. These must be purified from the plant biomass but chromatography steps are hindered by the high concentrations of host cell proteins (HCPs) in plant extracts. However, most HCPs irreversibly aggregate at temperatures above 60 °C facilitating subsequent purification of the target protein. Here, three methods are presented to achieve the heat precipitation of tobacco HCPs in either intact leaves or extracts. The blanching of intact leaves can easily be incorporated into existing processes but may have a negative impact on subsequent filtration steps. The opposite is true for heat precipitation of leaf extracts in a stirred vessel, which can improve the performance of downstream operations albeit with major changes in process equipment design, such as homogenizer geometry. Finally, a heat exchanger setup is well characterized in terms of heat transfer conditions and easy to scale, but cleaning can be difficult and there may be a negative impact on filter capacity. The design-of-experiments approach can be used to identify the most relevant process parameters affecting HCP removal and product recovery. This facilitates the application of each method in other expression platforms and the identification of the most suitable method for a given purification strategy.
Keywords: Plant Biology, Issue 114, Host cell protein depletion, design of experiments (DoE), downstream processing, heat precipitation, plant extract clarification, plant-derived pharmaceuticals
Introduction
Modern healthcare systems increasingly depend on biopharmaceutical proteins 1. Producing these proteins in plants is advantageous due to the low pathogen burden and greater scalability compared to conventional expression systems 2-4. However, the downstream processing (DSP) of plant-derived pharmaceuticals can be challenging because the disruptive extraction procedures result in a high particle burden, with turbidities exceeding 5,000 nephelometric turbidity units (NTUs), and host cell protein (HCP) concentrations often exceeding 95% [m/m] 5,6.
Elaborate clarification procedures are required to remove dispersed particles 7-9, but chromatography equipment is less expensive to operate in bind-and-elute mode during initial product recovery if there is an earlier step for the efficient removal of HCPs 10,11. This can be achieved either by precipitating the target protein using flocculants 12 or low pH 13,14, as well as by causing the HCPs to aggregate. The selective aggregation of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), the most abundant HCP in green plants such as tobacco (Nicotiana tabacum), can be promoted by adding polyethylene glycol 15, but this is expensive and incompatible with large-scale manufacturing. Heat treatment has been shown to denature and precipitate more than 95% of tobacco HCPs, while protein malaria vaccine candidates such as Vax8 remain stable in solution 16-18.
Three different approaches were used to achieve the heat-induced precipitation of tobacco HCPs: (i) blanching, i.e., the immersion of intact leaves in hot liquid, (ii) a temperature-controlled stirred vessel, and (iii) a heat exchanger (Figure 1) 16. For intact leaves, blanching achieved the rapid and efficient precipitation of HCPs and was also easy to scale up and compatible with existing large-scale manufacturing processes that include an initial step to wash the plant biomass 19. In contrast, temperature-controlled vessels are already available in some processes and can be used for the thermal treatment of plant extracts 20, but their scalability and energy transfer rate are limited because the surface-to-volume ratio of the tanks is progressively reduced and becomes unsuitable at process scale. A heat exchanger is a technically well-defined alternative to heated stirred vessels but requires an abundant supply of heating and cooling media, e.g., steam and cold water, as well as a tightly controlled volumetric flow rate that is adapted to the heat exchanger geometry and media properties, e.g., the specific heat capacity. This article shows how all three methods can be used for the heat-induced precipitation of tobacco HCPs, and plant HCPs in general. The establishment and operation of each method in a laboratory setting can be used to evaluate their suitability for larger-scale processes. The major challenge is to identify adequate scale-down models and running conditions for each operation that resemble the devices and conditions used during process-scale manufacturing. The data presented here refer to experiments conducted with transgenic tobacco plants expressing the malaria vaccine candidate Vax8 and fluorescent protein DsRed 16, but the method has also been successfully applied to N. benthamiana plants transiently expressing other biopharmaceutical proteins 21.
A design-of-experiments (DoE) approach 22 can facilitate process development, and flocculants 23 can also be beneficial in this context as previously described 8. The main difference between blanching, heated vessels and heat exchangers is that blanching is applied to intact leaves early in the process whereas the others are applied to plant extracts (Figure 1).
 Figure 1:Process Flow Scheme Illustrating the Implementation of Three Different Methods for Tobacco HCP Heat Precipitation. The plant material is washed and homogenized before clarification and purification. The equipment for the blanching step (red) can easily be added to the existing machinery. In contrast, using a stirred vessel (orange) and especially a heat exchanger (blue) requires one or several additional devices and tubing. Please click here to view a larger version of this figure.
Figure 1:Process Flow Scheme Illustrating the Implementation of Three Different Methods for Tobacco HCP Heat Precipitation. The plant material is washed and homogenized before clarification and purification. The equipment for the blanching step (red) can easily be added to the existing machinery. In contrast, using a stirred vessel (orange) and especially a heat exchanger (blue) requires one or several additional devices and tubing. Please click here to view a larger version of this figure.
Protocol
1. Cultivate the Tobacco Plants
Flush each mineral wool block with 1 to 2 L of deionized water and subsequently with 1 L of 0.1% [w/v] fertilizer solution. Place one tobacco seed in each mineral wool block and gently flush with 0.25 L of fertilizer solution without washing away the seed 16.
Cultivate the tobacco plants for 7 weeks in a greenhouse with 70% relative humidity, a 16 hr photoperiod (180 µmol sec−1 m−2; λ = 400 - 700 nm) and a 25/22 °C light/dark temperature regime.
Harvest all leaves except the four cotyledon leaves, which are located at the base of the plant stem.
2. Optional: Heat Precipitation by Blanching
NOTE: Carry out the steps described in steps 2.1 to 2.12 in order to precipitate tobacco HCPs by blanching. Skip the entire section 2, if the HCPs will be precipitated in a heated vessel (section 4) or using a heat exchanger (section 5).
Set aside 50 g of plant material and carry out extraction without blanching (section 3). Take a sample of this extract as an internal control during subsequent analysis (section 7).
Set up an 8-L working volume water bath, e.g., 50 x 40 x 40 cm, in a thermally-insulated polystyrene foam bucket or similar. Mount the adjustable thermostat on the water bath for subsequent temperature control (step 2.7).
Transfer the entire assembly onto a magnetic stir plate and place a magnetic stir bar in the water bath. Ensure that the magnetic field is strong enough to rotate the stir bar.
Surround the stir bar with at least four support tiles upon which a polypropylene basket (step 2.6) will be placed later during the blanching procedure. Make sure that the support tiles are made from a non-magnetic material (e.g., stainless steel alloy containing nickel) and that they are higher than the stir bar (Figure 2).
Add 8 L of deionized water to the water bath. NOTE: Using a buffer, e.g., 50 mM sodium phosphate (pH 7.5), can improve the target protein yields if low pH precipitation is an issue.
Place a 23 x 23 x 23 cm polypropylene basket on the support tiles and make sure that the basket does not interfere with the stir bar rotation and that it is fully submerged in the liquid. If necessary, add additional water/buffer until the basket is fully submerged, then remove the basket again. CAUTION: All subsequent steps up to 2.12 involve the handling of hot liquid. Wear appropriate personal protective equipment including thermally insulated gloves.
Use the adjustable thermostat to bring the water bath to 70 °C (or the temperature required for the experiments). Wait at least 15 min after the desired temperature is reached to ensure the entire assembly has reached a thermal equilibrium.
Prepare 150 g aliquots from the harvested tobacco leaves. Place one aliquot in the basket while avoiding irreversible compression and damage to the leaves, e.g., by tearing. Avoid overfilling the basket with plant material or a dense packing of the latter.
Carefully but quickly submerge the basket in the hot liquid and place it on the support tiles. Place a stainless steel block on top of the basket to prevent flotation.
Incubate the leaves for 5 min in the blanching fluid, or select a time suiting the experimental design. Monitor the liquid temperature during the entire incubation period.
Carefully remove the basket from the blanching fluid and let residual liquid drain from the leaves for 30 sec. Then take the plant leaves out of the basket, transfer them to the blender and immediately start the extraction (section 3). NOTE: Plants can be kept for extended periods of time after blanching and before the start of the extraction, e.g., more than 30 min on ice or frozen at -20 °C for several weeks has been successfully tested. However, product stability may decline with increasing storage times and thus immediate processing is recommended.
Repeat steps 2.8 to 2.11 with fresh leaf material until the entire harvested biomass is processed.
3. Protein Extraction from Tobacco Leaves
CAUTION: The next steps involve a blender with rotating blades. Do not work in the blender bucket while it is mounted on the blender motor.
Place 150 g (wet mass) of harvested (step 1.3) or blanched (step 2.11) leaves in the blender and add 450 ml of extraction buffer (50 mM sodium phosphate, 500 mM sodium chloride, 10 mM sodium disulfite, pH 8.0). NOTE: The extraction buffer composition depends on the protein to be extracted and can thus require adjustment, e.g., use of another pH or buffer component such as Tris.
Homogenize the leaves for 3 x 30 sec pulses with 30 sec interspersed breaks. Ensure that the leaves are homogenized and do not clog the blender bucket. Stop the blender and lift the leaves to prevent clogging if necessary, and then continue the homogenization.
Take a 1 ml sample of each extract that is produced for subsequent analysis (section 7). If the plant material is blanched, continue to section 6. Otherwise continue with heat precipitation in a vessel (section 4) or heat exchanger (section 5), depending on the selected experimental approach.
4. Optional: Heat Precipitation in a Stirred Vessel
NOTE: Conduct the steps described in sections 4.2 to 4.11 in order to precipitate tobacco HCPs in a stirred vessel. Skip the entire section 4, if HCPs have been precipitated by blanching (section 2) or will be precipitated using a heat exchanger (section 5).
Set up two 8-L working volume water baths, e.g., 50 x 40 x 40 cm, in thermally insulated polystyrene foam or similar. In the first bath, mount the adjustable thermostat for subsequent temperature control (step 4.6). In the second, add 5 L of deionized water and 2 kg of ice for subsequent cooling (step 4.9).
Transfer the first water bath onto a magnetic stir plate, and place the 2-L stainless steel vessel into the water bath such that the center of the vessel is aligned to the center of the stir plate.
Place a magnetic stir bar in the stainless-steel vessel. Ensure that the magnetic field is strong enough to rotate the stir bar.
Fill the water bath with deionized water to 5 cm below the upper edge of the stainless steel vessel. Then fill the vessel with extraction buffer. Place a polystyrene foam lid on the vessel. CAUTION: All subsequent steps up to 4.9 involve the handling of hot liquid. Wear appropriate personal protective equipment including thermally insulated gloves.
Insert a thermometer into the stainless steel vessel through a suiting hole in the polystyrene foam lid. Set the water bath temperature to 78 °C and incubate the entire assembly for 15 min to reach thermal equilibrium. Ensure that the temperature in the vessel is 70 °C, approximately 8 °C below the set point of the water bath.
If the temperature in the stainless steel vessel differs from 70 °C, adjust the temperature of the water bath accordingly and let the system equilibrate for another 15 min. Repeat this step until the temperature in the vessel is 70 °C or as required for the experiment, and empty the stainless steel vessel.
Pour 300 ml of extract (step 3.2) into the stainless-steel vessel while it is still in the water bath and start a timer. Stir the extract at 150 rpm and incubate for 5 min. Ensure that the extract reaches a temperature of 70 °C for at least 2 min during this incubation period.
Remove the hot water bath from the magnetic stirrer, take out the stainless steel vessel and place it in the ice-cold bucket. Place the latter onto the magnetic stirrer and remove the polystyrene foam lid.
Ensure that the plant homogenate is well agitated at 150 rpm, place the thermometer in the extract and incubate until it reaches a temperature of 20 °C or the temperature specified by the experimental design.
Repeat steps 4.7 to 4.9 for all aliquots. Take a 1 ml sample of each heat precipitated extract once it has reached the final temperature, then proceed with section 6.
5. Optional: Heat Precipitation in a Heat Exchanger
NOTE: Conduct the steps described in sections 5.2 to 5.12 in order to precipitate tobacco HCPs using a heat exchanger. Skip the entire section 5, if HCPs have been precipitated by blanching (section 2) or in a heated vessel (section 4).
Set up two 8-L working volume water baths, e.g., 50 x 40 x 40 cm, in thermally-insulated polystyrene foam buckets or similar. In the first bath, mount the adjustable thermostat for subsequent temperature control (step 5.3). In the second, add 5 L of deionized water and 2 kg of ice for subsequent cooling (step 5.10). CAUTION: All subsequent steps up to 5.9 involve the handling of hot liquid. Wear appropriate personal protective equipment including thermally insulated gloves.
Fill the first water bath with 8 L deionized water. Set the temperature to 74.5 °C using the thermostat. Incubate the assembly for 15 min to reach thermal equilibrium.
Prepare an insulated storage vessel by placing a 0.5-L plastic beaker into a 1-L plastic beaker and fill the gaps with cotton wool. Alternatively, use a dedicated thermally insulated vessel with a 0.5-L working volume.
Connect the heat exchanger to the peristaltic pump at one end and to an outlet hose on the other end using L/S 24 tubing. Place both tubing ends along with a thermometer in the insulated storage vessel and fill it with 300 ml extraction buffer (Figure 2).
Place the heat exchanger into the hot water bath and start the peristaltic pump at a rate of 300 ml min-1. Ensure that the resulting temperature in the vessel is 70 °C, approximately 4.5 °C below the set point of the water bath, after 3 min.
If the temperature in the insulated-vessel differs from 70 °C, adjust the temperature of the water bath accordingly and let the system equilibrate for another 15 min. Repeat this step until the temperature of the extraction buffer increases from ambient to 70 °C in less than 3 min, or equals that required for the experiment, and empty the stainless-steel vessel.
Discard the extraction buffer from the insulated vessel and prepare 300-ml aliquots of the plant extract. Fill the insulated vessel with one aliquot.
Pump the plant extract (section 3.3) through the heat exchanger at 300 ml min-1 for 5 min. Ensure that the extract temperature is 70 °C after 3 min, or equals the temperature defined in the experimental design.
After 5 min, place the heat exchanger into the ice-cold water bath while the extract is still being pumped. Incubate in this setting until the temperature reaches exactly 20 °C, or the temperature defined in the experimental design.
Remove the inlet hose connected to the peristaltic pump from the insulated vessel and continue pumping to collect residual heat-precipitated plant extract from within the heat exchanger. Then stop the pump.
Repeat steps 5.7 to 5.10 for all aliquots. Take a 1-ml sample of each heat-precipitated extract once it has reached the final temperature, then pass on the extracts to bag filtration (section 6). NOTE: After a first cycle through steps 5.7 to 5.10 there will be no extraction buffer to discard in step 5.7.
6. Bag Filtration of the Plant Extract
Mount a bag filter into the corresponding support basket which is fitted into the filter housing and provides mechanical support for the flexible filter material. Place a 1-L vessel beneath the basket and apply the extract aliquots (section 3.3, 4.10 or 5.11 depending on the selected heat treatment) to the bag at a rate of 150 ml min-1.
After filtration, measure the turbidity of a 1:10 dilution of filtrate in extraction buffer using the turbidimeter.
Take a 1 ml sample and process the bag filtrate as defined in the experimental design, e.g., depth filtration and chromatography 7,10.
7. Sample Analysis
- Measure the quantity of total soluble protein (TSP) using the Bradford method 24,25.
- In triplicate, pipette 2.5 µl of each sample into the single wells of a 96-well plate. Include eight bovine serum albumin (BSA) standards in triplicate covering the range 0 - 2,000 µg ml-1.
- Add 200 µl Bradford reagent to each well and mix thoroughly by pipetting up and down but gently enough to avoid forming bubbles.
- Incubate for 10 min at 22 °C and measure the absorbance at 595 nm in a spectrophotometer. Calculate the TSP concentration in the samples based on a standard curve through the BSA reference points.
- Quantify Vax8 by surface plasmon resonance spectroscopy 26.
- Setup the surface plasmon resonance instrument with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) running buffer (10 mM HEPES, 3 mM ethylenediaminetetraacetic acid (EDTA), 1,500 mM sodium chloride, 0.05% v/v polysorbate 20, pH 7.4) and mount a carboxymethylated dextran surface chip into the device. Use the prime function to flush the system and start a manual run with a flow rate of 30 µl min-1 over flow cells 1 and 2. Inject 30 mM hydrogen chloride twice for 60 sec with a 60 sec interspersed injection of 25 mM sodium hydroxide.
- Prepare 200 µl of a 500 µg ml-1 mAb 5.2 solution in 10 mM sodium acetate (pH 4.0). Thaw 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) vials and centrifuge them at 16,000 x g for 1 min. Mix 70 µl of EDC with 70 µl of NHS and transfer to a 7-mm plastic vial.
- Activate the carboxymethylated dextran surface chip surface by injecting the EDC/NHS mixture over flow cells 1 and 2 for 10 min with a flow rate of 10 µl min-1.
- Set the flow path to flow cell 2 only. Couple mAb 5.2 by injecting it for 15 min at a rate of 10 µl min-1.
- Switch the flow path to flow cells 1 and 2 and inject ethanolamine for 7 min at a rate of 5 µl min-1 to deactivate the surface. Then inject 30 mM hydrogen chloride twice for 60 sec with a 60 sec interspersed injection of 25 mM sodium hydroxide.
- Inject the samples and MSP1 - 19 standards at a rate of 30 µl min-1 for 180 sec. Ensure that the Vax8 concentration is 50 - 1,000 ng ml-1. Prepare pre-dilutions in HEPES buffered saline containing EDTA and polysorbate 20 (HBSEP) if necessary.
- Subtract the peak signal of flow cell 1 from that of flow cell 2 and use the difference to calculate the Vax8 concentration in samples based on the signal of the MSP 1 - 19 injection with a known protein concentration of 500 ng ml-1.
- Regenerate the chip surface after each sample or MSP 1 - 19 injection by exposure to 30 mM hydrogen chloride for 60 sec at a flow rate of 30 µl min-1.
- Quantify DsRed by fluorescence spectrometry
- In triplicate, pipette 50 µl of each sample into the single wells of a black half-area 96-well plate. Include six DsRed standards covering the range 0 - 225 µg ml-1.
- Measure the fluorescence in duplicate using a 530 ± 30 nm excitation filter and a 590 ± 35 nm emission filter in a spectrophotometer. Calculate the DsRed concentration in the samples based on a standard curve through the DsRed reference points.
- Determination of particle size distributions
- Wash a cuvette with 1 ml isopropanol and then with 2 ml of deionized water to remove dust particles. Pipette 850 µl of the sample into the cuvette.
- Place the cuvette into the zeta potential and particle size analyzer. Open the "Software" and start a manual measurement with "Protein" as the material and "PBS" as the dispersant. Select a temperature of 25 °C and an equilibration time of 180 sec.
- Choose the "DTS0012" cell and measure at 173° backscatter. Check the "auto" function for the measurement duration and select three measurements with no interspersed delay.
- Investigate the particle size distribution peak profile once the measurement is complete by selecting the three measurements of the sample in the experiment view. Choose the "Volume PSD" tab.
Representative Results
Heat precipitation of tobacco host cell proteins by blanching The blanching procedure described in section 2. was successfully used to precipitate HCPs from tobacco leaves with 70 °C, reducing the TSP by 96 ± 1% (n = 3) while recovering up to 51% of the Vax8 target protein, thus increasing its purity from 0.1% to 1.2% before chromatographic separation 16. It was also possible to recover 83 ± 1% (n =3) of the fluorescent protein DsRed, increasing its purity from 3.3% to 64.1%. The blanching procedure was easily integrated into a standard extraction and clarification scheme consisting of biomass washing, homogenization, bag filtration and depth filtration (Figure 1) 7. Preparing the blanching equipment (Figure 2) added about 5 min to the set-up time for the clarification devices, which routinely takes 20 min. Another 7 min was required to perform the blanching of intact leaves in addition to the typical extraction and clarification time of 45 min. However, only 2 min of the additional 7 min was actual "hands-on" time. Additionally, short incubation times of less than 1 min are possible, reducing the blanching time from 7 min to approximately 3 min. Therefore, blanching not only increases the initial purity of a product in crude plant extracts, but is also rapidly completed with no additional process equipment, thus offering the potential to replace at least an initial chromatography step. The blanching bath temperature remained constant, i.e., < 0.2 °C fluctuation, during all experiments even immediately after the addition of harvested leaves which were at ambient temperature. This ensured the process was repeatable, i.e., an average coefficient of variation of 17% (n = 24), in terms of TSP reduction, product yields and filter capacity in the subsequent clarification steps (Figure 3). However, the filter capacity declined at blanching temperatures > 63 °C, potentially increasing the costs of filter consumables. This can be addressed by adding flocculants after protein extraction 8 and filter aids after bag filtration 9, which can restore or even increase filter capacities. A temperature of 60 °C was sufficient to remove 80 ± 3% (n = 3) of HCPs and increase Vax8 purity 2.6 ± 0.1-fold (n = 3) without affecting filter capacity. Therefore, HCP removal by blanching is compatible with target proteins that have moderate heat stability, i.e., a melting temperature below 70 °C 27,28. However, increasing the temperature to 70 °C or more may result in an HCP removal of over 95% (Figure 3). It was useful to conduct the corresponding set of experiments in a well-designed manner using a statistical approach 22 because this allowed the rapid identification of the most relevant process parameters, i.e., heating time and temperature. At the same time, the DoE method generated a predictive model to facilitate process optimization 16.
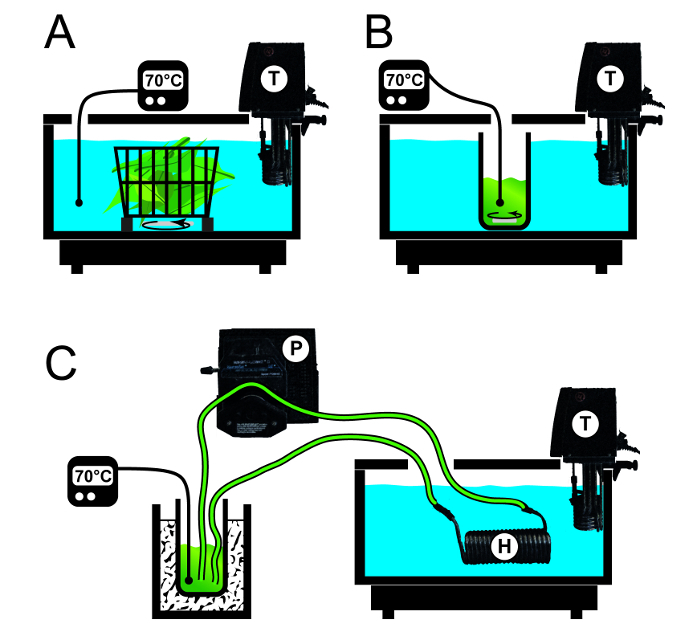 Figure 2:Schematic Setup of Three Methods to Precipitate Tobacco HCPs in Intact Leaves or Extracts Thereof.A. Blanching was carried out in a water bath heated with a thermostat (T) into which a basket containing intact leaves was submerged. The water bath was agitated to ensure a homogenous and constant temperature. B. A vessel containing a magnetic stir bar and leaf extract was submerged into a water bath. The temperature in the extract was monitored to ensure that the required temperature was achieved. C. A heat exchanger (H) was connected to a pump (P) and a thermally insulated storage vessel containing the plant extract. The heat exchanger was submerged in a water bath and the temperature of the heated extract was monitored. Please click here to view a larger version of this figure.
Figure 2:Schematic Setup of Three Methods to Precipitate Tobacco HCPs in Intact Leaves or Extracts Thereof.A. Blanching was carried out in a water bath heated with a thermostat (T) into which a basket containing intact leaves was submerged. The water bath was agitated to ensure a homogenous and constant temperature. B. A vessel containing a magnetic stir bar and leaf extract was submerged into a water bath. The temperature in the extract was monitored to ensure that the required temperature was achieved. C. A heat exchanger (H) was connected to a pump (P) and a thermally insulated storage vessel containing the plant extract. The heat exchanger was submerged in a water bath and the temperature of the heated extract was monitored. Please click here to view a larger version of this figure.
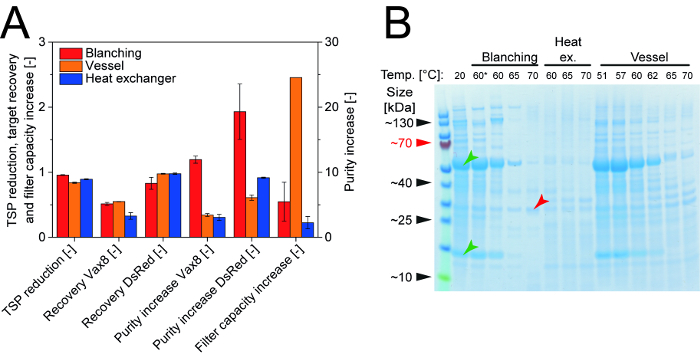 Figure 3:Comparison of Three Heat Precipitation Methods Showing Their Effect on Process Performance and the Purification of Two Target Proteins.A. Conditions supporting the removal of more than 90% of HCPs were identified for blanching, a stirred vessel and a heat exchanger setup, all of which increased the purity of the target proteins Vax8 and DsRed by a minimum of 2.5-fold and a maximum of 19-fold, with blanching performing best. In contrast, only the stirred vessel setup increased the capacity of the subsequent depth filtration step used for clarification of the heat treated plants or extracts. Error bars indicate the standard deviation (n = 3). B. The HCP content of samples after different heat treatment conditions can be analyzed and compared using Coomassie-stained polyacrylamide gels. RuBisCO (green arrows) is removed along with other HCPs as the temperature during heat treatment increases, whereas DsRed (red arrow) remains in solution. * indicates a samples that was exposed to heat for 0.5 min, all other samples were treated for 3.0 min or more. Re-print with permission from 16. Please click here to view a larger version of this figure.
Figure 3:Comparison of Three Heat Precipitation Methods Showing Their Effect on Process Performance and the Purification of Two Target Proteins.A. Conditions supporting the removal of more than 90% of HCPs were identified for blanching, a stirred vessel and a heat exchanger setup, all of which increased the purity of the target proteins Vax8 and DsRed by a minimum of 2.5-fold and a maximum of 19-fold, with blanching performing best. In contrast, only the stirred vessel setup increased the capacity of the subsequent depth filtration step used for clarification of the heat treated plants or extracts. Error bars indicate the standard deviation (n = 3). B. The HCP content of samples after different heat treatment conditions can be analyzed and compared using Coomassie-stained polyacrylamide gels. RuBisCO (green arrows) is removed along with other HCPs as the temperature during heat treatment increases, whereas DsRed (red arrow) remains in solution. * indicates a samples that was exposed to heat for 0.5 min, all other samples were treated for 3.0 min or more. Re-print with permission from 16. Please click here to view a larger version of this figure.
Heat precipitation of HCPs in a stirred vessel A stirred vessel for heat precipitation removed a maximum of 84 ± 1% (n = 3) HCPs, achieving a purity of 0.33 ± 0.02% (n = 3) for Vax8 and 20.2 ± 1.4% (n = 3) for DsRed (Figure 3). The heat treatment was carried out in a vessel separate from the homogenizer to prevent delays reflecting device occupancy when processing multiple samples in series. The handling effort required for the laboratory-scale process was similar to that for blanching but an additional stainless steel vessel and a dedicated cooling step were required. Furthermore, heat transfer to the extract was slower than during blanching, with incubation times of at least 5 min, i.e., 10 times longer than for blanching. The delayed heating was caused by the vessel, which posed an additional barrier to heat transfer, and the ~ 300% greater mass that was heated in the vessel due to the presence of extraction buffer in addition to the plant biomass. Precipitating proteins also adhered to the walls of the vessel, gradually building up an additional heat transfer barrier and increasing the effort required for subsequent cleaning. Setting the water bath temperature 8 °C above the temperature used for heat precipitation compensated for energy losses in the partially open system and achieved the desired extract temperature. In contrast to blanching (section 2) and the heat exchanger setup (section 5), HCP precipitation in a vessel increased the capacity of downstream depth filtration by 2.5-fold, reflecting the lower sheer forces in the vessel compared to pumping extract through the heat exchanger or homogenization after blanching, probably resulting in larger aggregates that were easier to remove in the bag filtration step.
Heat precipitation of HCPs in a heat exchanger Approximately 88.3 ± 0.7% (n = 12) of the HCP content was consistently removed from the extract using a heat exchanger within the temperature range 60 - 70°C, achieving a purity of 0.31 ± 0.01% (n = 12) for Vax8 and 27.6 ± 2.0% (n = 12) for DsRed (Figure 3). The average coefficient of variation was 13% (n = 24) indicating that the repeatability of this procedure was even better than blanching. The desired extract temperature was achieved after ~ 3 min if the water bath temperature was set 4.5 °C higher. As for the heated vessel, a dedicated cooling step was required after heat treatment. The heat exchanger involved more handling effort than the other methods because the pumping apparatus and heat exchanger required intensive cleaning due to the precipitate adhering to the walls of the narrow bore stainless-steel tubing. The heat exchanger also achieved the lowest downstream depth filter capacity, clarifying only 13.5 ± 6.0 (n = 3) L m-2 before clogging.
Discussion
The three methods for heat precipitation described above can effectively remove tobacco HCPs prior to any chromatographic purification step 16,17. They complement other strategies that aim to increase initial product purity, e.g., guttation 29, rhizosecretion 30 or centrifugal extraction 31,32, all of which are limited to secreted proteins. However, the heat-based methods can only be used in a meaningful way if the target protein to be purified can withstand the minimum precipitation temperature of ~ 60 °C for more than 1 min. Therefore, the first step in any of the three methods is to design a target molecule with a sufficiently high melting temperature, which has been described for several malaria vaccine candidate proteins consisting of different domains from several Plasmodium falciparum antigens 16,18,21. Once the thermal stability of the target protein has been demonstrated, one of the three methods can be selected based on the available equipment and media, anticipated final process scale and subsequent DSP operations 16.
Blanching was the fastest of the methods and additional equipment requirements were minimal, so it can easily be implemented into existing laboratory-scale purification protocols for plant-derived recombinant proteins. Thorough agitation of the blanching liquid is an important process parameter that affects the efficiency of HCP precipitation based on both empirical data and theoretical calculations 21,33. Failing to achieve good mixing can impair heat transfer and result in only partial HCP removal, which in turn can be detrimental to the product if host proteases remain active 21,34. Several other parameters can also affect HCP precipitation, e.g., the heating temperature and incubation time, and a DoE approach can therefore be useful to characterize the most relevant factors and provide predictive models to quantify their effects on responses such as product purity, recovery and the performance of subsequent DSP steps 22.
In the vessel setup, longer incubation times were required to achieve complete HCP precipitation and this may increase the likelihood of undesirable target protein denaturation reflecting the extended exposure to high temperatures. More thorough mixing in the vessel could improve the heat transfer and reduce the duration of heating. The long temperature ramp in this setup can also be challenging if proteases in the extract 21,34 become more active before final heat inactivation, causing product losses.
The increased depth filter capacity observed for the vessel setup can help to reduce consumables costs, allowing a larger number of samples to be handled in a project with a fixed budget or reducing the overall funding requirements for a given set of experiments. However, this benefit may be outweighed by the cost of the additional vessel, which is necessary in addition to the homogenizer to prevent the introduction of process hold steps if several extraction runs are required in a series of experiments, e.g., as part of a DoE approach. The positive effect on filter capacity may also diminish if a more intensive mixing regime is used to reduce heating times as suggested above.
A dedicated cooling step is necessary for both extract-based heat precipitation methods, requiring not only additional resources but also prolonging the overall processing time per sample, which can also conflict with fluent DoE procedures or experimental sequences in general. The heat exchanger setup is well characterized from an engineering perspective 35 and can easily be designed and scaled up for specific temperature differences, in contrast to the vessel, whose surface-to-volume ratio changes during scale up. However, once the heat exchanger size is defined, it can be difficult to adjust to alternative temperature differences because its length and thus the heat transfer area are fixed.
Changing other parameters, such as the residence time (or flow rate) and temperature of the heat exchanger medium, can restore flexibility to some degree, but only in small-scale experiments because these factors are typically operated in narrow windows in process scale operations due to restrictions imposed by the available equipment and media. The demand for a combination of short incubation times of 3 - 5 min and a temperature difference of 40 - 60 °C becomes increasingly difficult to solve at the device level as the process scale increases because the dimensions of the heat exchanger become larger. This is especially true for the cooling step because the temperature difference between the medium and desired extract temperature is often smaller (ΔT = 10−15 °C) than the heating step (ΔT = 20−40 °C) resulting in large equipment dimensions or longer cool-down times.
In the future, the protocol can be adapted to biopharmaceutical proteins other than vaccine candidates which in this study were specifically designed for thermal stability. Many antibodies can withstand temperatures of > 70 °C 36,37 which is already compatible with the current heat treatment protocol. This natural thermal stability can be increased further by engineering the different antibody domains 38, thereby increasing the number of proteins that can be subjected to the method (and its variations) presented here. The blanching method has already been applied to transgenic tobacco plants expressing a monoclonal antibody (2G12) 17 which has not been subject to selection for thermal stability or protein engineering. Heat treatment at 65 °C increased the purity of the antibody by a factor of two prior to chromatographic purification while the recovery was similar to that observed without blanching.
Additionally, characterizing the individual HCP melting temperatures of an expression system could facilitate the identification of a temperature with which the process could be conducted similar to pasteurization of milk: high temperature, short time 39. The heat treatment (except for blanching) may also be applied to other biological starting materials to remove HCPs if the product can withstand the necessary temperatures. The latter may deviate from the ones discussed here if other expression platforms such as mammalian cell culture supernatants are being processed. In any case, the cost-benefit-ratio should be taken into account, i.e., does the benefit of reduced HCP levels outweigh the cost for implementing a heat treatment step that causes additional investment costs, increases the process time and may reduce the product yield 16. A critical parameter in this context is the product activity. If it depends on the presence of linear epitopes as for some protein-based vaccines, then heat treatment is unlikely to have an effect 40. In contrast, if protein structure is important, e.g., for conformational epitopes, the precise orientation of amino acid side chains in an enzyme's active site or the correct folding of antibody complementarity determining regions, a heat treatment may interfere with protein activity 41,42. Therefore, suitable analysis assays should be established to monitor product performance before and after heat treatment.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
We would like to acknowledge Dr. Thomas Rademacher, Alexander Boes and Veronique Beiß for providing the transgenic tobacco seeds, and Ibrahim Al Amedi for cultivating the tobacco plants. The authors wish to thank Dr. Richard M. Twyman for editorial assistance as well as Güven Edgü for providing the MSP1-19 reference. This work was funded in part by the European Research Council Advanced Grant ''Future-Pharma'', proposal number 269110, the Fraunhofer-Zukunftsstiftung (Fraunhofer Future Foundation) and Fraunhofer-Gesellschaft Internal Programs under Grant No. Attract 125-600164.
References
- PhRMA. 2013 Report: Medicines in Development - Biologics. Washington, D.C., USA: Pharmaceutical Research and Manufacturers of America; 2013. [Google Scholar]
- Buyel JF. Process development strategies in plant molecular farming. Curr. Pharm. Biotechnol. 2015;16:966–982. doi: 10.2174/138920101611150902115413. [DOI] [PubMed] [Google Scholar]
- Stoger E, Fischer R, Moloney M, Ma JKC. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 2014;65:743–768. doi: 10.1146/annurev-arplant-050213-035850. [DOI] [PubMed] [Google Scholar]
- Melnik S, Stoger E. Green factories for biopharmaceuticals. Curr. Med. Chem. 2013;20:1038–1046. [PubMed] [Google Scholar]
- Buyel JF, Twyman RM, Fischer R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015;33:902–913. doi: 10.1016/j.biotechadv.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Wilken LR, Nikolov ZL. Recovery and purification of plant-made recombinant proteins. Biotechnol. Adv. 2012;30:419–433. doi: 10.1016/j.biotechadv.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Scale-down models to optimize a filter train for the downstream purification of recombinant pharmaceutical proteins produced in tobacco leaves. Biotechnol. J. 2014;9:415–425. doi: 10.1002/biot.201300369. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Flocculation increases the efficacy of depth filtration during the downstream processing of recombinant pharmaceutical proteins produced in tobacco. Plant Biotechnol. J. 2014;12:240–252. doi: 10.1111/pbi.12132. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Opdensteinen P, Fischer R. Cellulose-based filter aids increase the capacity of depth filters during the downstream processing of plant-derived biopharmaceutical proteins. Biotechnol. J. 2014;10:584–591. doi: 10.1002/biot.201400611. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Generic chromatography-based purification strategies accelerate the development of downstream processes for biopharmaceutical proteins produced in plants. Biotechnol. J. 2014;9:566–577. doi: 10.1002/biot.201300548. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Woo JA, Cramer SM, Fischer R. The use of quantitative structure-activity relationship models to develop optimized processes for the removal of tobacco host cell proteins during biopharmaceutical production. J. Chromatogr. A. 2013;1322:18–28. doi: 10.1016/j.chroma.2013.10.076. [DOI] [PubMed] [Google Scholar]
- Holler C, Vaughan D, Zhang CM. Polyethyleneimine precipitation versus anion exchange chromatography in fractionating recombinant beta-glucuronidase from transgenic tobacco extract. J. Chromatogr. A. 2007;1142:98–105. doi: 10.1016/j.chroma.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Downstream processing of biopharmaceutical proteins produced in plants: the pros and cons of flocculants. Bioengineered. 2014;5:138–142. doi: 10.4161/bioe.28061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, van Dolleweerd CJ, Ioakeimidis F, Keshavarz-Moore E, Ma JK. Considerations for extraction of monoclonal antibodies targeted to different subcellular compartments in transgenic tobacco plants. Plant Biotechnol. J. 2008;6:733–748. doi: 10.1111/j.1467-7652.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- Arfi ZA, Drossard J, Hellwig S, Fischer R, Buyel JF. Polyclonal antibodies can effectively detect tobacco host cell proteins after RuBisCO depletion and endotoxin removal. Biotechnol. J. 2015. [DOI] [PubMed]
- Buyel JF, Gruchow HM, Boes A, Fischer R. Rational design of a host cell protein heat precipitation step simplifies the subsequent purification of recombinant proteins from tobacco. Biochem. Eng. J. 2014;88:162–170. [Google Scholar]
- Buyel JF, Fischer R. A juice extractor can simplify the downstream processing of plant-derived biopharmaceutical proteins compared to blade-based homogenizers. Process Biochem. 2014;50:859–866. [Google Scholar]
- Beiss V, et al. Heat-precipitation allows the efficient purification of a functional plant-derived malaria transmission-blocking vaccine candidate fusion protein. Biotechnol. Bioeng. 2015;112:1297–1305. doi: 10.1002/bit.25548. [DOI] [PubMed] [Google Scholar]
- Ma JK, et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015;13:1106–1120. doi: 10.1111/pbi.12416. [DOI] [PubMed] [Google Scholar]
- Mahajan PV, Caleb OJ, Singh Z, Watkins CB, Geyer M. Postharvest treatments of fresh produce. Philos T R Soc A. 2014;372 doi: 10.1098/rsta.2013.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel S, et al. Optimized blanching reduces the host cell protein content and substantially enhances the recovery and stability of two plant derived malaria vaccine candidates. Front. Plant Sci. 2015. [DOI] [PMC free article] [PubMed]
- Buyel JF, Fischer R. Characterization of complex systems using the design of experiments approach: transient protein expression in tobacco as a case study. J. Vis. Exp. 2014. p. e51216. [DOI] [PMC free article] [PubMed]
- Buyel JF. Procedure to evaluate the efficiency of flocculants for the removal of dispersed particles from plant extracts. J. Vis. Exp. 2016. p. e53940. [DOI] [PMC free article] [PubMed]
- Simonian MH, Smith JA. Spectrophotometric and colorimetric determination of protein concentration. Curr. Protoc. Mol. Biol. 2006;76 doi: 10.1002/0471142727.mb1001as76. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Kaever T, Buyel JJ, Fischer R. Predictive models for the accumulation of a fluorescent marker protein in tobacco leaves according to the promoter/5'UTR combination. Biotechnol. Bioeng. 2013;110:471–482. doi: 10.1002/bit.24715. [DOI] [PubMed] [Google Scholar]
- Piliarik M, Vaisocherova H, Homola J. Surface plasmon resonance biosensing. Methods Mol. Biol. 2009;503:65–88. doi: 10.1007/978-1-60327-567-5_5. [DOI] [PubMed] [Google Scholar]
- Kim TD, Ryu HJ, Cho HI, Yang CH, Kim J. Thermal behavior of proteins: Heat-resistant proteins and their heat-induced secondary structural changes. Biochemistry-Us. 2000;39:14839–14846. doi: 10.1021/bi001441y. [DOI] [PubMed] [Google Scholar]
- Kwon S, Jung Y, Lim D. Proteomic analysis of heat-stable proteins in Escherichia coli. Bmb Rep. 2008;41:108–111. doi: 10.5483/bmbrep.2008.41.2.108. [DOI] [PubMed] [Google Scholar]
- Komarnytsky S, Borisjuk NV, Borisjuk LG, Alam MZ, Raskin I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 2000;124:927–933. doi: 10.1104/pp.124.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake PMW, et al. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J. 2009;23:3581–3589. doi: 10.1096/fj.09-131771. [DOI] [PubMed] [Google Scholar]
- Turpen TH. Tobacco mosaic virus and the virescence of biotechnology. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 1999;354:665–673. doi: 10.1098/rstb.1999.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury NJ, McDonald KA. Quantitative Evaluation of E1 Endoglucanase Recovery from Tobacco Leaves Using the Vacuum Infiltration-Centrifugation Method. Biomed. Res. Int. 2014. [DOI] [PMC free article] [PubMed]
- Buyel JF. Numeric simulation can be used to predict heat transfer during the blanching of leaves and intact. Biochem. Eng. J. 2015.
- Mandal MK, Fischer R, Schillberg S, Schiermeyer A. Inhibition of protease activity by antisense RNA improves recombinant protein production in Nicotiana tabacum cv. Bright Yellow 2 (BY-2) suspension cells. Biotechnol. J. 2014;9:1065–1073. doi: 10.1002/biot.201300424. [DOI] [PubMed] [Google Scholar]
- Welty JR, Wicks CE, Wilson RE. Fundamentals of momentum, heat, and mass transfer. Wiley; 1976. [Google Scholar]
- Lowe D, et al. Aggregation, stability, and formulation of human antibody therapeutics. Advances in protein chemistry and structural biology. 2011;84:41–61. doi: 10.1016/B978-0-12-386483-3.00004-5. [DOI] [PubMed] [Google Scholar]
- Gong R, et al. Engineered human antibody constant domains with increased stability. J. Biol. Chem. 2009;284:14203–14210. doi: 10.1074/jbc.M900769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet R, Lowe D, Christ D. Stability engineering of the human antibody repertoire. FEBS Lett. 2014;588:269–277. doi: 10.1016/j.febslet.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Stabel JR, Lambertz A. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp paratuberculosis in milk. J. Food Prot. 2004;67:2719–2726. doi: 10.4315/0362-028x-67.12.2719. [DOI] [PubMed] [Google Scholar]
- Wichers H. Ch. 12. In: Mills C, Wichers H, Hoffmann-Sommergruber K, editors. Managing Allergens in Food. Elsevier Science; 2006. p. 336. [Google Scholar]
- Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998;53:102–105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Dubois MF, Hovanessian AG, Bensaude O. Heat-shock-induced denaturation of proteins. Characterization of the insolubilization of the interferon-induced p68 kinase. J. Biol. Chem. 1991;266:9707–9711. [PubMed] [Google Scholar]


