Smallpox, a devastating infectious disease dreaded throughout much of recorded history, is caused by the variola virus, a member of the poxviridae family. In the 20th century alone, smallpox deaths worldwide numbered in the millions. In 1980, after an intensive program of immunization with vaccinia virus, a related but relatively nonpathogenic virus, the World Health Organization (WHO) declared the disease eradicated. By 1983, all known stocks of variola virus were in two WHO collaborating centers: the U.S. Centers for Disease Control and Prevention (CDC) in Atlanta and (after a transfer in 1994) the Russian State Research Center of Virology and Biotechnology (the Vektor Institute) in Novosibirsk. The WHO Committee on Orthopoxvirus Infections voted on several occasions to recommend destruction of the stocks, but each time the decision was deferred to permit more research on live variola virus. A 1999 National Academies report summarized and assessed scientific needs for live variola virus (1).
The concern that undeclared stocks of variola virus might exist and that they might be used as a bioterrorist weapon (2) was heightened in late 2001 by the deliberate release of Bacillus anthracis, the agent of anthrax, in the weeks after the September 11, 2001, attacks. That concern prompted a voluntary national-preparedness effort to vaccinate healthcare workers, first responders, and members of the military against smallpox. However, given the substantial side effects, the risks associated with the smallpox vaccine, and the absence of information about an imminent bioterrorist attack, vaccination was not accepted by all members of those groups, nor was it recommended for the general public by the government (3).
Whatever the likelihood of covertly held variola virus stocks, an intentional release of the virus would pose a serious health threat and would probably provoke a global health crisis. The lethality of the disease (up to 40%) and its ease of transmissibility place variola virus at the top of CDC's list of high-threat (Category A) agents. For that reason, the federal government rapidly increased its support of research related to the discovery of antiviral drugs against smallpox. In addition to providing potential therapy for infected people, the availability of antiviral drugs could decrease the risks associated with the smallpox vaccine by providing a treatment for vaccine-associated complications. Ultimately, the development of antiviral drugs against smallpox could deter rogue states and terrorists from releasing variola virus because its impact would be diminished. Moreover, the same new therapies are likely to be useful in other poxvirus diseases, such as monkeypox.
Scientific Opportunities
Introduction to Poxviruses.n The poxvirus family. Poxviruses are the largest known animal viruses, with ≈200 distinct genes (5). They are DNA viruses that replicate entirely in the cytoplasm. Thus, a subset of their gene products carries out the functions that are essential for the viruses to be independent of the host-cell nucleus. The other viral gene products use or modulate an astonishingly wide array of host-cell and immune-system processes.
Poxviruses infect most vertebrates and invertebrates, causing a variety of diseases of veterinary and medical importance. The one large family (Poxviridae) has two main subfamilies, the chordopoxvirinae, which infect vertebrates, and the entomopoxvirinae, which infect insects (Table 1). Each of the chordopoxviruses has a restricted and specific host array (Table 2). Humans are the sole hosts of two poxviruses, variola virus (smallpox virus) and molluscum contagiosum virus, although many members of Orthopoxvirus, Parapoxvirus, and Yatapoxvirus can infect both animals and humans (Table 2, zoonotic viruses). Vaccinia virus is the virus used in the variola virus vaccine, and it is widely used as a model poxvirus in the laboratory. Its origins are obscure.
Table 1. The poxvirus family: Poxviridae.
| Subfamily | Genus | Type species |
|---|---|---|
| Chordopoxvirinae (vertebrate hosts) | Orthopoxvirus | Vaccinia virus |
| Parapoxvirus | Orf virus | |
| Avipoxvirus | Fowlpox virus | |
| Capripoxvirus | Sheeppox virus | |
| Leporipoxvirus | Myxoma virus | |
| Suipoxvirus | Swinepox virus | |
| Molluscipoxvirus | Molluscum contagiosum virus | |
| Yatapoxvirus | Yaba monkey tumor virus | |
| Entomopoxvirinae (insect hosts) | Entomopoxvirus A | Melolontha melolontha entomopoxvirus (MMEV) |
| Entomopoxvirus B | Amsacta moorei entomopoxvirus (AMEV) | |
| Entomopoxvirus C | Chironomus luridus entomopoxvirus (CLEV) |
Table 2. Hosts of and infections caused by chordopoxvirinae.
| Virus | Animal reservoir | Geographic distribution | Transmission to other hosts |
|---|---|---|---|
| Genus Molluscipoxvirus | |||
| Molluscum contagiosum | Humans | Worldwide | No transmission to other hosts |
| Genus Orthopoxvirus | |||
| Zoonotic viruses | |||
| Monkeypox virus* | Squirrels | West and Central Africa | Monkeys, humans |
| Vaccinia virus | Unknown | Worldwide | Humans, buffaloes, rabbits, cows |
| Buffalopox virus† | Unknown | Asia | Buffaloes, humans |
| Camelpox virus | Camels | Africa, Asia | Camels |
| Cowpox virus‡ | Rodents | Europe, Asia | Cats, cows, zoo animals, humans |
| Elephantpox virus | Unknown | Asia | Elephants, humans |
| Nonzoonotic viruses | |||
| Variola virus | Humans | Previously worldwide | Only humans |
| Volepox virus | Voles | Western United States | None |
| Ectromelia virus | Rodents | Europe | None |
| Raccoonpox virus | Raccoons | Eastern United States | None |
| Skunkpox virus | Skunks | Eastern United States | None |
| Uasin Gishu disease virus | Unknown | East Africa | Horses |
| Taterapox virus | Gerbils | West Africa | None |
| Genus Parapoxvirus | |||
| Zoonotic viruses | |||
| Bovine papular stomatitis virus | Cattle | Worldwide | Humans |
| Orf virus | Sheep | Worldwide | Ruminants, humans |
| Pseudocowpox virus (milker's nodules) | Cattle | Worldwide | Humans |
| Sealpox virus | Seals | Worldwide | Humans |
| Nonzoonotic viruses | |||
| Auzduk disease virus | Camels | Africa, Asia | None |
| Parapoxvirus of red deer in New Zealand | Deer | New Zealand | None |
| Chamois contagious ecthyma virus | Chamois | — | — |
| Genus Yatapoxvirus | |||
| Tanapox virus | Rodents | East and Central Africa | Monkeys, humans |
| Yaba monkey tumor virus | Monkeys | West Africa | Humans |
—, no data available. Table data are taken from Krauss et al. (6).
Can be spread by exotic pets (brought to the U.S. via this route in 2003).
Closely related to vaccinia virus.
Probably identical to elephantpox virus.
The genomes of 30 poxviruses have been completely sequenced, including several strains of variola and vaccinia viruses. At least two variants of variola virus are known, and they cause two forms of smallpox: variola major, with a case fatality rate of 30–40%, and variola minor, with a much reduced fatality rate of ≈1%. At the genome level, the two variants are very similar. They differ in ≈2% of the roughly 185,000 unique DNA nucleotides; essentially all of the encoded proteins are nearly identical. These comparisons suggest that the high fatality rate associated with variola major may be attributable to a small number of genetic determinants, and they point to our ignorance of the causes of poxvirus pathogenesis in general and of variola virus pathogenesis in particular. Eradication of smallpox occurred at a time of limited knowledge of the molecular and cellular nature of the relevant host defense. Thus, during the times when it was possible to study human infections, the molecular tools for dissecting the host response were lacking.
Vaccinia virus has become the model of choice, and our knowledge of poxvirus biology is derived largely from studies of this virus. The tools available for its study now include large collections of temperature-sensitive mutant strains as well as recombinant strains with specific genes under inducible regulation. Several of the known gene products, such as its enzymes, can be expressed and studied in vitro, and new tools of cell biology are in place to dissect the virus–cell interactions.
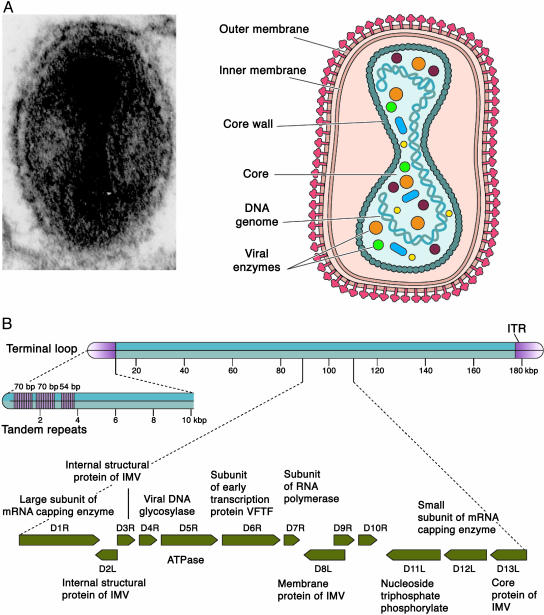
Poxvirus replication cycle. Poxviruses have a complex structure. Fig. 1A shows an electron micrograph of a cross section of the infectious intracellular mature vaccinia virion (IMV) and a schematic summary of the virion. The nature of the membrane envelope surrounding the IMV particle remains controversial. Some argue that there are two closely opposed lipid bilayer membranes; others argue that there is one. (The answer has important implications for viral entry and maturation, as will be discussed below). This IMV envelope forms the outer boundary of a 300-Å-thick surface layer that surrounds the inner core, which often appears dumbbell-shaped. The outer row of reproducibly observed knobs on the core wall has been termed the palisade. The core contains the double-stranded viral DNA genome and virion enzymes, including DNA-dependent RNA polymerase and RNA-processing enzymes. Pox virions contain no obvious helical or icosahedral nucleocapsid. A second infectious particle, the extracellular enveloped virion (EEV), contains an additional lipid bilayer membrane that is wrapped around the entire IMV particle.
Fig. 1.
Vaccinia virus, a representative poxvirus: virion structure (A) and genome organization with an expanded view of the HindIII D restriction enzyme fragment (B). The presence of an inner membrane in the IMV form of the virion shown in A is controversial. See Poxvirus replication cycle for a detailed description. [Reproduced with permission from ref. 4 (Copyright 2003, ASM Press).]
The genomes of poxviruses are double-stranded DNA molecules. Fig. 1B summarizes information obtained from the completely sequenced Copenhagen and WR strains of vaccinia virus. The 191-kbp genome is a double-stranded DNA molecule whose ends are covalently connected by single-stranded hairpin loops of 101 nucleotides. The sequences that form the hairpins are AT-rich and lie at the ends of 12-kbp inverted terminal repetition (ITR) elements that contain short direct repeats and several ORFs. The genome sequence reveals some 185 putative protein-coding sequences and provides a detailed genetic map.
The basis for naming the viral proteins is illustrated by the coding sequences of HindIII fragment D, which are shown in the expanded view in Fig. 1B. The ORFs are depicted by the arrows indicating their orientation in the genome, and each is named with the letter of the HindIII fragment in which the first ATG of the reading frame lies, followed by a number indicating its order and its orientation, either left (L) or right (R). The identities of the encoded proteins, where known, also are listed.
As illustrated in Fig. 1B, the vaccinia coding sequences are densely packed, and the template for RNA synthesis may be present in either strand. The sequences encoding structural proteins and essential enzymes are clustered in roughly the central 120 kb, whereas genes encoding virulence proteins, host-range proteins, or immunomodulators are found predominantly near the ends. This arrangement is also true for all other poxviruses.
Proteins that fulfill different functions are synthesized during different phases of the infectious cycle. For example, the mRNA capping enzyme, uracil DNA glycosylase (UDG), and most RNA polymerase subunits are products of early genes, whereas the genes encoding structural proteins are expressed only during the late phase of infection. In addition, a small but distinct class of proteins including the late transcription factors is encoded by intermediate genes.
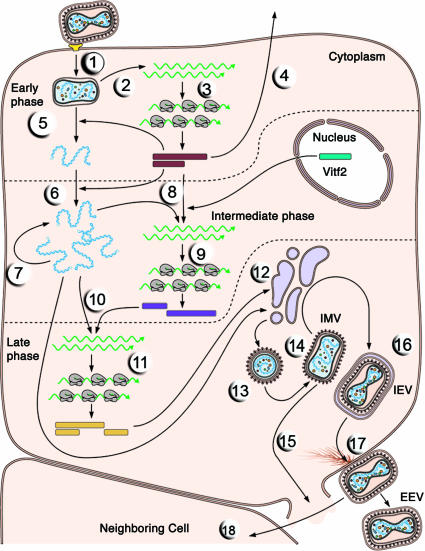
Even though they are DNA viruses, poxviruses replicate in the cytoplasm. Accordingly, they are only minimally dependent on the host cell for DNA and RNA replication, and they encode their own proteins for these processes. A simplified version of the single-cell reproductive cycle of vaccinia virus is illustrated in Fig. 2. EEV entry is illustrated in Fig. 2, step 1. The mechanisms by which either the IMV or the EEV infectious forms of vaccinia virus attach to and enter susceptible host cells are not well understood and will be constrained by the number of membranes enveloping the virus. After fusion of the viral and cellular membranes, primary uncoating takes place, and the viral core is released into the cytoplasm. All later steps in the infectious cycle take place in this cellular compartment. The core contains, in addition to the viral genome, the viral DNA-dependent RNA polymerase, the “initiation” proteins necessary for specific recognition of the promoters of viral early genes, and several RNA-processing enzymes that modify viral transcripts. On release into the host-cell cytoplasm, the core synthesizes viral early mRNAs (Fig. 2, step 2), which exhibit the features typical of cellular mRNAs and are translated by the cellular protein-synthesizing machinery (Fig. 2, step 3). Approximately half of the viral genes are expressed during this early phase of infection.
Fig. 2.
The single-cell reproductive cycle of vaccinia virus. The entry and replication of an EEV are illustrated. RNA molecules are green. See Poxvirus replication cycle for a detailed description of each illustrated step. [Reproduced with permission from ref. 4 (Copyright 2003, ASM Press).]
Some early proteins are secreted from the cell (Fig. 2, step 4) and have sequence similarity to cellular growth factors, which can induce proliferation of neighboring host cells, or are proteins that counteract host immune defense mechanisms. The synthesis of early proteins also induces a second uncoating reaction in which the core wall opens and a nucleoprotein complex containing the genome is released from the core (Fig. 2, step 5). This core disassembly leads to cessation of viral early gene expression. Other early proteins catalyze the replication of the viral DNA genome (Fig. 2, step 6). Newly synthesized viral DNA molecules can serve as templates for additional cycles of genome replication (Fig. 2, step 7), and they are the templates for transcription of viral intermediate-phase genes (Fig. 2, step 8). The activation of transcription of intermediate genes also requires specific viral proteins (the products of early genes) that confer specificity for intermediate promoters on the viral RNA polymerase, as well as a host-cell protein (Vitf2) that relocates from the infected cell nucleus to the cytoplasm. The proteins encoded by intermediate mRNAs (Fig. 2, step 9) include those necessary for transcription of late-phase genes (Fig. 2, step 10). The latter genes encode the proteins from which virions are built as well as the virion enzymes and other essential proteins, such as the early initiation proteins, that must be incorporated into virus particles during assembly. Once these proteins are synthesized by the cellular translation machinery (Fig. 2, step 11), the assembly of progeny virus particles begins. The viral membrane proteins are unglycosylated, and the role of cellular membranes in early stages of assembly is controversial (Fig. 2, step 12).
The initial assembly reactions result in formation of the immature virion (Fig. 2, step 13), which is a spherical particle delimited by a membrane that may be acquired from an early compartment of the cellular secretory pathway. This virus particle matures into the brick-shaped IMV (Fig. 2, step 14), which is released only on cell lysis (Fig. 2, step 15). However, the particle can acquire a second, double membrane from a trans-Golgi or early endosomal compartment to form the intracellular enveloped virion (IEV) (Fig. 2, step 16). The IEVs move to the cell surface on microtubules where fusion with the plasma membrane forms cell-associated virions (CEV) (Fig. 2, step 17). These CEV induce an actin polymerization that promotes a direct transfer to surrounding cells (Fig. 2, step 18); they can also dissociate from the membrane as EEV.
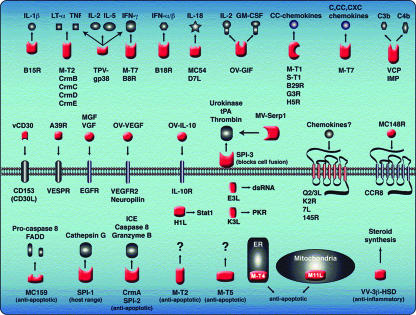
Although studies of viral entry and morphogenesis have revealed striking mechanisms by which the viral proteins interact with the actin and microtubule cytoskeleton, they also have left us with several unresolved puzzles in membrane biogenesis. In addition, there is an extensive molecular dialogue between the viral gene products and the vertebrate host's innate and adaptive immune systems, as illustrated by the known targets of many of the virus-encoded immune modulators and regulators of apoptosis (Fig. 3). These include secreted “virokines” that mimic host cytokines, secreted “viroceptors” that mimic host cytokine receptors, and intracellular proteins that interfere with apoptosis and signaling pathways.
Fig. 3.
Schematic representation of selected poxvirus immunomodulators and regulators of apoptosis. Proteins shown in red represent poxvirus proteins; host proteins are shown in black and gray. Secreted poxvirus viroceptor proteins (top row) function as soluble or cell surface decoys that bind host-cell cytokines or chemokines in the cell exterior. Poxvirus virokines also are secreted; they function as agonistic or antagonistic ligands for host cellular receptors (middle row). A number of poxvirus proteins function inside the cell to modulate apoptosis, cytokine processing, and host range (red proteins in cell interior). [Reproduced with permission from ref. 7 (Copyright 2003, Annual Reviews, www.annualreviews.org).]
In short, it is clear that poxviruses constitute an underexploited tool for probing fundamental processes in both cell biology and immunology. By taking advantage of this tool, we will certainly increase our understanding of important biological mechanisms; we are also very likely to discover unexpected ways to control poxvirus infections.
Routes to New Antiviral Therapeutic Agents: Poxvirus Enzymes as Candidate Drug Targets. Essential viral enzymes have frequently proved to be good targets for antiviral drugs (for example, herpesvirus thymidine kinase, HIV reverse transcriptase and protease, and influenza neuraminidase). Initial efforts to identify inhibitors should therefore focus on enzymes that have essential roles in poxvirus replication and for which atomic structures have been determined or can be determined promptly. These criteria narrow the field of candidate targets considerably. So far, the atomic structures of five poxvirus proteins have been determined: topoisomerase I, cap-specific 2′O-methyltransferase/poly(A) polymerase stimulatory subunit, complement-control protein, CrmA immune modulator, and an inhibitor of the RNA-dependent protein kinase. A poxvirus structural-biology initiative would be a valuable component of an organized effort to discover inhibitors of viral enzymes. The enzymes listed in Table 3 have at least some of the desired characteristics.
Table 3. Potential drug targets: Selected poxvirus enzymes.
| Drug target | Poxvirus enzymes |
|---|---|
| DNA replication and recombination (Fig. 2, steps 6 and 7) | |
| E9 | DNA polymerase (target of cidofovir) |
| A20 | Polymerase processivity factor |
| A22 | Holliday junction resolvase |
| D4 | Uracil DNA glycosylase |
| D5 | Nucleoside triphosphatase |
| mRNA synthesis and modification (Fig. 2, steps 2, 8, and 10) | |
| Nine subunits | RNA polymerase (J6, A24, A29, E4, J4, A5, D7, G5.5, and H4) |
| E1 (+J3) | Poly(A) polymerase [catalytic (+stimulatory)] |
| D1 (+D12) | mRNA capping enzyme [catalytic (+stimulatory)] |
| H6 | Topoisomerase I |
| H1 | Protein phosphatase (dual specificity) |
| Protein modification and virus assembly (Fig. 2, step 14) | |
| F10 | Protein kinase (dual specificity) |
| I7 | Cysteine protease |
| E10, A2.5, G4 | Thiol oxidases |
Drugs that target topoisomerases and cause them to damage DNA are among the most successful therapeutics developed for both infectious disease (quinolones) and cancer (etoposide, adriamycin, and camptothecins). Indeed, drug screening “in silico” (that is, by computer modeling) is already under way for poxvirus topoisomerase I, a 314-aa monomer comprising two domains joined by a flexible molecular hinge. A recent study indicates that the enzyme is not absolutely essential but has an important role in enhancing early transcription in the virus core (13).
H1 protein phosphatase, a dual-specificity cysteine phosphatase encapsidated in the virus particle, is required in order for the encapsidated viral genome to be transcribed by the viral transcriptase. Dual-specificity phosphatases (DSPs) participate in a number of cellular signaling pathways. Their activities are relevant to various disease states, and there has been substantial work on their pharmacology. The DSP domain of H1 is small (171 aa), however, and lacks unique structural features. There are also highly conserved “vaccinia H1-like” homologues in humans. Thus, a better candidate for an antiviral might be the F10 dual-specificity protein kinase, which also is critical for the earliest steps of virus morphogenesis. It has no sequence similarity to known cellular kinases, with the exception of a 110-aa region of a protein known as human H1 protein phosphatase. The F10 protein sequences in variola and monkeypox viruses are 98% identical.
The I7 cysteine protease, encapsidated in the virus particle, processes the major virion core proteins at a cleavage motif, AlaGly↑X. A loss in functionality of the enzyme leads to arrest of virion morphogenesis at a point after the formation of spherical immature particles. This protein of 432 aa is 99% identical in variola and monkeypox viruses; it appears to have only a very distant relationship to the cellular SUMO-specific protease Ulp1 and to adenovirus and African swine fever virus (ASFV) proteases.
The A22 Holliday junction (HJ) resolvase is a small, 187-aa, metal-dependent nuclease. The encapsidated enzyme is synthesized late in infection and participates in the resolution of concatemeric DNA replication intermediates before genome packaging. A distant relative of the bacterial HJ resolvase RuvC and fungal mitochondrial resolvase Cce1, the nuclease is peculiar to poxviruses and extocarpus siliculosus virus; no human orthologues have been identified. Little is known about possible HJ nuclease inhibitors and their potential as drugs.
Uracil DNA glycosylase (UDG) is a 218-aa protein that is required for DNA replication. The UDG has an essential role in viral DNA replication that is independent of its catalytic activity. However, viruses encoding a catalytically inactive UDG are attenuated in vivo.
The poxvirus capping enzyme is a multisubunit complex responsible for adding a 7-methyl guanosine “cap” to the 5′ end of viral messenger RNA. Although they accomplish the same function, there are important biochemical differences between the capping complexes of poxviruses and those of their mammalian hosts.
The virion encapsidated RNA polymerase (RNAP) holds great potential as a drug target. This nine-subunit enzyme, packaged in the viral core, transcribes genes expressed early in replication. Many of the subunits are not found in cellular RNA polymerases. RNAP appears to have a unique initiation mechanism that requires early transcription factors (ETFs) found only in poxviruses. Attempts to target pox RNAP so far have taken two approaches. The first is to use small molecules to block promoter recognition or ATP hydrolysis by ETFs. The second is to devise an “mRNA poison”: here the virus is provided with a substrate analogue (for example, adenosine N1 oxide) that is incorporated into the viral mRNA by poxvirus RNAP, but the mRNA cannot be translated into viral proteins.
The poxvirus poly(A) polymerase is a heterodimer composed of catalytic and processivity subunits. The processivity subunit, for which the atomic structure is known, also methylates the ribose of mRNA caps and works as a transcription elongation factor.
The E9 DNA polymerase, required for DNA replication, acts in concert with accessory proteins to attain efficient processive synthesis. Accessory proteins include the A20 protein, D4 UDG, and possibly others. The D5 NTPase and the viral SSB (the I3 protein) also are essential for viral replication. The NTPase has homology to helicases, although it is not yet known to act in this capacity. Viral DNA polymerases are established drug targets, as exemplified by azidothymidine (AZT), which inhibits the HIV reverse transcriptase, and acyclovir, which is efficiently phosphorylated by the herpes simplex virus viral thymidine kinase, resulting in a triphosphate that preferentially inhibits viral DNA polymerase. The DNA polymerase inhibitor cidofovir inhibits poxvirus DNA polymerases, including that of variola virus, and it has been shown to have antiviral effects in animal models. The drug currently is used to treat molluscipoxvirus infection in AIDS patients and is recommended by CDC for use in treating complications of vaccination with existing smallpox vaccines.
Three conserved poxvirus thiol oxidases, only distantly related to cellular proteins, are required for formation of the disulfide bonds in several viral membrane proteins. Deletion or mutation of any of these proteins prevents virus assembly. Thus, each of these redox proteins would provide a good target for developing an antiviral drug.
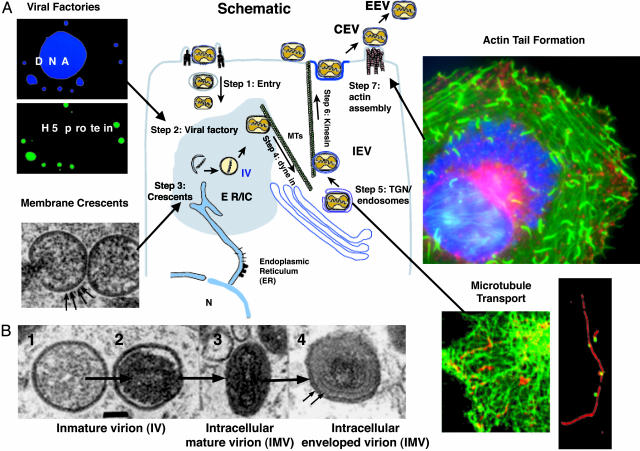
Cell Biology of Poxviruses. Six aspects of poxvirus research are particularly ripe for investigation and offer substantial opportunities for discovery. They are: (i) the mechanism of entry into host cells; (ii) the regulation and intracellular organization of DNA replication, DNA transcription (RNA synthesis), and translation (protein synthesis); (iii) the viral manipulation of host-cell membrane traffic; (iv) the viral subversion and exploitation of host-cell signaling pathways; (v) the viral exploitation of motor proteins for movement within a cell and for propulsion toward another cell; and (vi) the mechanism of cell-level host-range restriction. This list of topics indicates the wide array of advanced scientific expertise that should be used in future studies of poxvirus biology to increase our understanding of the infectious process. Fig. 4 highlights features of poxvirus cell biology and some of the unanswered questions for future research.
Fig. 4.
Major questions in the cell biology of poxvirus infection. (A Center) The schematic portrays some events in vaccinia infection and morphogenesis. The specific steps and unanswered questions are numbered and discussed in the text. Stages of virus maturation shown are immature virion (IV), intracellular mature virion (IMV), intracellular enveloped virion (IEV), cell-associated enveloped virion (CEV), and extracellular enveloped virion (EEV). Images surrounding the schematic illustrate some of these events. (Upper Left) Fluorescent micrographs of viral factories, which are detected together with the cell nucleus by the DNA binding dye Hoescht (blue) and, more specifically, by antibodies to the viral protein encoded by the H5 gene (green) [Reproduced with permission from ref. 8 (Copyright 2001, American Society for Cell Biology).] (Lower Left) A thin-section electron micrograph of membrane crescents, an early stage in viral assembly. The derivation of the membrane from either the endoplasmic reticulum (ER) or intermediate compartment (IR) is uncertain, although regularly spaced small spikes can be seen on the outer membrane (arrows). [Reproduced with permission from ref. 9 (Copyright 2002, American Society for Microbiology).] (Lower Near Right) Superimposed frames from a time-lapsed video showing microtubule-based movement of vaccinia virus that appears as red streaks along GFP-labeled microtubule tracks (green). [Reproduced with permission from ref. 10 (Copyright 2001, Nature Publishing Group, www.nature.com).] (Lower Far Right) Viral particles (green) remain stably associated with microtubules (red) even after extensive extraction of infected cells. [Reproduced with permission from ref. 11 (Copyright 2002, Society for General Microbiology).] (Upper Right) A fluorescent micrograph of actin tail formation (green) juxtaposed to and triggered by cell-surface associated CEV (red), which function to propel the virus particle away from the cell and/or into adjacent cells. 4′,6-Diamidino-2-phenylindole (DAPI) staining (blue) reveals a large viral factory adjacent to the cell nucleus (Photograph courtesy of Tim Newsome and Michael Way). (B) Thin-section electron micrographs of sequential stages of viral maturation. [Reproduced with permission from ref. 12 (Copyright 2001, American Society for Microbiology).]
Entry into host cells. Vaccinia virus can invade and replicate in a wide array of host-cell lines, but the identity of host-cell receptors and the routes of entry into the cytosol remain unknown (Fig. 4, step 1). There are two infectious forms of the virus, IMV and EEV, with either one or two (IMV) or two or three (EEV) lipoprotein bilayers surrounding the nucleoprotein core. The identity of putative viral fusion proteins is unknown, but the unusual arrangement of the poxvirus envelope may imply that its fusogens are mechanistically distinct from more familiar viral fusion proteins. Identification and characterization of the entry, fusion, and uncoating mechanisms of poxviruses should therefore broaden our understanding of the general mechanism of membrane fusion, which is central to a wide variety of critical processes in cells.
Regulation and organization of DNA replication, transcription, and translation. Poxviruses are unique among animal DNA viruses in carrying out DNA replication and transcription in the interphase cytoplasm. Expression of the viral genome thus includes opportunities for types of regulation unfamiliar elsewhere in animal cell biology and hence opportunities to identify specific antiviral targets. Only the bare essentials of the regulation of poxvirus gene expression are understood. Promoter sequences, the virus-encoded RNA polymerase, and transcription factors have been identified (14), but the mechanisms of transcription initiation, elongation, and termination are not known in any molecular detail. Data from in vitro studies indicate a role of host proteins in transcription, but no supporting in vivo data have been reported. It has long been known that intermediate and late transcripts have nontemplated 5′ poly(A) leader sequences, but their role has not been clarified. After virus infection, the turnover of all mRNAs is greatly increased by an unknown mechanism. With regard to DNA replication, several essential proteins have been identified by genetic screens, but their functions, except for that of the DNA polymerase, are not well defined (5). Other features of poxvirus DNA replication deserve attention. The linear DNA genome is terminated by hairpin loops at each end and is thus a covalently closed, single-stranded DNA. How does replication begin on this template? A focused effort on developing an in vitro DNA replication system composed of purified poxvirus proteins capable of replicating double-stranded DNA templates could provide a particularly fruitful test bed for the screening of potential antiviral drugs. [For a review of other such systems, see Bell et al. (15)].
There are cytologic hints that poxviruses establish some type of membrane-enclosed “virus factory” in the cytoplasm where replication takes place (Fig. 4, step 2). What is the nature of this putative compartment, and how does the virus set it up? Transcription and translation of at least some viral products are spatially controlled in the host cell, and some kinds of directed transport must take place during virus-particle assembly (Fig. 4, step 4). What is the molecular basis of these processes, and what roles do host-cell structural components have in scaffolding or directing them?
Viral manipulation of host-cell membrane traffic. To what extent does the virus exploit host-cell structures for organization of its assembly process, and to what extent does it remodel the cell scaffold to fit its needs (16)? Host membrane alterations are closely coupled to spatial targeting, transport, and assembly of virus-particle components. An early event in morphogenesis is the appearance within virus-factory regions of membrane “crescents,” which may be closely apposed lipid bilayers derived from endoplasmic reticulum membrane (Fig. 4, step 3). How do the viral proteins target, recruit, and grossly rearrange the host membranes to form these unusual structures, and how are viral genomes delivered to and inserted into them? Is there any relationship of crescent formation to autophagy? At later stages, the viral particles undergo a second layer of membrane wrapping, this time recruiting trans-Golgi or endosomal membranes (Fig. 4, step 5). Analysis of mutants and recombinant strains has enabled identification of viral proteins that are involved in many of these membrane manipulations, creating a diverse molecular toolkit, both for exploring cell membranes and organelles and for providing a diverse array of targets for inhibitors.
Viral exploitation of host-cell signaling pathways. Before the assembled particle becomes infectious, an essential virally encoded protease must cleave several proteins in a process that is coupled to condensation of the core. A virus-specific group of thiol transferase enzymes also catalyzes formation of disulfide bonds on the intracytoplasmic tails of virus membrane, enabling this oxidation to occur in an apparently reducing environment (17). The initial, immature particle, the immature virion (IV), is noninfectious, but the final result of the maturation steps, the IMV, is both highly infectious and stable. How does morphogenesis occur, and what molecular and mechanical events are involved? How is protein processing by cleavage and disulfide formation linked to the morphologic alterations? What key differences determine the infectivity of the IMV as opposed to the IV?
Viral exploitation of motor proteins. The assembled particles use microtubule motors to get to the perinuclear region for envelopment in the Golgi membrane (Fig. 4, step 4) and subsequently move along microtubules to the cell surface (Fig. 4, step 6) (18). Moreover, budded extracellular virions still attached to the cell membrane direct assembly of intracellular actin bundles at positions just beneath their membrane attachment point, driving formation of a membrane stalk that bears the virion (CEV) at its tip (Fig. 4, step 7). How do viral proteins establish these assemblies? Which proteins are essential for spread of the infection in a tissue?
Mechanism of host-range restriction in vitro and in vivo. There are a number of molecularly defined mutant vaccinia virus strains that replicate in some mammalian lines but not in others. Those strains provide an opportunity to define the role of host-cell factors in viral replication and morphogenesis. These mutant viruses also may facilitate the development of safer vaccines.
Understanding the complex molecular interactions that occur between the replicating virus and the host cell should reveal novel targets for antiviral therapy.
Research Needs in Pathogenesis and Host Response. Animal models. Because of the host-range specificity and containment requirements of variola virus, closely related orthopoxviruses that replicate in rodents (such as cowpox virus, ectromelia virus, and vaccinia virus, including the rabbitpox strain) are usually used for studies of viral pathogenesis. Monkeypox in the cynomolgus monkey is the best nonhuman-primate model, although large doses of virus are required. Monkeypox virus is a select agent (a hazardous biological agent subject to safety regulations), and Biosafety Laboratory 3 facilities are required, but primate models are essential for testing vaccines and therapeutics at late stages in their development. More accessible and less expensive animal models will be critical for rapid work at earlier stages, and development of realistic animal models for orthopoxvirus infection and disease (such as models for which the innoculum required for infection is not heroic and for which the endpoint is not necessarily death) should be emphasized. Research across the entire spectrum of poxvirus genera should be strongly encouraged, because the history of virology has shown that key insights into one group of viruses often come from the study of an apparently divergent group.
Proteomics. There is a clear need for a complete analysis of the structural and nonstructural proteins of variola virus and related orthopoxviruses supported by the requisite bioinformatics tools. Neither infectious variola virus nor intact variola virus DNA can be transferred from the two authorized smallpox facilities, but WHO regulations allow other laboratories to work with DNA representing up to 20% of the variola virus genome in nonpoxvirus vectors. A coordinated international effort to study variola virus proteins therefore will be necessary if we are to acquire a proper understanding of the viral proteome.
Viral and cellular determinants of host response. As is the case with many other viruses, little is known with certainty about immune characteristics that correlate with protective immunity against variola viruses or other poxviruses. Consequently, all aspects of both the innate and acquired immune response must be studied. It is important to define the innate immune response to a poxvirus infection, including cytokine production and regulation of cellular receptors involved in activation of elements of the innate immune system. The capabilities of modern DNA-array technology should be brought to bear on this analysis, with a focus on primate models (7). Fortunately, the available human arrays appear to be useable for nonhuman-primate samples as well.
If an animal model (such as monkeypox in cynomolgus monkeys) is deemed an adequate surrogate for variola virus, it will be of the utmost importance to track the various immune characteristics known to correlate with B cell and T cell activation and with emergence of memory B and T cells. That goal will require establishment (in the BL4 facility for variola virus and in appropriate containment facilities for other poxviruses) of the appropriate cell-sorting instruments, allowing researchers to readily obtain the relevant leukocyte subsets from infected animals (B cells, T cells, macrophages, dendritic cells, and so forth). Similarly, obtaining purified leukocyte subsets in this way will be essential for evaluating cytokine production. Wherever a mouse model can be used, advantage can be taken of the outstanding sets of reagents that are available (antibodies, nucleic acids, and knockout animals). Those experiments should be started now.
Immunotherapeutics. Protective human or humanized monoclonal antibodies to variola virus for use in immunotherapy should be developed with modern technologies. These antibodies would be directed to the viral surface proteins and possibly to secreted products. Many of the antibodies would crossreact with other orthopoxviruses and could be tested in animal models. The perceived importance of a “cytokine storm” (in which the production and release of inflammatory cytokines and stress mediators overwhelms the host) as a major determinant of the pathologic condition offers further opportunities for immune intervention (for example, with tumor necrosis factor antagonists).
Safer vaccines. Further knowledge of orthopoxvirus proteins, the mechanisms of infection, and the correlates of immunity may enable the design of safer smallpox vaccines. Possible approaches include the development of attenuated strains of vaccinia virus, recombinant proteins, and vectors. Any antipoxvirus therapeutics developed will make it possible to treat the rare complications of vaccination, making vaccinations even safer.
Discussion. Naturally occurring variola virus has been eradicated from the planet. Indeed, the last reported case of natural smallpox occurred in Somalia in 1977, long before recent advances in molecular biology, cell biology, and immunology would have allowed researchers to study the variola virus and the pathogenesis of human infection in fine detail. Studies on the closely related vaccinia virus have provided a relatively thorough outline of poxvirus replication in mammalian cells, but a great deal remains to be understood and little is known about how the human immune system responds to variola virus. Given the virulence of this virus (up to 40% mortality) and its ability to spread in a population, the consequences of an intentional release of variola virus could be devastating. Official stocks of the virus are closely held, but it is not known whether undeclared stocks exist, so it is difficult to assess the current degree of risk. Safer vaccines and therapeutics that can mitigate the consequences of infection would together provide a strong deterrent to any intentional release. Effective development of such deterrents will benefit greatly from a deeper understanding of the biology of poxviruses and of how they interact with their hosts. There is an enormous range of opportunities: because variola is a large virus, it requires many specific viral-encoded proteins to infect humans, each of which represents a potential target for an antiviral drug.
We recommend a research-and-development response at three levels. First, we propose an immediate focus on currently identifiable therapeutic targets, particularly essential poxvirus-encoded enzymes, through a combination of academic and industrial research. The pharmaceutical industry has enormous experience in the discovery, design and development of enzyme inhibitors, and each of the enzymes listed in Table 3 is a potential antiviral target. We recommend that the National Institutes of Health (NIH) use the mechanisms outlined in Incentives, Logistics, and Policies below (see especially Recommendation 1) to initiate, both in pharmaceutical and biotechnology companies and in some collaborating academic laboratories, immediate work on each of these enzymes. Second, we suggest that rapid, short-term progress toward deeper understanding of poxvirus biology will result from active collaborations between government or academic laboratories already engaged in poxvirus research and others with specific biologic or technologic expertise. As examples of such collaborations, we recommend NIH-sponsored initiatives in the structural biology of poxvirus proteins, in the cell biology of poxvirus infection, and in the interaction between poxviruses and the host immune system. Third, we observe that longer-term progress will require recruitment and training of a new cohort of outstanding young investigators, and we suggest that the initiatives just listed be designed with this long-term goal in mind. In the second part of this report, Incentives, Logistics, and Policies, we consider the organizational requirements for achieving each of those objectives and propose specific ways to meet them.
There is a pressing need for new antiviral drugs and new vaccines, whether to counter potential bioterrorist threats, to treat HIV, or to deal with emerging pathogens like severe acute respiratory syndrome (SARS) coronavirus. Collaborations that bring poxvirologists together with outstanding scientists in other fields of biology are not only likely to accelerate the search for ways to control infection by variola virus but also to produce discoveries relevant to diverse pathogens, including other DNA viruses and intracellular bacteria. Such collaborations will also expand our understanding of the fundamental cellular processes that poxviruses exploit.
In the broadest sense, directing the attention of a larger number of outstanding scientists with expertise in cell biology, structural biology, computational biology, and chemical biology to the complexities of viral pathogenesis can be a fruitful effort for increasing both national and international security. In the long run, a well conceived, concerted program to discover treatments for smallpox (should it reemerge) will provide models for how to motivate interdisciplinary groups to solve other major problems in public health. It will likewise demonstrate how to create effective incentives for participation of the biotechnology and pharmaceutical industries in the production of new types of protective agents against viruses and other threat agents, whether these are introduced intentionally or through natural processes.
Incentives, Logistics, and Policies
It is not known whether there are stocks of variola virus other than those sanctioned by the WHO or whether smallpox will someday become a weapon used by terrorists or rogue states. If the latter were to occur, the reemergence of smallpox would create a major international health crisis. To counter the potential use of variola virus as a weapon and to protect the public in the event of an intentional release, a concerted international effort is needed to tackle the scientific challenges described in the first part of this report and to translate the results of that research into new therapeutic candidates.
It is important to recognize that the eradication of naturally occurring smallpox was achieved through a global effort. Therefore, although many of the recommendations related to those issues are formulated here in the context of U.S. institutions (for example, NIH) the committee urges that its proposals be taken in an international context and that the initiatives recruit broad international participation.
Engaging the Pharmaceutical and Biotechnology Industries. The importance of industrial participation at early stages in the discovery of smallpox antivirals. If the discovery and development of antiviral therapeutics to treat poxvirus infections is to be a high priority, it is essential that pharmaceutical and biotechnology companies be engaged from the outset. Academic researchers eventually may acquire the resources, equipment, compound libraries, and experience required to participate successfully in drug discovery, but that process will certainly take years. Meanwhile, there are many pharmaceutical and biotechnology companies already poised to discover poxvirus-specific antiviral drugs, beginning with the candidate targets listed in Table 3. These companies currently have the equipment and compound libraries required to screen more than a million compounds per month against any drug target peculiar to poxviruses. Moreover, these for-profit companies have the expertise needed to assess the attributes or deficits of compounds emerging at early stages from high-throughput drug screens; to synthesize the hundreds of analogues of early drug candidates required to move such compounds from the early “hit” stage into bona fide drug leads; to perform the pharmacologic and toxicologic assessments essential for the generation of substantive, preclinical drug leads; to perform the detailed, regulated preclinical studies needed for submission of an investigational new drug (IND) application to the Food and Drug Administration (FDA); and to perform clinical trials at the safety and efficacy stages of drug development.
The success of both traditional pharmaceutical companies and biotechnology companies in discovering, developing, and marketing novel therapeutics specific for the treatment of HIV demonstrates the power of these organizations for producing clinically valuable antiviral therapeutics. Poxviruses offer a far broader spectrum of therapeutic targets than does HIV, and it is reasonable to expect that finding poxvirus-specific drugs will be less demanding than the challenges presented by HIV. We therefore believe that, with the engagement of appropriate pharmaceutical and biotechnology groups, clinically valuable products will emerge.
A program for engaging the pharmaceutical industry. Engaging pharmaceutical and biotechnology companies in a poxvirus drug-discovery effort will require significant changes in the ways that NIH, CDC, and other federal agencies have traditionally interacted with industry. First, because the government is likely to be the sole purchaser of any products, their development will require incentives and resources at critical stages. Second, the way in which programs and proposals are evaluated and reviewed will require advisory structures and procedures quite different from the peer-review system that works well for academic research awards.
The committee suggests the following outlines of a program to meet the challenges of industry incentives. First, senior government health officials should make a direct and open invitation to the highest levels of pharmaceutical and biotechnology management. A widely announced, high-profile program would allow companies to be seen as contributing to an important public-health response. Second, NIH should provide broad support for the early stages of drug discovery, beginning with currently identifiable targets and continuing as basic research uncovers new targets and novel strategies. Third, several awards (perhaps five or six in total) should be made to companies that have demonstrated their ability to deliver the most attractive preclinical candidate drugs, derived from work in the previous stage. These awards would support accelerated preclinical drug development, including “good manufacturing process” production, full evaluation of drug metabolism and pharmacokinetics, toxicology and animal efficacy tests, and appropriate regulatory compliance leading to an investigational new drug submission to the FDA.
The fourth step of the proposed antipoxvirus drug development program includes clinical studies and preparation of a new drug application (NDA) for approval by the FDA. This will be the most expensive phase of the process, and it is particularly for this step that new government/industry relationships will need to emerge. One potential strategy, which is incorporated in the pending BioShield legislation (see below), involves the concept of a guaranteed market, with prenegotiated prices contingent on successful product development (as defined by FDA licensing) within a fixed time frame. Key decisions will need to be made about the negotiation of a guaranteed market and price and about the timing and amount of government support during the development process. A possible drawback of this process is that it tends to reward the first company to bring forward a drug rather than the one that produces the best drug.
An alternative strategy for step four would be to create a “public pharmaceutical company,” either specifically for development of smallpox antivirals or more broadly for other bioweapons countermeasures. Such a course would, however, require the duplication of talent and resources already available within the pharmaceutical industry. The long timescale for drug development also might be inappropriate for a single mission-oriented facility.
It would be desirable to have the participation of pharmaceutical companies of various sizes in the discovery and development process. The committee found, in discussions with individuals acquainted with both large pharmaceutical companies and smaller biotechnology firms, that engaging companies in these two categories might require different approaches. For example, the contract program outlined as steps two and three in the preceding paragraph would probably work better for smaller companies, whereas larger firms might respond better to a single contract covering both these steps, specifying appropriate milestones for continuation.
The government also might consider assembling a consortium of pharmaceutical and biotechnology companies to share knowledge as discovery proceeds. In this setting, the exchange of information would reduce individual opportunity costs. Moreover, if one company were to discover a particularly favorable synthetic scaffold or series of compounds that inhibit a specific target, it would make sense for the entire community to focus on it. Alternatively, it might be appropriate to distribute work on different targets among different participating companies. Because the government is not the customary market for pharmaceutical companies, and because knowledge-sharing might lead to advances in understanding of antivirals in general, pharmaceutical companies might be more willing than usual to exchange information. The issues of antitrust regulation and intellectual property protection raised by such information-sharing would need to be considered, but if the program were deemed a sufficiently critical national priority, they should not be insurmountable.
The committee believes that in setting up and implementing a program such as the one outlined above, it is especially important that wise decisions be made by using the best scientific judgment and astute management considerations. Moreover, the ways in which programs are implemented will need to respond to the uncertainties and surprises of discovery and development. The concept of large, long-term contractual relationships with for-profit companies is relatively new to NIH and to the Department of Health and Human Services (DHHS). However, the National Institute of Allergy and Infectious Diseases (NIAID) has had some experience in this type of decision-making, both through its AIDS vaccine research effort and through the creation of the intramural Vaccine Research Center.
The high-level advisory and oversight panel described below in the section Implementation should have a major role in guiding the interaction of NIH and industry. In addition, the leadership of NIH and DHHS should examine how other branches of the federal government, such as the Department of Defense, interact with the private sector, as it develops its own model for such collaboration.
NIH will be defining goals and steering research. It therefore will be essential to develop effective modalities of internal and external review so that NIH can respond rapidly to proposals under the multistep program outlined above and so that it can have effective input from individuals with experience and good judgment in practical drug discovery and development. The mechanisms of peer review used by NIH to set priorities for support of academic science will be inadequate for evaluating programs that require large, long-term funding of private-sector research and development, both because conventional academic review groups tend to be relatively conservative and because the contract mechanism requires different qualities than a grant.
The committee notes that Anthony Fauci, director of NIAID, already has broached the subject of antivirals against smallpox with the heads of several leading pharmaceutical and biotechnology firms, initiating what we define here as step one.
As a rough measure of investment costs, the committee offers the following outline. Recent research, based on a survey of a large number of companies, finds that development of a single new drug costs ≈$800 million (year 2000 dollars) in out-of-pocket expenses, capitalized to the point of marketing approval (19). This estimate, which may be the high end of a spectrum of possible values, includes the costs of failed candidates at various stages in the development process. Clinical trials will be less expensive in the case of smallpox antivirals, because efficacy tests will involve animal rather than human subjects, but the cost savings might be offset by the requirements of a shorter-than-average timeline. The committee therefore chose to use the estimate $800 million for the overall cost of developing a single drug. Procurement, stockpiling, and renewal costs are, of course, additional. (Renewal costs refer to expenses required to ascertain potency of stockpiled materials and to replace them as needed.) It remains to be determined whether contract support for all or a substantial fraction of the $800 million would offset opportunity costs sufficiently to induce companies to participate, with procurement of an ultimate product as the long-term payoff. In any case, the committee believes that $1.5 to $2.5 billion can serve as a guide for the cost of developing two to three antiviral drugs.
For comparison, the economic cost of the 2001 anthrax attacks, including $220 million to decontaminate mail facilities in Maryland and New Jersey, have been estimated in the billions (20). The release of an infectious agent such as smallpox would certainly have an even greater impact. For example, one estimate of the cost in 2003 of SARS for the world economy as a whole (including both the direct costs experienced by health-care systems and the indirect costs associated with disrupted commerce, travel, and education) was close to $40 billion (21).
The average time from initiation of a discovery program to approval of a new drug frequently is cited as 10–15 years (22). Streamlining and knowledge-sharing might reduce this time somewhat, and animal rather than human efficacy trials might reduce it further. The committee thus believes that 7–10 years is a reasonable estimate of the time needed to achieve an approved product. Production and stockpiling times would add to this interval before treatment of an exposed public could be realized.
Current and expected initiatives. NIAID has released two broad research solicitations for product development that require a major commitment from industry. One, “Biodefense Partnerships: Vaccines, Adjuvants, Therapeutics, Diagnostics, and Resources,” involves partnerships between pharmaceutical companies and the government (23). Academic investigators also may be involved. Continued funding of such partnership projects depends on meeting predetermined milestones. Three of the 28 awards made in fiscal year 2003 were for the development of poxvirus therapeutics, including one to inhibit poxvirus phosphatases, another exploring the efficacy of high-titer vaccine immune globulin, and a third for development of an orally administered lipid-ether conjugate of cidofovir. Cidofovir is an acyclic nucleoside phosphonate with broad-spectrum activity against a large number of viruses, and it is likely to be useful in treating smallpox infections and complications from smallpox vaccination. Because the characteristics of cidofovir as a poxvirus therapeutic are not well understood, it is important for NIH to direct step-two and step-three programs in search of other treatments, engaging the most competitive biotechnology and pharmaceutical companies as rapidly as possible.
A second research solicitation that currently targets industry is the “Small Business Biodefense Program” (24). One of the 19 fiscal year 2003 awards targets poxvirus therapeutics. This program cannot support poxvirus research at our most substantial pharmaceutical or biotechnology companies, because they do not qualify as “small.”
The BioShield initiative, proposed in legislation that is before Congress at the time of this writing, may help to address step four. BioShield is intended to provide funds for creating and stockpiling countermeasures against potential bioweapons; smallpox is foremost among these. Its central concept is that NIH should be able to assure pharmaceutical companies that there will be a market if an effective product is discovered, developed, and delivered. As emphasized above, companies are unlikely to invest the human and technologic resources necessary for the discovery of important new smallpox therapeutics without incentives such as those that BioShield may offer. Even if properly funded in steps two, three, and four of a long-term antipoxvirus discovery and development program, companies must be able to expect with confidence that there will be a market for a successful product. BioShield, as the committee understands current plans, envisions that funding for step four will be included in prenegotiated procurement agreements, contingent on successful development of candidate drugs that result from direct funding of steps two and three. The committee believes this to be a useful model.
Liability Issues. In addition to giving the pharmaceutical and biotechnology industries adequate research support and a guarantee of an eventual market for the antismallpox therapeutics that they develop, the issue of product liability also should be addressed. As discussed in a National Academies report on countermeasures to biowarfare agents (25), the concern over liability risks might significantly deter some firms from applying their expertise to the development of new antivirals. That report noted that, under the Homeland Security Act of 2002 (Public Law 107–296), manufacturers of the current smallpox vaccine would be deemed employees of the Public Health Service for the purposes of liability claims (other than for gross negligence, willful misconduct, or illegal conduct) should the vaccine be administered in response to a declaration by the Secretary of the DHHS. This model and others should be considered by the Congress to address the issue of liability in the development of new smallpox antivirals.
Attracting Academic Investigators. An overriding goal is not simply to increase the quantity of work but also to attract researchers of high quality and strong commitment. We distinguish mechanisms for making rapid, short-term progress from those designed for longer-term enhancement of poxvirus research. A good way to start will be to partner vaccinia experts with established investigators who have expertise in other fields of biology. To expedite such interactions, NIH should immediately provide administrative supplements to established investigators for specific collaborative projects with clearly defined, short-term objectives. The supplements should stipulate exchange of technology or personnel between laboratories to disseminate expertise as rapidly as possible. To provide for longer-term collaborations, coinvestigator-initiated grants could pair poxvirologists with outstanding investigators in cell biology, structural biology, chemistry, immunology, and other fields.
For the longer term, attracting new, young investigators into poxvirus-related research must have high priority. The committee proposes three mechanisms by which this goal might be accomplished: (i) exchange of students and postdoctoral fellows between laboratories of complementary expertise through specially awarded 1- or 2-year fellowships; (ii) increased support for institutional training grants in viral pathogenesis, specifically to focus attention of graduate programs and their students on relevant fields; and (iii) a prestigious, competitive fellowship program (appointing, for example, a group of NIH Fellows in Pathogenesis) to encourage the most creative young investigators to build careers in virology. To promote a career commitment, the fellowship award should include a 3-year postdoctoral fellowship followed by an additional 3 years of faculty support that facilitates an effective transition into an independent position.
Other training opportunities should take advantage of proven mechanisms, such as advanced courses at the Cold Spring Harbor Laboratory and the Marine Biological Laboratory at Woods Hole. Those courses reach both new and established investigators and are generally 1–3 weeks long (some are longer) with a combination of lectures and practical laboratories. Enrollment is small (15–20 investigators) and includes a mixture of graduate students, postdoctoral fellows, young faculty, and the occasional experienced investigator seeking to venture into a new field. Generally, one or two course directors are selected to establish a curriculum, invite other faculty to give lectures or set up laboratory exercises, and negotiate with the host facility for appropriate space and physical resources. For a course on poxviruses, lectures might cover the viral life cycle or host–virus interactions at the cellular and organismal level, and it would inform the prospective researchers about the available reagents. By using vaccinia virus as a model, laboratory exercises might introduce participants to basic techniques of virology, including selection and mutagenesis, as well as cell-based and in vitro assays of viral activity. Organizing two such courses each summer, for example, one emphasizing the molecular and cellular biology of poxviruses, the other emphasizing pathogenesis and virus–host interactions, would provide a relatively inexpensive, rapid way for poxvirologists to recruit and identify committed collaborators, as well as to generate new interactions and ideas.
The Role of Federal Agencies and the Research Infrastructure. If the academic initiatives outlined in the preceding paragraphs are to succeed, an enhanced research infrastructure will be vital. A principal role of NIH is to foster academic research and industrial development, as outlined in the preceding paragraphs. NIAID has recently established eight regional centers of excellence in biodefense and awarded one-time grants for two national biocontainment laboratories [biosafety level (BSL)-3/4]. These new centers and laboratories should become major resources for the biology community studying poxviruses. The unique, in-house research at CDC also will play a key role in the development of new drug candidates against smallpox, and CDC must maintain its poxvirus program at a high level.
Repositories, databanks, and compound libraries. Research tools and infrastructure. A common theme throughout the committee's discussions was a need to go beyond a simple repository of virus strains to the creation of centralized resources, pulled together by an organization that could commission the production of reagents (chemical libraries, monoclonal antibodies against an extensive set of proteins, and so on), oversee their judicious distribution, and streamline the fulfillment of regulatory requirements. Obtaining suitable permits is a necessary but major barrier to undertaking research on certain pathogens. In our view, the central body should facilitate the process of obtaining approvals from WHO and CDC for using such reagents as variola-virus genes and variola-specific proteins. For example, it might set up a system for approving and periodically inspecting participating laboratories so that individual investigators need deal with only one agency.
Informatics tools. Some of the informational infrastructure that can enable new academic laboratories to engage in poxvirus research already is being developed. Support and expansion of these efforts would expedite their utility. The Poxvirus Bioinformatics Resource Center provides a relational database of poxvirus genomic sequences and their annotation and analysis, web-based data-mining and sequence-analysis tools, software for genome analysis, a poxvirus literature resource, a repository of poxvirus species and strains at the American Type Culture Collection (ATCC), and a discussion forum. This database could profitably be augmented with information on mutants and phenotypes, as well as on pharmacologic effectors.
Biodefense repository. A contract has recently been awarded to ATCC for creation of a repository of existing laboratory tools for biodefense-related research based on the AIDS repository model. Current plans envision the distribution of poxvirus strains, proteins, and DNA, but the extent of what will be distributed has not yet been established. A priority should be placed on the development and distribution of new poxvirus tools, including polyclonal and monoclonal antibodies to viral ORFs, glutathione S-transferase (GST)-fusion expression clones (pGEX), Gateway PCR clones (validated by sequence), GFP-labeled ORFs, and tandem affinity purification (TAP)-tagged proteins. Vaccinia virus is the model system of choice, on which most of this work should focus. But for biochemical studies and ultimately for screening, consideration should be given to generating a set of variola-virus PCR products for individual ORFs, (such as in Gateway vectors); here, of course, it will be important to observe regulatory safeguards.
Compound libraries. Specific inhibitors of poxvirus targets will be useful as probes of the viral life cycle. Academic scientists with access to the relevant expertise and to biochemical or cellular assays generally lack access to suitable chemical libraries. The committee proposes that NIH consider establishing a National Compound Library, which would expand on existing compound repositories, such as those held by the National Cancer Institute. A National Compound Library should contain 0.5–1 million drug-like compounds, and sufficient amounts should be collected to ensure longevity and a capacity to serve qualified groups throughout the country. Such groups would be expected to have high-throughput screening capability, secondary assay technologies for determining the selectivity of identified compounds, and expertise in synthetic organic chemistry. Because the cost of maintaining such a library could be substantial, it should probably serve other programs in addition to antiviral-drug discovery.
Coordination of facilities. The NIAID regional centers of excellence are an excellent way to coordinate and implement the committee's recommendations for national facilities. A group that includes the principal investigators of these centers and of the regional and national laboratories is being established by NIH to oversee their activities. This body, and the regional centers themselves, can provide mechanisms for carrying out the recommendations in the preceding paragraphs. The availability of BSL-3 and BSL-4 facilities in the regional and national laboratories also might afford the opportunity for establishing training programs in BSL-3/4 techniques and in the administrative procedures, protocols, and permissions needed to work with variola genes.
CDC facilities. CDC in Atlanta and Vektor in Novosibirsk are the only laboratories where research on variola is permitted by WHO. CDC has two BSL-4 facilities, each of which can accommodate up to 16 monkeys (and larger numbers of rodents). No other BSL-4 agent work can be conducted in a laboratory where variola virus is in use, and the current and planned studies on variola virus occupy fully one-half of CDC's space capacity for such pathogens. The BSL-4 facilities are available no more than 8 months per year, because of the need for preventive maintenance and safety procedures. At present, some needed equipment (such as telemetry, flow cytometers, and mass spectrometers) is unavailable in the maximal containment laboratory, and there is insufficient room to accommodate it. Some of these deficiencies may be corrected when new maximal containment laboratories, now under construction,a come online at CDC in 2–3 years. However, because of the pivotal role that this laboratory will have in testing promising drug candidates against variola virus, CDC must establish long-term goals for its poxvirus research program and take measures to ensure its continuing function.
Regulatory Questions. FDA rules on efficacy evaluation. The Animal Efficacy Rule, finalized by FDA in 2002, allows FDA to approve drugs and vaccines when it is not ethical or feasible to conduct human efficacy studies, as is the case for a smallpox antiviral drug. FDA requirements are not clear, however, because the animal models of smallpox have not been studied in the detail that usually accompanies work on other viral diseases. At the very least, scientists must understand the pathophysiology of the disease process and define the mechanism by which a proposed countermeasure would reverse it. The degree of detail required is pivotal to proper study design. The few monkey model studies completed to date measured many parameters, some of which may not be necessary. For example, data are collected on viral quantization, lesion counts, hematology, clinical chemistry, cytokine profiles, cDNA microarray analysis, and cellular and humoral immunologic responses. In general, FDA prefers end points based on mortality rather than morbidity. A morbidity end point more closely approximates human smallpox (up to 40% mortality), but these experiments require the use of many animals. Uniform mortality (the animal survives or it does not) can be achieved only with a model that resembles hemorrhagic, rather than common, smallpox. These issues are complex, but FDA must resolve them soon so that the experimental design for determination of efficacy can be defined.
WHO restrictions. WHO guidelines limit the extent of experimental work that is permitted on variola virus. Those guidelines, formulated over 2 decades ago, were based on the assumption that smallpox had been eradicated and that research on the virus, its genes, and its proteins should be restricted to guard against the possibility of an accidental release. Do recent events necessitate a reappraisal? If other countries or other entities covertly hold stocks of the virus, should WHO reassess its rules? These issues deserve debate in an international setting because a smallpox outbreak would create an international health crisis.
Continued compliance with international accords is, of course, essential, although the restriction of all variola research to the two WHO collaborating centers does limit research in the field (as was intended). Unfortunately, the site in Russia (Vektor) is under-equipped and inadequate to the task of contemporary studies, and CDC facilities are presently unequipped to conduct detailed pathogenesis studies in animals in BSL-4 containment. Variola virus could be handled safely in any BSL-4 facility that has a proven track record of biosafety in the use of pathogens for which no vaccines or therapies are available. Thus, one question is whether the importance of the research program recommended in the first part of this report and the evaluation of the likelihood of covert stocks outside Vektor and CDC make it necessary to discuss altering the two-site restriction. The committee suggests that CDC explore with WHO whether other qualified laboratories under its control might be deemed part of its site. It also suggests that CDC dedicate a larger fraction of its BSL-4 facilities to variola virus research and move other programs to sites elsewhere.
WHO currently prohibits any genetic manipulation of the variola genome. The prohibition extends to the incorporation of reporter genes, such as GFP, which would greatly facilitate high-throughput antiviral drug screens. GFP has been introduced into numerous viruses without affecting virulence. A white paper proposing GFP insertion has been presented to WHO on two occasions, most recently in November 2002. It has not yet been considered by the WHO orthopoxvirus committee, however, in part because of concern that approval of this proposal would open the door to more worrisome or dangerous genetic manipulations.
WHO restrictions pertain not only to variola virus but also to possession and expression of its genes. No laboratory outside CDC and Vektor may hold more than 20% of the total genome or hold even that if it is using other orthopoxviruses. Although currently not permitted, the introduction of single variola virus genes into vaccinia virus could be useful for testing antivirals and monoclonal antibodies in small-animal models without the hazard posed by working with variola virus.
In summary, WHO should be encouraged to reevaluate its rules in light of an independent estimation of the bioterrorist threat and of the contemporary scientific breakthroughs that could be exploited to address it.
Implementation. Implementing the various components (academic and industrial, national and international) of a smallpox antiviral program will require a consistent sense of urgency and a focused decision-making process. A high-level advisory and oversight panel, analogous to the AIDS Vaccine Research Working Group, should be created immediately, reporting to the heads of NIH, CDC, and any other federal agencies involved in this effort (including branches of the Department of Homeland Security). Membership on this committee should represent experience in drug discovery and development, poxvirus expertise at the highest level, and international efforts in smallpox antiviral research. Its first, urgent task will be to establish a specific timeline for drug discovery and development, including prioritization of currently feasible targets and approaches. A second important task will be to work out a blue-print for an effective international effort.
Other Issues. Some regulatory, political, and ethical issues are important but outside the scope of discussions of this group. The potential for misuse of the knowledge gained in further poxvirus research is of concern, just as the potential for dual use of knowledge is of concern in much other scientific research. Who should decide the auspices under which particular experiments should be done? How should the results be analyzed and disseminated? The National Academies report “Biotechnology Research in an Age of Terrorism: Confronting the `Dual Use' Dilemma” presents a scholarly discussion of some of the issues (26).
Another question has to do with how regulatory restrictions are enforced. Suitably talented researchers always have options in their choice of problems to study. A climate of apprehension about how even inadvertent lapses might be handled will drive away precisely the sorts of investigators whom we hope to attract into research on poxviruses or other topics relevant to biodefense. Apparent lapses in correct research practice should be dealt with by the oversight mechanisms that already govern handling of pathogens and hazardous or radioactive materials, not by the criminal justice system.
For additional reading, see refs. 27 and 28.
Summary of Recommendations. Based on the preceding discussion, the committee presents three categories in which actions taken by NIH and other branches of the federal government would enhance the prospects for the development of antiviral drugs against smallpox. These are: (i) establishing novel contractual relationships with drug companies (Recommendation 1, below); (ii) invigorating poxvirus research in academic and government laboratories (Recommendations 2, 3, and 4, below); and (iii) forging consensus on the criteria for safety and effectiveness of bioweapons countermeasures and on the regulations that will govern their development (Recommendations 5, 6, and 7, below).
Recommendations
-
Pharmaceutical and biotechnology company engagement.
-
We propose an immediate focus on currently identifiable therapeutic targets that are essential enzymes of poxviruses (see Table 3). To gain the participation of major companies, we suggest the following program:
- direct contact between senior government official and leaders of major pharmaceutical and biotechnology companies to initiate and encourage participation;
- targeted solicitation of proposals to carry out the screening of chemical libraries beginning with currently identifiable targets and elaboration of an early lead discovery phase, followed by federal support of preclinical development in those companies that present the most promising discovery-phase data; and
- support of full clinical development of the best preclinical candidates through negotiated guarantees of purchase contingent on meeting predetermined product specifications.
-
Successful engagement of industry through the process just outlined will require:
- high-level planning and oversight of the process by the advisory panel proposed in Recommendation 6;
- new procedures for rapid, insightful review, including participation of individuals with extensive experience in drug discovery and development;
- addressing the issue of indemnification, through legislation to limit the liability of companies that develop smallpox antivirals under this program; and
- consortium arrangements that permit companies to share information and thereby reduce risks.
- Therapeutics against smallpox will need to be licensed under the FDA's Animal Efficacy Rule. To promote sound application of this rule, NIH, the U.S. Army Medical Research Institute of Infectious Diseases, CDC, and the scientific community at large should engage in research on appropriate animal models for poxvirus infections and on the kinds of studies needed to show efficacy of antiviral drugs. The FDA itself also should receive sufficient funding to support its own work on these issues, so that the criteria for licensing smallpox antivirals can be established as early as possible.
-
Focused, short-term basic research. Rapid progress toward deeper understanding of poxvirus biology will result from active collaborations between academic laboratories already engaged in poxvirus research and those with specific biologic or technologic expertise. NIH should support established investigators, nationally and internationally, to work with poxvirologists on well defined, short-term projects, in which technology and personnel are exchanged, and also on longer-term, coinvestigator-initiated collaborations. The structural biology of poxvirus proteins, the cell biology of poxvirus infection, and the interaction between poxviruses and the host immune system are three areas in which such collaborations should be encouraged. Establishing the credibility of animal models for variola infection is also a high priority.
-
Recruiting and training a new cohort of young investigators. Long-term progress will require “new blood,” and research initiatives should be designed with this point in mind.
- NIH should attract new talent into poxvirology by granting 1- to 2-year fellowships that permit students and postdoctoral scientists to move between laboratories with complementary expertise and by awarding additional institutional training grants in viral pathogenesis.
- NIH should support one or more laboratory courses in poxvirus research at sites such as Cold Spring Harbor, NY, or Woods Hole, MA.
- A high-profile fellowship (NIH Fellow in Viral Pathogenesis) should be established to support the most outstanding students during postdoctoral studies and their first few years of faculty research.
Centralized resources. NIH, with the collaboration of CDC, should establish repositories and databanks. Large libraries of chemical compounds will be particularly useful for academic investigators who have the experience and facilities to undertake large-scale screening. NIH and CDC also should create an accessible central mechanism for facilitating WHO and CDC approvals and for coordinating access to the use of national laboratory facilities. The research centers of excellence being established by NIAID's Biodefense Program can provide mechanisms for responding to some of these needs.
WHO restrictions. NIH and CDC should encourage WHO to reevaluate its prohibition of certain manipulations of variola virus that would greatly facilitate novel screening methods, for example, the introduction of reporter genes into variola virus and the use of variola virus genes in other vectors. Mechanisms should be developed for undertaking such studies in CDC poxvirus research laboratories. CDC also should explore with WHO whether other qualified laboratories under its control might be deemed part of its site and otherwise take measures to ensure the continuing, vigorous function of its poxvirus research effort.
Implementation. In view of the importance of including expert scientific guidance throughout the steps recommended in this report, a high-level advisory and oversight panel, analogous to the AIDS Vaccine Research Working Group, should be created immediately, reporting to the heads of NIH, CDC, and any other federal agencies involved in this effort. Membership on this committee should represent experience in drug discovery and development, poxvirus expertise at the highest level, and international efforts in smallpox antiviral research. Its first, urgent task will be to establish a specific timeline for drug discovery and development, including prioritization of currently feasible targets and approaches. A second important task will be to work out a blueprint for an effective international effort (see Recommendation 7).
International participation. The prospect of an intentional release of the variola virus is a global concern. Research on bioweapon countermeasures therefore should involve broad international collaborations. There should be no undue restrictions on the participation of non-U.S. citizens in work at U.S. laboratories, and grant and contract funding should extend, where appropriate for support of the best science, to laboratories outside the U.S.
Acknowledgments
We extend our gratitude to the individuals who attended the National Academies' June 15–16, 2003, workshop on “Transforming Biological Information into New Therapies: Smallpox Antivirals,” and acknowledge NIAID for its support of this activity. In addition, we thank A. Mahmoud, D. Bloom, and E. Cordes for their useful insights on the pharmaceutical industry, and T. Caskey, M. Collett, J. Henney, N. Nathanson, R. Moyer, V. Sato, P. Palese, and P. Traktman for helpful comments on the draft of this article.
Abbreviations: WHO, World Health Organization; CDC, U.S. Centers for Disease Control and Prevention; IMV, intracellular mature vaccinia virion; EEV, extracellular enveloped virion; ITR, inverted terminal repetition; IEV, intracellular enveloped virion; CEV, cell-associated virion; UDG, uracil DNA glycosylase; IV, immature virion; NIH, National Institutes of Health; SARS, severe acute respiratory syndrome; FDA, Food and Drug Administration; DHHS, Department of Health and Human Services; NIAID, National Institute of Allergy and Infectious Diseases; BSL, biosafety level.
Footnotes
Adapted with permission from ref. 4 (Copyright 2003, ASM Press).
References
- 1.Institute of Medicine (1999) Assessment of Future Scientific Needs for Variola Virus, ed. Briere, R. (Natl. Acad. Press, Washington DC).
- 2.Lane, H. C., Montagne, J. L. & Fauci, A. S. (2001) Nat. Med. 7, 1271–1273. [DOI] [PubMed] [Google Scholar]
- 3.White House (December 13, 2002) Protecting Americans: Smallpox Vaccination Program, press release, www.whitehouse.gov/news/releases/2002/12/20021213-1.html.
- 4.Flint, S. J., Enquist, L. W., Racaniello, V. R. & Skalka, A. M. (2003) Principles of Virology: Molecular Biology, Pathogenesis, and Control of Animal Viruses (ASM Press, Washington DC), 2nd Ed.
- 5.Moss, B. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadelphia), pp. 2849–2883.
- 6.Krauss, H., Weber, A., Appel, M., Enders, B., Isenberg, H., Schiefer, H., Slenczka, W., von Graevenitz, A. & Zahner, H., eds. (2003) Zoonoses: Infectious Diseases Transmissible from Animals to Humans (ASM Press, Washington, DC), 3rd Ed.
- 7.Seet, B. T., Jonhston, J. B., Brunetti, C. R., Barrett, J. W., Everett, H., Cameron, C., Sypula, J., Nazarian, S. H., Lucas, A. & McFadden, G. (2003) Annu. Rev. Immunol. 21, 377–423. [DOI] [PubMed] [Google Scholar]
- 8.Tolonen, N., Doglio, L., Schleich, S. & Krijnse Locker, J. (2001) Mol. Biol. Cell 12, 2031–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risco, C., Rodriguez, J. R., Lopez-Iglesias, C., Carrascosa, J. L., Esteban M. & Rodriguez, D. (2002) J. Virol. 76, 1839–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rietdorf, J., Ploubidou, A., Reckmann, I., Holmstrom, A., Frischknecht, F., Zettl, M., Zimmermann, T. & Way, M. (2001) Nat. Cell Biol. 3, 992–1000. [DOI] [PubMed] [Google Scholar]
- 11.van Eijl, H., Hollinshead, M., Rodger, G., Zhang, W. H. & Smith, G. L. (2002) J. Gen. Virol. 83, 195–207. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths, G., Roos, N., Schleich, S. & Locker, J. K. (2001) J. Virol. 75, 11056–11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Fonseca, F. & Moss, B. (2003) Proc. Natl. Acad. Sci. USA 100, 11291–11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broyles, S. S. (2003) J. Gen. Virol. 84, 2293–2303. [DOI] [PubMed] [Google Scholar]
- 15.Bell, S. P. & Dutta, A. (2002) Annu. Rev. Biochem. 71, 333–375. [DOI] [PubMed] [Google Scholar]
- 16.Sodeik, B. & Krijnse-Locker, J. (2002) Trends Microbiol. 10, 15–24. [DOI] [PubMed] [Google Scholar]
- 17.Senkevich, T. G., White, C. L., Koonin, E. V. & Moss, B. (2002) Proc. Natl. Acad. Sci. USA 99, 6667–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, G. L., Murphy, B. J. & Law, M. (2003) Ann. Rev. Microbiol. 57, 323–342. [DOI] [PubMed] [Google Scholar]
- 19.DiMasi, J. A., Hansen, R. W. & Grabowski, H. G. (2003) J. Health Econ. 22, 151–185. [DOI] [PubMed] [Google Scholar]
- 20.Kestin, S. (December 16, 2001) South Florida Sun-Sentinel, Section A, pp. 1, 9.
- 21.Lee, J.-W. & McKibbin, W. J. (2004) in Institute of Medicine and National Research Council Forum on Microbial Threats: Learning from SARS: Preparing for the Next Disease Outbreak–Workshop Summary (Natl. Acad. Press, Washington, DC). [PubMed]
- 22.Pharmaceutical Research and Manufacturers Association (2003) Insights: Highlights from the Pharmaceutical Industry Profile 2003 (PhRMA, Washington, DC).
- 23.National Institute of Allergy and Infectious Diseases (November 14, 2002) Biodefense Partnerships: Vaccines, Adjuvants, Therapeutics, Diagnostics, and Resources, program announcement PAR-03-025.
- 24.National Institute of Allergy and Infectious Diseases (August 15, 2002) Small Business Biodefense Program, program announcement PAS-02-149.
- 25.Institute of Medicine and National Research Council (2004) Giving Full Measure to Countermeasures: Addressing Problems in the DoD Program to Develop Medical Countermeasures Against Biological Warfare Agents (Natl. Acad. Press, Washington, DC). [PubMed]
- 26.National Research Council (2004) Biotechnology Research in an Age of Terrorism: Confronting the “Dual Use” Dilemma (Natl. Acad. Press, Washington, DC).
- 27.De Clercq, E. (2002) Nat. Rev. 11, 13–25. [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine, Board on Health Care Services, Committee on the Evaluation of Vaccine Purchase Financing in the United States (2003) Financing Vaccines in the 21st Century: Assuring Access and Availability (Natl. Acad. Press, Washington, DC).