Abstract
Although several previous studies have demonstrated navigational deficits in early-stage symptomatic Alzheimer's disease (AD), navigational abilities in preclinical AD have not been examined. The present investigation examined the effects of preclinical AD and early-stage symptomatic AD on spatial navigation performance. Performance on tasks of wayfinding and route learning in a virtual reality environment were examined. Comparisons were made across the following three groups: Clinically normal without preclinical AD (n = 42), clinically normal with preclinical AD (n = 13), and early-stage symptomatic AD (n = 16) groups. Preclinical AD was defined based on cerebrospinal fluid Aβ42 levels below 500 pg/ml. Preclinical AD was associated with deficits in the use of a wayfinding strategy, but not a route learning strategy. Moreover, post-hoc analyses indicated that wayfinding performance had moderate sensitivity and specificity. Results also confirmed early-stage symptomatic AD-related deficits in the use of both wayfinding and route learning strategies. The results of this study suggest that aspects of spatial navigation may be particularly sensitive at detecting the earliest cognitive deficits of AD.
Keywords: Aging, allocentric, amyloid, caudate nucleus, egocentric, hippocampus
INTRODUCTION
Early in the course of symptomatic Alzheimer's disease (AD), individuals frequently lose their way in unfamiliar and familiar places [1–3]. These navigational deficits present a danger to individuals with AD as they may wander away from home and get lost [1]. In addition, getting lost while driving can be associated with significant negative consequences, including injury and death [4, 5]. Given that these deficits appear to emerge early during the course of the disease, it is important to gain a better understanding of the specific time course and features of these navigational deficits.
A current conceptualization of the process of AD is that it occurs in stages from a preclinical phase to mild cognitive impairment (MCI) to symptomatic AD [6, 7]. Two neuropathological features of AD are the formation of neurofibrillary tangles and amyloid plaques, which occur early in the hippocampus and neocortex (e.g., parietal regions), respectively [8, 9]. These neuropathological processes are thought to begin at least a decade or more before AD can be diagnosed clinically [10, 11]. Several biomarkers associated with these neuropathological processes have been identified. In particular, previous research indicates that reduced cerebrospinal fluid (CSF) levels of Aβ42 reflect the aggregation of amyloid into plaques in the brain at autopsy [12] and in vivo [13]. Fibrillar amyloid-β deposition can be detected through positron emission tomography (PET) studies using radiotracers such as Pittsburgh compound-B (PIB) with plaque formation indicated by higher uptake of the radiotracer [13]. Cross-sectional research using these neuroimaging and CSF biomarkers indicate that clinically normal (CN) individuals with evidence of the presence of AD biomarkers show alterations in brain structure and function [14–17]. In addition, CN individuals with amyloid deposition demonstrate greater atrophy as well as an increased risk of cognitive decline and development of symptomatic AD [18–22].
MCI is conceptualized as occurring subsequent to the preclinical phase [6, 7], and is characterized by cognitive impairment that minimally interferes with activities of daily living [7, 23, 24]. Prior research indicates that MCI individuals frequently possess neuropathological signs of AD and progress to a diagnosis of symptomatic AD at faster rates than CN individuals [23]. Within this framework it is recognized that not all individuals designated as preclinical AD or MCI will progress to a diagnosis of symptomatic AD. However, it provides a useful framework for cross-sectional and longitudinal investigations of the earliest manifestations of the AD process, as well as potential predictors of progression.
In addition to the formation of amyloid plaques and neurofibrillary tangles, MCI and preclinical AD are also characterized by volumetric decline of the hippocampus [25–27]. Although there is also some limited evidence of caudate atrophy during the MCI stage [28], previous research demonstrates relatively preserved caudate volume in individuals with preclinical AD [26, 27]. The cognitive changes associated with this early phase are less clear. Considering that the hippocampus is affected early during the course of the disease, cognitive abilities associated with this structure may be expected to be impaired. Indeed, a recent meta-analysis indicated significant, but relatively small, associations between episodic memory and amyloid burden [29]. Spatial navigation is another cognitive function supported in part by the hippocampus that may be deficient in preclinical AD.
Spatial navigation is considered a complex skill involving vestibular, proprioceptive, and visual sensory input. In addition, spatial navigation involves multiple cognitive processes, including knowledge of environmental features, decision-making based on current goals, path integration, and maintenance of one's location within the environment [30]. Despite this complexity, research from the non-human animal literature suggests that spatial navigation may involve the use of two distinct learning strategies. Response learning involves the development of a fixed, action-based representation coded from the navigator's perspective (i.e., egocentric representation). Conversely, place learning involves the development of a more flexible internal representation of the spatial relationships among landmarks in an environment (i.e., allocentric representation) (also known as a“cognitive map”) [31]. Previous research indicates that navigation performance is supported by a network of brain regions, including prefrontal, parietal, striatal, and medial temporal regions [32–34]. In terms of regions specifically contributing to each strategy, response learning is particularly associated with the caudate nucleus, and place learning tends to rely on hippocampal circuits [31, 35–37]. Thus, we may expect preclinical AD to be characterized by deficits on place learning tasks, but not response learning tasks.
Although no study to date has examined spatial navigation in preclinical AD, it has been investigated in MCI and symptomatic AD. Prior research provides some evidence for deficits in the use of both place [38–40] and response learning [41–43] strategies in both MCI and symptomatic AD (for a review see [44]), which is consistent with observations of hippocampal and caudate atrophy in these conditions [28, 45]. In addition, associations of hippocampal integrity with place learning and striatal integrity with response learning have been observed in these populations [46, 47]. There is also evidence for MCI and symptomatic AD-related deficits in learning or retrieving critical features of studied routes and environments. More specifically, both MCI and symptomatic AD individuals appear to have difficulty with locating landmarks on maps of the environment, recalling the order of landmarks, and determining the relative location of depicted scenes from the environment [42, 43]. Furthermore, individuals with symptomatic AD may also have deficits in the recall and recognition of landmarks [41].
The primary purpose of the current study was to investigate both place and response learning strategies in preclinical and early-stage symptomatic AD. A supplementary goal was to examine the degree to which individuals retain detailed environmental knowledge that may facilitate successful navigation. Performance on tasks of wayfinding and route learning in a virtual reality environment were administered to estimate place and response learning, respectively. The tasks were adapted from previous research [36, 48]. The wayfinding task required individuals to learn the spatial layout of the environment in order to find the locations of landmarks from various start points, and the route learning task required individuals to learn a specified path in the environment through a series of stimulus-response associations. Previous research indicates that performance on such wayfinding and route learning tasks are associated with the hippocampus and caudate, respectively [36, 48], which is consistent with the contributions of these regions to place and response learning. Based on previous research demonstrating that preclinical AD may be characterized by hippocampal atrophy and relatively preserved caudate volume [26, 27], we hypothesized greater performance deficits in wayfinding compared to route learning in preclinical AD. For early-stage symptomatic AD individuals, we expected to extend previous findings demonstrating deficits in both wayfinding and route learning.
MATERIALS AND METHODS
Participants
Seventy-nine participants were recruited from the Knight Alzheimer's Disease Research Center (ADRC) at Washington University. The ADRC uses a variety of methods to aid in recruitment including community events, seminars, lectures, newsletters, registry flyers, brochures, word-of-mouth, local television and radio reports, and their website. All participants were screened for major medical conditions (e.g., Parkinson's, Huntington's, stroke, seizures, head injury). Two individuals failed the visuomotor expertise test (see below, Practice and Visuomotor Expertise) and 6 clinically normal participants did not have CSF data, leaving 71 participants for this study. Participants had normal vision or wore corrective lenses.
The Clinical Dementia Rating Scale (CDR) [49] was used to determine the presence or absence of dementia, and the severity of dementia when present. CDR scores of 0, 0.5, and 1 correspond to clinical normality, very mild dementia, and mild dementia, respectively. In this research cohort, clinical diagnosis of symptomatic AD for the individuals with a CDR of 0.5 or greater was made in accordance with criteria reported by the NINCDS-ADRDA [50]. The clinical diagnosis of AD by the ADRC has been confirmed with the presence of AD pathology determined postmortem in 93% of cases [51], and this includes individuals meeting criteria for MCI [52]. 55 participants were classified as CN, 11 were classified as very mildly demented, and 5 were classified as mildly demented. The distinction between clinical normality and early-stage symptomatic AD was supported by an independent psychometric assessment (see Supplementary Table 1).
The predictor variable of AD status was categorical and consisted of three separate groups: CN biomarker−, CN biomarker+ (i.e., preclinical AD), and early-stage symptomatic AD. Although preclinical AD is characterized by both the formation of neurofibrillary tangles and amyloid plaques [10, 11], previous research indicates that changes in CSF Aβ42 may precede changes in CSF p-tau and tau, suggesting that CSF Aβ42 may be an earlier biomarker of AD pathology [53–56]. As a result, preclinical AD (i.e., CN biomarker+) was defined as CN with CSF Aβ42 levels <500 pg/ml [13]. CN biomarker− was defined as CN with CSF Aβ42 levels ≥500 pg/ml. Finally, early-stage symptomatic AD status was defined as CDR = 0.5 or 1. Demographic data are presented in Tables 1A and 1B. Participants consented to participation in accordance with the guidelines of the Washington University Human Research Protection Office.
Table 1A.
Sample description: Wayfinding
| CN Biomarker− | CN Biomarker+ | Symptomatic AD | |
|---|---|---|---|
| N | 42 | 12 | 15 |
| Age, years (mean/std)+ | 69/9 | 73/9 | 77/10 |
| Age Range (years) | 50–84 | 54–84 | 55–90 |
| Gender (M/F) | 19/23 | 5/7 | 9/6 |
| Education, years (mean/std) | 16/2 | 17/2 | 16/3 |
| Education Range (years) | 12–20 | 12–20 | 12–20 |
| Health Composite (mean/std) | 0.86/1.05 | 0.92/0.67 | 1.53/1.30 |
| Computer Experience Composite (mean/std) | 7.38/3.16 | 6.95/3.51 | 6.47/3.85 |
| Visuomotor Time: Wayfinding (mean/std)* | 53.00/3.40 | 55.50/3.23 | 55.13/5.60 |
| Selective Reminding Task (mean/std)+^ | 34.90/4.59 | 32.25/8.08 | 20.08/8.49 |
Indicates significant difference between CN biomarker− and CN biomarker+ individuals at p < 0.05.
Indicates significant difference between CN biomarker− and early-stage symptomatic AD individuals at p < 0.05.
Indicates significant differences between CN biomarker+ and symptomatic AD individuals at p < 0.05.
CN, clinically normal; AD, Alzheimer's disease.
Table 1B.
Sample description: Route learning
| CN Biomarker− | CN Biomarker+ | Symptomatic AD | |
|---|---|---|---|
| N | 39 | 12 | 16 |
| Age, years (mean/std)+ | 70/9 | 72/8 | 77/10 |
| Age Range (years) | 50–84 | 54–84 | 55–90 |
| Gender (M/F) | 18/21 | 5/7 | 10/6 |
| Education, years (mean/std) | 16/2 | 17/2 | 16/3 |
| Education Range (years) | 12–20 | 12–20 | 12–20 |
| Health Composite (mean/std) | 0.87/1.08 | 0.92/0.67 | 1.50/1.26 |
| Computer Experience Composite (mean/std) | 7.54/3.11 | 7.08/3.55 | 6.44/3.72 |
| Visuomotor Time: Route Learning (mean/std) | 54.13/4.09 | 55.58/4.83 | 53.38/4.69 |
| Selective Reminding Test (mean/std)+^ | 35.00/4.45 | 34.00/4.57 | 20.08/8.49 |
*Indicates significant difference between CN biomarker− and CN biomarker+ individuals at p < 0.05.
Indicates significant difference between CN biomarker− and early-stage symptomatic AD individuals at p < 0.05.
Indicates significant differences between CN biomarker+ and symptomatic AD individuals at p < 0.05.
CN, clinically normal; AD, Alzheimer's disease.
CSF collection and processing
CSF was collected as described previously [13]. Briefly, CSF free from blood contamination was collected by lumbar puncture in polypropylene tubes at 8:00 AM after overnight fasting. Samples were gently inverted to avoid gradient effects, briefly centrifuged at low speed to pellet any cellular elements, and aliquoted (500 μl) into polypropylene tubes before freezing at −84°C. Analyses for Aβ42 were completed using commercial enzyme-linked immunosorbant assay (INNOTEST; Fujirebio, formerly Innogenetics, Ghent, Belgium). Samples were kept continuously on ice with only a single thaw after initial freezing before assaying. CSF collection was on average 2.13 years prior to the experimental session. Only CSF data from CN individuals were included in this study. All CN individuals had a CDR rating of 0 at both the time of the CSF collection and the experimental session.
Experimental procedures
General
Participants completed separate 1.5–2 h sessions for wayfinding and route learning conditions. Sessions occurred about 1 week apart with order of administration counterbalanced across participants. WorldViz Vizard and Autodesk 3ds Max software were used to create two non-immersive desktop virtual maze environments for the wayfinding and route learning conditions. These mazes were also counterbalanced across participants. Previous research indicates that navigation performance in virtual environments is equivalent to performance in real-world environments [38, 42, 57]. Mazes consisted of a series of interconnected hallways with 20 landmarks and 4 wallpaper patterns throughout. Tasks were adapted from past work [36, 48] and presented on an Alienware laptop with a 17-inch monitor. See Supplementary Figure 1 for aerial perspectives of the two environments used for this study, as well as an example landmark. A joystick was used to maneuver through the environment. The computer recorded time and distance in virtual units.
Practice and visuomotor expertise
Participants completed a practice session and a visuomotor expertise test in a separate virtual environment of a long hallway with several bends. The experimenter provided instructions on joystick use and maneuvering through the environment during practice. For the visuomotor expertise test, participants traversed to a blue area at the end of the hallway within 65 s. Participants were given a maximum number of three attempts to complete the visuomotor expertise test within 65 s. Two participants were unable to complete the visuomotor expertise test within 65 s on the third attempt and, as a result, were not included in the study. Following the visuomotor expertise test, participants did not receive any feedback.
Wayfinding
For the learning phase, participants completed three study-test trials. During study, participants freely explored the environment for 7 min. Participants were encouraged to fully explore the environment and the experimenter provided prompts if there were parts of the environment not being explored. During test, which occurred immediately after each study phase, participants placed an ”X” at all landmark locations on a blank 2-D map of the maze (total correct: 0–20). The study-test trials were designed to generate relatively similar acquisition of an allocentric representation of the environment across participants. A similar method has been used previously to examine development of a cognitive map [58]. The delay phase, which was designed to assess the use of a cognitive map, occurred following a 10-min delay. Participants were presented with a picture of a landmark and required to find the landmark in the environment using the shortest path possible for a total of 12 landmarks, across 6 different start locations. A maximum of 3 min was allowed to reach each landmark. The dependent variable for the delay phase was the average distance traveled for the 12 landmarks.
Next, participants completed a series of supplementary tasks assessing environmental knowledge. First, participants recalled as many landmarks as possible (Landmark Free Recall; total correct: 0–20). The experimenter queried any responses not clearly associated with landmarks in the maze after participants completed their free recall. Participants were then asked to place an ”X” for all landmark locations on a blank 2-D map of the maze (Landmark Location Memory; total correct: 0–20). They were next presented with a 2-D map with ”Xs” in the locations of landmarks and asked to recall the identity of the landmarks (Landmark Identification Memory; total correct: 0–20). The experimenter clarified any responses that were unclear or ambiguous. Finally, participants completed a yes/no Scene Recognition task consisting of 20 images from the maze and 20 foils. The score was the total correct minus false alarms (score: 0–20). The supplementary tasks (Landmark Free Recall, Landmark Location Memory, Landmark Identification Memory, and Scene Recognition; see Supplementary Table 2 for descriptive statistics) were moderately to highly correlated (rs = 0.479–0.738, ps < 0.001; mean r = 0.615; Cronbach's alpha = 0.861), and an Environmental Knowledge Composite was created from the average of the z-scores for the supplementary tasks.
Route learning
For the learning phase, participants completed 4 study-test trials. For study, participants followed the same route repeatedly marked by arrows for 5 min. None of the participants deviated from the designated route. The route was approximately 4700 virtual units long and took about 90 s to complete without errors. During test, which occurred immediately after each study phase, participants drew the path on a blank 2-D map of the environment (proportion correct: 0–1). The proportion correct was the number of correctly drawn turns at intersections relative to the total number of intersections for the route. These multiple study-test trials were designed to generate relatively similar learning of the route across participants. The delay phase, designed to assess route learning, occurred after a 10-min delay. Participants traversed the learned path without arrows across 3 trials. A maximum of 5 min was allowed for each trial. The dependent variable for the delay phase was the average distance traveled across the 3 trials.
Next, participants completed a series of supplementary tasks assessing environmental knowledge. First, participants were asked to draw the path on a 2-D map of the environment (Route Drawing; proportion correct: 0–1). Participants then completed Landmark Free Recall, Landmark Location Memory, and Landmark Identification Memory tasks (total correct for each: 0–14). Next, participants completed a computerized test of landmark temporal order (Temporal Order Memory). Fourteen landmarks were presented at the top of the computer monitor in a different random order for each participant and empty boxes were on the bottom half. Participants used the mouse to move the landmarks into the empty boxes in the order in which the landmarks were encountered along the path traversed during the learning phase. The index of performance was the Spearman rank-order correlation between the actual order and the participant's order (range: 0–1). Finally, participants completed the Landmark Direction Knowledge task in which they indicated whether each landmark was associated with a right, left, or no turn for 14 landmarks (proportion correct: 0–1). The supplementary tasks (Route Drawing, Landmark Free Recall, Landmark Location Memory, Landmark Identification Memory, Temporal Order Memory, and Landmark Direction Knowledge; see Supplementary Table 2 for descriptive statistics) were moderately to highly correlated (rs = 0.538–0.811, ps < 0.001; mean r = 0.674; Cronbach's alpha = 0.924), and an Environmental Knowledge Composite was created from the average of the z-scores for the supplementary tasks.
Computer experience questionnaire
Participants indicated on a Likert scale (0 to 7) experience with computers, computer games, and virtual reality games. A computer experience composite was created from the sum of these experiences (score: 0–21).
Data analysis
Missing data
Due to computer error or participant withdrawal, data were unavailable for one individual with early-stage symptomatic AD and one CN biomarker+ individual for the wayfinding condition, as well as one CN biomarker+ and three CN biomarker− individuals for the route learning condition. As a result, the sample sizes for the route learning and wayfinding conditions were different (see Tables 1A and 1B). Data was also unavailable for one of the four tasks comprising the Wayfinding Environmental Knowledge Composite for one individual with early-stage symptomatic AD. The Wayfinding Environmental Knowledge Composite consisted of the average performance on the available three tasks for this individual.
Control variables
Age, gender, visuomotor maze time, computer experience, and the health composite were included as control variables in all analyses. The health composite was the sum of the presence or history of: Brief head trauma, heart attack, atrial fibrillation, angioplasty, bypass surgery, pacemaker, congestive heart failure, history of depression, hypertension, and diabetes (total: 0–10). Comparisons between the groups were conducted using independent samples t-tests for the continuous variables and chi-squares for the categorical variables (see Tables 1A and 1B).
Outliers
Univariate outliers were defined as values >3 STD from the group mean. Multivariate outliers were defined based on Mahalanobis and Cook's distance [59]. All analyses were conducted with and without outliers. Unless otherwise specified in the results section, results were unchanged when outliers were removed.
Statistical analyses
For the learning phases, repeated measures ANCOVAs were conducted in SPSS 21 with the test trial performance as the dependent variables and polynomial contrasts were used to examine main effects of trial. Total correct on the 3 test trials was the dependent variable for the wayfinding condition. Proportion correct on the 4 test trials was the dependent variable for the route learning condition. For the delay phase and the Environmental Knowledge Composite, ANCOVAs were conducted in SPSS 21. Average distance traveled to locate the 12 landmarks was the dependent variable for the wayfinding condition. Average distance traveled across the 3 trials was the dependent variable for the route learning condition. For all ANCOVA analyses, the Greenhouse-Geisser correction was used in instances where the homogeneity of variance assumption was violated. Planned pairwise comparisons were conducted to follow-up significant main effects of AD status, and were corrected for multiple comparisons using the Holm-Bonferroni approach [60, 61]. Contrast estimates and their standard errors were used to calculate the reported t-values.
A post-hoc ANCOVA with CSF tau and the CSF tau × Group interaction in the model was conducted in order to examine the potential contributions of neuronal injury to any observed differences between CN biomarker− and CN biomarker+ groups.
Receiver operating characteristic (ROC) analyses were conducted post-hoc to assess the diagnostic utility of the wayfinding task, and to compare that to both an episodic memory task and the route learning task. The free recall from the Selective Reminding Task [62] was used as the measure of episodic memory. An ROC analysis is used to determine the accuracy of a test in discriminating healthy individuals from diseased individuals [63]. An ROC curve plots the sensitivity (the proportion of diseased cases correctly classified) against the specificity (the proportion of healthy cases correctly classified) at different cutoff values of the test. The area under the ROC curve (AUC) indicates numerically how well a specific test distinguishes between healthy individuals and those suffering from a disease, with higher values indicating greater accuracy. In the current study, the method of DeLong, DeLong, and Clarke-Pearson [64] was used to compare areas under the curve using the Sigmaplot program. The difference of each area pair and its standard error and 95% confidence interval are computed. This is then followed by the chi-square statistic for the area comparison and its associated p-value.
RESULTS
Wayfinding
Learning phase
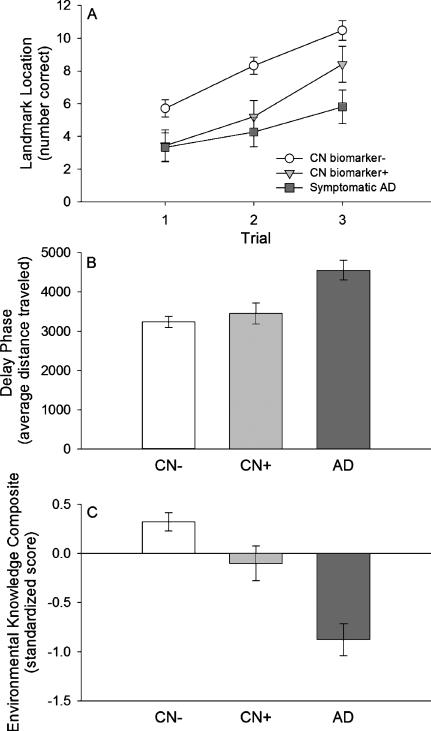
There was a significant main effect of trial for the learning phase (F(2, 118) = 76.335, p < 0.001; partial η2 = 0.564, 95% CI: 0.441–0.645; Fig. 1A) with a significant linear increase in performance across trials (F(1, 59) = 116.469, p < 0.001; partial η2 = 0.664, 95% CI: 0.513–0.750). There was also a significant main effect of AD status across the three learning trials (F(2, 59) = 7.805, p = 0.001; partial η2 = 0.209, 95% CI: 0.042–0.361; Fig. 1A). There was better performance for the CN biomarker− group compared to both the CN biomarker+ group (t = 2.319, p = 0.048; d = 0.759, 95% CI: 0.102–1.416) and the early-stage symptomatic AD group (t = 3.673, p = 0.003; d = 1.105, 95% CI: 0.481–1.728). There was not a significant difference between the CN biomarker+ and the early-stage symptomatic AD groups (t = 1.019, p = 0.313; d = 0.395, 95% CI: –0.372–1.161). The effect of AD status was not likely due to differences in the exploration of the maze as there were not significant group differences in distance traveled during the exploration phase (F(2, 66) = 0.179, p = 0.836; partial η2 = 0.005, 95% CI: 0.000–0.056), and the main effect of AD status remained significant when additionally controlling for distance traveled during the exploration phase (F(2, 58) = 8.094, p = 0.001; partial η2 = 0.218, 95% CI: 0.046–0.371). There was not a significant Trial × AD status interaction for the learning phase (F(4, 118) = 2.043, p = 0.093; partial η2 = 0.065, 95% CI: 0.000–0.137; Fig. 1A).
Fig. 1.
Wayfinding performance. A) learning phase performance; B) delay phase performance; C) Environmental Knowledge Composite. White circles = clinically normal individuals who are biomarker negative (CN biomarker−), light gray triangles = clinically normal individuals who are biomarker positive (CN biomarker+), dark gray squares = early-stage symptomatic AD individuals. Data represent estimated marginal means (controlling for covariates; see text for details), and error bars are standard error of the mean.
Delay phase
There was a significant main effect of AD status for the delay phase (F(2, 59) = 9.723, p < 0.001; partial η2 = 0.248, 95% CI: 0.067–0.399; Fig. 1B). There was a not a significant difference between the CN biomarker− and CN biomarker+ groups in terms of distance traveled to reach landmarks (t = 0.687, p = 0.494; d = 0.225, 95% CI: −0.418–0.868). Both the CN biomarker− group (t = 4.456, p < 0.001; d = 1.340, 95% CI: 0.702–1.979) and the CN biomarker+ group (t = 3.043, p = 0.006; d = 1.179, 95% CI: 0.357–2.000) traveled shorter distances to reach landmarks compared to the early-stage symptomatic AD group.
When additionally controlling for learning performance, there was not a significant difference between the CN biomarker− and CN biomarker+ groups (t = 0.584, p = 0.560; d = 0.191, 95% CI: −0.451–0.834). The CN biomarker− group (t = 3.885, p < 0.001; d = 1.169, 95% CI: 0.541–1.796) and CN biomarker+ group (t = 2.961, p = 0.008; d = 1.147, 95% CI: 0.328–1.965) traveled shorter distances compared to the early-stage symptomatic AD group after additionally controlling for learning performance.
Environmental knowledge composite
There was a significant main effect of AD status (F(2, 59) = 19.301, p < 0.001; partial η2 = 0.395, 95% CI: 0.191–0.531; Fig. 1C). The CN biomarker− group acquired more knowledge about the environment compared to the CN biomarker+ group (t = 2.099, p = 0.040; d = 0.687, 95% CI: 0.033–1.342). Both the CN biomarker− group (t = 6.180, p < 0.001; d = 1.859, 95% CI: 1.178–2.540) and the CN biomarker+ group (t = 3.256, p = 0.002; d = 1.261, 95% CI: 0.431–2.091) acquired more knowledge about the environment compared to the early-stage symptomatic AD group.
When additionally controlling for learning performance, there was not a significant difference between the CN biomarker+ and the CN biomarker− groups (t = 1.111, p = 0.273; d = 0.364, 95% CI: −0.282–1.009). Both the CN biomarker− group (t = 4.523, p < 0.001; d = 1.361, 95% CI: 0.720–2.001) and the CN biomarker+ group (t = 3.088, p = 0.006; d = 1.196, 95% CI: 0.373–2.019) acquired more knowledge about the environment compared to early-stage symptomatic AD group after additionally controlling for learning performance.
Route learning
Learning phase
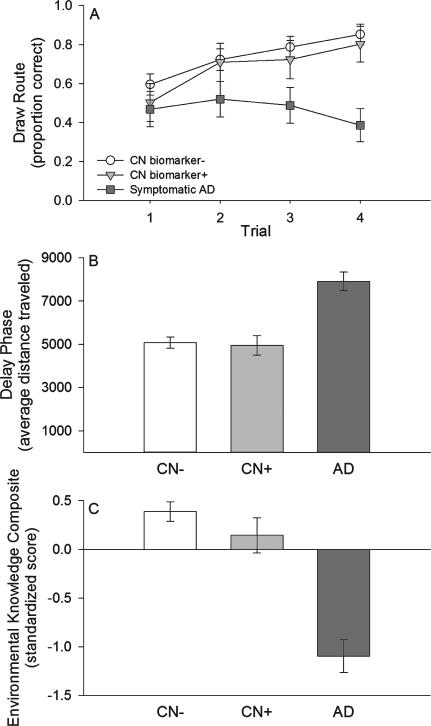
There was a significant main effect of trial for the learning phase (F(3, 171) = 8.177, p < 0.001; partial η2 = 0.125, 95% CI: 0.038–0.209; Fig. 2A) with significant linear improvement across the trials (F(1, 57) = 12.858, p = 0.001; partial η2 = 0.184, 95% CI: 0.037–0.350). There was a significant main effect of AD status across the four learning trials (F(2, 57) = 5.062, p = 0.009; partial η2 = 0.151, 95% CI: 0.011–0.302; Fig. 2A). There was not a significant difference between the CN biomarker− and the CN biomarker+ groups (t = 0.438, p = 0.658; d = 0.145, 95% CI: −0.503–0.792). Performance was significantly greater for both the CN biomarker− group (t = 3.115, p = 0.009; d = 0.925, 95% CI: 0.318–1.532) and CN biomarker+ group (t = 2.139, p = 0.072; d = 0.817, 95% CI: 0.038–1.595) compared to the early-stage symptomatic AD group. There was a non-significant trend for a Trial × AD status interaction for the learning phase (F(4.732, 134.862) = 2.145, p = 0.067; partial η2 = 0.070, 95% CI: 0.000–0.136; Fig. 2A).
Fig. 2.
Route learning performance. A) learning phase performance; B) delay phase performance; C) Environmental Knowledge Composite. White circles = clinically normal individuals who are biomarker negative (CN biomarker−), light gray triangles = clinically normal individuals who are biomarker positive (CN biomarker+), dark gray squares = early-stage symptomatic AD individuals. Data represent estimated marginal means (controlling for covariates; see text for details), and error bars are standard error of the mean.
Delay phase
There was a significant main effect of AD status for the delay phase (F(2, 57) = 14.775, p < 0.001; partial η2 = 0.341, 95% CI: 0.137–0.487; Fig. 2B). There was not a significant difference between the CN biomarker+ and CN biomarker− groups in terms of distance traveled during the delay phase (t = 0.247, p = 0.806; d = 0.082, 95% CI: −0.566–0.729). Both the CN biomarker− group (t = 5.523, p < 0.001; d = 1.640, 95% CI: 0.982–2.297) and CN biomarker+ group (t = 4.674, p < 0.001; d = 1.785, 95% CI: 0.902–2.667) traveled shorter distances compared to the early-stage symptomatic AD group.
When additionally controlling for learning performance, there was not a significant difference between the CN biomarker+ and CN biomarker− groups (t = 0.413, p = 0.681; d = 0.136, 95% CI: −0.511–0.784). Both the CN biomarker− group (t = 4.371, p < 0.001; d = 1.298, 95% CI: 0.667–1.928) and CN biomarker+ group (t = 4.007, p < 0.001; d = 1.530, 95% CI: 0.681–2.379) traveled shorter distances when compared to the early-stage symptomatic AD group after additionally controlling for learning performance.
Environmental knowledge composite
There was a significant main effect of AD status for the Environmental Knowledge Composite (F(2, 57) = 26.237, p < 0.001; partial η2 = 0.479, 95% CI: 0.275–0.602; Fig. 2C). There was not a significant difference between the CN biomarker+ and CN biomarker− groups (t = 1.184, p = 0.241; d = 0.391, 95% CI: −0.261–1.042). Both the CN biomarker− group (t = 7.305, p < 0.001; d = 2.169, 95% CI: 1.460–2.878) and the CN biomarker+ group (t = 4.956,p < 0.001; d = 1.893, 95%CI:0.995–2.790) acquired more knowledge about the environment compared to the early-stage symptomatic AD group.
When additionally controlling for learning performance, there was not a significant difference between the CN biomarker+ and the CN biomarker− groups for the Environmental Knowledge Composite (t = 1.123, p = 0.266;d = 0.371,95%CI:–0.280–1.022).Boththe CN biomarker− group (t = 6.204, p < 0.001; d = 1.788, 95% CI: 1.117–2.460) and the CN biomarker+ group (t = 4.335, p < 0.001; d = 1.656,95%CI:0.791–2.520) acquired more knowledge about the environment compared to the early-stage symptomatic AD group.
Post-Hoc analyses: CSF Tau
A post-hoc ANCOVA was conducted with average performance across the wayfinding learning trials as the dependent variable, Group (CN biomarker+ and CN biomarker−) as a predictor and with CSF tau and the Group × CSF tau interaction included in the model. CSF tau was not significantly associated with performance (F(1, 45) = 0.197, p = .659; partial η2 = 0.005, 95% CI: 0.000–0.1061). The main effect of group was significant (F(1, 45) = 5.702, p = 0.021; partial η2 = 0.112, 95% CI: 0.001–0.291). The Group × CSF tau interaction was not significant (F(1, 45) = 1.321, p = 0.256; partial η2 = 0.029, 95% CI: 0.000 –0.171)
Post-Hoc analyses: ROC
CN biomarker− versus CN biomarker+
ROC analyses were conducted to assess the diagnostic utility of the wayfinding task, and to compare that to the route learning task and an episodic memory task for distinguishing CN biomarker+ from CN biomarker− individuals. Episodic memory data were available for 41 CN biomarker− and 12 CN biomarker+ participants. For the standardized composite for the wayfinding task (learning and delay phases combined), there was a significant area under the curve (AUC; see Table 2). There was a non-significant trend for the wayfinding AUC to be greater than the AUC for the episodic memory task(χ2 = 3.12, p = 0.077), and the difference was significant with an outlier removed (χ2 = 4.00, p = 0.045; outlier removed: Wayfinding AUC = 0.80 (SE = 0.08), sensitivity = 0.83, specificity = 0.63). The way finding AUC was significantly better than the AUC for the route learning task (learning and delay phases combined; χ2 = 4.40, p = 0.036). The AUCs for the episodic memory task and route learning composite were not significantly different (χ2 = 0.003, p = 0.957).
Table 2.
ROC analyses
| Wayfinding | Route Learning | Selective Reminding | |
|---|---|---|---|
| CN biomarker– versus CN biomarker+ | |||
| AUC (SE) | 0.77 (0.08) | 0.55 (0.08) | 0.56 (0.10) |
| p-value | 0.004 | 0.57 | 0.52 |
| Youden Index | 0.49 | 0.32 | 0.20 |
| Sensitivity | 0.92 | 0.92 | 0.83 |
| Specificity | 0.57 | 0.39 | 0.37 |
| CN biomarker– versus Early-stage Symptomatic AD | |||
| AUC (SE) | 0.89 (0.06) | 0.77 (0.08) | 0.91 (0.07) |
| p-value | <0.001 | 0.005 | <0.001 |
| Youden Index | 0.62 | 0.53 | 0.83 |
| Sensitivity | 0.92 | 0.67 | 0.83 |
| Specificity | 0.71 | 0.87 | 1.0 |
| CN biomarker+ versus Early-Stage Symptomatic AD | |||
| AUC (SE) | 0.79 (0.09) | 0.79 (0.09) | 0.82 (0.10) |
| p-value | 0.01 | 0.01 | 0.007 |
| Youden Index | 0.58 | 0.50 | 0.68 |
| Sensitivity | 0.67 | 0.75 | 0.83 |
| Specificity | 0.92 | 0.75 | 0.85 |
SE, standard error; CN, clinically normal; AD, Alzheimer's disease.
CN biomarker− versus early-stage symptomatic AD
Episodic memory data were available for 41 CN biomarker− and 12 early-stage symptomatic AD participants. For the wayfinding composite, there was a significant AUC (see Table 2), and this was not significantly different than the AUC for the episodic memory task (χ2 = 0.03, p = 0.858). The wayfinding AUC was not significantly different than the AUC for route learning (χ2 = 1.29, p = 0.274). The AUC for the episodic memory task was significantly better than the AUC for route learning (χ2 = 5.39, p = 0.020).
CN biomarker+ versus early-stage symptomatic AD
Episodic memory data were available for 12 CN biomarker+ and 12 early-stage symptomatic AD participants. For the wayfinding composite, there was a significant AUC (see Table 2), and this was not significantly different than the AUC for the episodic memory task (χ2 = 0.06, p = 0.813). The wayfinding AUC was not significantly different than the AUC for route learning (χ2 < 0.01, p = 0.986). The AUC for the episodic memory task was not significantly better than the AUC for route learning (χ2 = 0.20, p = 0.656).
DISCUSSION
Preclinical AD-related deficits were observed on a wayfinding task indexing the use of a place learning strategy (i.e., allocentric frame of reference). The preclinical AD (CN biomarker+) group evidenced lower performance than the CN biomarker− group during the learning phase, and their performance did not differ significantly from that of the early-stage symptomatic AD group. While this effect of preclinical AD on learning was present when controlling for group differences in basic visuomotor processing in the virtual environment, it is still possible that the latter difference may have contributed to some degree to impaired learning. As the learning phase required the ability to learn the locations of objects in the environment in relation to each other, these results suggest preclinical AD-related deficits in forming a cognitive map.
In contrast, preclinical AD individuals were not significantly different from CN biomarker− individuals in terms of distance traveled to locate landmarks during the delay phase. The ability to reach landmarks efficiently after a delay depends upon the ability to form, retain, and utilize a mental representation of the environment. Although the rate of improvement did not significantly differ between groups, examination of Figure 1 suggests that by the 3rd trial preclinical AD individuals were approaching the performance of the CN biomarker− group. In fact, a post-hoc analysis revealed only a non-significant trend for group differences on the 3rd trial (p = 0.16). Thus, preclinical AD individuals may require additional training to learn a new environment to the same level as CN biomarker− individuals, but then retain sufficient information to use a cognitive map in a generally effective manner.
The preclinical AD individuals did evidence lower performance on the Environmental Knowledge Composite during the delay phase. However, the difference was no longer significant after controlling for learning phase performance. This suggests that the difficulties observed after a delay were in part related to trouble acquiring the environmental information. In addition, although the preclinical AD group's environmental knowledge may have been less detailed than that of the CN biomarker− group to some degree during the delay phase, it was generally sufficient for them to navigate the environment to locate landmarks.
Collectively, the current findings indicate that pre-clinical AD is associated with declines in acquiring the spatial information relevant for wayfinding performance. Moreover, post-hoc analyses indicated that wayfinding performance had high sensitivity and moderate specificity. Notably, there was evidence that wayfinding performance was more powerful in discriminating between preclinical AD and CN biomarker− groups than an episodic memory task. Given the mixed findings and generally small effect sizes for the influence of preclinical AD on standard psychometric tasks in the existing literature (e.g., [27, 29, 65]), further research on the robustness of the sensitivity of wayfinding tasks for preclinical AD is warranted.
In contrast to wayfinding, there were no significant effects of preclinical AD status on any aspect of the route learning task indexing use of a response learning strategy (i.e., an egocentric frame of reference). Additionally, route learning performance was significantly less powerful than the wayfinding task in discriminating between preclinical AD and CN biomarker− groups. We have previously demonstrated associations of similar versions of the wayfinding and route learning tasks with hippocampal and caudate volumes, respectively [48], which is consistent with place learning being a hippocampus-dependent task and response learning being a caudate-dependent task [31–37]. Thus, the behavioral pattern observed here aligns with previous research indicating preclinical AD is characterized by hippocampal atrophy, but relatively preserved caudate volume [26, 27]. Additional research is needed to determine whether preclinical AD-related deficits in wayfinding performance are mediated by structural brain differences.
Early-stage symptomatic AD-related deficits were observed for all of the wayfinding and route learning tasks relative to CN biomarker− individuals, and for all but the wayfinding learning task relative to CN biomarker+ individuals. These findings are consistent with previous work comparing symptomatic AD to CN individuals [41–44, 66–68]. However, the current study extends past work by demonstrating that early-stage symptomatic AD individuals generally perform worse than CN individuals with and without evidence of preclinical AD. Thus, early-stage symptomatic AD appears to be associated with deficits in acquiring, utilizing, and retaining the environmental information necessary for successful navigation performance across both strategies.
This pattern of more generalized spatial navigation deficits is consistent with evidence of both hippocampal and caudate atrophy in early-stage symptomatic AD, though hippocampal atrophy may be greater [28, 45]. This generalized pattern extends to the observation that neither wayfinding nor route learning performance was significantly better at discriminating groups than an episodic memory task (but see [67] and [68] for evidence of the superiority of navigation-related tasks to standard psychometric tasks). In addition, individuals with early-stage symptomatic AD generally demonstrated deficits on some aspects of both the wayfinding and route learning tasks in comparison to preclinical AD individuals. There is also evidence that MCI is associated with hippocampal and caudate atrophy, as well as decrements in both egocentric and allocentric frames of reference [28, 39, 40, 66, 69]. Overall, these observations suggest a progression such that preclinical AD is characterized by hippocampal atrophy and associated wayfinding difficulties, particularly during the learning phase. As the disease progresses, wayfinding deficits worsen, the caudate becomes involved, and route learning deficits emerge. Further research should be conducted to substantiate this conceptualization.
Importantly, although we have emphasized the potential contributions of the hippocampus to the observed wayfinding deficits, these impairments may also be related to changes in other brain regions impacted early during the course of AD. For example, there is evidence of very early changes in medial parietal regions, including the posterior cingulate and retrosplenial cortex [27, 70, 71]. These regions also play an important role in successful wayfinding performance (e.g., [32, 37, 72]). Subsequent neuroimaging research is needed in order to better determine the structural and functional brain changes that may account for preclinical AD-related deficits on wayfinding tasks.
Several limitations of the current work must be considered. Our operationalization of preclinical AD status as CN with low CSF Aβ42 levels is generally consistent with current recommendations, which are based in part on longitudinal demonstrations of increased risk for cognitive decline and progression to dementia [7]. However, the recently proposed NIA-AA criteria outline three stages of preclinical AD [7]: Cognitive normality with abnormal amyloid (stage 1), cognitive normality with abnormal amyloid and neuronal injury (stage 2), and subtle cognitive deficits in conjunction with abnormal amyloid and neuronal injury (stage 3). While our sample size did not permit examination of the separate stages, post-hoc analyses indicated that there was not a significant association between CSF tau (a marker of neuronal injury) and the learning phase of the wayfinding task and the effect of preclinical AD remained significant when controlling for CSF tau. These findings suggest that CSF Aβ42 may be contributing more to the impaired learning performance in this sample. Nonetheless, future research should systematically examine the effects of each preclinical stage on spatial navigation performance.
In addition, our operationalization of preclinical AD did not incorporate psychometric data. The use of a psychometric evaluation for distinguishing cognitive normality from very mild cognitive impairment could lead to differing results. It is conceivable that some individuals in the CN groups could have met a psychometric definition of MCI despite the lack of mean differences in psychometric task performance (see Supplementary Table 1). Thus, our current results must be interpreted within the context of the specific operationalization of preclinical AD, and may not generalize to classifications based on psychometric data. Importantly, the degree to which individuals conceptualized as in the preclinical phase in the current study will eventually develop symptomatic AD prior to death is currently unknown. As these individuals are participating in longitudinal research at the Knight ADRC, future research can examine the degree to which the “preclinical” AD individuals develop symptomatic AD. Current results are still consistent with the interpretation that cerebral amyloid deposition in CN individuals is associated with impaired performance on a hippocampally-supported navigation task, regardless of whether these individuals go on to develop symptomatic AD.
There was also a relatively long delay between CSF Aβ42 collection and the subsequent experimental tasks. Thus, there is the potential that some CN individuals may have possessed lower CSF Aβ42 levels at the time of the experimental session. However, this would work against finding significant differences between those designated as preclinical AD and CN biomarker− individuals. Nonetheless, future studies should incorporate concurrent measurement of amyloid deposition and navigation performance. Furthermore, participants did not undergo MRI scanning prior to enrollment to exclude brain pathology and/or severe vascular lesions and therefore it is unknown whether cerebrovascular changes may have contributed to observed findings. Although hippocampal atrophy but relatively preserved caudate volume has been observed in a prior study by our group [27], the lack of sufficient structural MRI data within a reasonable timeframe for the current study precluded examination of associations between regional brain volumes and navigation. Lastly, the sample sizes for the preclinical and early-stage symptomatic AD groups were relatively small, which may limit power as well as the generalizability of the findings.
Within the context of these limitations, the current investigation demonstrated significant preclinical AD-related deficits in aspects of wayfinding with relative preservation in route learning. In contrast, early-stage symptomatic AD was associated with deficits in both tasks when compared to CN biomarker− individuals. This pattern is consistent with decrements in hippocampal integrity prior to changes in the caudate. Importantly, these findings suggest that navigational tasks designed to assess a wayfinding strategy could represent a powerful tool for detecting the very earliest AD-related changes in cognition. Future research should examine whether wayfinding deficits in individuals with preclinical AD are associated with an increased risk of developing symptomatic AD.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants P50 AG05861, P01 AG03991, and P01 AG026276. S. Allison was supported by National Institute on Aging 5T32AG00030. We thank our lumbar puncture physicians for obtaining our CSF samples, M. Amos and A. Shah for processing and analyzing the CSF samples, S. Sathyan for scheduling the lumbar punctures, and the Clinical Core of the Knight Alzheimer's Disease Research Center for participant assessments. We thank Chauncey Scott and Tyler Blazey for assistance with the development of the maze environments and programming the maze tasks.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0855r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-150855.
REFERENCES
- 1.Henderson VW, Mack W, Williams BW. Spatial disorientation in Alzheimer's disease. Arch Neurol. 1989;46:391–394. doi: 10.1001/archneur.1989.00520400045018. [DOI] [PubMed] [Google Scholar]
- 2.Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:250–255. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- 3.Tu MC, Pai MC. Getting lost for the first time in patients with Alzheimer's disease. Int Psychogeriatr. 2006;18:567–570. doi: 10.1017/S1041610206224025. [DOI] [PubMed] [Google Scholar]
- 4.Dubinsky RM, Stein AC, Lyons K. Practice parameter: Risk of driving and Alzheimer's disease (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54:2205–2211. doi: 10.1212/wnl.54.12.2205. [DOI] [PubMed] [Google Scholar]
- 5.Hunt LA, Brown AE, Gilman IP. Drivers with dementia and outcomes of becoming lost while driving. Am J Occup Ther. 2010;64:225–232. doi: 10.5014/ajot.64.2.225. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association work-groups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 9.Selkoe DJ. The origins of Alzheimer disease: A is for amyloid. JAMA. 2000;283:1615–1617. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 10.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 13.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 14.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drzezga A, Becker JA, Van Dijk KR, Sreenivasan A, Talukdar T, Sullivan C, Schultz AP, Sepulcre J, Putcha D, Greve D, Johnson KA, Sperling RA. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM, Alzheimer's Disease Neuroimaging Initiative CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer's disease. J Neurosci. 2010;30:2088–2101. doi: 10.1523/JNEUROSCI.3785-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chételat G, Villemagne VL, Pike KE, Ellis KA, Ames D, Masters CL, Rowe CC, Australian Imaging Biomarkers and Lifestyle Study of Ageing Research Group Relationship between memory performance and β-amyloid deposition at different stages of Alzheimer's disease. Neurodegener Dis. 2012;10:141–144. doi: 10.1159/000334295. [DOI] [PubMed] [Google Scholar]
- 19.Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Clark CM, Joshi AD, Mintun MA, Skovronsky DM, Pontecorvo MJ, AV45-A11 Study Group Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: A multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Boeve BF, Petersen RC. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, Sutphen CL, Benzinger TL, Mintun MA, Holtzman DM, Morris JC. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM. Preclinical Alzheimer's disease and its outcome: A longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 26.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ, Alzheimer's Disease Neuroimaging Initiative Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: Cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen SK, Ho AJ, Hua X, Saharan PS, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM, Alzheimer's Disease Neuroimaging Initiative 3D maps localize caudate nucleus atrophy in 400 Alzheimer's disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol Aging. 2010;31:1312–1325. doi: 10.1016/j.neurobiolaging.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffat SD. Aging and spatial navigation: What do we know and where do we go? Neuropsychol Rev. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. University Press; Oxford: 1978. [Google Scholar]
- 32.Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25:890–899. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- 33.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi T, Matsuda H, Hirakata M, Ugawa Y. Navigation ability dependent neural activation in the human brain: An fMRI study. Neurosci Res. 2007;55:361–369. doi: 10.1016/j.neures.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 35.de Bruin JP, Swinkels WA, de Brabander JM. Response learning of rats in a Morris water maze: Involvement of the medial prefrontal cortex. Behav Brain Res. 1997;85:47–55. doi: 10.1016/s0166-4328(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 36.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 37.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and cau-date nucleus in human navigation: Variability and change with practice. J Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalová E, Vlcek K, Jarolímová E, Bures J. Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer's disease: Corresponding results in real space tests and computer tests. Behav Brain Res. 2005;159:175–186. doi: 10.1016/j.bbr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Laczó J, Andel R, Vlcek K, Macoška V, Vyhnálek M, Tolar M, Bojar M, Hort J. Spatial navigation and APOE in amnestic mild cognitive impairment. Neurodegener Dis. 2011;8:169–177. doi: 10.1159/000321581. [DOI] [PubMed] [Google Scholar]
- 40.Hort J, Laczó J, Vyhnálek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherrier MM, Mendez M, Perryman K. Route learning performance in Alzheimer disease patients. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:159–168. [PubMed] [Google Scholar]
- 42.Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.deIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69:986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- 44.Lithfous S, Dufour A, Després O. Spatial navigation in normal aging and the prodromal stage of Alzheimer's disease: Insights from imaging and behavioral studies. Ageing Res Rev. 2013;12:201–213. doi: 10.1016/j.arr.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, Thompson P, Fox NC. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009;30:1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, Sheardova K, Bures J, Hort J. Spatial navigation impairment is proportional to right hippocampal volume. Proc Natl Acad Sci U S A. 2012;109:2590–2594. doi: 10.1073/pnas.1121588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49:518–527. doi: 10.1016/j.neuropsychologia.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Head D, Isom M. Age effects on wayfinding and route learning skills. Behav Brain Res. 2010;209:49–58. doi: 10.1016/j.bbr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 50.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 51.Berg L, McKeel DW, Miller JP, Miller P, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Northon J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: Relation of histologic markers to dementia severity, age, sex, and apoE genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 52.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original and revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 55.Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 56.Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 57.Richardson AE, Montello DR, Hegarty M. Spatial knowledge acquisition from maps and from navigation in real and virtual environments. Mem Cognit. 1999;27:741–750. doi: 10.3758/bf03211566. [DOI] [PubMed] [Google Scholar]
- 58.Iaria G, Palermo L, Committeri G, Barton JJS. Age differences in the formation and use of cognitive maps. Beh Brain Res. 2009;196:187–191. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlations Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Mahwah: 2003. [Google Scholar]
- 60.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6:65–70. [Google Scholar]
- 61.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharm Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 62.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1998;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 63.Zweig MH, Campbell G. Receiver operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 64.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1998;44:837–845. [PubMed] [Google Scholar]
- 65.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 66.Laczó J, Andel R, Vyhnálek M, Vlcek K, Magerova H, Varjassyova A, Tolar M, Hort J. Human analogue of the Morris water maze for testing subjects at risk of Alzheimer's disease. Neurodegener Dis. 2010;7:148–152. doi: 10.1159/000289226. [DOI] [PubMed] [Google Scholar]
- 67.Bellassen V, Iglói K, de Souza LC, Dubois B, Rondi-Reig L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer's disease diagnosis. J Neurosci. 2012;32:1942–1952. doi: 10.1523/JNEUROSCI.4556-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pengas G, Patterson K, Arnold RJ, Bird CM, Burgess N, Nestor PJ. Lost and found: Bespoke memory testing for Alzheimer's disease and semantic dementia. J Alzheimers Dis. 2010;21:1347–1365. doi: 10.3233/jad-2010-100654. [DOI] [PubMed] [Google Scholar]
- 69.Shen L, Saykin AJ, Kim S, Firpi HA, West JD, Risacher SL, McDonald BC, McHugh TL, Wishart HA, Flashman LA. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging Behav. 2010;4:86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 71.Walhovd KB, Fjell AM, Brewer J, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jr, Jennings RG, Karow D, Dale AM, Alzheimer's Disease Neuroimaging, Initiative Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolbers T, Hegarty M, Büchel C, Loomis JM. Spatial updating: How the brain keeps track of changing object locations during observer motion. Nat Neurosci. 2008;11:1223–1230. doi: 10.1038/nn.2189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.