Abstract
Background:
The fronto-striatal circuits are the common neurobiological basis for neuropsychiatric disorders, including schizophrenia, Parkinson’s disease, Huntington’s disease, attention deficit hyperactivity disorder, obsessive-compulsive disorder, and Tourette’s syndrome. Fronto-striatal circuits consist of motor circuits, associative circuits, and limbic circuits. All circuits share 2 common features. First, all fronto-striatal circuits consist of hyper direct, direct, and indirect pathways. Second, all fronto-striatal circuits are modulated by dopamine. Intracellularly, the effect of dopamine is largely mediated through the cyclic adenosine monophosphate/protein kinase A signaling cascade with an additional role for the cyclic guanosine monophosphate/protein kinase G pathway, both of which can be regulated by phosphodiesterases. Phosphodiesterases are thus a potential target for pharmacological intervention in neuropsychiatric disorders related to dopaminergic regulation of fronto-striatal circuits.
Methods:
Clinical studies of the effects of different phosphodiesterase inhibitors on cognition, affect, and motor function in relation to the fronto-striatal circuits are reviewed.
Results:
Several selective phosphodiesterase inhibitors have positive effects on cognition, affect, and motor function in relation to the fronto-striatal circuits.
Conclusion:
Increased understanding of the subcellular localization and unraveling of the signalosome concept of phosphodiesterases including its function and dysfunction in the fronto-striatal circuits will contribute to the design of new specific inhibitors and enhance the potential of phosphodiesterase inhibitors as therapeutics in fronto-striatal circuits.
Keywords: fronto-striatal circuits, dopamine, phosphodiesterase, phosphodiesterase inhibitors, cyclic adenosine monophosphate
Introduction
Several neuropsychiatric disorders, including Parkinson’s disease, Huntington’s disease, attention-deficit hyperactivity disorder (ADHD), Tourette’s syndrome, schizophrenia, and obsessive-compulsive disorder, share the fronto-striatal circuits, also known as cortico-striatal-thalamic circuits, as their neurobiological basis. The fronto-striatal circuits comprise motor, cognitive, and limbic circuits (Alexander et al., 1986, 1990; Alexander and Crutcher, 1990). These circuits operate in a very complex manner that is extensively described elsewhere (Surmeier et al., 2007; Haber and Rauch, 2010; Gerfen and Surmeier, 2011; Surmeier et al., 2011; Calabresi et al., 2014). Dysfunction of these circuits produces the wide range of motor, cognitive, and affective symptoms observed in related neuropsychiatric disorders. One prominent feature of the complex functioning of the fronto-striatal circuits is their modulation by dopamine, both at the level of the frontal cortex as well as the striatum. As a result, dopaminergic receptors are strongly expressed throughout all fronto-striatal circuits (Gerfen and Surmeier, 2011; Nishi et al., 2011; Kuroiwa et al., 2012). Unsurprisingly, dopaminergic medication has been the first-line therapy for several disorders related to dysfunctional fronto-striatal circuits; however, efficacy is often moderate at best and accompanied by severe side effects (e.g., ADHD, schizophrenia, and Parkinson’s disease).
Dopamine originating from substantia nigra pars compacta (SNc) and/or ventral tegmental area (VTA) (nigrostriatal and mesolimbic pathways) binds to both dopamine type1 (D1) receptors and dopamine type2 (D2) receptors on medium spiny neurons (MSNs) in the striatum (Gerfen and Surmeier, 2011). D1 receptors are mainly found on MSNs of the direct pathway, and D2 receptors are mainly found on MSNs of the indirect pathway where they establish antagonistic interactions with adenosine A2a receptors (Gerfen et al., 1990; Ferre et al., 2011). Additionally, dopamine released from VTA (mesocortical pathway) also binds to D1 receptors in the frontal cortex (Kuroiwa et al., 2012). D1 receptors activate the Gαs/olf family of G proteins to stimulate cyclic adenosine monophosphate (cAMP) production and thereby striatonigral and frontal signaling (Sibley et al., 1993; Beaulieu and Gainetdinov, 2011). In contrast, the D2 receptors couple to the Gαi/o family of G proteins and thus induce inhibition of cAMP production, thereby inhibiting striatopallidal signaling that eventually leads to disinhibition of the frontal cortex (Figure 1). Actions of the dopamine receptors in both pathways can be viewed as synergistically or complementary.
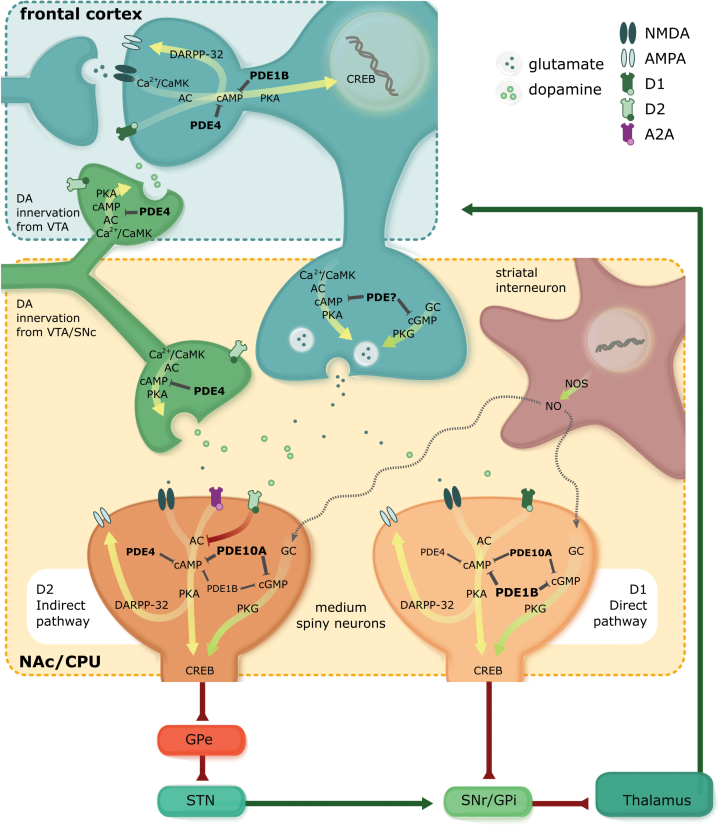
Figure 1.
Fronto-striatal circuits originate in the frontal cortex and pass through the basal ganglia, which project via the thalamus back to frontal brain areas. Output neurons in the striatum are medium spiny neurons (MSNs), which consist of direct pathway and indirect pathway neurons. The direct pathway neurons inhibit tonically active neurons in globus pallidus interna (GPi)/substantia nigra pars reticulata (SNr). The indirect pathway neurons activate neurons in GPi/SNr via inhibition of the globus pallidus externa (GPe) and activation of the subthalamic nucleus (STN). Direct and indirect pathway neurons induce opposing effects on the output neurons in GPi/SNr, resulting in disinhibition and proinhibition of output, respectively. Within the basal ganglia all projections are GABAergic except those from the STN. Main phosphodiesterases (PDEs) expressed in fronto-striatal circuits are PDE1B, PDE4, and PDE10A. PDE1B is generally colocalized with dopamine (DA) D1 receptors in the brain and thought to represent a major inactivation mechanism of D1 receptors. By acting like a DA D1 agonist PDE1B-Is can enhance phosphorylation of cAMP response element binding protein (CREB) as well as Dopamine- and cAMP-Regulated PhosphoProtein MR 32kDa (DARPP-32) enhancing synaptic transmission (e.g., AMPA receptors), neuron excitability, and synapto- and neurogenesis, resulting in neuroplasticity and neuroprotective effects at glutamatergic frontal and fronto-striatal synapses. Regarding fronto-striatal signaling, the effect of PDE4 inhibition on cAMP/protein kinase A (PKA) signaling, is linked to indirect pathway adenosine A2a receptor signaling and has no major role in D1 receptor direct pathway signaling. An opposite situation is observed at frontal dopaminergic signaling. In the frontal cortex, PDE4 is –just as PDE1B- localized at DARPP-32 expressing neurons. In contrast to the striatum, PDE4 inhibition enhances DA D1 receptor-induced phosphorylation of DARPP-32 in the frontal cortex, indicating a prominent role of PDE4 in frontal DA receptor signaling. Finally, DA release from DAergic midbrain terminals can be influenced with a PDE4 inhibitor as DA is expressed at DAergic terminals in neurons of the SNc in which cAMP has been reported to be a strong inducer of tyrosine hydroxylase (TH) gene transcription rate and mRNA affecting DA synthesis and release. In direct pathway neurons, PDE10A inhibition activates cAMP/PKA signaling related to D1 receptor signaling, whereas in indirect pathway neurons PDE10A inhibition activates cAMP/PKA signaling by simultaneous potentiation of adenosine A2A receptor signaling and inhibition of D2 receptor signaling. Effects of PDE10A inhibition are suggested to predominate the indirect pathway. In contrast to PDE4 inhibition, PDE10A inhibition does not increase TH phosphorylation and therefore has no effects on DA synthesis and release. Nevertheless, it cannot be ruled out that selective PDE inhibitors (PDE-Is) might influence both the direct and indirect pathway via enhancing the release of DA from frontal DAergic projections depending on the –to be determined- presence of PDEs in these terminals.
In striatal interneurons containing nitric oxide synthase (NOS), nitric oxide (NO) is produced and diffuses into dendrites of MSNs which contain high levels of guanylate cyclase (GC), which, when activated, lead to the synthesis of cyclic guanosine monophosphate (cGMP). In the striatum, transient elevations in intracellular cGMP, next to cAMP, primarily act to increase neuronal excitability and to facilitate glutamatergic fronto-striatal transmission. Thus, inhibition of selective PDE subtypes can also target the cGMP/protein kinase G (PKG) pathway and have an effect on fronto-striatal functioning.
Intracellularly, the effect of dopamine on striatonigral, striatopallidal, and frontal neurons is largely mediated through the cAMP-activated cascade (Nishi et al., 2008, 2011; Nishi and Snyder, 2010; Kuroiwa et al., 2012). cAMP is synthesized from adenosine triphosphate by adenylyl cyclase, which is activated directly by activated G-protein coupled receptors or by calmodulin (CaM)-dependent protein kinase (PK)/ after Ca2+ influx. cAMP affects synaptic plasticity through both presynaptic neurotransmitter release and postsynaptic intracellular pathways (Figure 1). The former might be mediated via a presynaptic calcium (Ca2+)/CaM-dependent PK/cAMP/cAMP-dependent PKA cascade and elevation of cAMP has been found to result in the synthesis and/or release of several neurotransmitters, including 2 main players in the fronto-striatal circuits: glutamate and dopamine (Schoffelmeer et al., 1985; Imanishi et al., 1997; Rodriguez-Moreno and Sihra, 2013).
The influence on postsynaptic intracellular pathways occurs through activation of postsynaptic PKA by cAMP produced by adenylyl cyclase stimulated by either glutamatergic-induced Ca2+ influx or dopamine signaling-stimulated Gs. PKA exerts several effects related to neuroplasticity and neuroprotection. The fastest postsynaptic response in relation to neuroplasticity mediated by cyclic nucleotides is the activation and insertion of stored receptors by PKA through phosphorylation of GluR1 subunits promoting α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking into the postsynaptic membrane for potentiation of glutamatergic transmission (Song et al., 2013). In addition to the mobilization and membrane insertion of stored receptors, the process of protein synthesis (e.g., AMPA receptors) further increases neuroplasticity (Carew and Sutton, 2001; Izquierdo et al., 2006).
PKA also phosphorylates cAMP response element- binding protein (CREB) (Mayr and Montminy, 2001) and Dopamine- and cAMP-Regulated PhosphoProtein MR 32kDa (DARPP-32) (Greengard, 2001; Svenningsson et al., 2004). Phosphorylated CREB is also involved in neuroplasticity (e.g., synthesis of other proteins) (Impey et al., 1996; Lu et al., 1999; Sakamoto et al., 2011) and neuroprotection (e.g., neuronal arborization, synaptogenesis, and neurogenesis) (Mantamadiotis et al., 2002; Bruel-Jungerman et al., 2006; Sakamoto et al., 2011). One of the genes transcribed by phosphorylated CREB is bdnf (Scott Bitner, 2012). After release, the protein BDNF binds to the tropomyosin-related kinase B receptor, which is the receptor with the highest affinity for BDNF. BDNF is involved in the proliferation, survival, and differentiation of new neurons (i.e., neurogenesis in the brain) (Minichiello, 2009).
In addition, the activity-dependent release of BDNF and subsequent tropomyosin-related kinase B-mediated activation of CREB is also an important mechanism of enhancing neuronal communication, specifically in active neurons of the brain. For instance, BDNF increases synaptic strength with adjacent neurons by processes like long-term potentiation (LTP), thus ameliorating their connectivity (Lu et al., 2008; Minichiello, 2009). Interestingly, LTP itself has been linked to both synaptogenesis and neurogenesis (Bruel-Jungerman et al., 2006).
DARPP-32 is phosphorylated at Thr34 in both striatal and frontal neurons. DARPP-32 thereby converts into a potent inhibitor of protein phosphatase-1 (PP-1). DARPP-32 is also phosphorylated at Thr75 by Cdk5 and this converts DARPP-32 into an inhibitor of PKA. Thus, DARPP-32 has the unique property of being a dual-function protein, acting either as an inhibitor of PP-1 or of PKA influencing neuroplasticity (Svenningsson et al., 2004). The inhibition of PP-1 controls the phosphorylation state and activity of many downstream physiological effectors, including various neurotransmitter receptors (e.g., AMPA receptor GluR1 subunit, N-methyl-D-aspartate receptor NR1 subunit), ion channels and pumps (e.g., N/P-type Ca2+ channels, Na+ channel, Na+, K+-ATPase), and transcription factors (e.g., CREB, c-Fos, ΔFosB) (Greengard et al., 1999). Striatal LTP and long-term depression are dependent on cAMP and DARPP-32 phosphorylation (Calabresi et al., 2000).
The cAMP/PKA cascade is thus a potential target for pharmacological intervention in neuropsychiatric disorders related to dopaminergic frontal and striatal dysfunction. cAMP is degraded by cAMP-specific phosphodiesterases (PDEs) and dual substrate PDEs. Eleven PDE families have been described, distinguished by molecular properties, substrate specificity, and regulation (Bender and Beavo, 2006). These enzymes are expressed in unique and overlapping patterns throughout the body and central nervous system (Lakics et al., 2010; Table 1). Selective PDE inhibitors (PDE-Is) prevent the degradation of cyclic nucleotides leading to increased concentrations of cAMP. Due to the differential expression of PDE subtypes in one or more of the frontal and striatal pathways or dopaminergic terminals, different subtype-specific PDE-Is enable stimulation of dopamine synthesis, inhibtion of D2 receptor signaling or stimulation of D1 receptor signaling (Nishi et al., 2011). However, the level of expression of different PDE family members in these fronto-striatal circuits in both physiological and pathological conditions is incompletely understood and a subject of intense investigation. In the fronto-striatal circuits, the main therapeutic mechanism of PDE inhibition is enhanced neuroplasticity and neuroprotection through previously discussed CREB and DARPP-32 signaling cascades (Figure 1). However, known effects of PDE-Is on neuroinflammation and cytokine-mediated responses may play additional roles (Hebb and Robertson, 2008; Wilson and Brandon, 2015).
Table 1.
Localization of the Different PDEs in the Brain of Rodents and Humans in Adulthood
| PDE | Localization in the Body | Localization in the Brain |
|---|---|---|
| PDE1A-C | Heart, smooth muscles, lungs | Hippocampus, cortex, olfactory bulb, striatum (highest expression levels), thalamus, amygdala, cerebellum; expression levels are in general highest for 1A and lowest for 1C |
| PDE2A | Heart, adrenal cortex, platelets | Hippocampus, cortex, striatum, hypothalamus, amygdala, midbrain |
| PDE3A-B | Heart, smooth muscles, kidneys, platelets | Throughout the brain low expression levels |
| PDE4A-D | Wide variety of tissues: e.g., smooth muscles, lungs, kidneys, testes |
Hippocampus, cortex, olfactory bulb, striatum, thalamus, hypothalamus, amygdala, midbrain, cerebellum; expression levels are in general highest for 4A-4D (differs per brain structure) and lowest for 4C |
| PDE5A | Smooth muscles, skeletal muscles, lungs, kidneys, platelets |
Hippocampus, cortex, cerebellum |
| PDE6A-C | Rod and cone cells in retina | Pineal gland |
| PDE7A-B | Heart, skeletal muscles, liver, kidneys, testes, pancreas |
Hippocampus, cortex, olfactory bulb, striatum, thalamus, hypothalamus, midbrain; expression levels are in general highest for 7B |
| PDE8A-B | Heart, liver, kidneys, lungs, testes, thyroid | Hippocampus, cortex, olfactory bulb, striatum, thalamus, hypothalamus, midbrain; expression levels are in general highest for 8B |
| PDE9A | Kidneys, spleen, prostate, various gastrointestinal tissues |
Hippocampus, cortex, olfactory bulb, striatum, thalamus, hypothalamus, amygdala, midbrain, cerebellum |
| PDE10A | Heart, skeletal muscles, lungs, liver, kidneys, testes, pancreas, thyroid |
Hippocampus, cortex, striatum (highest expression levels), midbrain, cerebellum |
| PDE11A | Skeletal muscles, liver, kidneys, testes, prostate, thyroid |
Throughout the brain low expression levels |
Abbreviation: PDE, phosphodiesterase.
Note that this table does not provide information with respect to the level of expression (protein or mRNA) of the different PDEs. Adapted from Prickaerts, 2015, based on Lakics et al, 2010; Pérez-Torres et al, 2010.
The integration of individual PDEs into specific signalosomes within different functional compartments has revealed the functional roles of these PDEs and linked the large number of PDE isoforms to the compartmentalized regulation of specific cyclic nucleotide signaling pathways and biological responses. This compartmentalization therefore contributes to both the fine-tuning and specificity of cyclic nucleotide signaling (Jurevicius and Fischmeister, 1996; Zaccolo et al., 2000; Mongillo et al., 2006; Maurice, 2011; Stangherlin et al., 2011; Stangherlin and Zaccolo, 2012). More in detail, compartmentalization provides spatially distinct pools of PKA and PKG to be activated in different ways. This idea was confirmed by the observation of accumulation of cAMP in localized pools (Houslay, 1995). These pools are created by physical interactions between different components of signaling cascades and structural elements of the cell. Localization and activation of both cyclases and PDEs are important determinants in the process of cyclic nucleotide homeostasis by modulating fluctuations in the compartments. For PDEs, sequestration and anchoring is the principal mechanism to create cyclic nucleotide gradients (Houslay and Milligan, 1997; Houslay and Adams, 2003). Subsequently, different PKA isoforms are anchored at specific intracellular sites by A-kinase anchoring proteins (AKAPs) (Rubin, 1994). AKAPs control the gradients of cAMP in the cell and modify localized target proteins, thereby causing sequestration of PKA into distinct cellular compartments. This also applies to the fronto-striatal circuits and is considered the main determinant of the target PDE isoform within a specific neuropsychiatric disorder. In addition, different PDE isoforms can integrate multiple distinct cellular inputs and allow crosstalk between cyclic nucleotides and other signaling networks and systems (Dodge-Kafka et al., 2005; Mongillo et al., 2006; Houslay et al., 2007; Stangherlin et al., 2011; Wilson et al., 2011; Kritzer et al., 2012).
Since the focus of this review is on frontal and striatal dopaminergic regulation, PDE1B, PDE2A, PDE4, PDE7B, PDE9A, and PDE10A in particular are of special interest (Lakics et al., 2010). PDE1B, PDE7B, and PDE10A are highly enriched in striatum and/or frontal cortex. PDE2A, PDE4 (A, B, D), and PDE9A are more widely distributed but are also expressed in striatum and/or frontal cortex. There are only very limited preclinical data on PDE2A, PDE7A, and PDE9A inhibition (e.g., Duinen et al., 2015). To date, most research has been devoted to the potential of PDE1B, PDE4, and PDE10A for regulation of dopaminergic frontal and striatal signaling, and therefore these subtypes will be discussed below.
PDE1
PDE1 and Dopamine Signaling
PDE1 hydrolyzes both cAMP and cGMP. PDE1, unlike any other class of PDE, is uniquely activated by the binding of a complex of Ca2+ and CaM. PDE1 is encoded by 3 separate genes: PDE1A, PDE1B, and PDE1C. PDE1B is highly colocalized with D1 receptors in the brain and is particularly rich in the striatum, hippocampus, and prefrontal cortex (Lakics et al., 2010). PDE1 activity was first described as cytosolic; however, it now appears that PDE1A is not restricted to the cytosol but is also present in the nucleus where it contributes to the regulation of transcription factors (Nagel et al., 2006). This opens a new field of research in transcriptional regulation. Changes in PDE1 location associated with cell differentiation might contribute to compartmental signaling (Nagel et al., 2006).
Since PDE1B is strongly and selectively expressed in the striatum and frontal cortex, it could be a relevant target for modulating fronto-striatal behaviors. However, only a few studies have been published with PDE1-Is (Medina, 2011; Nunes et al., 2011). Indeed, as noted by the authors, in these studies no potent and selective inhibitors of PDE1 isoforms were available for research. Vinpocetine, often referred to as a PDE1-I, has substantial other activities, including inhibition of Na2+ channels and IκB kinase. Vinpocetine therefore should not be considered as a selective PDE1-I. Since PDE1B is activated by Ca2+ and CaM, it provides a mechanism for crosstalk between Ca2+ and cyclic nucleotide signaling (Nishi et al., 2008, 2011; Nishi and Snyder, 2010). PDE1B was localized to all DARPP-32-positive MSNs indicating expression in both striatal pathways (Nishi et al., 2011). Behavioral profiles of PDE1B knockout mice showed rather mild behavioral effects. The authors show an increased spontaneous locomotor activity in the presence of methamphetamine administration. Cognitive aspects are reported as similar to wild type. Overall, the data suggest predominant effects of PDE1B-Is would be seen in the striatonigral direct pathway (Reed et al., 2002; Ehrman et al., 2006; Siuciak et al., 2007; Zhang, 2010). However, regarding effects on frontal and striatal dopamine release or effects on cAMP/PKA signaling in the frontal cortex, these areas have so far been understudied.
Implications and Clinical Overview of PDE1B-Is
PDE1B is generally colocalized with dopamine D1 receptors in the brain and thought to represent a major inactivation mechanism of D1 receptors. PDE1B is not membrane bound but contained mainly in a soluble intracellular compartment (Fusco and Giampa, 2015). Targeting this subfamily of PDEs is therefore considered a promising therapeutic strategy in disorders characterized by frontal cognitive dysfunction, like schizophrenia and ADHD. Negative and cognitive symptoms of schizophrenia are associated with reduced dopamine (D1) function in the prefrontal cortex, also referred to as hypofrontality (Liemburg et al., 2012; Arnsten, 2013). The reduced prefrontal dopamine function disturbs the balance of excitatory to inhibitory synaptic interactions in this area (Winterer, 2006). Thus, the decreased ratio of D1/D2 signaling in schizophrenia would favor unstable cortical representation of internal and external stimuli (Winterer and Weinberger, 2004) and as such, affect cognition. Likewise, dopaminergic hypofrontality is observed in ADHD patients (Pliszka, 2005; Sagvolden et al., 2005; Arnsten and Pliszka, 2011) and linked to inattentiveness, hyperactivity, and impulsivity. By acting supposedly like a dopamine D1 agonist, a PDE1B-I can enhance phosphorylation of GluR1 subunits to potentiate glutamatergic fronto-striatal signaling. In addition, potentiation of dopamine receptors will increase phosphorylation of DARPP-32 and CREB, subsequently inducing gene expression in the prefrontal cortex and benefiting clinical symptoms by activation of neuroplasticity at prefrontal synapses.
Regarding striatal disorders like movement disorders and positive symptoms in schizophrenia, not much data is available. Assuming effects of PDE1B are indeed preferentially induced in the dopamine D1 direct pathway, subsequent DARPP-32 and CREB phosphorylation will enhance synaptic transmission, neuron excitability, and synapto-neurogenesis inducing neuroplasticity and neuroprotection at glutamatergic fronto-striatal synapses. In theory, neuropsychiatric disorders related to striatal hypofunction (like hypokinetic movement disorders such as Parkinson’s disease) would benefit from stimulated plasticity in striatonigral neurons (Nishino et al., 1993; Heckman et al., 2015). This is in contrast to the desired mechanism of action of treatment for hyperkinetic movement disorders (like Huntington’s disease) and antipsychotic treatment, as both preferably target the D2 receptor striatopallidal pathway (Strange, 1998; Walker, 2007). Support for this hypothesis comes from studies that found impaired cyclic nucleotide signaling mechanisms to occur in human Parkinson’s disease as well as in experimental animals (Belmaker et al., 1978; Volicer et al., 1986; Nishino et al., 1993; Sancesario et al., 2004). Additional upregulation in PDE1B activity has also been observed following 6-hydroxydopamine (6-OHDA) lesions (as a Parkinson’s model) in rats (Sancesario et al., 2004). The opposite has been observed in Huntington’s disease (Luthi-Carter et al., 2000; Nucifora et al., 2001). These studies found decreased cyclic nucleotide (cAMP) levels in the deafferented striatum accompanied by decreased PDE1B activity. The decreased PDE1B activity may be compensatory to the decrease in cyclic nucleotide levels. CREB-mediated transcriptional dysregulation has also been reported to occur during Parkinson’s disease pathology. PDE1-Is have been tested in an animal model of haloperidol-induced catalepsy. As haloperidol is a potent D2 dopamine receptor antagonist, interference with D2 dopamine receptors causes significant motor disturbances seen frequently with schizophrenics treated with antipsychotic medicines. The haloperidol-induced catalepsy model is capable of testing agents for exacerbation or lessening of these motoric effects. The potent and selective PDE1-I, ITI-214 (see below), reversed the haloperidol-induced catalepsy, indicating the potential use of this mechanism to reverse such motoric effects (Wennogle et al., 2010).
BDNF has been demonstrated to exert protective actions on nigral dopaminergic neurons in in vivo and in vitro models of Parkinson’s disease (Hyman et al., 1991; Levivier et al., 1995; Shults et al., 1995; Hung and Lee, 1996; Feng et al., 1999; Mohapel et al., 2005; Sun et al., 2005), whereas inhibition of nigral BDNF expression has been reported to cause dopaminergic neuronal loss (Porritt et al., 2005). Postmortem studies have demonstrated reduced levels of BDNF within the SNc in Parkinson’s disease patients (Mogi et al., 1999; Parain et al., 1999; Howells et al., 2000; Chauhan et al., 2001). Furthermore, during in vitro experiments, BDNF has been demonstrated to promote the survival and differentiation of mesencephalic dopaminergic neurons (Hyman et al., 1991; Feng et al., 1999). The enhancement of cerebral cyclic nucleotide levels by PDE1B inhibition would improve CREB-mediated signaling mechanisms providing therapeutic effects in Parkinson’s disease.
Recently, a set of 4 clinical studies were performed with a truly selective and potent PDE1-I, ITI-214 (Li et al., 2016a). With the exception of work performed with vinpocetine, a nonselective agent as noted above, ITI-214 is the first selective PDE1-I studied in humans. Clinical evaluations included a series of Phase I single- and multiple ascending-dose studies performed in the US and Japan. ITI-214 was given orally to healthy volunteers and patients using once-a-day dosing and was shown to be safe and well tolerated, with a linear pharmacokinetic profile. This study has been reported in a press release (Intra-Cellular Therapies, 2014), where the company concludes that “these studies represent a significant milestone as the first demonstration of the safety of a potent and highly specific PDE1-I in humans.”
PDE4
PDE4 and Dopamine Signaling
PDE4, which is cAMP specific, is encoded by 4 distinct genes in mammals, PDE4A, PDE4B, PDE4C, and PDE4D, and is expressed as at least 25 splice variants. Each of these variants has a modular structure consisting of a variant-specific N-terminal domain, regulatory domains (upstream conserved region 1 and 2 [UCR1 and UCR2]), a conserved catalytic domain, and an isoform-specific C-terminal domain (McCahill et al., 2008; Gurney et al., 2011; Richter et al., 2013). Transcription of a number of PDE4 genes is activated by the cAMP/PKA/CREB cascade (D’Sa et al., 2002; Le Jeune et al., 2002), and PKA induction of PDE4 genes serves as a long-term feedback mechanism. The N-terminal domain and UCR1/2 interact with variant-specific binding proteins to direct the subcellular targeting of PDE4 variants (McCahill et al., 2008). Various targeting proteins have been identified, including arrestin, AKAPS, receptor for activated C kinase 1, disrupted in schizophrenia 1, Src, and extracellular receptor kinase (ERK) (see Nishi and Snyder, 2010).
Nishi and colleagues (2008) showed that the inhibition of PDE4 by rolipram weakly enhanced cAMP/PKA signaling both in neostriatal slices and in vivo (Nishi et al., 2008). Rolipram increased the phosphorylation of DARPP-32 but only at high concentrations. Rolipram treatment enhanced adenosine A2a receptor-mediated phosphorylation of DARPP-32 but had no effect on D1 receptor/cAMP/PKA-mediated phosphorylation at the level of DARPP-32. Enhanced adenosine A2a receptor-mediated signaling is expected to oppose actions of the dopamine D2 receptor in striatopallidal neurons. These findings may suggest that PDE4 is exclusively expressed in indirect pathway neurons. However, immunohistochemical analysis of previously mentioned neostriatal slices revealed that PDE4B expression can be found in both pathways but with a higher expression in indirect pathway neurons. Regarding striatal dopaminergic signaling, it seems that the effect of PDE4 inhibition on cAMP/PKA signaling is linked to adenosine A2a receptor signaling and has no major role in striatal dopamine signaling. An opposite situation is observed at frontal dopaminergic signaling. In the frontal cortex, several PDE isoforms are expressed in cortical neurons (Cherry and Davis, 1999; Pérez-Torres et al., 2000). For the mouse frontal cortex, it has been described that PDE4B is localized at DARPP-32-expressing neurons (Nishi and Snyder, 2010). In contrast to the striatum, rolipram enhanced dopamine D1 receptor-induced phosphorylation of DARPP-32 in the frontal cortex, indicating a prominent role of PDE4 in frontal dopamine receptor signaling. Finally, dopamine is known to be expressed at dopaminergic terminals in neurons of the SNc (Cherry and Davis, 1999), where cAMP has been reported to be a strong inducer of tyrosine hydroxylase (TH) gene transcription rate and mRNA affecting dopamine synthesis (Kumer and Vrana, 1996; Chen et al., 2008). Rolipram enhanced haloperidol-induced phosphorylation of TH at Ser40 in presynaptic dopamine terminals with a proportional increase in dopamine synthesis though failed to do so in the absence of haloperidol. Also, rolipram enhanced levels of 3,4-Dihydroxyphenylacetic acid and the 3,4-Dihydroxyphenylacetic acid/dopamine ratio, indicating an increased dopamine metabolism. However, no increase in the level of dopamine itself was found, indicating the absence of a direct effect on dopamine release (Nishi et al., 2008).
Implications and Clinical Overview of PDE4-Is
Compared with PDE1, much more is known regarding the role of PDE4 in frontal and striatal dopaminergic functioning. However, by no means have all the effects been unraveled and all the questions been resolved for PDE4. PDE4 inhibition has been shown to increase dopaminergic tone in striatal neurons by increasing both synthesis and metabolism, though lacking a direct effect on release. It is known that basal ganglia functioning depends on specific amounts of dopamine in order to function at peak performance. Low levels of dopamine cause movement difficulties, while excessive dopamine causes involuntary movements. Even if PDE4 inhibition itself may not be involved in the process of releasing dopamine, the enhanced production of the dopamine precursor levodopa by enhanced cAMP-stimulated TH gene transcription may result in enhanced stimulus-driven dopamine release. PDE4-Is may therefore constitute an interesting treatment for neuropsychiatric disorders involving hypofunctioning striatal dopamine systems, like Parkinson’s disease. Indeed, rolipram has been reported to attenuate MPTP-induced dopamine depletion in the striatum and reduce the loss of nigral TH-positive neurons in vitro (Hulley et al., 1995a; Yamashita et al., 1997a, 1997b) and in vivo (Hulley et al., 1995b; Yang et al., 2008). Next, it remains to be seen if these effects on synthesis and metabolism also apply to frontal dopaminergic terminals arriving from the VTA. If so, PDE4 would be an interesting target for disorders characterized by frontal dopaminergic dysfunction, like ADHD or schizophrenia (cognitive and negative symptoms). Frontal dopaminergic hypofunctioning can not only be opposed by PDE4 inhibition via augmented release of neurotransmitters at dopaminergic terminals but also, like previously discussed for PDE1B, by means of increased DA D1 receptor/cAMP/PKA signaling inducing DARPP-32 and CREB phosphorylation, leading to concomitant gene transcription related to neuronal plasticity. Because PDE4 inhibition affects both these mechanisms, PDE4-Is are particularly interesting as a potential treatment for ADHD and schizophrenia. Finally, in the striatum, PDE4 inhibition regulates adenosine A2a signaling and is therefore often viewed as exerting dopamine D2 antagonistic effects, although it may also be viewed as mimicking the effect of an adenosine A2a agonist. Both would indicate antipsychotic potential of PDE4-Is by counteracting hyperdopaminergia, which has been confirmed by several studies over the years, including early clinical trials (Casacchia et al., 1983; Parkes et al., 1984; Siuciak, 2008). Based on results of rolipram, this latter mechanism is also applicable to Huntington’s disease. Rolipram exerted neuroprotective effects in 2 rodent Huntington’s disease models via increased CREB phosphorylation and subsequent targets like BDNF (DeMarch et al., 2007, 2008; Fusco and Giampa, 2015). Neuroprotective effects of rolipram were induced by sparing of striatal neurons, prevention of intranuclear inclusion formation, and attenuation of microglial reactivity (DeMarch et al., 2008). Furthermore, rolipram was effective in preventing CREB binding protein sequestration into striatal neuronal intranuclear inclusions, sparing interneurons of R6/2 mice and rescuing motor coordination and activity deficits (Giampa et al., 2009b; but see Hannan, 2009). However, there are a range of molecular and cellular mechanisms implicated in the pathogenesis of Huntington’s disease (Gil and Rego, 2008).
When examining actual clinical data, the first clinical trials into PDE4 were done in the field of depression research (Esposito et al., 2009). These first clinical studies showed a good antidepressant response to rolipram treatment (Zeller et al., 1984; Fleischhacker et al., 1992). However, rolipram produces severe dose-limiting side effects, including emesis, headache, gastric hyper secretion, nausea, and vomiting. This has put a serious hold on the further development of rolipram and other related PDE4-Is. It also prevented rolipram from reaching the market. Yet, a clinical Phase II trial started in 2006 to reevaluate the antidepressant properties of rolipram (estimated study completion date: December 2013). No details are yet available to the scientific community. Another PDE4-I, ND1251, was reported to improve memory in a group of 8 depressed subjects (Outsourcing Pharma, 2014).
Although rolipram was primarily developed for treating depression (Zeller et al., 1984; Fleischhacker et al., 1992), rolipram has also been investigated in early clinical trials as a treatment for Parkinson’s disease (Casacchia et al., 1983; Parkes et al., 1984). Some positive effects of rolipram were observed, however not exceeding the efficacy of levodopa or other dopaminergic drugs. At the moment, second-generation PDE4-Is are being developed, which are supposed to have less-emetic side effects and are being studied for other disorders besides depression. As a result, roflumilast was approved by the Food and Drug Administration (FDA) in 2011 as an antiinflammatory drug for the treatment of Chronic Obstructive Pulmonary Disease exacerbations.
PDE4-Is were also tested in clinical trials as treatment for schizophrenia. Takeda has recently finished a proof of mechanism Phase I clinical study with the PDE4-I roflumilast in combination with second-generation antipsychotics in schizophrenia patients (ClinicalTrials.gov Identifier: NCT02079844).
PDE4-Is were also examined as a treatment for Huntington’s disease. The new experimental PDE4-I GSK356278 was tested by GlaxoSmithKline as a new treatment for Huntington’s disease in 2 subsequent Phase I studies. In 2012, the first Phase I study was completed investigating the safety, tolerability, pharmacokinetics, and pharmacodynamics of GSK356278 (ClinicalTrials.gov Identifier: NCT01573819). GSK356278 was well tolerated when it was given as a single dose to healthy people, and in this study the objective was to observe effects of GSK356278 after daily intake. Subsequently, a second Phase I positron emission tomography brain occupancy study of GSK356278 was conducted in male healthy volunteers (ClinicalTrials.gov Identifier: NCT01602900; no results are disclosed). It is currently unclear whether this PDE4-I treatment is aimed at the motor or cognitive symptoms observed in Huntington’s disease.
Of note, Ibudilast (or AV-411) is another PDE4-I in development as an antiinflammatory drug to treat, for instance, Amyotrophic Lateral Sclerosis (ClinicalTrials.gov Identifier: NCT02238626). However, this compound not only inhibits PDE4 but also serves as a glial activator. Central nervous system applications of AV-411 are being explored in clinical Phase II studies, that is, pain and drug abuse (ClinicalTrials.gov Identifier: NCT00723177, NCT01217970, NCT02025998, NCT01860807).
Additionally, different genetic studies have shown a positive relationship between PDE4B polymorphisms and schizophrenia, which likely results in significantly decreased PDE4B levels as detected in postmortem brain tissue (Fatemi et al., 2008a; Guan et al., 2012). Low PDE4B levels, which might be considered as a compensatory mechanism, do not necessarily result in increased cAMP levels, as several mechanisms can also be activated that counteract the decreased degradation of cAMP by PDE4B. Another genetic link is related to the gene Disrupted-in Schizophrenia-1 (DISC1; Harrison and Weinberger, 2005). A chromosomal translocation of this gene increases susceptibility for schizophrenia (Millar et al., 2000; Sachs et al., 2005), and, interestingly, binding of DISC1 to PDE4B is disrupted, which might result in an overactivity of the latter (Millar et al., 2005; Murdoch et al., 2007).
PDE10
PDE10 and Dopamine Signaling
PDE10, which is encoded by PDE10A, is a dual substrate PDE, hydrolyzing both cAMP and cGMP. PDE10A is present both in striatonigral direct and striatopallidal indirect pathway MSNs (Xie et al., 2006; Nishi et al., 2008). Additionally, PDE10A regulates cAMP/PKA signaling (Nishi et al., 2008) and gene expression (Strick et al., 2010) in the MSNs of both pathways. Interestingly, PDE10A hydrolyzes both cAMP and cGMP, but it has an approximate 20-fold higher affinity for cAMP (Bender and Beavo, 2006), making it an interesting target for disorders involving the fronto-striatal circuits. In the striatum, PDE10A is expressed in both direct and indirect pathway MSNs, but not in interneurons (Xie et al., 2006; Nishi et al., 2008; Sano et al., 2008). Of the 3 splice variants, PDE10A2 is associated with the membrane, whereas PDE10A1 and PDE10A3 are found in the cytosol (Kotera et al., 2004). In the striatum, mainly PDE10A2 is expressed, and it is found at membranes in dendrites and spines of MSNs (Xie et al., 2006). PDE10A2 is phosphorylated by PKA at Thr16 within the N-terminal region (Kotera et al., 2004). Nishi and colleagues argue that this seems to induce the translocation of PDE10A2 from membrane to cytosol, thereby controlling cAMP/PKA signaling within the spines (Nishi and Snyder, 2010; see also Wilson and Brandon, 2015).
Through this effect on cAMP/PKA signaling, PDE10A inhibition by papaverine showed enhanced phosphorylation of CREB and ERK (Rodefer et al., 2005; Siuciak et al., 2006; Becker and Grecksch, 2008) and of their downstream targets DARPP-32 and GluR1 (Nishi et al., 2008) at PKA sites in striatal MSNs both in vitro and in vivo. More specifically, in both direct and indirect pathway neurons, PDE10A shows equal expression patterns (Xie et al., 2006; Nishi et al., 2008; Sano et al., 2008), regulation of cAMP/PKA signaling (Nishi et al., 2008), and gene expression (Strick et al., 2010). However, distinguishing between both pathways, in direct pathway neurons, PDE10A inhibition activates cAMP/PKA signaling related to D1 receptor signaling (Nishi et al., 2008), whereas in indirect pathway neurons, PDE10A inhibition activates cAMP/PKA signaling by simultaneous potentiation of adenosine A2A receptor signaling and inhibition of D2 receptor signaling. A study of neuronal type-specific regulation of DARPP-32 phosphorylation at Thr34 using neostriatal slices showed that papaverine increased DARPP-32 phosphorylation by 6-fold in indirect pathway neurons, whereas it increased DARPP-32 phosphorylation by only 2-fold in direct pathway neurons, indicating that effects of PDE10A inhibition predominate the indirect pathway (Bateup et al., 2008; Nishi et al., 2008). Recent electrophysiological results support this conclusion (Threlfell et al., 2009). More support is provided by recent behavioral studies published by different groups; however, these latter studies also show substantial D1 direct pathway effects of PDE10A-Is (Megens et al., 2014a, 2014b; Gentzel et al., 2015; Suzuki et al., 2016).
In vivo, PDE10A-Is are studied mostly for effects on spontaneous or stimulated behaviors providing evidence for predominant indirect pathway effects (similar to effects of D2 receptor blockers). This would include inhibition of spontaneous or stimulant-induced behavior, inhibition of conditioned avoidance behavior, reversal of stimulant-induced sensory gating deficits, and preferential activity against apomorphine-induced climbing (Schmidt et al., 2008; Grauer et al., 2009; Kehler and Nielsen, 2011; Gresack et al., 2013; Megens et al., 2014b). However, concomitant D1 receptor stimulation causes reduced efficiency against behavioral stimulants via direct pathway activation (Menniti et al., 2007; Sotty et al., 2009; Gresack et al., 2013; Megens et al., 2014b). D1 receptor stimulation is also responsible for the cognition-enhancing effects (Rodefer et al., 2005; Grauer et al., 2009) and socializing effects (Grauer et al., 2009) of PDE10A-Is. So indeed, there is compelling evidence for substantial direct pathway activation of PDE10A-Is. This latter notion is supported by recent work from Megens and coworkers (2014a) in suppressed behavior via D1 receptor blockade, D2 receptor blockade or dopamine depletion. Their results indicate that PDE10A-Is reverse behavioral suppression after D1 receptor blockade (hypolocomotion) via direct pathway activation (next to suppressing stimulant behavior via indirect pathway activation). These effects are indicative of substantial D1 agonistic effects of PDE10A-Is (next to their D2 antagonistic effects). Still, the main effects of PDE10A-Is are suggested to be exerted trough the indirect pathway. By this route, PDE10A-Is can cause extrapyramidal side effects, resembling D2 receptor blockers. The latter may explain why PDE10A-Is have not yet reached the market as antipsychotic treatment. This notion is supported by the recent failure of the Pfizer PDE10A-I MP-10 (or PF-02545920) in a Phase II clinical trial as antipsychotic treatment, where it showed no efficacy on positive and negative symptoms and produced motor side effects (akathisia and dystonia) in patients with schizophrenia (DeMartinis et al., 2012).
Finally, in contrast to PDE4 inhibition by rolipram, PDE10A inhibition by papaverine showed no increases on TH phosphorylation at Ser40 (PKA site), suggesting no effects of PDE10A inhibitors on dopamine synthesis. Of note, only at high concentrations did papaverine show an effect on TH phosphorylation. Also, results for papaverine should be confirmed by using the more potent PDE10A-Is TP-10 and MP-10. Additionally, PDE10A inhibition showed no effects on dopamine metabolism (Nishi et al., 2008). Therefore, in contrast to PDE4, it is assumed that PDE10A does not play a major role at dopaminergic terminals.
Implications and Clinical Overview of PDE10A-Is
PDE10A is even more extensively studied in relation to the fronto-striatal circuits than the previously discussed PDE4 and PDE1B subtypes. Due to the hypothesis of a higher expression in indirect pathway neurons, PDE10A-Is have received much attention as potential dopamine D2 antagonists and as such for their antipsychotic properties. Historically, positive symptoms in schizophrenia have been linked to overstimulation of dopamine receptors in the striatum (Baumeister and Francis, 2002), which is attenuated by (PDE10A inhibition-induced) dopamine D2 receptor antagonism. Because of the expected predominant effects in the indirect pathway, PDE10A is also hypothesized as a therapeutic target in Huntington’s disease. Increases in cAMP are expected to drive CREB-dependent signaling pathways, known to be dysregulated in Huntington’s disease mouse models (Choi et al., 2009). In line, like rolipram, TP-10 was shown to be neuroprotective in the quinolinic acid model of Huntington’s disease through CREB-mediated neuroprotection (Giampa et al., 2009a). In a follow-up study, PDE10A-I treatment of R6/2 mice showed significant delays in development of the motor deficits measured in this model accompanied by reduced striatal and cortical cell loss (Giampa et al., 2010). This was accompanied by increased CREB phosphorylation, suggesting that increased cAMP signaling in these brain regions could slow progression of neurodegeneration. Additionally, gene expression studies have implicated PDE10A and cAMP signaling as a therapeutic strategy for Huntington’s disease (Hebb et al., 2004). Chronic treatment of wild-type mice with TP-10 resulted in an increase in gene expression of members of the ERK and PKA signaling pathways as well as an increase in ERK and MSK phosphorylation (Roze et al., 2008; Kleiman et al., 2011; Martin et al., 2011). Both these effects have proven to be neuroprotective in models of Huntington’s disease. Hypothetically, in Parkinson’s disease, PDE10A-Is could be used in the same way to treat dopamine agonist- or levodopa-induced dyskinesias. Chronic treatment with both classes of drugs leads to improvement in symptoms but causes unwanted side effects. These unwanted symptoms are thought to be due to D1 receptor functional supersensitivity, abnormal cAMP signaling, and enhanced ERK signaling (Bezard et al., 2001; Aubert et al., 2005; Santini et al., 2007). Cyclic nucleotide levels were found to be decreased in the brains of rats treated with a combination of levodopa and 6-OHDA (Giorgi et al., 2008). Consistent with this finding, treatment of levodopa-induced dyskinesias with TP-10 reduced the severity of dyskinesias observed in 6-OHDA rats. In this way, PDE10A-Is rescue decreases in cyclic nucleotide levels and prolong the use of levodopa (Wilson and Brandon, 2015).
Preclinical antipsychotic effects of PDE10A-Is may have initiated fronto-striatal disorder-related research, though lack of clinical efficacy and possible extrapyramidal side effects are hampering PDE10A-Is in reaching the market as antipsychotic treatment. An example of the latter is provided by the failure of the Phase II clinical trial of the Pfizer PDE10A-I MP-10 (or PF-02545920). MP-10 showed no efficacy and produced motor side effects. Despite the serious challenges, there remains interest in PDE10A-Is as an antipsychotic treatment. For instance, Takeda is currently recruiting participants for a clinical Phase II study to evaluate the efficacy, safety, and tolerability of TAK-063 compared with placebo in treatment of acutely exacerbated schizophrenia. Efficacy was explained as determining whether cognitive impairment associated with schizophrenia would be attenuated (ClinicalTrials.gov Identifier: NCT02477020). Also, a Phase I study by Hoffmann-La Roche has just been completed in which the safety, tolerability, and pharmacokinetics of RO5545965 in patients with schizophrenia on risperidone was tested (no results have been posted; ClinicalTrials.gov Identifier: NCT02019329). Of note, in 2012 Amgen started and terminated a Phase I study to assess the safety and tolerability of their PDE10-I AMG 579 following a single oral dose administration in healthy subjects and patients with schizophrenia or stable schizoaffective disorder (ClinicalTrials.gov Identifier: NCT01568203).
A recent study found no difference in PDE10A mRNA expression between schizophrenia patients and comparison subjects in any of the brain regions studied (thalamus, caudate, putamen, nucleus accumbens, globus pallidus, and substantia nigra). This is the first in vivo assessment of PDE10A expression in patients with schizophrenia. However, this should not be interpreted as a case against developing PDE10A drugs in schizophrenia. The study of intracellular signaling pathways makes a persuasive case for how PDE10A-Is could influence the overall signaling in a therapeutic direction, regardless of whether there is an intrinsic change in PDE10A in schizophrenia (Marques et al., 2016).
Pharmaceutical companies have also started to redesignate their PDE10A-Is to Huntington’s disease. A Phase II proof-of-concept trial is now being initiated in which Pfizer’s PDE10A-I MP-10 will be tested for safety and efficacy in subjects with Huntington’s disease (ClinicalTrials.gov Identifier: NCT02197130). Omeros initiated a Phase II clinical trial in Huntington’s disease patients with OMS824 after an earlier Phase II trial in schizophrenia patients (no results disclosed; ClinicalTrials.gov Identifier: NCT01952132). The Huntington’s disease trial is a sequential-cohort dose escalation study that evaluates the safety and tolerability of OMS824 over 4 weeks (ClinicalTrials.gov Identifier: NCT02074410). In parallel with the clinical OMS824 trial, Omeros is conducting preclinical rat studies to support clinical trials of longer duration. However, based on that data, there might be a safety issue and based on follow-up communications with the FDA, Omeros has suspended the ongoing Huntington’s disease trial. The FDA has requested that Omeros further evaluates the preclinical data in order to characterize the compound more fully prior to reinitiating the clinical trial (Omeros, 2014). Additional support for the use of PDE10A-Is in Huntington’s disease comes from a recent study that shows that PDE10A levels are lowered early before symptom onset in Huntington’s disease (Niccolini et al., 2015b). Whether this is cause or consequence remains to be determined; however, it most likely resembles a consequence of the degeneration of striatal cells and therefore the PDE10A enzymes within. These results were recently confirmed by a study with the radioligand [18F] MNI-659A (Russell et al., 2016). A comparable large-scale Phase 0 study is currently recruiting new participants. The aim of this study is to measure the availability of the PDE10A enzyme in Huntington’s disease gene expansion carriers using the recently developed radioligand [18F] MNI-659. The study will be cross-sectional, examining Huntington’s disease gene expansion carriers at different stages of the disease (premanifest, stage 1, and stage 2) compared with healthy controls (ClinicalTrials.gov Identifier: NCT02061722).
Of note, Niccolini et al. (2015a) also demonstrated striatal and pallidal loss of PDE10A expression in Parkinson’s disease patients, which is associated with Parkinson’s disease duration and severity of motor symptoms and complications. These results suggest that dopaminergic nigrostriatal degeneration affects the expression of PDE10A in striatum and pallidum. Hypothesizing, it most likely resembles a compensatory mechanism. Less dopaminergic input from the SNc equals less cAMP activation in striatal and pallidal areas decreasing the required levels of PDE10A. In another, more implausible scenario, the decrease is causative. The decrease in PDE10A levels reflects the overall expression of PDE10A in these brain areas not specified for the direct and indirect pathway. Because of the stronger expression of PDE10A in the indirect pathway compared with the direct pathway, PDE10A degeneration will affect the indirect pathway more strongly. Subsequently, reduced PDE10A expression results in enhanced activation of the indirect pathway, resulting in increased inhibition of movement. In both Parkinson’s disease and Huntington’s disease, altered PDE10 levels are likely compensatory/consequential instead of causative. In hyperkinetic movement disorders like Huntington’s disease, PDE10A may thus be a promising target for pharmacological agents (PDE10A-Is enhance the little cAMP signaling that is left in the indirect pathway).
Conclusion
Clinical trials investigating the effects of PDE-Is in neuropsychiatric disorders are overall very sparse, and the wealth of positive preclinical data could not yet be translated into clinical efficacy. As a result, no definitive conclusions can be drawn merely based on clinical trial outcomes. Therefore, the current review provides a discussion of the role of PDEs in dopaminergic frontal and striatal signaling and the potential of their associated inhibitors in specific disorders of the fronto-striatal circuits. Subsequently, an overview is provided of the current clinical status.
The fronto-striatal circuits compose the neurobiological basis for several neuropsychiatric disorders, including Parkinson’s disease, Huntington’s disease, ADHD, Tourette’s syndrome, schizophrenia, and obsessive-compulsive disorder. The fronto-striatal circuits constitute a plurality of parallel segregated circuits, which can be clustered together in motor circuits, associative/cognitive circuits, and limbic circuits (Krack et al., 2010). Together, dysfunctions in these circuits produce the wide range of symptoms observed in related neuropsychiatric disorders.
Intracellularly, direct and indirect pathway signaling in the striatum is largely mediated through the cAMP/PKA cascade (Nishi et al., 2008, 2011; Nishi and Snyder, 2010). Cyclic nucleotide cascades are involved in synaptic transmission, neuron excitability, neuroplasticity, and neuroprotection in all types of fronto-striatal circuits (Figure 1). Additionally, all fronto-striatal circuits are modulated by dopamine. Next to the effects of cAMP/PKA pathways on glutamatergic and GABAergic signaling in the fronto-striatal circuits, these cyclic nucleotide pathways also play a major role in the dopaminergic modulation of the circuits. The intracellular effect of dopamine is mediated through dopamine receptor-regulated activation of cAMP/PKA and subsequent DARPP-32 and CREB phosphorylation in both striatal and frontal neurons.
In the last decades, PDEs have therefore received increased attention for their possible role in disorders involving the fronto-striatal circuits. Based on overall expression patterns in frontal and striatal dopaminergic terminals, indirect pathway neurons, and direct pathway neurons, PDE1B, PDE2A, PDE4, PDE7B, PDE9A, and PDE10A seem to be the most interesting targets (Lakics et al., 2010), although most attention and resources have thus far been devoted to the potential of PDE1B, PDE4, and PDE10A due to their role in dopaminergic signaling. The main site of action and expression of PDE1B, PDE4, and PDE10A as discussed in this clinical review is inferred from biochemical analyses of striatal cAMP/PKA effectors, behavioral phenotypes of knockout mice, and the observation of effects of subtype-specific PDE-Is on dopamine-related behavior. The different PDE subtypes, and more specifically their splice variants, can be related to different disorders due to their differential expression in one or more of the frontal and striatal pathways or dopaminergic terminals inducing stimulation of dopamine synthesis, the inhibtion of D2 receptor signaling or the stimulation of D1 receptor signaling. The different PDE isoforms contain a multiplicity of structural and biochemical properties and are located in specific subcellular compartments, with specific transcriptional and posttranscriptional regulation (Keravis and Lugnier, 2012). Therefore, expression of a PDE subtype in a brain area does not make it an interesting target per se. Their particular involvement in dopaminergic modulation of fronto-striatal signaling is what makes them an interesting target for related disorders. Preferably, the targeted cyclic nucleotide signaling cascade is involved in the pathology of the disorder or contributes to the reduction of the pathology. However, even if this is not the case, PDE inhibition could still influence the overall signaling in a therapeutic direction. Currently, researchers are just beginning to unravel the precise subcellular localization and the role of functional compartmentalization in physiological and pathological conditions of the fronto-striatal circuits (e.g., PDE10A: Russwurm et al., 2015; Li et al., 2016b; MacMullen et al., 2016). Another important consideration is that, in general, PDE-I research involving fronto-striatal disorders is based on the classical view of basal ganglia direct and indirect pathway functioning. Considerable evidence is accumulating to challenge this classical view (Cui et al., 2013; Calabresi et al., 2014; Keeler et al., 2014).
From a therapeutic perspective, inhibition of PDEs with increased expression appears most promising. This way, cognition and plasticity deficits resulting from impaired cAMP/PKA signaling might be improved by inhibiting specific PDE isoforms. However, PDE inhibition might have negative effects on cognition and plasticity when PDEs are already downregulated and cAMP levels and PKA activity are high. In this scenario, elevated cAMP levels might go over a physiological level and disrupt signaling. Along this line, high doses of rolipram impaired prefrontal cognitive function in aged but not young monkeys, likely due to overstimulation of the already disinhibited cAMP/PKA signaling pathway in the aged prefrontal cortex (Ramos et al., 2003; Arnsten et al., 2005). This argues to specifically target PDEs that are overexpressed (Table 2).
Table 2.
Changes in Human Phosphodiesterase mRNA Levels in Several Fronto-Striatal Disorders
| Schizophrenia | Parkinson’s Disease | Huntington’s Disease | ||||
|---|---|---|---|---|---|---|
| Cerebellum | Striatum | Pallidum | Striatum | Pallidum | Thalamus | |
| PDE1 | + (1C) |
NS | NS | NS | NS | NS |
| PDE2 | NS | NS | NS | NS | NS | NS |
| PDE3 | NS | NS | NS | NS | NS | NS |
| PDE4 | = (4A1, 4A5, 4A8) - (4B1, 4B2, 4B3, 4B4) |
NS | NS | NS | NS | NS |
| PDE5 | NS | NS | NS | NS | NS | NS |
| PDE6 | ||||||
| PDE7 | NS | NS | NS | NS | NS | NS |
| PDE8 | + (8B) |
NS | NS | NS | NS | NS |
| PDE9 | NS | NS | NS | NS | NS | NS |
| PDE10 | = (10A) |
- (10A) |
- (10A) |
- (10A) |
- (10A) |
+ (10A) |
| PDE11 | NS | NS | NS | NS | NS | NS |
+, increased, -, decreased, =, no change; NS, not studied; (Fatemi et al., 2008a, 2008b, 2010; Niccolini et al., 2015a, 2015b; Marques et al., 2016; Russell et al., 2016).
In addition to the backbone formed by frontal neurons, MSNs and their dopaminergic modulation, the importance of interneurons in physiological and pathological fronto-striatal functioning is becoming increasingly apparent. Several types of interneurons can be found in the striatum, like cholinergic and different GABAergic interneurons (Gerfen and Surmeier, 2011). In particular, nitric oxide synthase containing GABAergic interneurons we would like to highlight. These nitric oxide-producing interneurons play an important role in fronto-striatal functioning (West and Tseng, 2011). Nitric oxide diffuses from these interneurons into dendrites of MSNs that contain high levels of guanylate cyclase, which, when activated, lead to the synthesis of cGMP (Figure 1). In the intact striatum, transient elevations in intracellular cGMP primarily act to increase neuronal excitability and to facilitate glutamatergic fronto-striatal transmission (West and Tseng, 2011; Threlfell and West, 2013). Although the main focus in the fronto-striatal system has been on cAMP signaling, several PDE-Is (also) target cGMP (e.g., PDE1-Is and PDE10-Is) and may exert their effects (additionally) on the cGMP signaling cascade (Padovan-Neto et al., 2015).
Summarizing, increased understanding of the subcellular localization and unraveling of the signalosome concept of PDEs including its function and dysfunction in the fronto-striatal circuits will contribute to the design of new specific inhibitors and enhance the potential of PDE-Is as therapeutics in fronto-striatal circuits.
Statement of Interest
L.W. is an employee of Intra-Cellular Therapies, which has a financial interest in the PDE1 inhibitor ITI-214. M.A.v.D., A.B., and J.P. have a proprietary interest in the PDE4 inhibitor roflumilast.
Acknowledgments
PRAH is financially supported by the Human Enhancement and Learning (HEaL) initiative of Maastricht University.
References
- Alexander GE, Crutcher MD. (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. (2013) The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937–2003. Cereb Cortex 23:2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. (2011) Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav 99:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. (2005) Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med 11:121–128. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. (2005) Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister AA, Francis JL. (2002) Historical development of the dopamine hypothesis of schizophrenia. J Hist Neurosci 11:265–277. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G. (2008) Phosphodiesterase inhibitors--are they potential neuroleptic drugs? Behav Brain Res 186:155–160. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Ebstein RP, Biederman J, Stern R, Berman M, van Praag HM. (1978) The effect of L-dopa and propranolol on human CSF cyclic nucleotides. Psychopharmacology (Berl) 58:307–310. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520. [DOI] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE. (2001) Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci 2:577–588. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. (2006) Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci 26:5888–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. (2000) Dopamine and cAMP-regulated phosphoprotein 32kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci 20:8443–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022–1030. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Sutton MA. (2001) Molecular stepping stones in memory consolidation. Nat Neurosci 4:769–771. [DOI] [PubMed] [Google Scholar]
- Casacchia M, Meco G, Castellana F, Bedini L, Cusimano G, Agnoli A. (1983) Therapeutic use of a selective cAMP phosphodiesterase inhibitor (Rolipram) in Parkinson’s disease. Pharmacol Res Commun 15:329–334. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. (2001) Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat 21:277–288. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu L, Radcliffe P, Sun B, Tank AW. (2008) Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Mol Pharmacol 73:1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. (1999) Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol 407:287–301. [PubMed] [Google Scholar]
- Choi YS, Lee B, Cho HY, Reyes IB, Pu XA, Saido TC, Hoyt KR, Obrietan K. (2009) CREB is a key regulator of striatal vulnerability in chemical and genetic models of Huntington’s disease. Neurobiol Dis 36:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. (2013) Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Sa C, Tolbert LM, Conti M, Duman RS. (2002) Regulation of cAMP-specific phosphodiesterases type 4B and 4D (PDE4) splice variants by cAMP signaling in primary cortical neurons. J Neurochem 81:745–757. [DOI] [PubMed] [Google Scholar]
- DeMarch Z, Giampa C, Patassini S, Martorana A, Bernardi G, Fusco FR. (2007) Beneficial effects of rolipram in a quinolinic acid model of striatal excitotoxicity. Neurobiol Dis 25:266–273. [DOI] [PubMed] [Google Scholar]
- DeMarch Z, Giampa C, Patassini S, Bernardi G, Fusco FR. (2008) Beneficial effects of rolipram in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis 30:375–387. [DOI] [PubMed] [Google Scholar]
- DeMartinis N, Banerjee A, Kumar V, Boyer S, Schmidt C, Arroyo S. (2012) Poster #212 results of a phase 2a proof-of-concept trial with a PDE10A Inhibitor in the treatment of acute exacerbation of schizophrenia. Schizophr Res 136:S262. [Google Scholar]
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duinen MV, Reneerkens OA, Lambrecht L, Sambeth A, Rutten BP, Os JV, Blokland A, Prickaerts J. (2015) Treatment of cognitive impairment in schizophrenia: potential value of phosphodiesterase inhibitors in prefrontal dysfunction. Curr Pharm Des 21:3813–3828. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Williams MT, Schaefer TL, Gudelsky GA, Reed TM, Fienberg AA, Greengard P, Vorhees CV. (2006) Phosphodiesterase 1B differentially modulates the effects of methamphetamine on locomotor activity and spatial learning through DARPP32-dependent pathways: evidence from PDE1B-DARPP32 double-knockout mice. Genes Brain Behav 5:540–551. [DOI] [PubMed] [Google Scholar]
- Esposito K, Reierson GW, Luo HR, Wu GS, Licinio J, Wong ML. (2009) Phosphodiesterase genes and antidepressant treatment response: a review. Ann Med 41:177–185. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS. (2008. a) PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res 101:36–49. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Lee S. (2008. b) Phosphodiesterase-4A expression is reduced in cerebella of patients with bipolar disorder. Psychiatr Genet 18:282–288. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Vazquez G. (2010) Phosphodiesterase signaling system is disrupted in the cerebella of subjects with schizophrenia, bipolar disorder, and major depression. Schizophr Res 119:266–267. [DOI] [PubMed] [Google Scholar]
- Feng L, Wang CY, Jiang H, Oho C, Mizuno K, Dugich-Djordjevic M, Lu B. (1999) Differential effects of GDNF and BDNF on cultured ventral mesencephalic neurons. Brain Res Mol Brain Res 66:62–70. [DOI] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Orru M, Guitart X, Navarro G, Cortes A, Casado V, Canela EI, Lluis C, Franco R. (2011) Adenosine A2A receptors and A2A receptor heteromers as key players in striatal function. Front Neuroanat 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wolf R, Gerlach W, Jaklitsch H, Sastre-y-Hernandez M, et al. (1992) A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology 26:59–64. [DOI] [PubMed] [Google Scholar]
- Fusco FR, Giampa C. (2015) Phosphodiesterases as therapeutic targets for Huntington’s disease. Curr Pharm Des 21:365–377. [DOI] [PubMed] [Google Scholar]
- Gentzel RC, Toolan D, Roberts R, Koser AJ, Kandebo M, Hershey J, Renger JJ, Uslaner J, Smith SM. (2015) The PDE10A inhibitor MP-10 and haloperidol produce distinct gene expression profiles in the striatum and influence cataleptic behavior in rodents. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampa C, Laurenti D, Anzilotti S, Bernardi G, Menniti FS, Fusco FR. (2010) Inhibition of the striatal specific phosphodiesterase PDE10A ameliorates striatal and cortical pathology in R6/2 mouse model of Huntington’s disease. PLoS One 5:e13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampa C, Middei S, Patassini S, Borreca A, Marullo F, Laurenti D, Bernardi G, Ammassari-Teule M, Fusco FR. (2009. b) Phosphodiesterase type IV inhibition prevents sequestration of CREB binding protein, protects striatal parvalbumin interneurons and rescues motor deficits in the R6/2 mouse model of Huntington’s disease. Eur J Neurosci 29:902–910. [DOI] [PubMed] [Google Scholar]
- Giampa C, Patassini S, Borreca A, Laurenti D, Marullo F, Bernardi G, Menniti FS, Fusco FR. (2009. a) Phosphodiesterase 10 inhibition reduces striatal excitotoxicity in the quinolinic acid model of Huntington’s disease. Neurobiol Dis 34:450–456. [DOI] [PubMed] [Google Scholar]
- Gil JM, Rego AC. (2008) Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci 27:2803–2820. [DOI] [PubMed] [Google Scholar]
- Giorgi M, D’Angelo V, Esposito Z, Nuccetelli V, Sorge R, Martorana A, Stefani A, Bernardi G, Sancesario G. (2008) Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: new aspects in the pathogenetic mechanisms. Eur J Neurosci 28:941–950. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Pulito VL, Navarra RL, Kelly MP, Kelley C, Graf R, Langen B, Logue S, Brennan J, Jiang L, Charych E, Egerland U, Liu F, Marquis KL, Malamas M, Hage T, Comery TA, Brandon NJ. (2009) Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J Pharmacol Exp Ther 331:574–590. [DOI] [PubMed] [Google Scholar]
- Greengard P. (2001) The neurobiology of dopamine signaling. Biosci Rep 21:247–269. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23:435–447. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Seymour PA, Schmidt CJ, Risbrough VB. (2014) Inhibition of phosphodiesterase 10A has differential effects on dopamine D1 and D2 receptor modulation of sensorimotor gating. Psychopharmacology (Berl) 231:2189–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan F, Zhang C, Wei S, Zhang H, Gong X, Feng J, Gao C, Su R, Yang H, Li S. (2012) Association of PDE4B polymorphisms and schizophrenia in Northwestern Han Chinese. Hum Genet 131:1047–1056. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Burgin AB, Magnusson OT, Stewart LJ. (2011) Small molecule allosteric modulators of phosphodiesterase 4. Handb Exp Pharmacol:167–192. [DOI] [PubMed] [Google Scholar]
- Haber SN, Rauch SL. (2010) Neurocircuitry: a window into the networks underlying neuropsychiatric disease. Neuropsychopharmacology 35:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ. (2009) Towards a therapy for Huntington’s disease (Commentary on Giampa et al.). Eur J Neurosci 29:901. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68; image 45. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Robertson HA. (2008) PDEs as drug targets for CNS immune disorders. Curr Opin Investig Drugs 9:744–753. [PubMed] [Google Scholar]
- Hebb AL, Robertson HA, Denovan-Wright EM. (2004) Striatal phosphodiesterase mRNA and protein levels are reduced in Huntington’s disease transgenic mice prior to the onset of motor symptoms. Neuroscience 123:967–981. [DOI] [PubMed] [Google Scholar]
- Heckman PR, Blokland A, Ramaekers J, Prickaerts J. (2015) PDE and cognitive processing: beyond the memory domain. Neurobiol Learn Mem 119:108–122. [DOI] [PubMed] [Google Scholar]
- Houslay MD. (1995) Compartmentalization of cyclic AMP phosphodiesterases, signalling ‘crosstalk’, desensitization and the phosphorylation of Gi-2 add cell specific personalization to the control of the levels of the second messenger cyclic AMP. Adv Enzyme Regul 35:303–338. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. (2003) PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. (2007) cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res 100:950–966. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Milligan G. (1997) Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci 22:217–224. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA. (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166:127–135. [DOI] [PubMed] [Google Scholar]
- Hulley P, Hartikka J, Lubbert H. (1995. a) Cyclic AMP promotes the survival of dopaminergic neurons in vitro and protects them from the toxic effects of MPP+. J Neural Transm Suppl 46:217–228. [PubMed] [Google Scholar]
- Hulley P, Hartikka J, Abdel’Al S, Engels P, Buerki HR, Wiederhold KH, Muller T, Kelly P, Lowe D, Lubbert H. (1995. b) Inhibitors of type IV phosphodiesterases reduce the toxicity of MPTP in substantia nigra neurons in vivo. Eur J Neurosci 7:2431–2440. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EH. (1996) The mesolimbic dopaminergic pathway is more resistant than the nigrostriatal dopaminergic pathway to MPTP and MPP+ toxicity: role of BDNF gene expression. Brain Res Mol Brain Res 41:14–26. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–232. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Sawa A, Ichimaru Y, Miyashiro M, Kato S, Yamamoto T, Ueki S. (1997) Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol 321:273–278. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. (1996) Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron 16:973–982. [DOI] [PubMed] [Google Scholar]
- Intra-Cellular Therapies (2014) Products and technology: PDE inhibitor platform Available at http://www.intracellulartherapies.com/products-technology/pde-inhibitor-platform.html.
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. (2006) Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci 29:496–505. [DOI] [PubMed] [Google Scholar]
- Jurevicius J, Fischmeister R. (1996) cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci U S A 93:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler JF, Pretsell DO, Robbins TW. (2014) Functional implications of dopamine D1 vs. D2 receptors: A ‘prepare and select’ model of the striatal direct vs. indirect pathways. Neuroscience 282C:156–175. [DOI] [PubMed] [Google Scholar]
- Kehler J, Nielsen J. (2011) PDE10A inhibitors: novel therapeutic drugs for schizophrenia. Curr Pharm Des 17:137–150. [DOI] [PubMed] [Google Scholar]
- Keravis T, Lugnier C. (2012) Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol 165:1288–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman RJ, Kimmel LH, Bove SE, Lanz TA, Harms JF, Romegialli A, Miller KS, Willis A, des Etages S, Kuhn M, Schmidt CJ. (2011) Chronic suppression of phosphodiesterase 10A alters striatal expression of genes responsible for neurotransmitter synthesis, neurotransmission, and signaling pathways implicated in Huntington’s disease. J Pharmacol Exp Ther 336:64–76. [DOI] [PubMed] [Google Scholar]
- Kotera J, Sasaki T, Kobayashi T, Fujishige K, Yamashita Y, Omori K. (2004) Subcellular localization of cyclic nucleotide phosphodiesterase type 10A variants, and alteration of the localization by cAMP-dependent protein kinase-dependent phosphorylation. J Biol Chem 279:4366–4375. [DOI] [PubMed] [Google Scholar]
- Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. (2010) Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 33:474–484. [DOI] [PubMed] [Google Scholar]
- Kritzer MD, Li J, Dodge-Kafka K, Kapiloff MS. (2012) AKAPs: the architectural underpinnings of local cAMP signaling. J Mol Cell Cardiol 52:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. (1996) Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 67:443–462. [DOI] [PubMed] [Google Scholar]
- Kuroiwa M, Snyder GL, Shuto T, Fukuda A, Yanagawa Y, Benavides DR, Nairn AC, Bibb JA, Greengard P, Nishi A. (2012) Phosphodiesterase 4 inhibition enhances the dopamine D1 receptor/PKA/DARPP-32 signaling cascade in frontal cortex. Psychopharmacology (Berl) 219:1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakics V, Karran EH, Boess FG. (2010) Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59:367–374. [DOI] [PubMed] [Google Scholar]
- Le Jeune IR, Shepherd M, Van Heeke G, Houslay MD, Hall IP. (2002) Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5 in human airway smooth muscle cells. Identification and characterization of a novel PDE4D5 promoter. J Biol Chem 277:35980–35989. [DOI] [PubMed] [Google Scholar]
- Levivier M, Przedborski S, Bencsics C, Kang UJ. (1995) Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci 15:7810–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zheng H, Zhao J, Zhang L, Yao W, Zhu H, Beard JD, Ida K, Lane W, Snell G, Sogabe S, Heyser CJ, Snyder GL, Hendrick JP, Vanover KE, Davis RE, Wennogle LP. (2016. a) Discovery of potent and selective inhibitors of Phosphodiesterase 1 for the treatment of cognitive impairment associated with neurodegenerative and neuropsychiatric diseases. J Med Chem 59:1149–1164. [DOI] [PubMed] [Google Scholar]
- Li YW, Seager MA, Wojcik T, Heman K, Molski TF, Fernandes A, Langdon S, Pendri A, Gerritz S, Tian Y, Hong Y, Gallagher L, Merritt JR, Zhang C, Westphal R, Zaczek R, Macor JE, Bronson JJ, Lodge NJ. (2016. b) Biochemical and behavioral effects of PDE10A inhibitors: relationship to target site occupancy. Neuropharmacology 102:121–135. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, Knegtering H, Klein HC, Kortekaas R, Aleman A. (2012) Antipsychotic medication and prefrontal cortex activation: a review of neuroimaging findings. Eur Neuropsychopharmacol 22:387–400. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. (1999) Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci 19:10250–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, Ross CA, Borchelt DR, Tapscott SJ, Young AB, Cha JH, Olson JM. (2000) Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Hum Mol Genet 9:1259–1271. [DOI] [PubMed] [Google Scholar]
- MacMullen CM, Vick K, Pacifico R, Fallahi-Sichani M, Davis RL. (2016) Novel, primate-specific PDE10A isoform highlights gene expression complexity in human striatum with implications on the molecular pathology of bipolar disorder. Translational psychiatry 6:e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. (2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31:47–54. [DOI] [PubMed] [Google Scholar]
- Marques TR, Natesan S, Niccolini F, Politis M, Gunn RN, Searle GE, Howes O, Rabiner EA, Kapur S. (2016) Phosphodiesterase 10A in schizophrenia: a PET study using [C]IMA107. Am J Psychiatry. Advance online publication. doi: appiajp201515040518. [DOI] [PubMed] [Google Scholar]
- Martin E, Betuing S, Pages C, Cambon K, Auregan G, Deglon N, Roze E, Caboche J. (2011) Mitogen- and stress-activated protein kinase 1-induced neuroprotection in Huntington’s disease: role on chromatin remodeling at the PGC-1-alpha promoter. Hum Mol Genet 20:2422–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH. (2011) Subcellular signaling in the endothelium: cyclic nucleotides take their place. Curr Opin Pharmacol 11:656–664. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609. [DOI] [PubMed] [Google Scholar]
- McCahill AC, Huston E, Li X, Houslay MD. (2008) PDE4 associates with different scaffolding proteins: modulating interactions as treatment for certain diseases. Handb Exp Pharmacol:125–166. [DOI] [PubMed] [Google Scholar]
- Medina AE. (2011) Therapeutic utility of phosphodiesterase type I inhibitors in neurological conditions. Front Neurosci 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megens AA, Hendrickx HM, Mahieu MM, Wellens AL, de Boer P, Vanhoof G. (2014. a) PDE10A inhibitors stimulate or suppress motor behavior dependent on the relative activation state of the direct and indirect striatal output pathways. Pharmacol Res Perspect 2:e00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megens AA, Hendrickx HM, Hens KA, Fonteyn I, Langlois X, Lenaerts I, Somers MV, de Boer P, Vanhoof G. (2014. b) Pharmacology of JNJ-42314415, a centrally active phosphodiesterase 10A (PDE10A) inhibitor: a comparison of PDE10A inhibitors with D2 receptor blockers as potential antipsychotic drugs. J Pharmacol Exp Ther 349:138–154. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Chappie TA, Humphrey JM, Schmidt CJ. (2007) Phosphodiesterase 10A inhibitors: a novel approach to the treatment of the symptoms of schizophrenia. Curr Opin Investig Drugs 8:54–59. [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. (2000) Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9:1415–1423. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. (2005) DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310:1187–1191. [DOI] [PubMed] [Google Scholar]
- Minichiello L. (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850–860. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. (1999) Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett 270:45–48. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. (2005) Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience 132:767–776. [DOI] [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98:226–234. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. (2007) Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci 27:9513–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, Berk BC, Yan C. (2006) Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res 98:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolini F, Foltynie T, Reis Marques T, Muhlert N, Tziortzi AC, Searle GE, Natesan S, Kapur S, Rabiner EA, Gunn RN, Piccini P, Politis M. (2015. a) Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain 138:3003–3015. [DOI] [PubMed] [Google Scholar]
- Niccolini F, Haider S, Reis Marques T, Muhlert N, Tziortzi AC, Searle GE, Natesan S, Piccini P, Kapur S, Rabiner EA, Gunn RN, Tabrizi SJ, Politis M. (2015. b) Altered PDE10A expression detectable early before symptomatic onset in Huntington’s disease. Brain 138:3016–3029. [DOI] [PubMed] [Google Scholar]
- Nishino N, Kitamura N, Hashimoto T, Tanaka C. (1993) Transmembrane signalling systems in the brain of patients with Parkinson’s disease. Rev Neurosci 4:213–222. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Shuto T. (2011) Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. (2008) Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci 28:10460–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL. (2010) Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J Pharmacol Sci 114:6–16. [DOI] [PubMed] [Google Scholar]
- Nucifora FC, Jr, Sasaki M, Peters MF, Huang H, Cooper JK, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson VL, Dawson TM, Ross CA. (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291:2423–2428. [DOI] [PubMed] [Google Scholar]
- Nunes F, Ferreira-Rosa K, Pereira Mdos S, Kubrusly RC, Manhaes AC, Abreu-Villaca Y, Filgueiras CC. (2011) Acute administration of vinpocetine, a phosphodiesterase type 1 inhibitor, ameliorates hyperactivity in a mice model of fetal alcohol spectrum disorder. Drug Alcohol Depend 119:81–87. [DOI] [PubMed] [Google Scholar]
- Omeros (2014) Oremos provides update on PDE10 inhibitor program Available at http://investor.omeros.com/phoenix.zhtml?c=219263&p=irol-newsArticle_Print&ID=1979683.
- Outsourcing Pharma (2014) PDE4 re-emerges as depression therapy Available at http://www.outsourcing-pharma.com/Preclinical-Research/PDE4-re-emerges-as-depression-therapy.
- Padovan-Neto FE, Sammut S, Chakroborty S, Dec AM, Threlfell S, Campbell PW, Mudrakola V, Harms JF, Schmidt CJ, West AR. (2015) Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways. J Neurosci 35:5781–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R. (1999) Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 10:557–561. [DOI] [PubMed] [Google Scholar]
- Parkes JD, Thompson C, Brennan L, Gajraj N, Howcroft B, Ruiz J. (1984) Rolipram in Parkinson’s disease. Adv Neurol 40:563–565. [PubMed] [Google Scholar]
- Pérez-Torres S, Miró X, Palacios JM, Cortés R, Puigdoménech P, Mengod G. (2000) Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and [3H]rolipram binding autoradiography: Comparison with monkey and rat brain. J Chem Neuroanat 20:349–374. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. (2005) The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1385–1390. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, Batchelor PE, Howells DW. (2005) Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol 192:226–234. [DOI] [PubMed] [Google Scholar]
- Prickaerts J. (2015) Phosphodiesterase inhibitors. In: Encyclopedia of psychopharmacology (Stolerman I, ed), pp1306–1315. Berlin, Heidelberg: Springer. [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. (2003) Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron 40:835–845. [DOI] [PubMed] [Google Scholar]
- Reed TM, Repaske DR, Snyder GL, Greengard P, Vorhees CV. (2002) Phosphodiesterase 1B knock-out mice exhibit exaggerated locomotor hyperactivity and DARPP-32 phosphorylation in response to dopamine agonists and display impaired spatial learning. J Neurosci 22:5188–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Menniti FS, Zhang HT, Conti M. (2013) PDE4 as a target for cognition enhancement. Expert Opin Ther Targets 17:1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Murphy ER, Baxter MG. (2005) PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur J Neurosci 21:1070–1076. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Sihra TS. (2013) Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+-calmodulin and PKA in cerebrocortical synaptosomes. FEBS Lett 587:788–792. [DOI] [PubMed] [Google Scholar]
- Roze E, Betuing S, Deyts C, Marcon E, Brami-Cherrier K, Pages C, Humbert S, Merienne K, Caboche J. (2008) Mitogen- and stress-activated protein kinase-1 deficiency is involved in expanded-huntingtin-induced transcriptional dysregulation and striatal death. FASEB J 22:1083–1093. [DOI] [PubMed] [Google Scholar]
- Rubin CS. (1994) A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim Biophys Acta 1224:467–479. [PubMed] [Google Scholar]
- Russell DS, Jennings DL, Barret O, Tamagnan GD, Carroll VM, Caille F, Alagille D, Morley TJ, Papin C, Seibyl JP, Marek KL. (2016) Change in PDE10 across early Huntington disease assessed by [18F]MNI-659 and PET imaging. Neurology 86:748–754. [DOI] [PubMed] [Google Scholar]
- Russwurm C, Koesling D, Russwurm M. (2015) Phosphodiesterase 10A is tethered to a synaptic signaling complex in striatum. J Biol Chem 290:11936–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL. (2008) Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci 28:625–632. [DOI] [PubMed] [Google Scholar]
- Rutten K, Wallace TL, Works M, Prickaerts J, Blokland A, Novak TJ, Santarelli L, Misner DL. (2011) Enhanced long-term depression and impaired reversal learning in phosphodiesterase 4B-knockout (PDE4B-/-) mice. Neuropharmacology 61:138–147. [DOI] [PubMed] [Google Scholar]
- Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL. (2005) A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry 10:758–764. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. (2005) Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1239–1247. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancesario G, Giorgi M, D’Angelo V, Modica A, Martorana A, Morello M, Bengtson CP, Bernardi G. (2004) Down-regulation of nitrergic transmission in the rat striatum after chronic nigrostriatal deafferentation. Eur J Neurosci 20:989–1000. [DOI] [PubMed] [Google Scholar]
- Sano H, Nagai Y, Miyakawa T, Shigemoto R, Yokoi M. (2008) Increased social interaction in mice deficient of the striatal medium spiny neuron-specific phosphodiesterase 10A2. J Neurochem 105:546–556. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G. (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci 27:6995–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, Proulx-LaFrance C, Majchrzak MJ, Ramirez AD, Schmidt K, Seymour PA, Siuciak JA, Tingley FD III, Williams RD, Verhoest PR, Menniti FS. (2008) Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther 325:681–690. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Wardeh G, Mulder AH. (1985) Cyclic AMP facilitates the electrically evoked release of radiolabelled noradrenaline, dopamine and 5-hydroxytryptamine from rat brain slices. Naunyn Schmiedebergs Arch Pharmacol 330:74–76. [DOI] [PubMed] [Google Scholar]
- Scott Bitner R. (2012) Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics. Biochem Pharmacol 83:705–714. [DOI] [PubMed] [Google Scholar]
- Shults CW, Kimber T, Altar CA. (1995) BDNF attenuates the effects of intrastriatal injection of 6-hydroxydopamine. Neuroreport 6:1109–1112. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ, Jr, Shen Y. (1993) Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol 35:391–415. [DOI] [PubMed] [Google Scholar]
- Siuciak JA. (2008) The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 22:983–993. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, Shrikhande A, Wong S, Menniti FS, Schmidt CJ. (2006) Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology 51:386–396. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. (2007) Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology 53:113–124. [DOI] [PubMed] [Google Scholar]
- Song RS, Massenburg B, Wenderski W, Jayaraman V, Thompson L, Neves SR. (2013) ERK regulation of phosphodiesterase 4 enhances dopamine-stimulated AMPA receptor membrane insertion. Proc Natl Acad Sci U S A 110:15437–15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Montezinho LP, Steiniger-Brach B, Nielsen J. (2009) Phosphodiesterase 10A inhibition modulates the sensitivity of the mesolimbic dopaminergic system to D-amphetamine: involvement of the D1-regulated feedback control of midbrain dopamine neurons. J Neurochem 109:766–775. [DOI] [PubMed] [Google Scholar]
- Stangherlin A, Gesellchen F, Zoccarato A, Terrin A, Fields LA, Berrera M, Surdo NC, Craig MA, Smith G, Hamilton G, Zaccolo M. (2011) cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ Res 108:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangherlin A, Zaccolo M. (2012) cGMP-cAMP interplay in cardiac myocytes: a local affair with far-reaching consequences for heart function. Biochem Soc Trans 40:11–14. [DOI] [PubMed] [Google Scholar]
- Strange PG. (1998) Pathology and drug action in schizophrenia: insights from molecular biology. Essays Biochem 33:105–116. [DOI] [PubMed] [Google Scholar]
- Strick CA, James LC, Fox CB, Seeger TF, Menniti FS, Schmidt CJ. (2010) Alterations in gene regulation following inhibition of the striatum-enriched phosphodiesterase, PDE10A. Neuropharmacology 58:444–451. [DOI] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. (2005) Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Res 1052:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, Bargas J. (2011) Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 198:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Harada A, Suzuki H, Miyamoto M, Kimura H. (2016) TAK-063, a PDE10A inhibitor with balanced activation of direct and indirect pathways, provides potent antipsychotic-like effects in multiple paradigms. Neuropsychopharmacology. Advance online publication. doi: 10.1038/npp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Sammut S, Menniti FS, Schmidt CJ, West AR. (2009) Inhibition of phosphodiesterase 10A increases the responsiveness of striatal projection neurons to cortical stimulation. J Pharmacol Exp Ther 328:785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, West AR. (2013) Review: modulation of striatal neuron activity by cyclic nucleotide signaling and phosphodiesterase inhibition. Basal Ganglia 3:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volicer L, Beal MF, Direnfeld LK, Marquis JK, Albert ML. (1986) CSF cyclic nucleotides and somatostatin in Parkinson’s disease. Neurology 36:89–92. [DOI] [PubMed] [Google Scholar]
- Walker FO. (2007) Huntington’s disease. Lancet 369:218–228. [DOI] [PubMed] [Google Scholar]
- Wennogle LP, Snyder G, Li P, Vanover K, Davis R, Fienberg A, Hendricks J, Mates S. (2010) Enhancement of cognition in schizophrenia via inhibition of phosphodiesterase-1B and potentiation of dopamine D1 receptor signaling. Schizophrenia Res 117:489. [Google Scholar]
- West AR, Tseng KY. (2011) Nitric oxide-soluble guanylyl cyclase-cyclic GMP signaling in the striatum: new targets for the treatment of Parkinson’s Disease? Front Syst Neurosci 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LS, Baillie GS, Pritchard LM, Umana B, Terrin A, Zaccolo M, Houslay MD, Maurice DH. (2011) A phosphodiesterase 3B-based signaling complex integrates exchange protein activated by cAMP 1 and phosphatidylinositol 3-kinase signals in human arterial endothelial cells. J Biol Chem 286:16285–16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LS, Brandon NJ. (2015) Emerging biology of PDE10A. Curr Pharm Des 21:378–388. [DOI] [PubMed] [Google Scholar]
- Winterer G. (2006) Cortical microcircuits in schizophrenia--the dopamine hypothesis revisited. Pharmacopsychiatry 39:S68–71. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. (2004) Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 27:683–690. [DOI] [PubMed] [Google Scholar]
- Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, Menniti FS. (2006) Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Hayashi A, Baba J, Sawa A. (1997. a) Rolipram, a phosphodiesterase-4-selective inhibitor, promotes the survival of cultured rat dopaminergic neurons. Jpn J Pharmacol 75:155–159. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Miyashiro M, Baba J, Sawa A. (1997. b) Rolipram, a selective inhibitor of phosphodiesterase type 4, pronouncedly enhanced the forskolin-induced promotion of dopamine biosynthesis in primary cultured rat mesencephalic neurons. Jpn J Pharmacol 75:91–95. [DOI] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Lorenzo BJ, Beal MF. (2008) Attenuation of MPTP neurotoxicity by rolipram, a specific inhibitor of phosphodiesterase IV. Exp Neurol 211:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. (2000) A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol 2:25–29. [DOI] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. (1984) Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17:188–190. [DOI] [PubMed] [Google Scholar]
- Zhang HT. (2010) Phosphodiesterase targets for cognitive dysfunction and schizophrenia--a New York Academy of Sciences Meeting. IDrugs 13:166–168. [PubMed] [Google Scholar]