Abstract
Background:
Increasing evidence supports a role for appetite-regulating pathways like ghrelin, insulin, and leptin in alcoholism. We previously reported that intravenous (i.v.) exogenous ghrelin increases alcohol craving. We also reported i.v. ghrelin reduces endogenous serum leptin, whose levels, in turn, negatively correlated with alcohol craving. Exogenous ghrelin administration decreases insulin secretion both in vitro and in vivo experiments. This study tested the hypothesis that i.v. ghrelin may also decrease endogenous serum insulin levels in alcoholic individuals. Additionally, we explored possible correlations between serum insulin and alcohol craving, since a correlation between insulin and alcohol craving was previously reported.
Methods:
This was a double-blind, placebo-controlled human laboratory study (n=43). Non-treatment-seeking, alcohol-dependent, heavy drinkers were randomized to receive i.v. ghrelin or placebo, followed by an alcohol cue-reactivity procedure.
Results:
There was a main effect for i.v. ghrelin, compared to placebo in reducing serum insulin (P<.05). There was also a time effect (P<.001) but not ghrelin x time interaction (P>.05). We did not find a correlation between the reduction of serum insulin and alcohol craving (P>.05). The change in serum insulin was consistent with a parallel reduction in serum connective-peptide in the ghrelin group compared with placebo, although this difference did not reach statistical significance (P=.076). No similar effects were found for other glucose-regulating hormones analyzed i.e. glucagon, glucagon-like peptide-1, and gastric inhibitory peptide (Ps>.05).
Conclusions:
These findings indicate i.v. ghrelin administration has an effect on reducing serum insulin in alcohol-dependent individuals; however, the reduction of insulin did not correlate with changes in alcohol cue-elicited craving. We speculate that, unlike for leptin, the interactions between ghrelin and insulin relationship are limited at the peripheral level. However, mechanistic studies are needed to investigate this hypothesis.
Keywords: ghrelin, insulin, incretins, glucagon, leptin, alcohol craving, alcoholism
Introduction
Increasing evidence supports the role of appetite-regulating pathways in addiction (Leggio et al., 2011; Engel and Jerlhag, 2014). Among these, recent attention has been paid to the potential role of ghrelin in alcoholism. Ghrelin, a 28-amino acid (aa) residue peptide produced primarily by the stomach, plays a key role in increasing appetite, food intake, and reward (Skibicka and Dickson, 2013). Ghrelin also plays a role in alcohol reward, alcohol consumption, and other alcohol-seeking behaviors in rodents (for reviews, see Leggio, 2010; Engel and Jerlhag, 2014). A longitudinal clinical study revealed a positive correlation between blood ghrelin levels and both alcohol craving and risk of relapse in alcoholic patients (Leggio et al., 2012). More recently, our group provided direct clinical translational evidence of the role of ghrelin in alcohol craving in heavy-drinking alcoholic individuals by showing that i.v. administration of exogenous ghrelin acutely increased cue-induced alcohol craving (Leggio et al., 2014). Furthermore, both preclinical and clinical work on alcoholism has investigated the appetitive hormone leptin (Kiefer et al., 2001, 2005; Hillemacher et al., 2007; Haass-Koffler et al., 2015). Leptin has inverse physiological effects compared with ghrelin, for example, leptin decreases appetite, and reward, whereas ghrelin exhibits opposite roles on these behaviors (Elmquist et al., 1998; Traebert et al., 2002). Notably, an interaction (“cross-talk”) between ghrelin and leptin in regulating appetite and reward signaling has been described (Traebert et al., 2002; Haass-Koffler et al., 2015).
Insulin is a 51-aa peptide hormone secreted by the pancreatic β-cells together with the connective-peptide (C-peptide), a 31-aa residue cleaved off from proinsulin. It has been hypothesized that ghrelin may have a role in β-cell function disorders (for review, see Meyer, 2010). Ghrelin receptors are expressed in the human pancreatic β-cell (Granata et al., 2007). However, data on the role of ghrelin in insulin secretion are conflicting. For example, endogenous ghrelin acts on rat islet β-cells, via calcium signaling, and restricts glucose-induced insulin release (Dezaki et al., 2004). Exogenous ghrelin administration decreases insulin secretion both in vitro β-cell lines (Colombo et al., 2003, Wierup et al., 2004) and in vivo in the pancreas of animal models (Egido et al., 2002). However, ghrelin increases insulin secretion from glucose-incubated (glucose-stimulated condition) rat pancreatic islet cells (Date et al., 2002). Ghrelin increased insulin secretion from the pancreas of both normal and diabetic rat models (Adeghate and Ponery, 2002). The effects of ghrelin on insulin have not been extensively investigated yet in humans. Indeed, the few human data available are conflicting and limited to studies with healthy volunteers. Specifically, a significant reduction in serum insulin levels after an i.v. ghrelin injection was reported in 11 male healthy volunteers (Broglio et al., 2001), an observation not replicated in another study with 8 healthy subjects who received an i.v. ghrelin infusion (Lucidi et al., 2005). Finally, a recent study in 12 healthy volunteers indicated that i.v. ghrelin inhibited glucose-stimulated insulin secretion (Tong et al., 2010).
Preclinical studies have shown a role of insulin on dopamine-related reward processing (for review, see Figlewicz, 2003). In humans, while moderate alcohol consumption may be protective against the risk of diabetes (for review, see Liu et al., 2008)], heavy drinking has been associated with higher glucose levels, therefore increasing the risk of diabetes (Athyros et al., 2007; Leggio et al., 2009). In alcohol-dependent individuals, a significant positive correlation between insulin, C-peptide concentrations, and alcohol craving was previously described (Leggio et al., 2008).
The present study was aimed at investigating the effects of exogenous i.v. ghrelin administration on endogenous serum insulin levels in nontreatment-seeking, alcohol-dependent heavy drinkers. Consistent with previous work in healthy volunteers (Broglio et al., 2001; Tong et al., 2010), our hypothesis was that i.v. exogenous ghrelin administration acutely decreases endogenous serum insulin levels. Additionally, we also explored if changes in insulin levels correlated with alcohol craving, since we previously reported that insulin correlates with craving in alcohol-dependent individuals (Leggio et al., 2008). Since insulin and C-peptide are coproduced and cosecreted by the β-cells, we also measured the C-peptide to confirm our findings with insulin. Our hypothesis was that the results for C-peptide should be in the same direction as for those for insulin. Finally, to explore if the hypothesized effect of i.v. ghrelin on insulin was specific for insulin or nonspecific for other glucose-regulating hormones, we also measured glucagon, as well as the gastrointestinal derived incretin hormones glucagon-like peptide (GLP-1) and gastric inhibitory peptide (GIP).
Materials and Methods
Setting
This study was conducted at the Brown University Center for Alcohol and Addiction Studies, Providence, RI. The study was approved by the Brown University Institutional Review Board. The use of synthetic human ghrelin was approved under the FDA Investigational New Drug regulations (IND# 109,242). All participants signed an informed consent prior to participation.
Summary of the Parent Study
The parent study was a double-blind, placebo-controlled, randomized, proof-of-concept human laboratory study (ClinicalTrial.gov: NCT01190085). Participants were screened and enrolled using the DSM-IV diagnosis of alcohol dependence. According to the NIAAA guidelines, heavy drinking was defined as consuming on average ≥4 standard drinks/d for women or ≥5 standard drinks/d for men during the 90-day period before screening, using the Timeline Follow-Back. Individuals with diabetes or obesity (body mass index [BMI] >30kg/m2) were excluded from the study. Forty-five non-treatment-seeking, heavy drinking, alcohol-dependent individuals were randomized to three groups (Leggio et al., 2014). After obtaining a breath alcohol concentration of 0.00, an i.v. cannula was inserted in the nondominant arm. To avoid significant between-subject variability in the baseline levels of ghrelin and other hormones (e.g., insulin and leptin), a fixed light breakfast (~700 kJ; approximately 62% carbohydrate, 13% protein, and 25% fat) (Cummings et al., 2001; Vestergaard et al., 2007; Paulo et al., 2008) was served and consumed by all subjects. Thirty minutes after breakfast was consumed, exogenous ghrelin was administered i.v. over 10 minutes at a dose of either 3 mcg/kg, 1 mcg/kg, or 0 mcg/kg (placebo). Participants then underwent a cue-reactivity procedure, during which they were exposed to two trials, where each trial included neutral plus appetitive-control (juice) cues and then alcohol cues. Urge was measured by using either an Alcohol Visual Analogue Scale or a Juice Visual Analogue Scale (Rohsenow et al., 2000).
In brief, the parent study showed that there was a significant effect of ghrelin 3 mcg/kg vs placebo in increasing alcohol cue-elicited craving (P < .05). We also found a significant positive correlation between postinfusion blood ghrelin concentration and the increase in urge to drink alcohol (P < .05). No similar results were found for the juice craving (P > .05). For additional details, see (Leggio et al., 2014).
Blood Samples Analysis
Intravenous Ghrelin or placebo was infused as a 10-minute bolus. Blood samples for this analysis were collected at the following time points: at baseline (-15min), after the juice trial (+23min), the alcohol trial (+29min), and postexperiment (+48min). Blood samples were centrifuged and stored at −80°C. Serum insulin, C-peptide, glucagon, GLP-1, and GIP levels were determined using a fluorescent bead-based Bio-Plex assay (Bio-Rad, Hercules, CA). Results were expressed as pg/mL.
Data Analytic Strategy
Consistent with a previous analysis we recently reported where we investigated the effects of i.v. ghrelin on serum leptin levels (Haass-Koffler et al., 2015), we collapsed the two i.v. ghrelin groups (1 mcg/kg and 3 mcg/kg) and compared them with placebo. Additionally, we included in the analyses only the blood samples collected at baseline (-15min), during the second juice and alcohol trials (+23 and +29min), and before discharge (+48min). In fact, consistent with our parent study (Leggio et al., 2014), the previous analysis we recently reported (Haass-Koffler et al., 2015) and with other human laboratory studies (Monti et al., 1999) the cue-reactivity procedure indicated stronger elicited craving response during the second alcohol trial.
Serum hormones levels were normalized (Sloan et al., 2011) to reduce individual and gender-related variability (Werner et al., 2008), evaluated as the change from baseline, and expressed as means (M) and standard errors (SEMs). Serum insulin had a skewness and kurtosis slightly in excess; consequently, the data were transformed using log transformation to improve the nature of the distribution. We included all the available data points within the standardized curves. Outliers were taken into account consistent with previous recommendations (Tabachnick and Fidell, 2001). The statistical method used was repeated-measures ANOVA. The delta (Δ) from baseline was used to evaluate potential correlations with craving scores (Alcohol Visual Analogue Scale or Juice Visual Analogue Scale scales) via regression analysis using Pearson’s correlation coefficient. This latter analysis was only conducted with the hormones for which a significant i.v. ghrelin effect was detected. All statistical tests were 2-sided, and statistical significance was accepted if an alpha value P <.05 was obtained. SPSS (v.22) (Armonk, NY) was used to conduct the analysis and GraphPad Prism (v.5) was used to generate figures (La Jolla, CA).
Results
Description of the Sample
Demographics and baseline characteristics are described in the parent study (Leggio et al., 2014). The serum insulin values of two participants were outside the standard curve and were excluded from this analysis, therefore leaving a sample of 43 subjects. There was no significant difference in the demographics and basal serum ghrelin levels between groups in the parent study and the sample utilized for this analysis. We found no correlation between BMI and baseline serum insulin, C-peptide, glucagon, GLP-1, and GIP levels (Ps > .05).
Endogenous Serum Insulin and C-Peptide Levels After Exogenous Ghrelin i.v. Infusion
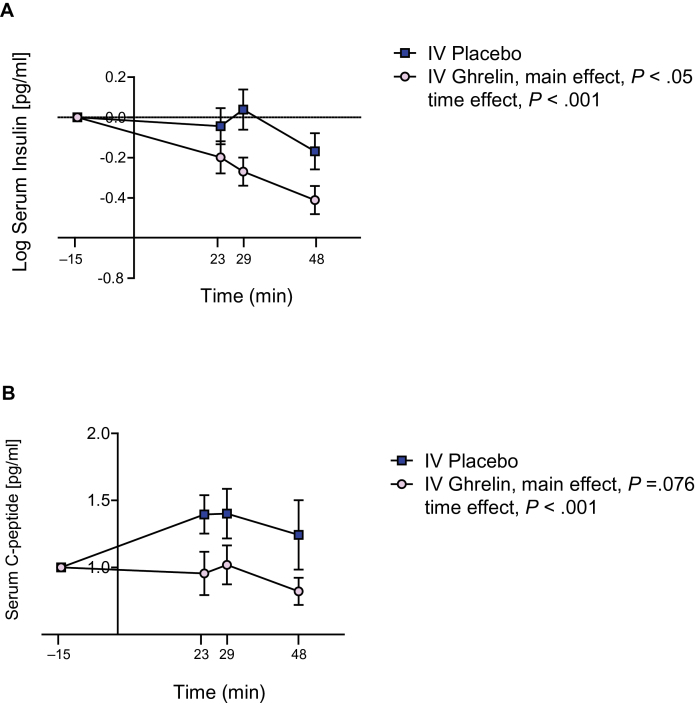
Serum insulin values in the two groups (i.v. ghrelin vs placebo) were normalized, log-transformed to produce a normal distribution, and evaluated as change from baseline (Figure 1A). There was a main effect for i.v. ghrelin administration, compared with placebo, in reducing serum insulin levels (F 1,42 = 4.15, P < .05). There was also a significant time effect (F 1,42 = 10.88, P < .001) but no significant ghrelin by time interaction for this outcome (P > .05). Serum C-peptide values in the two groups (i.v. ghrelin vs placebo) were normalized and evaluated as change from baseline (Figure 1B). There was a trend main effect for i.v. ghrelin administration in reducing serum C-peptide levels (F 1,36 = 3.34, P =.076). There was also a significant time effect (F 1,36 = 9.23, P < .001) but no significant ghrelin by time interaction for this outcome (P > .05).
Figure 1.
Changes in endogenous serum insulin and C-peptide levels after i.v. infusion of exogenous ghrelin vs placebo. (A) There was a significant main effect for i.v. ghrelin administration, compared with placebo, in reducing serum insulin levels (F1,42 = 4.15, P < .05). There was also a significant time effect (F1,42 = 10.88, P < .001) but no significant ghrelin by time interaction for this outcome (P >.05). (B) There was a trend main effect for i.v. ghrelin administration compared with placebo in reducing serum C-peptide levels (F1,36 = 3.34, P =.076) and a significant time effect on reduced serum insulin levels (F1,36 = 9.23, P < .001), but no significant ghrelin by time interaction for this outcome (P >.05). All data are reported M ± SEM, P>.05, NS, not significant.
Endogenous Serum Glucagon, GLP-1, and GIP Levels After Exogenous i.v. Ghrelin Infusion
Serum glucagon, GLP-1, and GIP values were normalized to be distributed on the same scale and evaluated as change from baseline. There was a significant time effect on glucagon (F 1,40 = 9.13, P <.001), but no significant ghrelin effect or ghrelin by time interaction (Ps >.05). Finally, there were no main i.v. ghrelin administration or time effects nor i.v. ghrelin administration by time interactions for either GLP1 or GIP (Ps >.05).
Relationship between Serum Insulin Levels and Urges to Drink Alcohol or Juice
There was no significant correlation between the change from baseline in serum insulin levels and the change from baseline in the urge to drink alcohol or urge to drink juice (Ps >.05).
Relationship between Serum Ghrelin and Endogenous Serum Insulin Levels
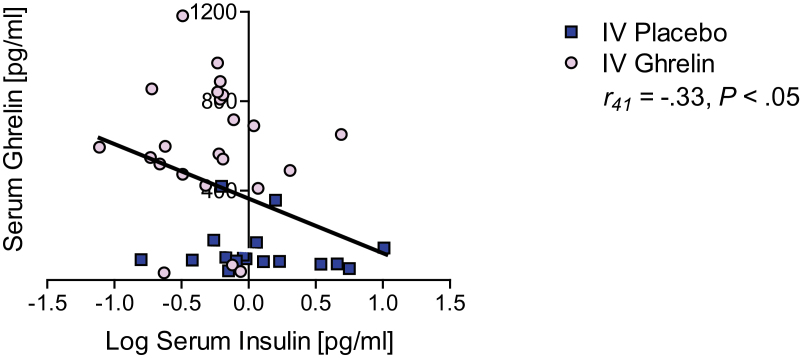
Finally, we assessed whether there was a correlation between serum ghrelin and endogenous insulin levels at the time of the alcohol trial. We found a negative correlation between serum ghrelin and insulin levels at the time of the alcohol trial (r 41 = -.33, P < .05) (Figure 2).
Figure 2.
Relationship between endogenous serum ghrelin and insulin levels relationship after exogenous i.v. ghrelin infusion. We found a negative correlation between serum ghrelin and endogenous insulin levels (r41 = -.33, P < .05) at the time of the alcohol trial. All data are reported M ± SEM, P>.05, NS, not significant.
Relationship between Endogenous Serum Insulin and Leptin Levels After Exogenous i.v. Ghrelin Infusion
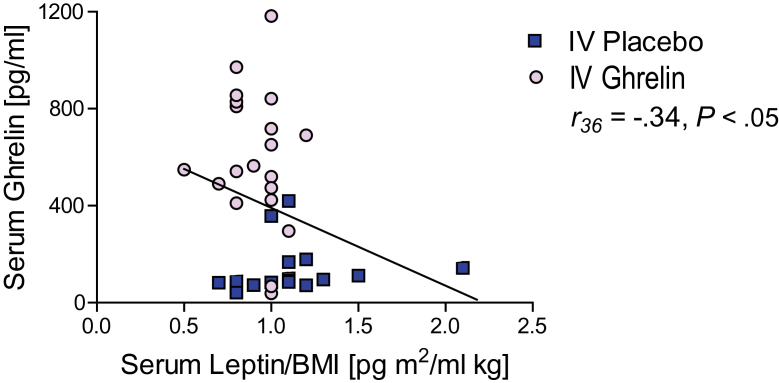
In our previous study (Haass-Koffler et al., 2015), we had reported that i.v. ghrelin, compared with placebo, significantly reduced endogenous leptin levels. Based on the findings reported above, where i.v. ghrelin reduced endogenous insulin levels and insulin inversely correlated with serum ghrelin levels, we further analyzed the leptin-related data. This additional analysis also revealed a significant negative correlation between serum ghrelin and leptin levels at the time of the alcohol trial (r 36 = -.34, P < .05) (Figure 3). As such, our final step in this study was to analyze potential correlations between serum insulin and leptin levels at the time of the alcohol trial. We did not find, however, a correlation between serum insulin and leptin levels at the time of the alcohol trial (P > .05).
Figure 3.
Relationship between endogenous serum leptin and insulin levels relationship after exogenous i.v. ghrelin infusion. We found a negative correlation between serum endogenous leptin and insulin levels (r36 = -.34, P < .05) at the time of the alcohol trial. All data are reported M ± SEM, P>.05, NS, not significant.
Discussion
In this study, consistent with our hypothesis, i.v. ghrelin administration, compared with placebo, significantly decreased serum insulin levels. This is the first study in which the effects of exogenous i.v. ghrelin administration on endogenous serum insulin levels were assessed in heavy-drinking alcohol dependent individuals.
Previous work shows an effect of i.v. ghrelin in reducing serum insulin levels (Broglio et al., 2001; Tong et al., 2010). However, that study was conducted in healthy volunteers, while here we employed alcohol-dependent heavy drinkers, a population with several associated metabolic disorders (Kim and Kim, 2012). While we specifically excluded individuals with obesity (i.e., BMI > 30) and/or diabetes, it was important to test whether the findings observed in healthy volunteers replicated in this population of alcohol-dependent heavy drinkers. Although we did not obtain blood glucose levels during the experiment, it is conceivable that, as insulin levels decreased during the cue-reactivity experiment, by contrast glucose levels increased. Our pharmacological challenge with i.v. ghrelin was hypothesized to increase alcohol craving, as we in fact observed and previously reported (Leggio et al., 2014). Additionally, ghrelin administration increases alcohol-seeking behaviors in rodents, as reported by independent laboratories using different animal models (reviewed in Leggio, 2010; Engel and Jerlhag, 2014). As such, we speculate that hyperglycemic states (including but not limited to states of hyperghrelinemia) may be associated with increased drinking behaviors. Consistent with this hypothesis, we previously reported that blood glucose levels correlated with heavy drinking levels in alcohol-dependent heavy drinkers (Leggio et al., 2009). However, this conclusion is only speculative and is limited by the fact that blood glucose levels were not available in this experiment and by the fact that we only studied the acute effects of i.v. ghrelin; therefore, our results cannot be generalized to a chronic condition of heavy drinking. Nonetheless, it is important to note that, within the endocrine pathways investigated here, our results suggest a potential specific effect of ghrelin on insulin. In fact, the i.v. ghrelin administration effect on reducing serum insulin levels were corroborated by a parallel, yet not statistically significant, reduction in the serum C-peptide, the endopeptide cleaved off from pro-insulin. By contrast, similar results were not found for glucagon, GLP-1, or GIP. The reason why the reduction in serum C-peptide levels, unlike that for serum insulin, did not reach statistical significance may be due to the short duration of i.v. ghrelin administration and to the C-peptide half-life (t 1/2 = 30 minutes), which is significantly longer compared with the insulin half-life (t 1/2 = 5 minutes). As such, i.v. ghrelin infusion may not have affected C-peptide levels as fast as insulin serum level.
Our parent study (Leggio et al., 2014) showed that i.v. ghrelin administration resulted in an acute increase in cue-induced urge to drink alcohol. During the experiment, there was also a significant positive correlation between the postinfusion serum ghrelin levels and the increase in urge to drink alcohol. In this analysis, while i.v. ghrelin reduced serum endogenous insulin levels, the reduction in insulin did not correlate with the increase in alcohol craving. These results are different compared to previous findings we reported with leptin. In fact, in the sample here investigated, i.v. ghrelin acutely decreased endogenous serum leptin levels, and changes in leptin levels negatively correlated with alcohol craving (Haass-Koffler et al., 2015). In other words, i.v. ghrelin reduced both leptin (Haass-Koffler et al., 2015) and insulin (present study), and postinfusion serum ghrelin levels were inversely correlated with both leptin and insulin levels. However, only leptin significantly correlated with the increased alcohol craving during the cue-reactivity experiment. Consistent with this observation, we found no relationship between serum insulin and leptin levels in this study. Additionally, the observation that, in our sample, the decrease of serum insulin levels did not correlate with the decrease of serum leptin levels further suggests that the changes in serum insulin were driven exclusively by the i.v. ghrelin administration, and serum leptin and insulin in alcohol-dependent individuals do not act together as homeostatic messengers as seen in previous studies with healthy individuals (Hallschmid et al., 2004).
Mechanistic studies are needed in order to shed light on the interactions of ghrelin with both insulin and leptin. In fact, our results are based on correlations and do not establish causality. Additionally, it is important to highlight that, while a previous study from our group reported a significant positive correlation between craving and insulin (Leggio et al., 2008), here we did not study the relationship of craving with physiological insulin per se. Rather, we investigated insulin serum levels in the context of an i.v. ghrelin challenge. Therefore our results on insulin and craving do not generalize outside the context of a pharmacologically induced placebo-controlled status of hyperghrelinemia. Additionally, craving measurements were different between the two studies. In the previous study, craving was measured using a retrospective measure, while here we employed a human laboratory paradigm where craving was measured in real time during an alcohol cue-reactivity procedure.
This study has several strengths: (1) it was the first human research to evaluate insulin response to an i.v. ghrelin infusion, compared with placebo, in heavy-drinking alcohol dependent individuals; (2) the ghrelin-insulin relationship was evaluated in a strict and well-controlled environment (i.e., real time cue-elicited craving and controlling for alcohol, food, and smoking); (3) i.v. ghrelin dose was calculated based on weight; and (4) normalization of serum insulin at baseline allowed us to account for individual variations in peripheral insulin levels, including sex-related differences. Limitations of this research study are also important to consider and include: (1) the lack of blood glucose levels during the experiment; (2) the small sample; and (3) the short duration of i.v. ghrelin administration. An additional consideration is that juice and alcohol cues were presented in a fixed order, which may be seen as a limitation. However, the use of a fixed order was done to allow for the most conservative assessment of alcohol cue-reactivity (Monti et al., 1987; Monti et al., 1999; Rohsenow et al., 2000), as previous studies reported a general lowering of cue-reactivity to any stimulus presented second (Monti et al., 1987). Finally, while a visual analogue scale was used to measure craving for alcohol, future larger studies might also employ alcohol craving questionnaires able to identify different phenotypes (Martinotti et al., 2013)
In spite of these important limitations, this study adds important novel information to the current literature as it represents the first human study to investigate the relationship between ghrelin and insulin after a pharmacological challenge with i.v. ghrelin in a clinically important population of alcohol-dependent heavy drinking individuals.
Statement of Interest
Dr. Swift has received travel and honorarium from D&A Pharma, Lundbeck and consultant fees from CT Laboratories. The other authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
The authors acknowledge the technical support from Samuel Fricchione, BA, and the nursing support from Tamara Sequeira, RN, and Julia Nadeau, RN, at the Brown University Center for Alcohol and Addiction Studies; and the technical support from Valerie Zabala, BS, and Ming Tong, MD, at Rhode Island Hospital and Chetram Deochand and Rosa Yu at Brown University. The authors also thank Karen Smith, National Institutes of Health (NIH) Library for bibliographic assistance.
This work was supported by the National Institutes of Health intramural funding (grant no. ZIA-AA000218; Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, to L.L.), jointly supported by the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research; and the National Institute on Drug Abuse Intramural Research Program. The parent study was funded by the National Institute on Alcohol Abuse and Alcoholism (grant no. R21-AA019709 to L.L. while at Brown University). Dr. Haass-Koffler’s current work is supported by the National Institute on Alcohol Abuse and Alcoholism (grant no. K01AA023867 to C.L.H.-K.) and previously by the National Institute on Alcohol Abuse and Alcoholism (training grant 5T32AA007459-28 to C.L.H.-K.).
References
- Adeghate E, Ponery AS. (2002) Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol 14:555–560. [DOI] [PubMed] [Google Scholar]
- Athyros VG, Liberopoulos EN, Mikhailidis DP, Papageorgiou AA, Ganotakis ES, Tziomalos K, Kakafika AI, Karagiannis A, Lambropoulos S, Elisaf M. (2007) Association of drinking pattern and alcohol beverage type with the prevalence of metabolic syndrome, diabetes, coronary heart disease, stroke, and peripheral arterial disease in a Mediterranean cohort. Angiology 58:689–697. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Bruning JC. (2010) CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 1212:97–113. [DOI] [PubMed] [Google Scholar]
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. (2001) Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86:5083–5086. [DOI] [PubMed] [Google Scholar]
- Colombo M, Gregersen S, Xiao J, Hermansen K. (2003) Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas 27:161–166. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S. (2002) Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 51:124–129. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Benoit SC. (2010) Insulin, leptin and reward. Trends Endocrinol Metab 21:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. (2004) Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 53:3142–3151. [DOI] [PubMed] [Google Scholar]
- Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. (2002) Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146:241–244. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. (1998) Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1:445–450. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E. (2014) Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS drugs 28:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP. (2003) Insulin, food intake, and reward. Semin Clin Neuropsychiatry 8:82–93. [DOI] [PubMed] [Google Scholar]
- Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghe C, Isgaard J, Papotti M, Ghigo E, Muccioli G. (2007) Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3’,5’-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology 148:512–529. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Aoun EG, Swift RM, de la Monte SM, Kenna GA, Leggio L. (2015) Leptin levels are reduced by intravenous ghrelin administration and correlated with cue-induced alcohol craving. Transl Psychiatry 5:e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. (2004) Intranasal insulin reduces body fat in men but not in women. Diabetes 53:3024–3029. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Bleich S, Frieling H, Schanze A, Wilhelm J, Sperling W, Kornhuber J, Kraus T. (2007) Evidence of an association of leptin serum levels and craving in alcohol dependence. Psychoneuroendocrinology 32:87–90. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D, Wiedemann K. (2001) Leptin: a modulator of alcohol craving? Biol Psychiatry 49:782–787. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Demiralay C, Wolf K, Wiedemann K. (2005) Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J Psychiatr Res 39:545–551. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim DJ. (2012) Alcoholism and diabetes mellitus. Diabetes Metab J 36:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Malandrino N, Miceli A, Capristo E, Gasbarrini G, Addolorato G. (2008) Insulin but not insulin growth factor-1 correlates with craving in currently drinking alcohol-dependent patients. Alcohol Clin Exp Res 32:450–458. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ray LA, Kenna GA, Swift RM. (2009) Blood glucose level, alcohol heavy drinking, and alcohol craving during treatment for alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Alcohol Clin Exp Res 33:1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, Swift RM, Addolorato G. (2012) Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 17:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L. (2010) Role of the ghrelin system in alcoholism: acting on the growth hormone secretagogue receptor to treat alcohol-related diseases. Drug News Perspect 23:157–166. [DOI] [PubMed] [Google Scholar]
- Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov-Polevoy AB, Swift RM. (2011) Role of feeding-related pathways in alcohol dependence: a focus on sweet preference, NPY, and ghrelin. Alcohol Clin Exp Res 35:194–202. [DOI] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA. (2014) Intravenous Ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang Y, Lam KS, Xu A. (2008) Moderate wine consumption in the prevention of metabolic syndrome and its related medical complications. Endocr Metab Immune Disord Drug Targets 8:89–98. [DOI] [PubMed] [Google Scholar]
- Lucidi P, Murdolo G, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Taglioni C, Fanelli C, Broglio F, Ghigo E, Bolli GB, Santeusanio F, De Feo P. (2005) Metabolic and endocrine effects of physiological increments in plasma ghrelin concentrations. Nutr Metab Cardiovasc Dis 15:410–417. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Callea A, Di Giannantonio M, Janiri L, Craving Study G (2013) Craving Typology Questionnaire (CTQ): a scale for alcohol craving in normal controls and alcoholics. Compr Psychiatry 54:925–932. [DOI] [PubMed] [Google Scholar]
- Meyer C. (2010) Final answer: ghrelin can suppress insulin secretion in humans, but is it clinically relevant? Diabetes 59:2726–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. (1987) Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol 96:122–126. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. (1999) Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcoholism, clinical and experimental research 23:1386–1394. [PubMed] [Google Scholar]
- Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD. (2008) Estrogen elevates the peak overnight production rate of acylated ghrelin. J Clin Endocrinol Metab 93:4440–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. (2000) Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol 109:738–742. [PubMed] [Google Scholar]
- Skibicka KP, Dickson SL. (2013) Enteroendocrine hormones: central effects on behavior. Curr Opin Pharmacol 13:977–982. [DOI] [PubMed] [Google Scholar]
- Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, Sempokuya T, Alegria L, Bugger H, Abel ED. (2011) Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes 60:1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. (2001) Using multivariate statistics. Boston, MA: Allyn and Bacon. [Google Scholar]
- Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D. (2010) Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59:2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. (2002) Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol 14:580–586. [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, Jorgensen JO. (2007) Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab 292:E1829–1836. [DOI] [PubMed] [Google Scholar]
- Werner M, Tonjes A, Stumvoll M, Thiery J, Kratzsch J. (2008) Assay-dependent variability of serum insulin levels during oral glucose tolerance test: influence on reference intervals for insulin and on cut-off values for insulin sensitivity indices. Clin Chem Lab Med 46:240–246. [DOI] [PubMed] [Google Scholar]
- Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. (2004) Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52:301–310. [DOI] [PubMed] [Google Scholar]