Abstract
Background:
Attention deficit/hyperactivity disorder has been shown to affect working memory, and fMRI studies in children and adolescents with attention deficit/hyperactivity disorder report hypoactivation in task-related attentional networks. However, studies with adult attention deficit/hyperactivity disorder patients addressing this issue as well as the effects of clinically valid methylphenidate treatment are scarce. This study contributes to closing this gap.
Methods:
Thirty-five adult patients were randomized to 6 weeks of double-blind placebo or methylphenidate treatment. Patients completed an fMRI n-back working memory task both before and after the assigned treatment, and matched healthy controls were tested and compared to the untreated patients.
Results:
There were no whole-brain differences between any of the groups. However, when specified regions of interest were investigated, the patient group showed enhanced BOLD responses in dorsal and ventral areas before treatment. This increase was correlated with performance across all participants and with attention deficit/hyperactivity disorder symptoms in the patient group. Furthermore, we found an effect of treatment in the right superior frontal gyrus, with methylphenidate-treated patients exhibiting increased activation, which was absent in the placebo-treated patients.
Conclusions:
Our results indicate distinct activation differences between untreated adult attention deficit/hyperactivity disorder patients and matched healthy controls during a working memory task. These differences might reflect compensatory efforts by the patients, who are performing at the same level as the healthy controls. We furthermore found a positive effect of methylphenidate on the activation of a frontal region of interest. These observations contribute to a more thorough understanding of adult attention deficit/hyperactivity disorder and provide impulses for the evaluation of therapy-related changes.
Keywords: fMRI, adult attention deficit/hyperactivity disorder, working memory, clinical trial, methylphenidate
Introduction
Attention deficit/hyperactivity disorder (ADHD) is not only found in children and adolescents but also in about 5% of young adults who meet full diagnostic criteria (Willcutt, 2012). The consequences of adult ADHD (aADHD) are severe, with affected individuals consistently showing lower educational and professional achievement as well as worse mental and physical health (Biederman et al., 2010, 2012; Gjervan et al., 2012; Brook et al., 2013). Some theories of ADHD etiology focus on impaired behavior inhibition presumably leading to executive function deficits (Barkley, 1997). In fact, overall executive function impairment in ADHD patients is clearly visible both for children (Willcutt et al., 2005) and adults (Boonstra et al., 2005) with effect sizes in the medium range. Specific executive function impairments were found for measures of working memory (Martinussen et al., 2005), which is considered an important component of higher order cognitive functioning.
In line with these results, a recent study reports more working memory deficits in children with ADHD than in healthy control children (Fried et al., 2016). Importantly, within this group of children with ADHD, those with pronounced working memory deficits showed worse cognitive functioning and poorer educational outcomes than those without pronounced deficits. Working memory functioning in ADHD thus seems to be of particular importance in understanding common impairments in patients with this disorder. In addition, studies examining the acute effects of stimulant medication on executive function show an improvement of behavioral executive function measures, including working memory manipulation and storage with small and medium effect sizes, respectively (Coghill et al., 2014).
Previous neuroimaging studies comparing the functional brain activation of aADHD patients to healthy controls during working memory tasks show alterations on the neuronal level with less prefrontal activation in the aADHD group than in a healthy control group (Ehlis et al., 2008; Valera et al., 2010; Schecklmann et al., 2013) as well as an overall decreased activation pattern in frontoparietal regions (Bayerl et al., 2010). Researchers employing meta-analytic techniques to investigate functional brain activity in aADHD patients from a network perspective also report hypoactivation in the frontoparietal network and, as a potential compensatory mechanism, hyperactivation in the dorsal attention network (Cortese et al., 2012).
So far, intervention studies on working memory exist only in children and adolescents and found an upregulation of frontoparietal network activity (Wong and Stevens, 2012; Cubillo et al., 2013) and connectivity (Wong and Stevens, 2012) through methylphenidate (MPH). There are no studies of working memory in adult patients that firstly compare patients and well-matched healthy controls, and secondly use placebo-controlled designs and span several weeks. To date, most studies investigate children and adolescents and rely on dispensing single doses of medication or using a naturalistic on/off design. A recent review (Spencer et al., 2013) of studies examining the effects of psychostimulants on fMRI-measured brain function reports only 4 studies with aADHD patients (Bush et al., 2008; O’Gorman et al., 2008; Schlochtermeier et al., 2011; Stoy et al., 2011). Only one of these studies (Bush et al., 2008) investigated task-related functional activation in a double-blind placebo-controlled design that spanned several weeks, and no study used a comparable working memory task.
The goal of this study was to therefore attempt to close this gap by examining activation differences between aADHD patients and healthy controls and to investigate the impact of placebo-controlled double-blind MPH treatment in aADHD patients during an n-back working memory task. Since the focus of this investigation was the task-related activation in frontal and parietal brain regions, fMRI was employed as the method of choice for superior spatial resolution. In line with previous findings (Ehlis et al., 2008; Bayerl et al., 2010; Cortese et al., 2012; Schecklmann et al., 2013), we expected the 2-back condition of this task to be associated with decreased frontal and parietal activation in aADHD patients compared to the 0-back control condition and healthy controls. In addition, we hypothesized that treatment with MPH for 6 weeks compared to placebo should increase activation in these areas for the treated aADHD patients.
Methods
Participants and Clinical Diagnosis
A total of 41 patients with aADHD were recruited from the ADHD outpatient clinic at the Department of Psychiatry, Psychosomatics, and Psychotherapy of the University of Wuerzburg. Diagnoses were made by an experienced psychiatrist according to DSM-IV-TR (2000). Patients had to be medication-naïve or without medication for at least 3 months prior to testing with no obvious comorbid disorders to be approached for participation. To corroborate the initial diagnosis, all patients were administered the Wender-Reimherr-Interview (WRI) (Corbisiero et al., 2010) and the Conners’ Adult ADHD Rating Scales (CAARS) (Conners et al., 1999). The Wender Utah Rating Scale (WURS) (Ward et al., 1993) was administered to retrospectively measure ADHD symptoms in childhood. To assess possible comorbid axis I disorders (an exclusion criterion), all patients were assessed with the Structured Clinical Interview for DSM-IV (Wittchen et al., 1997), the Hamilton Depression Rating Scale (Hamilton, 1960), and the Hamilton Anxiety Rating Scale (Hamilton, 1959). With exception of the WURS, all questionnaires were administered twice, before the first and the second fMRI appointment, in order to track possible treatment-related changes in symptomatology. Furthermore, all participants completed the Standard Progressive Matrices (SPM) (Kratzmeier and Horn, 1988) to obtain a nonverbal estimate of their intellectual functioning. All participants furthermore completed a second working memory/ selective attention task as well as the Digit Span subtest from the German version of the WAIS (Aster et al., 2006) and the Stroop Color Word Test (Bäumler, 1985), the results of which are published elsewhere (Biehl et al., 2014).
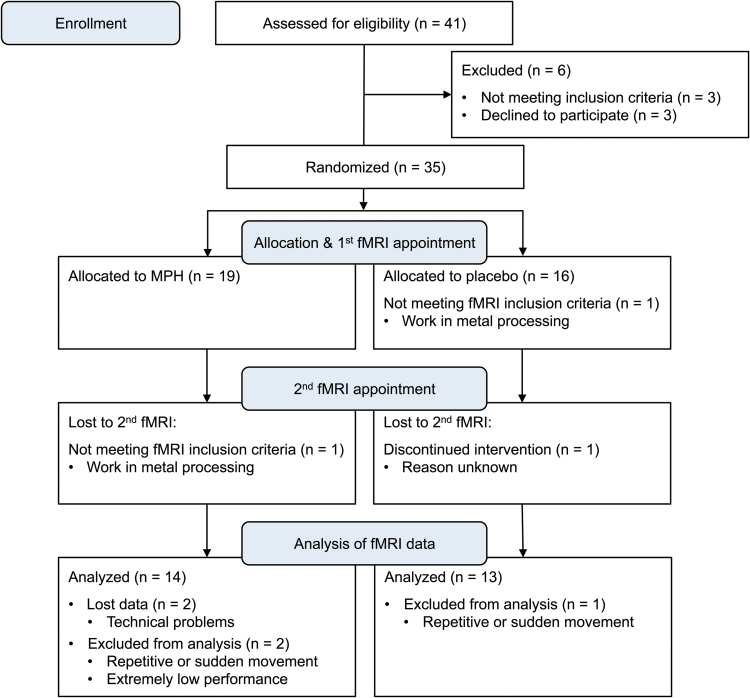
Of the 41 recruited aADHD patients, 3 did not meet full inclusion criteria and 3 more patients decided not to proceed with the study after inclusion, resulting in an initial sample of 35 patients. Subsequently, 1 patient decided to discontinue the study for unknown reasons after the first fMRI appointment. No or only one set of fMRI data was obtained from 2 patients who started working in metal processing and were excluded from further fMRI data collection in accordance with the health and safety regulations of the Research Center for Magnetic Resonance Bavaria. Two data sets of aADHD patients from the first fMRI appointment were lost because of technical problems. Two patients had to be excluded after preprocessing of the data because of excessive movement in the scanner (continuous repetitive movements and sudden movement of >2mm, respectively). One patient was excluded because her behavioral performance showed extremely low outlier values, resulting in a total of 29 patient data sets for the first and 27 patient data sets for the first as well as the second fMRI appointment. Sixteen of the 29 patients (55%) were classified as predominantly inattentive, 12 patients (41%) were classified as combined type, and one patient was classified as predominantly hyperactive/impulsive. Nineteen of these patients (66%) were medication naïve, while 7 patients (24%) had had previous treatment attempts with MPH and 1 patient had had a previous treatment attempt with atomoxetine. No reliable information about previous treatment attempts could be obtained for 2 patients.
In addition, 47 healthy control participants without a past or present diagnosis of ADHD and without any current neurological or psychiatric diseases were recruited from a previously established participant pool (see also Gschwendtner et al., 2012; Biehl et al., 2013) as well as through university advertisements. A subset of 29 healthy control participants was chosen to match the patient group most closely in a case-control design (P ≥ .2 for age, gender, and years of schooling) (Table 1). All participants completed the Adult ADHD Self-Report Scale to obtain a current estimate of their ADHD-related symptomatology (Kessler et al., 2005) and had normal or corrected-to-normal vision.
Table 1.
Overview of Mean Demographic Data and Questionnaire Scores for Healthy Controls and the aADHD MPH and Placebo Groups
| First Appointment | First Appointment | Second Appointment | |||||
|---|---|---|---|---|---|---|---|
| All Participants | Controls | ADHD | aADHD Group | Placebo | MPH | Placebo | MPH |
| Participants (male) | 29 (13) | 29 (18) | Participants (male) | 14 (8) | 15 (10) | 13 (7) | 14 (9) |
| Mean age | 33.3 (9.3) | 36.1 (9.9) | Mean age | 35.5 (10.1) | 36.7 (10.0) | 36.4 (9.9) | 37.3 (10.1) |
| Mean school years | 11.1 (1.8) | 10.8 (1.6) | Mean school years | 10.5 (1.4) | 11.2 (1.8) | 10.5 (1.5) | 11.4 (1.7) |
| SPM raw score | 50.7 (4.9) | 49.4 (7.0) | SPM raw score | 49.0 (8.0) | 49.8 (6.1) | 49.2 (8.2) | 49.6 (6.3) |
| ASRS | CAARS DSM-IV Symptoms | ||||||
| Inattention | 11.2 (4.6)*** | 24.0 (4.8)*** | Inattentive | 77.4 (10.0) | 80.0 (9.1) | 69.1 (12.8) | 61.6 (12.7) |
| Hyperactivity/Impulsivity | 9.7 (5.4)*** | 18.7 (6.2)*** | Hyperactive/impulsive | 65.3 (13.0) | 55.8 (15.1) | 56.1 (13.9) | 45.1 (11.9) |
| Total | 75.9 (11.3) | 71.5 (11.7) | 64.7 (13.8) | 54.5 (12.6) | |||
| HAM-A | 9.3 (8.5) | 8.5 (8.0) | 6.0 (5.1) | 7.0 (7.0) | |||
| HAM-D | 5.5 (5.8) | 4.6 (6.0) | 2.4 (2.3) | 3.9 (6.0) | |||
Abbreviations: ASRS: Adult ADHD Self-Report Scale; CAARS: Conners’ Adult ADHD Rating Scales; HAM-A: Hamilton Anxiety Rating Scale; HAM-D: Hamilton Depression Rating Scale; MPH: Methlyphenidate; SPM: Standard Progressive Matrices.
Standard deviation is noted in parentheses unless stated otherwise.
*** significant between-group differences, P ≤ .001.
Medication and Placebo Procedures
After inclusion in the study, all aADHD patients were randomly assigned to either immediate release MPH or placebo treatment in a double-blind design. Randomization lists were generated using Rancode 3.6 (Isi Medien GmbH, München, Germany). Block size was restricted to 4. Separate lists were used for male and female participants and for different genotypes (which are of no interest here). Treatment was selected according to these lists; the number of the respective medication box was subsequently assigned using a different list. The random allocation sequences were generated by Medice Arzneimittel Pütter GmbH & Co. KG (Iserlohn, Germany), and a medical laboratory assistant/study nurse assigned participants to the intervention. Neither the patients nor any researcher involved in data collection were aware of the assigned treatment. Medication and placebos were provided by Medice Arzneimittel Pütter GmbH & Co. KG. Medication was dispensed in a free titration design. The medication schedule started with an initial daily dose of 10mg, which was increased by 10mg every week up to a maximum daily dose of 60mg. Medication was only increased as long as the patient subjectively benefitted from the increase without suffering from any disturbing side effects. A psychiatrist saw each participating patient at least every 2 weeks to assess symptom response and side effects and adjust medication dosage if necessary. Patients were debriefed after the second fMRI appointment following 6 weeks of treatment with MPH medication or placebo and could subsequently decide to begin or continue MPH treatment (depending on their previous treatment). Furthermore, all patients were seen by a psychiatrist for a final follow-up assessment 4 weeks after the end of the double-blind medication phase (see supplementary Figure 1 for a diagram of the study design). There were no harmful or unintended effects for any of the patients.
After 6 weeks of medication, the average daily medication dose was 49mg (SD=15). Medication doses were significantly lower for patients in the MPH (M=44mg, SD=18) compared to patients in the placebo group (M=55mg, SD=8; t(32)=2.48, P = .02). In line with previous studies (Biederman et al., 2010; Medori et al., 2008; Rösler et al., 2009), clinically significant treatment response was defined as a fixed minimum reduction in T-scores, in this case on the CAARS DSM-IV Total ADHD Symptoms scale as well as on the CAARS DSM-IV Inattentive Symptoms scale and/or the CAARS DSM-IV Hyperactive/Impulsive Symptoms scale, from day 1 to day 42. Given that the CAARS scores in this study were based on patient self-report, which was previously found to lead to higher endpoint scores than investigator ratings in clinical trials (Adler et al., 2008), we deemed a minimum reduction of 20% adequate to classify patients as responders. Six patients (40%) in the placebo group and 12 patients (63%) in the MPH group responded to treatment. As mentioned above, not all patients could be reassessed at the second fMRI appointment, which resulted in a total of 27 patient data sets that comprised both the first fMRI appointment without medication and the second fMRI appointment after 6 weeks of MPH or placebo (see Figure 1 for the CONSORT 2010 Flow Diagram).
Figure 1.
CONSORT flow diagram illustrating the ADHD patients’ progress through the study.
Ethical approval was obtained through the Ethical Review Board of the medical faculty of the University of Wuerzburg; all procedures involved were in accordance with the 2008 Declaration of Helsinki. All participants gave written informed consent after full explanation of the procedures.
Experimental Paradigm
The modified version of the classic n-back task (Cohen et al., 1994) is well established in the literature (Egan et al., 2001; Goldberg et al., 2003; Mattay et al., 2003; Diaz-Asper et al., 2008). It requires participants to respond on every trial by indicating the number shown “n” trials earlier (numbers range from 1 to 4). The paradigm we used comprised a 0-back, a 1-back, and a 2-back condition, presented in blocks of 30 seconds each. The 0-back condition served as control condition as it constitutes a motor equivalent to the 1-back and 2-back conditions, but does not require higher cognitive functions of working memory. Numbers were presented for 500ms with 1 500-ms interstimulus interval, leading to a total of 15 number presentations per block. Fifteen blocks (i.e., 5 blocks per condition) were presented in pseudo-randomized order with the entire experiment lasting around 8 minutes. All participants were familiarized with the task beforehand and completed a practice run to ensure their understanding of the instructions.
Analysis of Behavioral Data
Potential differences in behavioral performance (correct responses, incorrect responses, and missed trials) between the aADHD group and the healthy control group were investigated using mixed-model ANOVAs with the between-subjects factor group (aADHD vs healthy controls) and the within-subjects factor task difficulty (0-back, 1-back, 2-back). To investigate medication effects on behavioral performance, mixed-model ANOVAs with the between-subjects factor treatment (MPH vs placebo) and the within-subjects factors time of measurement (first fMRI appointment vs second fMRI appointment) and task difficulty (0-back, 1-back, 2-back) were computed (see supplementary Table 1 for the complete performance data). If assumptions of sphericity were violated, degrees of freedom were adjusted according to Greenhouse-Geisser (Greenhouse and Geisser, 1959). For all behavioral data, outliers were identified using z-transformation of the data. Participants with any value exceeding z = ±3.29 were excluded from further data analysis.
To compute meaningful correlations of mean cluster activations for the contrast of the most demanding condition (2-back) minus the control condition (0-back) and performance data, performance indices were calculated as the ratio of 2-back percent correct responses to 0-back percent correct responses. To avoid missing values for the incorrect response ratios due to a lack of incorrect responses in any of the 2 conditions, percent incorrect responses was converted to its corresponding negative value. This procedure yielded 2 different performance indices, one based on the number of correct responses and the other based on the number of incorrect responses. The index based on correct responses should be seen as indicating performance quality. In contrast, the index based on incorrect responses should be understood as indicating performance monitoring. Higher values reflect better behavioral performance for both indices. Since responses in this paradigm were externally paced by the appearance of the next number stimulus, reaction times were not analyzed. For all analyses, P ≤ .05 was considered significant and P ≤ .1 was considered a trend.
Imaging Parameters and Analysis
Imaging data were acquired using a Siemens MAGNETOM Avanto MRI scanner with a magnetic field strength of 1.5 Tesla (Siemens AG, Erlangen, Germany) and a 12-channel head coil. The TR of the T2*-weighted gradient echo planar imaging (EPI) sequence was 3s; the echo time (TE) was 50ms. Further parameters were: flip angle 90°, in-plane resolution 3.6×3.6mm2, field of view (FOV) 230×230mm2, 32 axial slices (descending order), slice thickness 4mm (1 mm gap). Slices were aligned to the AC-PC line. The first three volumes of each sequence were discarded to allow for signal saturation. In addition, a high-resolution structural MPRAGE scan (TR 1.87s, TE 3.74ms, flip angle 15º, in-plane resolution 1.4×1mm2, FOV 250×250mm2, slice thickness 1mm) was acquired for each participant. The experimental task was presented via MRI-compatible goggles (VisuaStim Digital, Resonance Technology, Inc., Northridge, CA) using Presentation (version 11.3, Neurobehavioral Systems, Inc., Albany, NY). To minimize head movement, participants lay on a polyurethane foam head cushion with additional movement restraints mounted to the sides of the head coil.
All fMRI data were analyzed using Statistical Parametric Mapping (SPM8) (Wellcome Trust, 2009) implemented in MATLAB (The MathWorks). EPI images were realigned, and the MPRAGE scan was coregistered to the mean EPI image and segmented. EPI images were then normalized to 3 mm3 voxel size and smoothed with a 9 mm3 full-width at half maximum Gaussian smoothing kernel. Subsequently, first-level analyses were computed with a high-pass filter of 256s incorporating the conditions of interest (0-back, 1-back, 2-back) as well as the 6 movement parameters obtained during preprocessing. Results were whole-brain family-wise error (FWE) corrected with P < .05 unless specified otherwise. The extent threshold for a given cluster was set to a minimum of 5 voxels.
To examine task-induced activation, whole brain analyses were carried out across all participants using 1-sample t tests. To investigate differences between the aADHD and the control group, whole brain analyses were carried out using 2-sample t tests. Medication effects in aADHD patients were examined using mixed-model ANOVA implemented as flexible factorial models in SPM8 with the between-subjects factor treatment (MPH vs placebo) and the within-subjects factor time of measurement (first fMRI appointment vs second fMRI appointment).
Regions of Interest Analysis
Whole brain results for the contrast 2-back minus 0-back were examined for activation at peak voxels of interest belonging to the attention network as specified by Fox and colleagues (2006). Further analyses were then carried out by testing for differences between aADHD patients and healthy controls as well as between MPH- and placebo-treated aADHD patients using small volume correction (spheres with 9 mm diameter). For all ROI analyses, peak voxels with P FWE ≤ .05 were considered significant and peak voxels with P FWE ≤ .1 were considered trends. If the small volume correction showed between-group differences for the peak voxel of a given cluster, the contrast estimates of this cluster were exported for each participant using REX Toolbox (Whitfield-Gabrieli, 2009). These mean cluster activations were entered into SPSS Statistics 20 (IBM, New York, NY) to calculate correlations between mean cluster activations and behavioral as well as questionnaire data.
Results
Behavioral Data
Mixed-model ANOVAs for the first fMRI appointment with the between-subjects factor group and the within-subjects factor task difficulty yielded no significant main effect of group and no significant interaction of group and task difficulty for correct responses, incorrect responses, or missed trials (all P > .1; see supplementary Table 1 for all performance data). There was, however, a significant main effect of task difficulty (F (2,112) = 99.37, P < .001) for correct responses, for missed trials (F (2,112) = 52.22, P < .001), and for incorrect responses (F (2,112) = 47.75, P < .001) with all participants showing worse performance with increasing task difficulty.
Six patients (40%) in the placebo group and 12 patients (63%) in the MPH group responded to treatment, yielding a trend for a between-group difference (t(32) = 1.34, P one-sided = .095). Mixed-model ANOVAs for correct responses, incorrect responses, and missed trials for the patient group with the between-subjects factor medication and the within-subjects factors time of measurement and task difficulty showed significant main effects of time of measurement (F (1,25) = 9.14, P = .01) and task difficulty (F (2,50) = 59.41, P < .001) for correct responses: Patients performed better on the second compared to the first appointment and showed worse performance with increasing task difficulty. The same was true for missed trials (main effect time of measurement: F (1,25) = 12.67, P = .002; main effect task difficulty: F (2,50) = 23.52, P < .001). In contrast, for incorrect responses, only a main effect of task difficulty could be found (F (2,50) = 24.45, P < .001).
fMRI Data
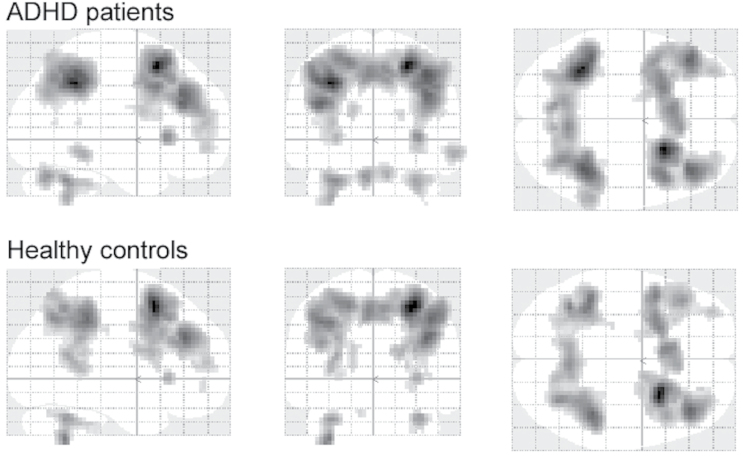
At the first fMRI appointment, whole brain analyses for the contrast of the most demanding condition (2-back) minus the control condition (0-back) showed strong activation patterns of frontal and parietal regions of the dorsal and ventral attention network as well as of the cerebellum and the caudate nuclei for both groups (Figure 2). An examination of attention network peak voxels as specified by Fox and colleagues (2006) across both groups revealed significant task-induced bilateral activation in the intraparietal sulcus as well as significant unilateral activation in the right superior, middle, and inferior frontal gyri, and the right precuneus. These peak voxels were subsequently examined for between-group differences as described above.
Figure 2.
Significantly activated voxels found in the whole brain analysis with P FWE < .05 (5 voxels extent threshold) for the contrast 2-back minus 0-back in the patient group (top) and the healthy control group (bottom).
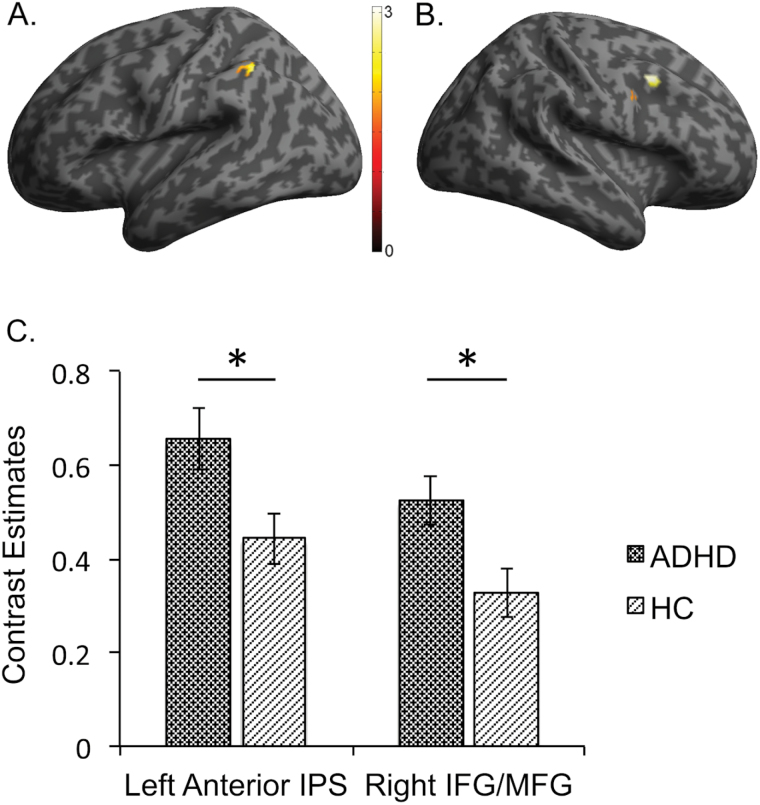
Comparisons between the aADHD and the healthy control group showed no significant whole brain differences during the first fMRI appointment. However, ROI analyses yielded significantly greater peak voxel activation in aADHD patients compared to healthy controls in the left anterior intraparietal sulcus (IPS; P FWE = .02, cluster size 43 voxels; Table 2; Figure 3) and the right inferior/middle frontal gyrus (IFG/MFG; P FWE = .050, cluster size 41 voxels). In addition, we found trends for greater activation in the patient group compared to the healthy controls in the right posterior IPS and the right anterior IPS (P FWE = .06, cluster size 19 voxels and P FWE = .09, cluster size 57 voxels, respectively).
Table 2.
Peak Voxel Differences Between Healthy Controls and ADHD Patients for the Contrast 2-Back Minus 0-Back
| Anatomical Region | MNI Coordinates (ROI Center) | MNI Coordinates (Peak Voxel) | Cluster Size | t (56) | P FWE |
|---|---|---|---|---|---|
| Right posterior IPS | 21 -73 52 | 15 -76 46 | 19 | 2.84 | .06 |
| Right anterior IPS | 36 -52 46 | 39 -49 49 | 57 | 2.57 | .09 |
| Left posterior IPS | -21 -73 46 | --- | |||
| Left anterior IPS | -42 -43 43 | -36 -49 40 | 43 | 3.36 | .02 |
| Right MFG | 36 38 25 | --- | |||
| Right SFG | 30 44 34 | --- | |||
| Right IFG/MFG | 48 14 35 | 45 11 37 | 41 | 2.96 | .050 |
| Right SFG | 3 17 55 | --- | |||
| Right precuneus | 3 -58 52 | --- |
Abbreviations: IFG: inferior frontal gyrus; IPS, intraparietal sulcus; MFG, middle frontal gyrus; SFG, superior frontal gyrus.
Specified are the examined anatomical regions and their MNI coordinates based on Fox et al. (2006), as well as the corresponding cluster sizes (in voxels), peak voxel MNI coordinates, and t- as well as P FWE-values.
P values ≤ .05 were considered significant, P values ≤ .1 were considered trends.
Figure 3.
Clusters found in the ROI analysis that showed significantly greater peak voxel activation for the contrast 2-back minus 0-back in the patient group (ADHD) compared to the healthy control group (HC) in the (A) left anterior intraparietal sulcus (IPS) and in the (B) right inferior/ middle frontal gyrus (IFG/MFG) as well as mean contrast estimates and standard error of measurement for these two clusters (C). *Significantly different, P ≤ .05.
As the mixed-model ANOVA yielded no significant whole brain interactions of treatment and time of measurement for the patient group, peak voxels of the attention network (Fox et al., 2006) were used for ROI analyses of treatment-induced differences. ROI analyses showed a significant interaction of treatment and time of measurement for the contrast 2-back minus 0-back in the right superior frontal gyrus (SFG; F (1,25) = 14.14, P FWE = .04, cluster size: 12 voxels). Subsequent 2-sample t tests for this ROI showed a trend for greater SFG activation in the MPH group compared to the placebo group (t (25) = 2.81, P FWE = .09) at the second fMRI appointment, while no significant differences were observed at the first appointment (all P FWE > .1).
Correlation coefficients between mean contrast estimates for clusters with significant or trend level peak voxel differences and performance indices at the first fMRI appointment revealed significant or trend level associations between correct response performance and activation in the left anterior IPS as well as the right anterior and posterior IPS across all participants (Table 3): The higher the activation in these areas, the better performance with regard to correct responses. Similarly, incorrect response performance correlated significantly with activation in the right IFG/MFG as well as the right anterior and posterior IPS: The higher the activation in these areas, the fewer incorrect responses were given in all participants.
Table 3.
Correlation Coefficients (r) and P Values for the Contrast Estimates (Contrast 2-Back Minus 0-Back) of the Investigated Clusters and the Two Performance Indices for the Entire Sample (Patient Group and Healthy Control Group), and Correlation Coefficients (r) and P Values for the Contrast Estimates (Contrast 2-Back Minus 0-Back) of the Investigated Clusters and the T-Scores for CAARS DSM-IV Hyperactive/Impulsive Symptoms and CAARS DSM-IV Total ADHD Symptoms Scales for the Patient Group at the First fMRI Appointment
| Anatomical Region | Performance (Correct) | Performance (Incorrect) |
CAARS
H-I Symptoms b |
CAARS
Total Symptoms |
|---|---|---|---|---|
| r (P Value) a | r (P Value) a | r (P Value) c | r (P Value) c | |
| Left anterior IPS | .25 (.06) | .22 (n.s.) | .46 (.01) | .54 (.002) |
| Right IFG/MFG | .20 (n.s.) | .32 (.01) | .41 (.03) | .44 (.02) |
| Right posterior IPS | .35 (.01) | .48 (<.001) | .46 (.01) | .40 (.03) |
| Right anterior IPS | .24 (.07) | .37 (.004) | .28 (n.s.) | .40 (.03) |
Abbreviations: IFG, inferior frontal gyrus; IPS, intraparietal sulcus; MFG, middle frontal gyrus.
a df = 56.
b Hyperactive/impulsive symptoms.
c df = 27; P values ≤ .05 were considered significant, P values ≤ .1 were considered trends.
For the patient sample at the first appointment, activation in some of the investigated areas correlated with symptom severity as measured with the CAARS (Table 3): Higher fMRI contrast estimates in the left anterior IPS, the right IFG/MFG, and the right posterior IPS correlated significantly with scores on the CAARS DSM-IV Hyperactive/Impulsive Symptoms and the CAARS DSM-IV Total ADHD Symptoms scales.
Discussion
The results of this study contribute to previous observations in an unexpected way: Based on a meta-analysis of fMRI studies investigating executive function (Cortese et al., 2012), we hypothesized to find a hypoactivation of attention network ROIs in aADHD patients compared to healthy controls. This was not the case, and our data did actually show a hyperactivation of several network ROIs in the frontal and parietal lobes for the aADHD group. To interpret this finding, the results from the above-mentioned meta-analysis as well as from two similar previous studies need to be reexamined. The first similar study employed a classic n-back task and reports hypoactivation particularly in the DLPFC in a group of children with ADHD compared to healthy controls (Cubillo et al., 2013). These 2 groups showed behavioral performance differences, with the ADHD group performing significantly worse than the healthy control group in the more demanding conditions. The same is true for a study with aADHD patients, which reports less network activation but also worse performance in the patient group (Bayerl et al., 2010). Interestingly, the meta-analysis of fMRI studies with adults reports hypoactivation in the frontoparietal network as well as hyperactivation in the dorsal attention network, which the authors interpret as possibly reflecting compensatory efforts (Cortese et al., 2012).
This latter interpretation is very compatible with our own data. On the one hand, the activation of network ROIs across all participants was unexpectedly increased, but on the other hand correlated positively with behavioral task performance. The absence of behavioral performance differences between the 2 groups thus suggests a successful compensation of possibly existing deficits by the aADHD group. In addition, activation of network ROIs correlated positively with the CAARS DSM-IV Hyperactive/Impulsive Symptoms and Total ADHD Symptoms scales in aADHD patients. This might indicate that aADHD patients with more severe symptoms had to apply more effort to successfully complete the task, which would be consistent with the interpretation of increased functional activation reflecting compensatory efforts. This interpretation is further supported by the observation of a medication effect in the right superior frontal gyrus of patients treated with MPH compared to patients treated with placebo. This is in line with previous reports of MPH upregulating network activity and frontal activation (Rubia et al., 2011; Wong and Stevens, 2012; Cubillo et al., 2013).
Our results thus seem well in line with models that suggest executive function impairment in ADHD (Barkley, 1997; Sonuga-Barke, 2005; Sonuga-Barke et al., 2010). Unlike the patients of previous fMRI studies of working memory function (Bayerl et al., 2010; Cubillo et al., 2013), however, the patients in our study seem to have been able to compensate for their executive function deficits in a cognitively demanding situation, showing equal performance, but increased fMRI activation. Our strict inclusion and exclusion criteria might have contributed to this result as the participating patients might thus have been particularly well adjusted. One could therefore argue that these patients had successfully learned to compensate for the deficits caused by their ADHD earlier in life, which is reflected in our results. However, it should be noted that this pattern of compensation through increased activation might have changed if task difficulty had been increased even further. Ko and colleagues (2013) report that increased frontoparietal activation in a group with aADHD during the 2-back condition of a phonological working memory task was reversed with the aADHD group showing decreased activation in the more demanding 3-back condition.
However, there are also several limitations to this study. Since inclusion and exclusion criteria were rather strict, it was not possible to select participants based on their ADHD subtype. This is especially important as there is some evidence that the childhood inattentive type might represent a disorder that is etiologically and neurobiologically distinct from the childhood hyperactive/impulsive and combined types (Goodyear and Hynd, 1992; Diamond, 2005). Thus, including inattentive and combined type aADHD patients in our investigated sample likely increased the variance within that sample, thereby reducing the probability of finding any significant differences. While we found correlations for the CAARS DSM-IV Hyperactive/Impulsive Symptoms as well as the Total ADHD Symptoms scales with activation parameters, no correlations were found for the CAARS DSM-IV Inattentive Symptoms scale. This could point to high heterogeneity of the inattentive ADHD symptoms in our sample.
In addition, much information could be gained by splitting the examined patients into responders and nonresponders based on an a priori criterion, and by investigating the obtained fMRI data separately for these 2 groups. However, the sample size in our study was not sufficient to allow any meaningful analyses of this kind. Another potential limitation concerns the very strict inclusion and exclusion criteria, which might have led to the inclusion of only relatively well-adjusted aADHD patients. Furthermore, no third party assessment by a parent or other relative was obtained to corroborate childhood symptoms of ADHD self-reported on the WURS (Ward et al., 1993). In addition, the SPM might not be an adequate test to obtain a nonverbal estimate of intellectual functioning in the patient population, as there is some overlap between symptoms of ADHD and autistic traits (Mulligan et al., 2009; Grzadzinski et al., 2011), which might have caused patients to achieve inflated scores (see also Hayashi et al., 2008).
This is, to our knowledge, the first study investigating working memory processes and methylphenidate effects in a sample of aADHD patients using a double-blind placebo-controlled design, and it also is the largest adult sample investigated to date with this type of design. The results show that the functional differences during task-completion and the effects of MPH in aADHD patients are subtle, but nevertheless present. They are consistent with the notion that MPH treatment in aADHD patients supports endogenous compensatory mechanisms by further increasing activation of frontal attention network ROIs. A common compensatory mechanism of psychological and drug interventions for the amelioration of aADHD symptoms provides the rationale for further studies integrating both approaches to optimize treatment of aADHD. Employing behavioral task performance combined with fMRI imaging may differentiate patients who profit from a given therapeutic approach from those who will not, even after a short treatment period. The present study may therefore contribute to the design of a priori stratification studies to define patients with additional treatment needs beyond the present standard treatment.
Statement of Interest
Medication and placebos were provided by Medice Arzneimittel Pütter GmbH & Co. KG (Iserlohn, Germany). C. P. Jacob is on the advisory board of Medice Arzneimittel Pütter GmbH & Co. KG. He has also given lectures for Medice and for Eli Lilly and Company (Indianapolis, IN). All other authors declare that they have no conflicts of interest.
Supplementary Material
Acknowledgments
The authors thank Inge Gröbner for coordinating the patient appointments and all medical residents who were present during the fMRI data collection.
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; grant nos. HE 4531/1-1 and RTG 1253/1-1). Thomas Dresler was partly supported by the LEAD Graduate School & Research Network (GSC1028), a project of the Excellence Initiative of the German federal and state governments. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
References
- Adler LA, Faraone SV, Spencer TJ, Michelson D, Reimherr FW, Glatt SJ, Marchant BK, Biederman J. (2008) The reliability and validity of self- and investigator ratings of ADHD in adults. J Atten Disord 11: 711–719 . [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders ( 4th ed., text rev.). Arlington, VA: : American Psychiatric Publishing; . [Google Scholar]
- Barkley RA. (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121: 65–94 . [DOI] [PubMed] [Google Scholar]
- Bayerl M, Dielentheis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, Stoeter P, Huss M, Konrad A. (2010) Disturbed brain activation during a working memory task in drug-naive adult patients with ADHD. Neuroreport 21: 442 . [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. (2010) How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiat Res 177: 299–304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. (2012) Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiat 73: 941–950 . [DOI] [PubMed] [Google Scholar]
- Biehl SC, Ehlis AC, Müller LD, Niklaus A, Pauli P, Herrmann MJ. (2013) The impact of task relevance and degree of distraction on stimulus processing. BMC Neurosci 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl SC, Gschwendtner KM, Guhn A, Müller LD, Reichert S, Heupel J, Reif A, Deckert J, Herrmann MJ, Jacob CP. (2014) Does adult ADHD interact with COMT val 158 met genotype to influence working memory performance? Atten Defic Hyperact Disord : 1–7 . [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. (2005) Executive functioning in adult ADHD: a meta-analytic review. Psychol Med 35: 1097–1108 . [DOI] [PubMed] [Google Scholar]
- Brook JS, Brook DW, Zhang CS, Seltzer N, Finch SJ. (2013) Adolescent ADHD and adult physical and mental health, work performance, and financial stress. Pediatrics 131: 5–13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. (2008) Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiat 65: 102–114 . [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. (2014) Effects of methylphenidate on cognitive functions in children and adolescents with ADHD: evidence from a systematic review and a meta-analysis. Biol Psychiat 76: 603–615 . [DOI] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. (1994) Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp 1: 293–304 . [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow EP. (1999) Conners’ Adult ADHD Rating Scales (CAARS) . North Tonawanda, NY: : Multi-Health Systems; . [Google Scholar]
- Corbisiero S, Buchli-Kammermann J, Stieglitz RD. (2010) Reliability and validity of the Wender-Reimherr-Interview (WRI) - an instrument for the diagnostic of the ADHD in adulthood. ZPPP 58: 323–331 . [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. (2012) Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiat 169: 1038–1055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer M, Simmons A, Rubia K. (2013) Drug-specific laterality effects on frontal lobe activation of atomoxetine and methylphenidate in attention deficit hyperactivity disorder boys during working memory. Psychol Med : 1–14 . [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. (2008) Genetic variation in catechol-O-methyltransferase: Effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol Psychiat 63: 72–79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. (2001) Effect of COMT val(108/158) met genotype on frontal lobe function and risk for schizophrenia. P Natl Acad Sci USA 98: 6917–6922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlis A-C, Baehne CG, Jacob CP, Herrmann MJ, Fallgatter AJ. (2008) Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: A functional near-infrared spectroscopy (fNIRS) study. J Psychiat Res 42: 1060–1067 . [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. P Natl Acad Sci USA 103: 10046–10051 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R, Chan J, Feinberg L, Pope A, Woodworth KY, Faraone SV, Biederman J. (2016) Clinical correlates of working memory deficits in youth with and without ADHD: a controlled study. J Clin Exp Neuropsyc 38: 487–496 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjervan B, Torgersen T, Nordahl HM, Rasmussen K. (2012) Functional impairment and occupational outcome in adults with ADHD. J Atten Disord 16: 544–552 . [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. (2003) Executive subprocesses in working memory - relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiat 60: 889–896 . [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. (1959) On methods in the analysis of profile data. Psychometrika 24: 95–112 . [Google Scholar]
- Grzadzinski R, Di Martino A, Brady E, Angeles Mairena M, O’Neale M, Petkova E, Lord C, Castellanos FX. (2011) Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord 41: 1178–1191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendtner KM, Biehl SC, Mühlberger A, Sommer C, Kübler A, Reif A, Herrmann MJ. (2012) The relationship between valence, task difficulty, and the COMT val(158)met polymorphism in disengagement processes. J Psychophysiol 26: 124–131 . [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety-states by rating. Brit J Med Psychol 32: 50–55 . [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosur Ps 23: 56–62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Kato M, Igarashi K, Kashima H. (2008) Superior fluid intelligence in children with Asperger’s disorder. Brain Cognition 66: 306–310 . [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Delmer O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, Ustun TB, Walters EE. (2005) The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol Med 35: 245–256 . [DOI] [PubMed] [Google Scholar]
- Ko C-H, Yen J-Y, Yen C-F, Chen C-S, Lin W-C, Wang P-W, Liu G-C. (2013) Brain activation deficit in increased-load working memory tasks among adults with ADHD using fMRI. Eur Arch Psy Clin N 263: 561–573 . [DOI] [PubMed] [Google Scholar]
- Kratzmeier H, Horn R. (1988) Standard Progressive Matrices . Weinheim: : Beltz Test Gesellschaft; . [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. (2005) A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Psy 44: 377–384 . [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. (2003) Catechol O-methyltransferase val(158)-met genotype and individual variation in the brain response to amphetamine. P Natl Acad Sci USA 100: 6186–6191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan A, et al. (2009) Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord 39: 197–209 . [DOI] [PubMed] [Google Scholar]
- O’Gorman RL, Mehta MA, Asherson P, Zelaya FO, Brookes KJ, Toone BK, Alsop DC, Williams SCR. (2008) Increased cerebral perfusion in adult attention deficit hyperactivity disorder is normalised by stimulant treatment: A non-invasive MRI pilot study. Neuroimage 42: 36–41 . [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad A-M, Brammer M, Taylor E. (2011) Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacol 36: 1575–1586 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Ehlis A-C, Plichta MM, Dresler T, Heine M, Boreatti-Huemmer A, Romanos M, Jacob CP, Pauli P, Fallgatter AJ. (2013) Working memory and response inhibition as one integral phenotype of adult ADHD? A behavioral and imaging correlational investigation. J Atten Disord 17: 470–482 . [DOI] [PubMed] [Google Scholar]
- Schlochtermeier L, Stoy M, Schlagenhauf F, Wrase J, Park SQ, Friedel E, Huss M, Lehmkuhl U, Heinz A, Stroehle A. (2011) Childhood methylphenidate treatment of ADHD and response to affective stimuli. Eur Neuropsychopharm 21: 646–654 . [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. (2005) Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiat 57: 1231–1238 . [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Bitsakou P, Thompson M. (2010) Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Psy 49: 345–355 . [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J. (2013) Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J Clin Psychiat 74: 902–917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Stroehle A. (2011) Reward processing in male adults with childhood ADHD-a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology 215: 467–481 . [DOI] [PubMed] [Google Scholar]
- Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Whitfield-Gabrieli S, Vitulano M, Schiller M, Seidman LJ. (2010) Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry 167: 86–94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. (1993) The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150: 885–890 . [DOI] [PubMed] [Google Scholar]
- Wellcome Trust (2009) Statistical Parametric Mapping (SPM8) . London, UK: . [Google Scholar]
- Whitfield-Gabrieli S. (2009) Region of Interest Extraction (REX) Toolbox . Boston, MA: . [Google Scholar]
- Willcutt EG. (2012) The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9: 490–499 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiat 57: 1336–1346 . [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Zaudig M, Fydrich T. (1997) SKID – Strukturiertes Klinisches Interview für DSM-IV. Achse I und II . Göttingen: : Hogrefe; . [Google Scholar]
- Wong CG, Stevens MC. (2012) The effects of stimulant medication on working memory functional connectivity in attention-deficit/hyperactivity disorder. Biol Psychiat 71: 458–466 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.