Abstract
Background:
Development of new strategies that can effectively prevent and/or treat alcohol use disorders is of paramount importance, because the currently available treatments are inadequate. Increasing evidence indicates that the lateral habenula (LHb) plays an important role in aversion, drug abuse, and depression. In light of the success of high-frequency stimulation (HFS) of the LHb in improving helplessness behavior in rodents, we assessed the effects of LHb HFS on ethanol-drinking behavior in rats.
Methods:
We trained rats to drink ethanol under an intermittent access two-bottle choice procedure. We used c-Fos immunohistochemistry and electrophysiological approaches to examine LHb activity. We applied a HFS protocol that has proven effective for reducing helplessness behavior in rats via a bipolar electrode implanted into the LHb.
Results:
c-Fos protein expression and the frequency of both spontaneous action potential firings and spontaneous excitatory postsynaptic currents were higher in LHb neurons of ethanol-withdrawn rats compared to their ethanol-naïve counterparts. HFS to the LHb produced long-term reduction of intake and preference for ethanol, without altering locomotor activity. Conversely, low-frequency electrical stimulation to the LHb or HFS applied to the nearby nucleus did not affect drinking behavior.
Conclusions:
Our results suggest that withdrawal from chronic ethanol exposure increases glutamate release and the activity of LHb neurons, and that functional inhibition of the LHb via HFS reduces ethanol consumption. Thus, LHb HFS could be a potential new therapeutic option for alcoholics.
Keywords: c-Fos immunohistochemistry, deep brain stimulation, drug abuse, locomotion
Introduction
Alcohol use disorders (AUD) represent one of the most serious health and socioeconomic concerns worldwide. To reduce or eliminate the desire to drink and dampen the harmful effects of alcohol, pharmacological, psychological, and social therapeutic interventions are employed. To date, however, a reliable and permanently effective treatment has not been identified.
Deep brain stimulation (DBS) is one of the neurosurgical interventions using implanted electrodes to deliver electrical pulses to areas in the brain, and has shown promising results as an experimental treatment of psychiatric disorders such as major depression (Morishita et al., 2014). Although both preclinical and clinical studies have suggested that DBS may be effective for AUD (Luigjes et al., 2012), it is not yet entirely clear which brain areas should be targeted. The lateral habenula (LHb) has received increasing attention in recent years for its role in processing reward, aversion, addiction, and depression (Matsumoto and Hikosaka, 2007, 2009; B Li et al., 2011; Jhou et al., 2013; Neumann et al., 2015; Meye et al., 2015). Functional modifications of the LHb by DBS reverse the depressive-like phenotype in the learned helpless rodent model (B Li et al., 2011). LHb electrical stimulation produces enduring inhibition of cocaine-seeking behavior (Friedman et al., 2010). We therefore hypothesized that DBS of the LHb will reduce alcohol consumption.
To test this hypothesis, in the current study we first trained rats to drink ethanol, and then measured changes in the LHb by examining c-Fos immunohistochemistry and electrophysiological events during withdrawal. Lastly, we examined changes in ethanol intake induced by LHb DBS. Our results showed that LHb neurons were activated during ethanol withdrawal. Inhibition of the LHb by high-frequency stimuli (HFS) reduced ethanol consumption. Thus, HFS of the LHb could be a potential new therapeutic option for AUD.
Materials and Methods
Animals and Housing
All experiments were performed in accordance with the National Institutes of Health Institutional Animal Care and Use Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Rutgers, the State University of New Jersey. Ex vivo electrophysiological recordings were performed on slices from male Sprague-Dawley (SD) rats, in vivo studies on male Long-Evans rats (both from Taconic Farm, NY). The rats were housed individually, in ventilated Plexiglas cages in a climate-controlled room (20–22℃), and allowed to acclimatize to the housing conditions and handling before the start of the experiments. All rats were kept on a 12-h light /dark cycle: lights off at 15:00 hours. Food and water were available ad libitum, or as otherwise indicated.
Intermittent Access to 20% Ethanol Two-Bottle Choice Drinking Procedure
Male two-month-old Long-Evans or SD rats were trained to drink alcohol under the intermittent access to 20% ethanol in two bottles free choice (IE2BC) paradigm, as previously described (Simms et al., 2008; J Li et al., 2010, 2011b, 2012b). Briefly, animals were given 24h concurrent access to one bottle of 20% (v/v) ethanol in water and one bottle of water, starting at 15:15 hours on Monday. After 24h, the ethanol bottle was replaced with a second water bottle that was available for the next 24h. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. On each ethanol-drinking day, the placement of the ethanol bottle was alternated to control for side preferences. The amount of ethanol or water consumed was determined by weighing the bottles before access and after 24h access. The weight of each rat was measured daily Monday through Friday. Ethanol consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. The preference ratio of ethanol intake was calculated by the following formula: preference ratio (%) = ethanol solution intake (ml)/total fluid intake (ml of ethanol solution + ml of water). Rats were maintained on the 20% ethanol intermittent access two-bottle choice paradigm for 8 weeks (24 ethanol sessions). We call these rats IE2BC rats.
Brain Slice Preparation
We have previously shown that SD rats in the IE2BC paradigm escalated their ethanol intake and subsequently became putatively dependent on ethanol (J Li et al., 2011a, 2011b). In the current study, we used SD rats for electrophysiological experiments to take the advantage of their calmness and ease of handling. Rats at 0h or 24h withdrawal from ethanol after eight weeks in the IE2BC paradigm or ethanol-naïve counterparts were anesthetized with ketamine/xylazine (80mg/10mg/kg i.p.) then decapitated. Brain slices were prepared as previously described (Ye et al., 2006; Zuo et al., 2013). Briefly, the brain was rapidly removed and placed in ice-cold glycerol-based artificial cerebrospinal fluid (GaCSF) containing the following (in mM): 252 glycerol, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 0.3 L-ascorbate, and 10 glucose, and oxygenated with 95% O2/5% CO2 (carbogen). Coronal slices (230–250 µm thick) were cut with a Compresstome VF-200 slicer (Precisionary Instruments Inc.), then immediately transferred to a holding chamber and incubated for at least 1h at room temperature (24–25°C) in carbogenated regular artificial cerebrospinal fluid (aCSF) of almost the same composition as GaCSF, the exception being that 252mM glycerol was replaced with 126mM NaCl. After equilibration, a single slice was transferred to a submersion-type recording chamber and mechanically stabilized with a platinum ring.
Electrophysiological Recordings
Electrophysiological recordings were conducted as previously described (Zuo et al., 2015). Cells were visualized using infrared differential contrast and fluorescence microscopy (Leica DM6000FS, Leica Microsystems). Electrical signals were recorded with an Axon 700B amplifier, a Digidata 1440A A/D converter, and Clampfit 10.3 software (Molecular Devices Co). Data were filtered at 2kHz and sampled at 5kHz. Throughout the experiments, the bath was continually perfused with warm (33°C) carbogenated aCSF (1.5–2.0ml/min). Patch pipettes (4–5 MΩ) for voltage-clamp were filled with a solution containing (in mM): 140 Cs-methanesulfonate, 5 KCl, 2 MgCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 MgATP, and 0.2 guanosine triphosphate. The internal solution for action potential recordings was similar, except that 140 K-gluconate replaced Cs-methanesulfonate. The pH was adjusted to 7.2 with Tris-base and the osmolality to 310 mOsmol/L with sucrose for all internal solutions. excitatory postsynaptic current (EPSCs) mediated by α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors were recorded at a holding potential of -70 mV in the presence of gabazine (10 µM) and strychnine (1 µM) to block GABAA and glycine receptors, respectively. We recorded the electrophysiological events, including EPSCs and spontaneous action potential firing, for 15 minutes and analyzed their frequency by averaging the data in the last 5 minutes.
Stereotaxic Surgery and Postsurgical Care
Six to eight weeks after the initial IE2BC drinking paradigm, when ethanol intake was stable, under isoflurane anesthesia and the use of a stereotaxic apparatus (David Kopf Instruments), each Long-Evans rat in the DBS group was implanted with one bipolar stimulating electrode (tip diameter 0.8mm) into the right LHb in the following coordinates (in mm): -3.7 Bregma, 0.75 mediolateral, and 5.2 ventral to the skull surface (Paxinos and Watson, 2007). In a subgroup of rats, a bipolar stimulating electrode was implanted in the lateral posterior thalamic nucleus, mediorostral (LPMR) in the following coordinates (in mm): -3.8 Bregma, 1.6–2.0 mediolateral, 4.8 ventral to the skull surface, which is within close proximity to the LHb. Rats resumed ethanol drinking one week after surgery. Two to three weeks later, when the drinking levels of rats were stable, DBS was performed. Before DBS, animals were taken from the colony, brought to the experimental room, and handled for 5min per day for four to five times until the experimental day. During this phase, animals became accustomed to the experimenter, the experimental room, and the manipulation procedure.
Deep Brain Stimulation
The DBS protocol consisted of episodes of seven stimuli at 130 Hz, separated by 40ms intervals at 150 µA intensity. It has been shown that this protocol can effectively reduce excitatory synaptic transmission onto ventral tegmental area (VTA)-projecting LHb neurons (B Li et al., 2011). Two DBS (each lasting for 15min) or sham (no stimulation) were applied, one at 4h and the second at 24h, respectively, after the removal of the ethanol bottle. After the second DBS, rats were given access to 20% ethanol for 20min. Ethanol consumption was measured for three consecutive drinking sessions. Only animals with the electrode correctly placed in the LHb were included for data analysis. To determine whether the effect of DBS depends on the frequency of stimulation, we also tested the effects of low-frequency stimulation (LFS: 7 stimuli at 10 Hz, 150 µA, followed with a 40 msec interval) in the LHb.
Measurements of Locomotor Activity
To determine whether the reduction in ethanol drinking was accompanied by motor impairments, we evaluated the effect of LHb HFS on locomotor activity. Two 15min HFS or sham stimuli were applied to the LHb, at 4h and 24h after the removal of the ethanol bottle. Twenty min after the second HFS, rats were placed in the locomotor chambers (TruScan Photobeam Activity Monitors, 41cm × 41cm × 41cm) for 60min sessions. Movement in terms of total distance travelled (cm) was recorded automatically using TruScan 2.0 software, as previously described (J Li et al., 2012b). Locomotion measurement was repeated the following week, when HFS or sham stimuli was reversed, so that all rats received both treatments.
Immunohistochemistry
To evaluate the changes in neuronal activity in the LHb induced by chronic (8–12 weeks) ethanol drinking and withdrawal, we examined c-Fos expression in the LHb in one group of ethanol-naïve rats and four groups of ethanol-drinking rats (each group had six rats), which were sacrificed at 0h, 24h, 72h, and 7 d after the final drinking session. Immunohistochemistry analysis was performed as described previously (J Li et al., 2010, 2012a). Briefly, rats were killed under deep anesthesia with sodium pentobarbital (50mg/kg, i.p.), then perfused transcardially with ice-cold saline, followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4). The brains were then removed and transferred to a solution containing 30% sucrose in 4% paraformaldehyde overnight at 4◦C for cryoprotection.
Coronal brain sections containing the LHb (30 µm) were cut on a freezing microtome (Microm HM550) and then processed for immunohistochemistry. Brain sections were first treated with 0.3% hydrogen peroxide solution to inhibit endogenous peroxidase activity and then incubated for 30 minutes in a solution containing 0.3% Triton X-100 and 3% normal goat serum to minimize nonspecific labeling. Next, the tissue sections were incubated overnight at 4◦C with anti-c-Fos rabbit pAb (EMD Chemicals Inc.), diluted 1:15,000 in 0.1M PBS containing 3% normal goat serum and 0.2% Triton X-100. After several rinses in PBS, sections were incubated first with biotinylated anti-rabbit antibody (1:200, Vector Laboratories), washed again and then incubated with avidin–biotin peroxidase complex from the ABC kit (Vector Laboratories) before visualization of the peroxidase activity in a solution of diaminobenzidine (Vector Laboratories).
Cell Counts and Quantification
Quantitative analysis of immunolabelled cells was performed by using an assisted image analysis system. This system consists of a Nikon Eclipse 80i bright field microscope (Micron Optics) interfaced with a color digital camera Nikon DS-Ri1 digital camera (Micron Optics), and a computer with a NIS-Elements BR 3.0 software (Micron Optics). NIS-Elements BR 3.0 software has the function of object account, which is designed for automated object detection and counting. Fos-positive cells, as indicated by the dark brown staining, were identified when the nuclear structure demonstrated clear immunoreactivity compared with the background level. We counted c-Fos positive cells in the LHb region (inside the black circle in the representative section shown in Figure 1). We identified the LHb region according to a previous report (Andres et al., 1999). The habenula complex, surrounded by the paraventricular, mediodorsal, and centrolateral thalamic nuclei in the right and left sides, was subdivided into the medial (MHb) and LHb. The area of the MHb is characterized by its small, densely-packed neurons, which form sharp demarcation against the LHb complex. The ventral border of the LHb area is distinguished by the formation of the medial and lateral roots of the fasciculus retroflexus. The lateral border of LHb is surrounded by the centrolateral thalamic nuclei. Counting of labelled cells was obtained as a means of positive labelling from LHb sections (every fifth section between -3.36 and -4.2 Bregma were collected and counted) from each animal (a total of six rats). Two investigators who were blind to the experimental protocols performed the visual counting of labelled cells in sections of six animals in each set of experimental conditions.
Figure 1.
Withdrawal from chronic voluntary ethanol drinking significantly increases the number of c-Fos immunoreactive neurons in the lateral habenula. Representative photomicrographs from (A, A’) ethanol-naïve rats and rats chronically drinking ethanol at (B, B’) 0h, (C, C’) 24h, (D, D’) 72h, or (E, E’) 7 days after the removal of the ethanol bottle. The small green squares in A, B, C, D, and E panels indicate the regions shown at higher magnification, respectively, in A’, B’, C’, D’, and E’. Scale bar = 100 µm in A, B, C, D, and E; scale bar = 50 µm in A’, B’, C’, D’, and E’. (F) Summary of the mean numbers of c-Fos-IR cells ± standard error of the mean (SEM). (G) Double staining with antibody to c-Fos (green) and antibody to EAAC1 (marker of glutamate neurons; red), and merged (white arrows). (H) Summary (mean ± SEM) of the percentage of glutamatergic neurons containing c-Fos-IR. Scale bar = 10 µm in G. n = 6 animals/each arm. *p < 0.001 vs. ethanol-naïve counterparts.
Immunofluorescence Staining
To verify that the c-Fos immunoreactivity (IR) positive neurons in the LHb were glutamatergic, we performed immunofluorescence double-staining for glutamate transporter excitatory amino acid carrier 1 (EAAC1) (also known as excitatory amino acid transporter 3 (EAAT3), which presents on neuronal stomata, dendrites, and axon-terminals) and c-Fos protein in the LHb in ethanol-naïve rats and rats at 24h withdrawal from chronic ethanol drinking. After blocking with 10% goat serum, the sections were incubated with one of the two primary antibodies overnight: anti-c-Fos rabbit pAb (1:15,000, EMD Chemicals Inc.) or goat anti-glutamate transporter (EAAC1, 1:6,000; EMD Millipore). After rinsing, the sections were stained with a relevant secondary antibody: fluorescent-conjugated anti-rabbit secondary antibody (1:200; Sigma-Aldrich) or DyLight red conjugated anti-goat secondary antibody (1:200; Vector Laboratories). Sections were washed in PBS and mounted.
Statistical Analysis
Ethanol drinking data were subjected to a two-way repeated measures analysis of variance (RM ANOVA). Parameters showing a significant overall primary effect were subjected to Tukey’s post hoc analysis. One-way ANOVA was used in LHb neuronal firing rate and spontaneous excitatory postsynaptic current (sEPSC) frequency locomotor activity, and immunohistochemical staining results. Statistical significance was set at p < 0.05.
Results
Withdrawal From Chronic Intermittent Voluntary Ethanol Drinking Increases LHb Neuronal Activity
c-Fos immunoreactivity (c-Fos-IR) was found only in a few LHb neurons in ethanol-naïve rats (Figure 1A and A’). One-way ANOVA revealed a main effect of treatment on c-Fos expression in the LHb neurons [F(4,29) = 8.58; p < 0.001]. Post hoc comparisons indicated that the number of c-Fos-IR neurons was robustly increased in rats at 24h withdrawal from chronic drinking (Figure 1B, B’, and F; p < 0.001 vs. ethanol naive). The increase was maintained at 72h withdrawal (Figure 1C, C’, and F; p < 0.001 vs. ethanol naive), and returned to the control level at 7 days of withdrawal (Figure 1E, E’, and F; p = 0.26 vs. ethanol naive). Moreover, double staining with anti-c-Fos and anti-EAAC1 (glutamate transporter, a marker of glutamate neuron) demonstrated that more than 50% of c-Fos-IR cells were glutamatergic neurons (Figure 1G and H). These results indicate that LHb glutamatergic neurons are activated during ethanol withdrawal.
Withdrawal From Chronic Intermittent Voluntary Ethanol Drinking Increases Firing Rate and sEPSC Frequency in LHb Neurons
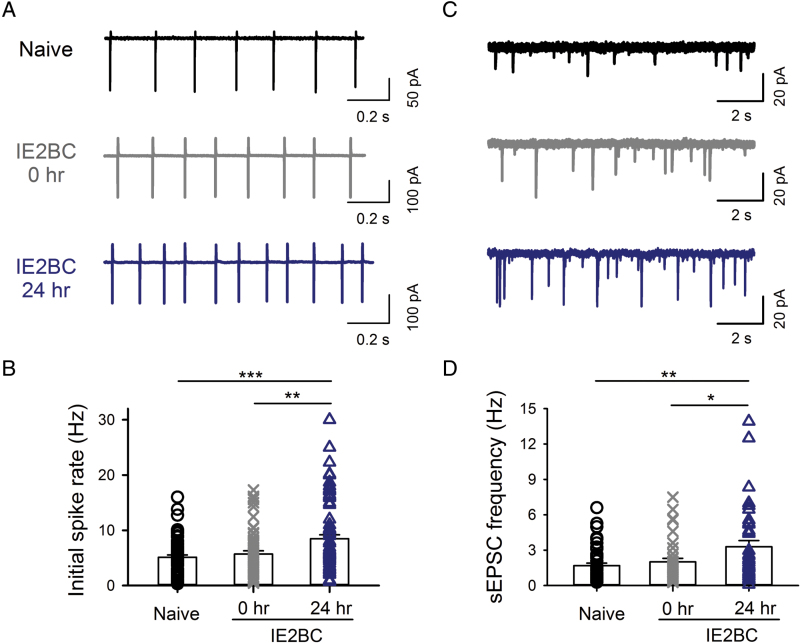
To further understand the change in LHb neurons after chronic ethanol drinking, we measured the electrophysiological events from LHb neurons in brain slices prepared at 0h or 24h after the final ethanol session from rats that had been in the IE2BC paradigm for 8 weeks and from the ethanol-naïve counterparts (n = 13–15 rats/group). The baseline-firing rate of LHb neurons was substantially higher [F(2,207) = 9.47, p < 0.001] in brain slices from rats in the 24h withdrawal from ethanol than those from the ethanol-naive group (p < 0.001) or at 0h ethanol withdrawal (p = 0.004; Figure 2A and B). There was also a substantial increase [F(2,120) = 6.0, p = 0.003] in basal sEPSC frequency in the 24h ethanol-withdrawn group than the naive group (p = 0.003) or the 0h ethanol-withdrawn group (p = 0.042; Figure 2C and D). Additionally, sEPSC amplitude was significant increased [F(2,120) = 6.2, p = 0.003] in the 0h withdrawal group (20.2±0.55 pA; p = 0.039) and 24h withdrawal group (20.9±0.87 pA; p = 0.004) compared with the ethanol-naïve group (17.7±0.64 pA). These data suggest that the glutamate transmission and excitability of LHb neurons increased after chronic ethanol drinking.
Figure 2.
Withdrawal from chronic voluntary ethanol drinking accelerates spontaneous firings and potentiates spontaneous excitatory postsynaptic currents (sEPSCs) in the lateral habenula (LHb) neurons. (A) Sample traces of initial spontaneous spikes, recorded in the cell-attached mode, of LHb neurons in slices of naïve rats and rats at 0h and 24h withdrawal from chronic intermittent access to ethanol in a two-bottle free choice (IE2BC) paradigm. Frequency of initial spikes was significantly higher in LHb neurons from rats at 24h withdrawal from chronic ethanol drinking. (B) Mean ± standard error of the mean (SEM). Data are from individual cells from ethanol-naive rats (5.1±0.4 Hz, n = 69 cells from 15 rats), at 0h withdrawal (5.7±0.6 Hz, n = 62 cells from 13 rats), and at 24h withdrawal of IE2BC rats (8.5±0.7 Hz, n = 79 cells from 15 rats). (C) sEPSC frequency was significantly higher in LHb neurons in slices from rats at 24h withdrawal from IE2BC (3.3±0.3 Hz, n = 39 cells from 15 rats) than the naive group (1.7±0.2 Hz, n = 48 cells from 15 rats) or at 0h withdrawal from IE2BC (2.0±0.5 Hz, n = 36 cells from 13 rats). (D) Mean ± SEM and individual values for ethanol naïve, 0h, or 24h withdrawal from IE2BC rats. *p < 0.05, **p < 0.01, ***p < 0.001, one-way analysis of variance followed by Tukey’s post hoc test.
High Frequency Stimulation of the LHb Produces Enduring Inhibition of Ethanol Consumption
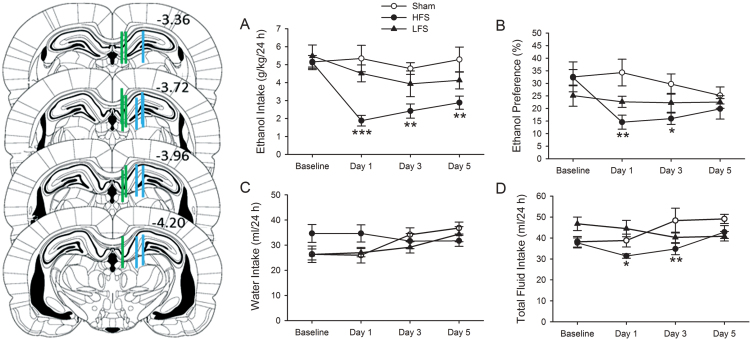
A previous rodent study has shown that electrical stimulation of the LHb with HFS (seven stimuli at 130 Hz, 150 µA, with a 40ms interval) for 1h suppresses excitatory synaptic transmission in the LHb and reverses a depressive-like phenotype in the learned helpless model (B Li et al., 2011). We examined whether the same HFS protocol can alter ethanol consumption. Histological verifications showed that the placement of the electrode tips in four of twelve rats were outside of the LHb; therefore, the data of these four rats were excluded from the statistical analysis. Figure 3 shows the location of the electrode tips within the LHb of the remaining eight rats.
Figure 3.
High-frequency stimulation, but not low-frequency stimulation, in the lateral habenula (LHb) significantly decreases ethanol consumption in rats chronically drinking ethanol. Left Panel: diagram of coronal sections showing the placements of electrode tips from individual rats with accurate placements in the LHb (green bars) or the lateral posterior thalamic nucleus, mediorostral (LPMR, blue bars). There was no significant difference regarding the baseline drinking levels between these three groups. LHb high-frequency stimulation (HFS), but neither low-frequency stimulation (LFS) nor sham stimuli, significantly reduced (A) ethanol consumption (g/kg/24h) on day 1, 3, and 5 and (B) ethanol preference on day 1 and day 3. (C) Water intake was not affected by HFS or LFS at the 24h time point on any test day. (D) HFS in the LHb significantly decreased total fluid intake on day 1. The values are expressed as mean ± standard error of the mean. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with sham (two-way repeated measures analysis of variance followed by Tukey’s post hoc analysis). n = 8 animals for sham and HFS groups, n = 7 for LFS group.
Examination of LHb DBS on alcohol consumption revealed a main effect of treatment [F(2,60) = 13.40; p < 0.001] and a main effect of day [F(3,60) = 4.70; p = 0.005]. Only a tendency for a significance treatment X day interaction was observed [F(6,60) = 1.93; p = 0.09]. Post hoc comparisons revealed that HFS, but not LFS, robustly reduced ethanol consumption. Ethanol intake of rats that received LHb HFS was substantially decreased starting on day 1 and was sustained up to day 5 after HFS, compared with those rats that received sham stimulation (p < 0.001, for day 1; p = 0.003 for day 3 and 5) or with baseline ethanol consumption (p < 0.001, for day 1; p = 0.004 for day 3 and p = 0.02 for day 5). In parallel, two-way RM ANOVA for ethanol preference also revealed a main effect of treatment [F(2,60) = 6.14; p = 0.008] and a strong tendency for a main effect of day [F(3,60) = 2.65; p = 0.05] with no effect of treatment X day interaction [F(6,60) = 1.64; p = 0.15]. Post hoc comparisons indicated that ethanol preference was significantly reduced starting on day 1 and sustained at day 3, but not at day 5, after LHb HFS, but not LHb LFS (p = 0.01 on day 1, p = 0.03 on day 3, p = 0.58 on day 5 HFS vs. sham; Figure 3B). Neither LHb HFS nor LHb LFS affected water intake on any of the test days, compared with sham stimulation (Figure 3C). Total fluid intake was significantly reduced on day 1 after LHb HFS compared with sham stimulation (Figure 3D; p = 0.04 on day 1 and p < 0.001 on day 3). LHb LFS did not significantly alter total fluid intake (Figure 3D) over the 24h access period on any of the test days.
Next, to test whether the effect of HFS is site-specific, we examined HFS on the LPMR, a region adjacent to the LHb. Histological verification found that the electrode tip placements for all animals were in the LPMR (Figure 3A, blue bars). HFS in the LPMR had no significant effect either on the intake of (sham: 6.0±0.5g/kg/24h; HFS: 6.1±0.8g/kg/24h) or preference for ethanol (sham: 30.5±2.1%; HFS: 28.3±2.6%).
To assess whether reduction of ethanol consumption is a result of motor impairment, we examined the effect of LHb HFS on locomotor activity in a separate group of rats. Three of eight animals in this experiment showed the placements of the electrode tips were outside of the LHb, and their data were excluded from the statistical analysis. The activity data for all 10min intervals and the accumulated data showed that LHb HFS did not significantly alter locomotor activity over the 60min analysis (Figure 4A and B). Two-way ANOVA showed a significant increase in the cumulative distance traveled over time (p < 0.001), but with no significant main effect of HFS treatment (p = 0.53) or treatment × time interaction (p = 0.52).
Figure 4.
Lateral habenula (LHb) deep brain stimulation (DBS) does not affect locomotion. (A) Total distance traveled (cumulative cm) across the 60min session after high-frequency stimulation LHb. (B) The time course of the distance traveled during the 60min after LHb DBS or sham (n = 6). The values are expressed as mean ± standard error of the mean. One-way or two-way repeated measures analysis of variance followed by Tukey’s post hoc test.
Discussion
We report here that withdrawal from chronic voluntary ethanol drinking increases the activity of LHb neurons, as evidenced by an increase in c-Fos-IR positive cell numbers, in the spontaneous firing rate and in the frequency and amplitude of sEPSCs in LHb neurons. Importantly, HFS of the LHb produced enduring reduction of ethanol consumption and preference in rats.
Previous studies in primates have shown that LHb neurons are excited by negative stimuli, such as aversive air puffs or cues predicting the absence of reward (Matsumoto and Hikosaka, 2007, 2009). The negative emotions experienced during withdrawal from cocaine can lead to increased excitability of LHb neurons (Neumann et al., 2015). In concordance with their findings, we demonstrated that the number of c-Fos protein-positive neurons was significantly increased at 24h, and lasted for 72h after withdrawal from chronic ethanol drinking (Figure 1). In keeping with this, our electrophysiological data showed that the firing rates and the frequency of sEPSCs of LHb neurons in slices prepared from rats at 24h ethanol withdrawal were significantly increased. Remarkably, there was not a significant change either in c-Fos-IR number or in the frequency of spontaneous firing and of sEPSCs in LHb neurons from rats at 0h ethanol withdrawal. These results indicate that the increase in pre-synaptic glutamatergic tone and in the activity of LHb neurons only occurs during withdrawal. Interestingly, the amplitude of sEPSCs was increased in 0h and 24h, suggesting that the function of the postsynaptic AMPA receptors in the LHb may be increased after chronic alcohol drinking. Further study is needed to confirm this possibility.
The hyperactivity of LHb neurons may inhibit midbrain dopaminergic neurons directly or via activating the inhibitory neurons in the rostromedial tegmental nucleus (Jhou et al., 2009, 2013). This may contribute to the dopamine hypofunction state during ethanol withdrawal (Diana et al., 1993; Weiss et al., 1996; Bailey et al., 2001; RY Shen, 2003; Karkhanis et al., 2015). This dopamine hypofunction is believed to contribute to the excessive drinking in alcohol dependent animals. Therefore, it is not surprising that functional inhibition of LHb through HFS reduced ethanol consumption in rats that have been drinking ethanol chronically. Notably, we failed to detect a significant change in the number of c-Fos-IR cells in the RMTg at 24h ethanol withdrawal, even though there was a pronounced increase after withdrawal from cocaine (data not shown). This observation suggests that the relationship between LHb and RMTg excitability may be more complex than previously thought. This is particularly important in the case of ethanol exposure, which is known to increase the release endogenous opioids, and it is well documented that µ-opioid receptors are richly expressed in the RMTg. Activation of these µ-opioid receptors could inhibit RMTg neurons, which may counteract the excitatory effects of the glutamate transmission from the LHb. Future studies on the relationship between LHb and RMTg during ethanol withdrawal are warranted.
DBS is an adjustable, reversible, non-destructive neurosurgical intervention using implanted electrodes to deliver electrical pulses to areas in the brain. DBS in selected brain regions has shown significant therapeutic benefits for the treatment of refractory obsessive–compulsive disorder, Tourette syndrome, and depressive disorder (Kringelbach et al., 2007). This therapy consists of delivering HFS to the LHb. In the current study, a HFS protocol that has proven effective in depressed patients was delivered through the stimulation electrode to the LHb in rats at 24h withdrawal from chronic ethanol drinking. This produced an enduring inhibition of ethanol consumption, as indicated by a significant decrease in ethanol intake and preference. Remarkably, the reduction lasted for 72h after HFS. This effect was dependent on both the frequency of the stimulation and the placement of the electrode. Whereas a high frequency (130 Hz) produced a strong inhibition of ethanol consumption, a low frequency (10 Hz) had no significant effect (Figure 3). HFS of the LHb did not change locomotion, indicating that the reduction of ethanol consumption is not a result of motor impairment. Moreover, HFS was effective only when the electrode was placed in the LHb, but not in a brain region adjacent to the LHb.
Interestingly, a previous study found that lesions of the LHb increase voluntary ethanol drinking and operant-self administration in rats (Haack et al., 2014), in contrast to the reduced drinking after DBS observed in our current study. The mechanisms underlying the disparity are unclear. However, the experimental conditions of these two studies are different. One major difference is the time in which the DBS or the lesion was applied to the LHb. In Haack’s study, the LHb was lesioned one week before the animal was exposed to ethanol. LHb lesions did not acutely increase voluntary ethanol consumption, but rather increased the rate of escalation of intake, leading to higher sustained levels of ethanol consumption. Based on this observation, these investigators concluded that loss of an ethanol-induced aversive signaling could contribute to the increased ethanol intake in LHb lesioned rats. By contrast, in our current study, LHb HFS was applied to the LHb of rats that have been drinking ethanol for more than two months, when the animals are probably dependent on ethanol (J Li et al., 2011b).
The neuro-circuitry mechanism underlying the long-lasting effect of LHb HFS on ethanol-drinking behavior remains unclear. Although the majority of LHb afferents arise from the forebrain, the LHb also receives a substantial projection from the VTA (Phillipson and Griffith, 1980; Skagerberg et al., 1984; Gruber et al., 2007). Electrical stimulation of the midbrain decreases the firing rate of LHb neurons (X Shen et al., 2012). Optogenetic activation of projections from a unique population of VTA neurons expressing dopaminergic markers to the LHb produced GABA-mediated inhibitory synaptic transmission, which suppressed the firing of postsynaptic LHb neurons in brain slices and promoted reward-related behavior (Stamatakis et al., 2013). Activation of glutamatergic projections from VTA neurons expressing vesicular glutamate transporter 2 (vGluT2) to the LHb produce aversion conditioning (Root et al., 2014). In support of our finding, a previous study has demonstrated that the excitatory synaptic transmission onto VTA-projecting LHb neurons was potentiated in the learned helplessness models of depression. This potentiation is due to an enhanced presynaptic release probability. Depleting transmitter release by repeated electrical stimulation of LHb afferents using the protocol similar to the one used in our study markedly suppressd the synaptic drive onto VTA-projecting LHb neurons and significantly reduced learned helplessness behavior in rats (B Li et al., 2011). Though we did not identify the sources of the excitatory synaptic inputs on LHb neurons, we found that repeated HFS of the LHb significantly reduced ethanol intake and preference. We propose that depleting transmitter release by repeated HFS may be one mechanism underlying LHb HFS-induced inhibition of alcohol drinking.
In summary, the most salient finding of the present study is that withdrawal from chronic voluntary ethanol drinking leads to a substantial increase in LHb neuronal activity. Functional inhibition of the LHb by tetanic HFS reduced ethanol intake in rats. This is the first demonstration of DBS in the LHb reducing ethanol drinking. HFS of the LHb could be a potential new therapeutic option for alcohol-dependent individuals.
Statement Of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by National Institutes of Health grants AA021657 and AA022292 (Dr Ye).
References
- Andres KH, von During M, Veh RW. (1999) Subnuclear organization of the rat habenular complexes. J Comp Neurol 407:130–150. [DOI] [PubMed] [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, Little HJ. (2001) Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology 41:989–999. [DOI] [PubMed] [Google Scholar]
- Diana M, Rossetti ZL, Gessa G. (1993) Rewarding and aversive effects of ethanol: interplay of GABA, glutamate and dopamine. Alcohol Alcohol Suppl 2:315–319. [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R, Yadid G. (2010) Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology 59:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. (2007) Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett 427:165–170. [DOI] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. (2014) Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLOS One 9:e92701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. (2013) Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci 33:7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. (2015) Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 150:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. (2007) Translational principles of deep brain stimulation. Nat Rev Neurosci 8:623–635. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. (2011) Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. (2010) Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res 34:1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye JH. (2011. a) Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res Bull 86:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. (2011. b) Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol 16:600–614. [DOI] [PubMed] [Google Scholar]
- Li J, Sun Y, Ye JH. (2012. a) Electroacupuncture decreases excessive alcohol consumption involving reduction of FosB/DeltaFosB levels in reward-related brain regions. PLOS One 7:e40347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. (2012. b) Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J Pharm Exp Ther 341:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, Mazaheri A, De Vries TJ, Denys D. (2012) Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry 17:572–583. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. (2009) Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Valentinova K, Lecca S, Marion-Poll L, Maroteaux MJ, Musardo S, Moutkine I, Gardoni F, Huganir RL, Georges F, Mameli M. (2015) Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nat Neurosci 18:376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. (2014) Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics 11:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PA, Ishikawa M, Otaka M, Huang YH, Schluter OM, Dong Y. (2015) Increased excitability of lateral habenula neurons in adolescent rats following cocaine self-administration. Int J Neuropsychop 18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2007) The rat brain in stereotaxic coordinates, 6th edn. New York: Elsevier/Academic Press. [Google Scholar]
- Phillipson OT, Griffith AC. (1980) The neurones of origin for the mesohabenular dopamine pathway. Brain Res 197:213–218. [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M. (2014) Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci 34:13906–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY. (2003) Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharm Exp Ther 307:566–572. [DOI] [PubMed] [Google Scholar]
- Shen X, Ruan X, Zhao H. (2012) Stimulation of midbrain dopaminergic structures modifies firing rates of rat lateral habenula neurons. PLOS One 7:e34323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O, Bjorklund A. (1984) Origin, course and termination of the mesohabenular dopamine pathway in the rat. Brain Res 307:99–108. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. (2013) A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16:3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. (2006) Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods 158:251–259. [DOI] [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye JH. (2013) Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology 70:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH. (2015) Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol. doi:10.1111/adb.12298. [Epub ahead of print], PMID: 26283508 [DOI] [PMC free article] [PubMed] [Google Scholar]