Abstract
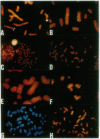
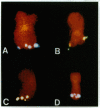
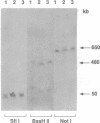
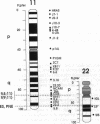
Chromosome translocations are associated with a variety of human leukemias, lymphomas, and solid tumors. To localize molecular markers flanking the t(11;22) (q24;q12) breakpoint that occurs in virtually all cases of Ewing sarcoma and peripheral neuroepithelioma, high-resolution chromosomal in situ suppression hybridization was carried out using a panel of cosmid clones localized and ordered on chromosome 11q. The location of the Ewing sarcoma translocation breakpoint was determined relative to the nearest two cosmid markers on 11q, clones 23.2 and 5.8, through the analysis of metaphase chromosome hybridization. By in situ hybridization to interphase nuclei, the approximate physical separation of these two markers was determined. In both Ewing sarcoma and peripheral neuroepithelioma, cosmid clone 5.8 is translocated from chromosome 11q24 to the derivative chromosome 22 and a portion of chromosome 22q12 carrying the leukemia inhibitory factor gene is translocated to the derivative chromosome 11. The physical distance between the flanking cosmid markers on chromosome 11 was determined to be in the range of 1000 kilobases, and genomic analysis using pulsed-field gel electrophoresis showed no abnormalities over a region of 650 kilobases in the vicinity of the leukemia inhibitory factor gene on chromosome 22. This approach localizes the Ewing sarcoma breakpoint to a small region on chromosome 11q24 and provides a rapid and precise technique for the molecular characterization of chromosomal aberrations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B. Solid tumor cytogenetics. Progress since 1979. Cancer Genet Cytogenet. 1989 Jul 1;40(1):3–12. doi: 10.1016/0165-4608(89)90140-4. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Budarf M., Emanuel B. S., Mohandas T., Goeddel D. V., Lowe D. G. Human differentiation-stimulating factor (leukemia inhibitory factor, human interleukin DA) gene maps distal to the Ewing sarcoma breakpoint on 22q. Cytogenet Cell Genet. 1989;52(1-2):19–22. doi: 10.1159/000132831. [DOI] [PubMed] [Google Scholar]
- Budarf M., Sellinger B., Griffin C., Emanuel B. S. Comparative mapping of the constitutional and tumor-associated 11;22 translocations. Am J Hum Genet. 1989 Jul;45(1):128–139. [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Le Beau M. M., Pitha P., Rowley J. D. Interferon and c-ets-1 genes in the translocation (9;11)(p22;q23) in human acute monocytic leukemia. Science. 1986 Jan 17;231(4735):265–267. doi: 10.1126/science.3455787. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Lewis K. A. Physical mapping of complex genomes by cosmid multiplex analysis. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5030–5034. doi: 10.1073/pnas.86.13.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Lewis K., Rothenberg B. E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989 Jun 30;79(1):9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Gearing D. P., King J. A., Willson T. A., Hilton D. J., Nicola N. A., Metcalf D. Molecular cloning and expression of the human homologue of the murine gene encoding myeloid leukemia-inhibitory factor. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2623–2627. doi: 10.1073/pnas.85.8.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Williams R. L. The pleiotropic actions of leukemia inhibitory factor. Cancer Cells. 1989 Nov;1(3):77–80. [PubMed] [Google Scholar]
- Griffin C. A., McKeon C., Israel M. A., Gegonne A., Ghysdael J., Stehelin D., Douglass E. C., Green A. E., Emanuel B. S. Comparison of constitutional and tumor-associated 11;22 translocations: nonidentical breakpoints on chromosomes 11 and 22. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6122–6126. doi: 10.1073/pnas.83.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska F. G., Tsujimoto Y., Croce C. M. Oncogene activation by chromosome translocation in human malignancy. Annu Rev Genet. 1987;21:321–345. doi: 10.1146/annurev.ge.21.120187.001541. [DOI] [PubMed] [Google Scholar]
- Henderson D. W., Leppard P. J., Brennan J. S., Mukherjee T. M., Swift J. G. Primitive neuroepithelial tumours of soft tissues and of bone: further ultrastructural and immunocytochemical clarification of 'Ewing's sarcoma', including freeze-fracture analysis. J Submicrosc Cytol Pathol. 1989 Jan;21(1):35–57. [PubMed] [Google Scholar]
- Julier C., Nakamura Y., Lathrop M., O'Connell P., Leppert M., Litt M., Mohandas T., Lalouel J. M., White R. A detailed genetic map of the long arm of chromosome 11. Genomics. 1990 Jul;7(3):335–345. doi: 10.1016/0888-7543(90)90167-s. [DOI] [PubMed] [Google Scholar]
- Koduru P. R., Offit K., Filippa D. A. Molecular analysis of breaks in BCL-1 proto-oncogene in B-cell lymphomas with abnormalities of 11q13. Oncogene. 1989 Jul;4(7):929–934. [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H., McNeil J. A. Interphase and metaphase resolution of different distances within the human dystrophin gene. Science. 1990 Aug 24;249(4971):928–932. doi: 10.1126/science.2203143. [DOI] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Tang C. J., Watkins P. C., Manuelidis L., Ward D. C. Rapid detection of human chromosome 21 aberrations by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9664–9668. doi: 10.1073/pnas.85.24.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Philip I., Wiels J., Philip T., Goridis C., Lenoir G. M., Tursz T. Neuroectoderm-associated antigens on Ewing's sarcoma cell lines. Cancer Res. 1987 Jan 1;47(1):183–187. [PubMed] [Google Scholar]
- Lipinski M., Hirsch M. R., Deagostini-Bazin H., Yamada O., Tursz T., Goridis C. Characterization of neural cell adhesion molecules (NCAM) expressed by Ewing and neuroblastoma cell lines. Int J Cancer. 1987 Jul 15;40(1):81–86. doi: 10.1002/ijc.2910400115. [DOI] [PubMed] [Google Scholar]
- McKeon C., Thiele C. J., Ross R. A., Kwan M., Triche T. J., Miser J. S., Israel M. A. Indistinguishable patterns of protooncogene expression in two distinct but closely related tumors: Ewing's sarcoma and neuroepithelioma. Cancer Res. 1988 Aug 1;48(15):4307–4311. [PubMed] [Google Scholar]
- Moreau J. F., Donaldson D. D., Bennett F., Witek-Giannotti J., Clark S. C., Wong G. G. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature. 1988 Dec 15;336(6200):690–692. doi: 10.1038/336690a0. [DOI] [PubMed] [Google Scholar]
- Reynolds C. P., Smith R. G., Frenkel E. P. The diagnostic dilemma of the "small round cell neoplasm": catecholamine fluorescence and tissue culture morphology as markers for neuroblastoma. Cancer. 1981 Nov 1;48(9):2088–2094. doi: 10.1002/1097-0142(19811101)48:9<2088::aid-cncr2820480929>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Turc-Carel C., Gemmill R. M. Chromosomes in solid tumors and beyond. Cancer Res. 1988 Mar 1;48(5):1049–1059. [PubMed] [Google Scholar]
- Selleri L., von Lindern M., Hermans A., Meijer D., Torelli G., Grosveld G. Chronic myeloid leukemia may be associated with several bcr-abl transcripts including the acute lymphoid leukemia-type 7 kb transcript. Blood. 1990 Mar 1;75(5):1146–1153. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R., Baker E., Hyland V. J., Callen D. F., Stahl J., Gough N. M. The gene for human leukemia inhibitory factor (LIF) maps to 22q12. Leukemia. 1989 Jan;3(1):9–13. [PubMed] [Google Scholar]
- Trask B., Pinkel D., van den Engh G. The proximity of DNA sequences in interphase cell nuclei is correlated to genomic distance and permits ordering of cosmids spanning 250 kilobase pairs. Genomics. 1989 Nov;5(4):710–717. doi: 10.1016/0888-7543(89)90112-2. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Aurias A., Mugneret F., Lizard S., Sidaner I., Volk C., Thiery J. P., Olschwang S., Philip I., Berger M. P. Chromosomes in Ewing's sarcoma. I. An evaluation of 85 cases of remarkable consistency of t(11;22)(q24;q12). Cancer Genet Cytogenet. 1988 Jun;32(2):229–238. doi: 10.1016/0165-4608(88)90285-3. [DOI] [PubMed] [Google Scholar]
- Udey J. A., Blomberg B. Human lambda light chain locus: organization and DNA sequences of three genomic J regions. Immunogenetics. 1987;25(1):63–70. doi: 10.1007/BF00768834. [DOI] [PubMed] [Google Scholar]
- Van den Berghe H., David G., Broeckaert-Van Orshoven A., Louwagie A., Verwilghen R., Casteels-Van Daele M., Eggermont E., Eeckels R. A new chromosome anomaly in acute lymphoblastic leukemia (ALL). Hum Genet. 1979 Jan 25;46(2):173–180. doi: 10.1007/BF00291919. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Lewis K. A., Ruiz J. C., Rothenberg B., Zhao J., Evans G. A. Cosmid vectors for rapid genomic walking, restriction mapping, and gene transfer. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2160–2164. doi: 10.1073/pnas.84.8.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Kozak C., Reeves R., Gearhart J., Nunn M. F., Nash W., Fowle J. R., 3rd, Duesberg P., Papas T. S. Conserved chromosomal positions of dual domains of the ets protooncogene in cats, mice, and humans. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1792–1796. doi: 10.1073/pnas.83.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Kao-Shan S., Tsai S., Israel M. A. Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet. 1986 Apr 1;21(3):185–208. doi: 10.1016/0165-4608(86)90001-4. [DOI] [PubMed] [Google Scholar]