Abstract
Intracoronary acetylcholine provocation testing (ACH-test) is an established method for assessment of epicardial coronary artery spasm in the catheterization laboratory which was introduced more than 30 years ago. Due to the short half-life of acetylcholine it can only be applied directly into the coronary arteries. Several studies have demonstrated the safety and clinical usefulness of this test. However, acetylcholine testing is only rarely applied in the U.S. or Europe. Nevertheless, it has been shown that 62% of Caucasian patients with stable angina and unobstructed coronary arteries on coronary angiography suffer from coronary vasomotor disorders that can be diagnosed with acetylcholine testing. In recent years it has been appreciated that the ACH-test not only assesses the presence of epicardial spasm but that it can also be useful for the detection of coronary microvascular spam. In such cases no epicardial spasm is seen after injection of acetylcholine but ischemic ECG shifts are present together with a reproduction of the patient's symptoms during the test. This article describes the experience with the ACH-test and its implementation in daily clinical routine.
Keywords: Medicine, Issue 114, Coronary spasm, coronary microvascular dysfunction, acetylcholine, coronary angiography, vascular smooth muscle cells, angina pectoris
Introduction
Angina pectoris is the hallmark of coronary artery disease and the concept of an epicardial stenosis causing myocardial ischemia and exercise-induced angina has been established for many years. However, many patients with angina pectoris do not have the typical triad of retrosternal pain, onset during exercise and relief by nitroglycerine or rest. Frequently patients report angina pectoris at rest or a combination of exertional and resting angina as well as shortness of breath upon exertion as a possible angina equivalent. In 1959 Prinzmetal was the first to introduce the concept of transient spasm of the coronary arteries causing angina at rest associated with ST-segment elevation on the electrocardiogram (ECG) but with preserved exercise capacity1. This hypothesis was confirmed later using coronary angiography and it appeared that coronary spasm can be present in patients with epicardial stenoses or normal coronary arteries2. In the 1980ies intracoronary acetylcholine provocation testing (ACH-test) for the detection of coronary artery spasm was established in Japan and subsequently the interested in clinical research for coronary spasm increased3.
However, after introduction of percutaneous coronary intervention in 1977 and the first stent implantation in 1986 the interest in coronary vasomotor disorders declined considerably at least in Europe and the United States. This may be also due to the invasive nature of the ACH-test (due to the short half-life of ACH it can only be administered into the coronary arteries for the assessment of coronary spasm). Still, many patients with signs and symptoms of myocardial ischemia do not have any relevant epicardial stenosis on coronary angiography4,5,6. In such patients intracoronary acetylcholine provocation testing is useful to detect a clinically relevant coronary vasomotor disorder and institute appropriate medical therapy7.
Acetylcholine is a neurotransmitter in the parasympathetic nervous system. It acts via nicotinergic as well as muscarinergic (mAChR) receptors. The latter are important for vascular homeostasis and acetylcholine binds on mAChR as a non-selective agonist. Activation of these receptors at the endothelial level leads to nitric oxide mediated vasodilatation whereas activation of mAChR on the vascular smooth muscle cells leads to vasoconstriction8. Depending on the integrity of the endothelium and the reactivity of the smooth muscle cells the net effect of intracoronary acetylcholine administration is vasodilatation or vasoconstriction. The physiologic response of coronary arteries in response to acetylcholine in human beings is not fully understood but it has been reported that vasodilatation as well as vasoconstriction of up to 25% of the vessel diameter may be physiologic as shown in patients with normal coronary arteries and no angina pectoris9.
Intracoronary acetylcholine provocation testing has been recommended by the European Society of Cardiology guidelines10 as well as the Japanese Circulation Society guidelines11 for assessment of epicardial and/or microvascular spasm in patients with angina pectoris and unobstructed coronary arteries. Intracoronary acetylcholine provocation testing has been established in daily clinical routine in the catheterization laboratory of our institution in 2003. Since 2006 a standardized protocol has been followed12. ACH-testing is generally performed in all patients with signs and symptoms of myocardial ischemia yet no relevant epicardial stenosis (<50%) on coronary angiography. ACH-testing is performed immediately after diagnostic coronary angiography according to the protocol described below. Ergonovine is another agent that is used for spasm provocation testing with a different mechanism of action. Detailed information on ergonovine testing can be found elsewhere12.
Protocol
NOTE: Intracoronary acetylcholine testing has been approved by the local ethics committee and the protocol follows the guidelines of our institution for human research.
1. Preparation of the Acetylcholine Solutions (See Materials Table)
Mix the 20 mg acetylcholine with the 2 ml solvent provided with the package.
Add the 2 ml ACH solution to 98 ml of NaCl 0.9 %. This corresponds to a dose of 0.2 mg/ml and is called stock solution 1. Add 9 ml of stock solution 1 to 91 ml of NaCl 0.9%, This corresponds to a dose of 18 µg/ml and is called stock solution 2.
Prepare 3 perfusion syringes (each of 50 ml) labeled as "high", "medium" and "low". Fill the perfusion syringe 1 labeled "high" with 40 ml of stock solution 2.
Fill the perfusion syringe 2 (labeled "medium") with 8 ml of the perfusion syringe 1 (high) and add 32 ml of NaCl 0.9%. This corresponds to a dose of 3.6 µg/ml.
Fill the perfusion syringe 3 (labeled "low") with 4 ml of the perfusion syringe 2 (medium) and add 36 ml of NaCl 0.9%. This corresponds to a dose of 0.36 µg/ml.
2. Preparation of the Syringes for Intracoronary Injection of Acetylcholine
Prepare 5 syringes for the intracoronary injection (4 syringes of 5 ml [#1, #2, #3, #4] and 1 syringe of 10 ml).
Fill the 5 ml syringe #1 with 6 ml from the perfusion syringe labeled "low" (see step 1.5). This corresponds to a dose of approximately 2 µg.
Fill the 5 ml syringe #2 with 6 ml from the perfusion syringe labeled "medium". This corresponds to a dose of approximately 20 µg.
Fill the 5 ml syringe #3 with 5.5 ml from the perfusion syringe labeled "high". This corresponds to a dose of approximately 100 µg.
Fill the 10 ml syringe with 11 ml from the perfusion syringe labeled "high". This corresponds to a dose of approximately 200 µg.
Fill the 5 ml syringe #4 with 4.5 ml from the perfusion syringe labeled "high". This corresponds to a dose of approximately 80 µg. Put this syringe aside and only use it for assessment of the right coronary artery.
3. Diagnostic Coronary Angiography
Inject local anaesthesia either in proximity to the right radial artery (usually 2 ml of mepivacain) or in proximity to the right femoral artery (usually 15 ml of mepivacain).
Confirm the success of local anesthesia is by pricking the anesthetized-skin with the needle and asking the patient if pain is still present.
Puncture the artery according to the Seldinger technique13 with a cannula, then insert the wire through the cannula and remove it. Insert the sheath (usually 5F) over the wire. Perform coronary angiography under sterile conditions.
Advance the wire through the sheath to the ascending aorta and place the diagnostic catheter above the aortic valve. Then remove the wire and connect the catheter with the contrast syringe. NOTE: The contrast agent contains Iomeprol, Trometamol, hydrochloric acid and water.
Place the diagnostic catheter for the left coronary artery in the left main stem by slightly pulling and turning the catheter. Confirm correct positioning of the catheter by injecting 2 ml of contrast agent.
Perform coronary angiography according to the Judkins technique14 with manual injections of approximately 10 ml of contrast to visualize the coronary arteries in different views. NOTE: Usually LAO 40° and RAO 35° are used for the right coronary artery and LAO 45°/CRAN 25°, RAO 30°/CRAN 30° and RAO 20°/CAUD 30° are used for the left coronary artery.
Start acetylcholine testing after exclusion of any relevant epicardial stenosis (≥50%) by visual assessment.
4. Intracoronary Injection of Acetylcholine
Inject 6 ml of the 2 µg syringe #1 into the left coronary artery. Inject this within 20 sec with continuous monitoring of the ECG and the patient's symptoms (e.g. chest pain, shortness of breath, dizziness). Perform coronary angiography of the left coronary artery (usually a RAO 20°/CAUD 30° projection is best projection) after injection of the 6 ml.
Perform coronary angiography by injecting 10 ml of contrast manually via the contrast syringe into the catheter. The 12-lead-ECG should be recorded and printed after every dose of acetylcholine. A pause of 1 min should lie between every dose.
Inject 6 ml of the 20 µg syringe #2 into the left coronary artery. Inject this within 20 sec with continuous monitoring of the ECG and the patient's symptoms. Perform coronary angiography of the left coronary artery after injection of the 6 ml as mentioned above.
Inject 5.5 ml of the 100 µg syringe #3 into the left coronary artery. Inject this within 20 sec with continuous monitoring of the ECG and the patient's symptoms. Perform coronary angiography of the left coronary artery after injection of the 5.5 ml as mentioned above. NOTE: Most patients report some symptoms, show ECG changes or epicardial vasoconstriction at this dose. Sometimes the speed of the manual injection needs to be slowed down. As mentioned below different protocols for ACH-testing are in use with a different speed of injection. A slower injection over a period of 3 min compared to the injection within 20 sec may also be feasible.
If no spasm (i.e. >90% vasoconstriction compared to the relaxed state of the vessel after 200 µg nitroglyercine injection) occurs at the 100 µg dose continue with the 200 µg (10 ml syringe) ACH dose. Inject the 11 ml within 20 sec with continuous monitoring of the ECG and the patient's symptoms.
Perform coronary angiography of the left coronary artery after injection of the 11 ml as mentioned above (section 3). NOTE: Although the 200 µg dose has been applied in many patients it should be mentioned that appropriate doses of intracoronary acetylcholine remain to be fully defined. Some authors suggest that 100 µg should be the maximum dose for the left coronary artery. NOTE: Often bradycardia occurs and the speed of the injection needs to be slowed down.
Inject 80 µg ACH (4.5 ml, syringe #4) into the right coronary artery if no abnormal findings are seen during testing of the left coronary artery. Inject this within 20 sec with continuous monitoring of the ECG and the patient's symptoms. Often bradycardia occurs and the speed of the injection needs to be slowed down to prevent prolonged bradycardia and/or asystole.
Perform coronary angiography of the right coronary artery after injection of the 4.5 ml (usually a LAO 40°/0° projection is best) as mentioned above (section 3).
Inject intracoronary nitroglycerine with a dose of 200 µg into each tested artery after the test or when severe symptoms (i.e. severe chest pain or severe shortness of breath), ischemic ECG shifts or epicardial spasm occurs.
Image the vessel as mentioned in step 4.2 after one minute to document reversion of spasm.
Representative Results
Interpretation of the acetylcholine test is based on three criteria. First, the patient is asked throughout the test whether or not symptoms occur. Frequently, patients report a reproduction of their usual symptoms such as chest pain, shortness of breath or other symptoms. This represents an important point for the overall interpretation of the test. Second, a 12-lead-ECG registration is continuously performed throughout the test with a special emphasis on ischemic ECG shifts such as ST-segment depression, ST-segment elevation and T-wave alternans. Third, the coronary angiogram is repeated after every dose of acetylcholine to assess the epicardial narrowing compared to the relaxed state after nitroglycerine administration. The latter is done visually but is also done quantitatively with dedicated software afterwards. A focal or diffuse epicardial diameter reduction 90% compared to the relaxed state after nitroglycerine administration is considered pathologic (previously a 75% criterion was applied but we chose to now follow the Japanese guideline recommendations11).
The acetylcholine test is uneventful if none of the above mentioned criteria is met. If one of the above criteria is met (e.g. solely reproduction of symptoms or ischemic ECG shifts only) the test is termed unequivocal. Epicardial coronary spasm is diagnosed when an epicardial vasoconstriction 90% compared to the relaxed state after nitroglycerine administration together with ischemic ECG shifts and reproduction of the patient's symptoms is seen (Figure 1 and 2). Microvascular spasm is diagnosed in case of a reproduction of the patient's symptoms with ischemic ECG shifts (usually ST-segment depression) during the ACH-test without epicardial vasoconstriction 90% (Figure 3)15.
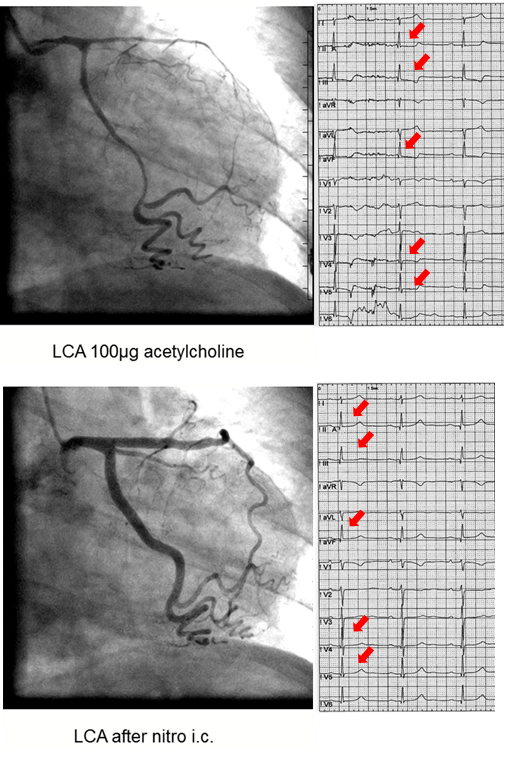 Figure 1:Diffuse Epicardial Spasm. Left coronary artery angiograms and ECGs of a 40 year old female patient with chest pain at rest showing diffuse epicardial spasm (mainly of the left anterior descending artery) after 100 µg of acetylcholine (top) with concomitant ST-segment depression (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
Figure 1:Diffuse Epicardial Spasm. Left coronary artery angiograms and ECGs of a 40 year old female patient with chest pain at rest showing diffuse epicardial spasm (mainly of the left anterior descending artery) after 100 µg of acetylcholine (top) with concomitant ST-segment depression (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
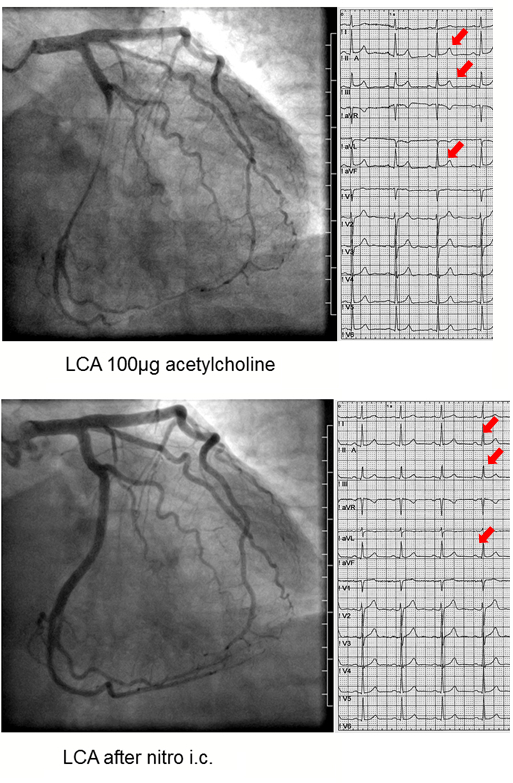 Figure 2:Prinzmetal-Type Epicardial Spasm. Left coronary artery angiograms and ECGs of a 43 year old male patient with chest pain at rest showing focal Prinzmetal-type epicardial spasm in the left circumflex artery after 100 µg of acetylcholine (top) with concomitant ST-segment elevation (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
Figure 2:Prinzmetal-Type Epicardial Spasm. Left coronary artery angiograms and ECGs of a 43 year old male patient with chest pain at rest showing focal Prinzmetal-type epicardial spasm in the left circumflex artery after 100 µg of acetylcholine (top) with concomitant ST-segment elevation (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
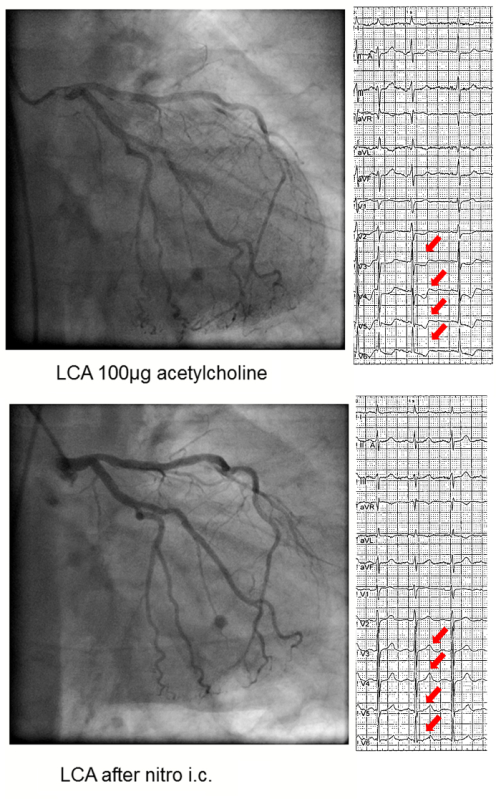 Figure 3:Coronary Microvascular Spasm. Left coronary artery angiograms and ECGs of a 66 year old woman with shortness of breath upon exertion and rest angina showing no epicardial spasm after 100 µg of acetylcholine but ST-segment depression (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
Figure 3:Coronary Microvascular Spasm. Left coronary artery angiograms and ECGs of a 66 year old woman with shortness of breath upon exertion and rest angina showing no epicardial spasm after 100 µg of acetylcholine but ST-segment depression (red arrows) and reproduction of the patient's usual symptoms. The latter findings resolved after intracoronary nitroglycerine injection (bottom). Please click here to view a larger version of this figure.
Discussion
It is feasible to implement the acetylcholine test in daily clinical routine in the catheterisation laboratory. Apart from the preparation of the ACH solutions there are several technical issues that have to be resolved before starting the test including radiolucent ECG leads for continuous 12-lead ECG registration. This is essential to be able to detect transient ischemic ECG changes during the test. Moreover, it is important to know that the ACH solutions can only be used for 2 hr. After that they should be newly prepared.
It is essential to interrogate the patient about any symptoms occurring during the test (known or unknown). One should not only ask for chest pain or dyspnoea but any symptoms that the patient may experience during the test. In addition, the patient must be asked if the symptoms have previously occurred in everyday life.
Frequently, ACH administration leads to bradycardia and atrio-ventricular (AV) block. However, due to the short half-life of acetylcholine this is only of short duration if the manual injection is slowed or shortly interrupted. After restoration of normal sinus rhythm the acetylcholine injection can be continued with a slower speed. However, the AV block is sometimes sustained and can cause severe bradycardia with Adams-Stokes attacks. Thus, insertion of a temporary pacing wire may be recommended in selected cases.
There is no current consensus regarding the speed of the injection. Previous protocols have applied manual injections over 3 min for each dose. However, the Japanese Guidelines recommend manual injections within 20 sec. Of note, these quicker injections may only be possible when a temporary pacing wire is in place due to the risk of bradycardia, especially when the right coronary artery is challenged.
If the patient experiences severe symptoms and/or significant ECG changes are seen the test should be stopped and the vessel should be imaged one more time. After that intracoronary nitroglycerine administration should be immediately performed (usually 200 µg). In most cases this will alleviate symptoms and revert the spasm and the ECG changes. Sometimes, often in patients with microvascular spasm, another nitroglycerine injection is necessary. In rare cases with refractory spasm despite intracoronary nitroglycerine administration atropine (e.g. 1 mg) should be administered intravenously as this is the antagonist of acetylcholine. This usually leads to vasodilatation and improvement of symptoms. Generally the complication rate is around 1 % including e.g. non-sustained ventricular tachycardia, bradycardia or coronary dissection. This rate corresponds with the complication rate described for diagnostic coronary angiography16.
Frequently assessment of the left and right coronary artery with acetylcholine may not be possible because of a pathologic test result during the assessment of the left coronary artery injection and the need of intracoronary nitroglycerine injection. This in our opinion may alter the results for the right coronary artery. However, assessment of severity of spasm (i.e. presence of multivessel spasm) may only be possible when all coronary arteries are challenged.
A frequent criticism concerns the specificity of the acetylcholine test, i.e. it is suggested that acetylcholine would induce coronary spasm in any individual if the dose is only high enough. Indeed, there is no data on the effect of intracoronary acetylcholine testing in human beings without any coronary pathology. However, around 30% of patients exhibit coronary microvascular spasm in response to acetylcholine without any relevant epicardial vasoconstriction indicating that despite the high dose of 200 µg of ACH in the left coronary artery epicardial spasm often cannot be provoked. Nevertheless, a study on healthy young volunteers assessing the effects of intracoronary acetylcholine administration would be desirable, although such a study may be difficult to perform due to the invasive nature of the acetylcholine test.
In recent years it has appeared that the ACH-test is not only a test for the assessment of epicardial coronary spasm but also for coronary microvascular spasm. Although the coronary microcirculation cannot be visualized in human beings in vivo at the moment, the combination of ischemic ECG-shifts during the ACH-test together with reproduction of the patient's symptoms without demonstrable epicardial spasm on angiography has been accepted as a definition for microvascular spasm. Thus, coronary microvascular spasm patients can be regarded as a subgroup of patients with coronary microvascular dysfunction.
Future applications of this protocol could include serial assessments with ACH as part of an investigation of new pharmacological substances. Depending on the pharmacokinetics of the respective drug ACH-testing could be performed at baseline and after administration of a study drug assessing coronary vasomotion before and after treatment. Due to the short half-life of ACH the test can only be performed invasively by injecting the ACH into the coronary arteries. Other substances such as ergonovine may be given intravenously but it has been debated if such an approach is equally effective. Moreover, other non-invasive tests for provocation of coronary spasm such as hyperventilation testing have shown limited sensitivity and are thus of limited use17.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project is supported in part by grant KKF-15-1.
References
- Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–388. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- Cheng TO, Bashour T, Kelser GA, Jr, Weiss L, Bacos J. Variant angina of Prinzmetal with normal coronary arteriograms. A variant of the variant. Circulation. 1973;47(3):476–485. doi: 10.1161/01.cir.47.3.476. [DOI] [PubMed] [Google Scholar]
- Yasue H, et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74(5):955–963. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- Bell MR, Berger PB, Holmes DR, Jr, Mullany CJ, Bailey KR, Gersh BJ. Referral for coronary artery revascularization procedures after diagnostic coronary angiography: evidence for gender bias? J Am Coll Cardiol. 1995;25(7):1650–1655. doi: 10.1016/0735-1097(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Patel MR, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):885–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Henderson RA, Seed P, Treasure T, Hampton JR. Quality of life, employment status, and anginal symptoms after coronary angioplasty or bypass surgery. 3-year follow-up in the Randomized Intervention Treatment of Angina. Circulation. 1996;94(2):135–142. doi: 10.1161/01.cir.94.2.135. [DOI] [PubMed] [Google Scholar]
- Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59(7):655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Lee JD, Ogawa K, Hara A, Nakamura T. Coronary artery vasoreactivity to intracoronary acetylcholine infusion test in patients with chest pain syndrome. Intern Med. 1992;31(1):22–27. doi: 10.2169/internalmedicine.31.22. [DOI] [PubMed] [Google Scholar]
- Montalescot G, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013) Circ J. 2014;78(11):2779–2801. doi: 10.1253/circj.cj-66-0098. [DOI] [PubMed] [Google Scholar]
- Ong P, Athanasiadis A, Sechtem U. Patterns of coronary vasomotor responses to intracoronary acetylcholine provocation. Heart. 2013;99(17):1288–1295. doi: 10.1136/heartjnl-2012-302042. [DOI] [PubMed] [Google Scholar]
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- Judkins MP. Selective coronary arteriography. I. A percutaneous transfemoral technic. Radiology. 1967;89(5):815–824. doi: 10.1148/89.5.815. [DOI] [PubMed] [Google Scholar]
- Mohri M, et al. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351(9110):1165–1169. doi: 10.1016/S0140-6736(97)07329-7. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. 2001;52:289–295. doi: 10.1002/ccd.1067. [DOI] [PubMed] [Google Scholar]
- Nakao K, et al. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80(5):545–549. doi: 10.1016/s0002-9149(97)00419-0. [DOI] [PubMed] [Google Scholar]


