Abstract
Objective
WHO and UNICEF recommend cup feeding for neonates unable to breastfeed in low-resource settings. In developed countries, cup feeding in lieu of bottle feeding in the neonatal period is hypothesized to improve breastfeeding outcomes for those initially unable to breastfeed. Our aim was to synthesize the entire body of evidence on cup feeding.
Methods
We searched domestic and international databases for original research.
Our search criteria required original data on cup feeding in neonates published in English between January 1990 and December 2014.
Results
We identified 28 original research papers. Ten were randomized clinical trials, 7 non-randomized intervention studies, and 11 observational studies; 11 were conducted in developing country. Outcomes evaluated included physiologic stability, safety, intake, duration, spillage, weight gain, any and exclusive breastfeeding, length of hospital stay, compliance, and acceptability. Cup feeding appears to be safe though intake may be less and spillage greater relative to bottle or tube feeding. Overall, slightly higher proportions of cup fed versus bottle fed infants report any breastfeeding; a greater proportion of cup fed infants reported exclusive breastfeeding at discharge and beyond. Cup feeding increases breastfeeding in subgroups (e.g. those who intend to breastfeed or women who had a Caesarean section). Compliance and acceptability is problematic in certain settings.
Conclusions
Further research on long-term breastfeeding outcomes and in low-resource settings would be helpful. Research data on high risk infants (e.g. those with cleft palates) would be informative. Innovative cup feeding approaches to minimize spillage, optimize compliance, and increase breastfeeding feeding are needed.
Keywords: Cup, cup feeding, Paladai, Neonates, Preterm, Feeding, Systematic Review
Introduction
Breastfeeding offers the best nutrition and is the optimal method for feeding neonates.(1, 2) Unfortunately, not all infants can be breastfed. Preterm infants, infants with an oral cleft or other anomalies, and infants with metabolic, neurologic, or developmental immaturities may encounter breastfeeding difficulties.(3, 4) In some cases, infants are unable to breastfeed because the mother is unavailable, sick, or has nipple damage.(4, 5)
Cup feeding has a long history of being a neonatal feeding option.(6–8) In high-resource settings, nasogastric tubes and bottles are the default tools of choice when an infant is unable to breastfeed. Feeding cups in high-resource settings are used by some in the short-term to deliver supplemental feeding and to avoid ‘nipple confusion’, a theory that exposure to artificial nipples interferes with a neonate’s ability to breastfeed.(9) WHO and UNICEF recommend cup feeding in low-resource settings where water quality is poor and electricity unreliable.(4, 10–12) In these settings, nasogastric tubes may not be available and bottles have crevices that promote infection.(13, 14) Cups are easier to keep clean and are less likely than bottles to be used for long-term storage of milk which can facilitate bacterial contamination. Cup feeding may supplement breastfeeding, minimize exposure to nasogastric tubes, or serve as a long-term feeding solution for those never able to breastfeed.(3, 4, 15) Advantages of cup feeding include enhanced bonding, a greater sense of maternal control and confidence, the ability to engage other family members in the infant’s care, and freeing up nursing staff when caregivers conduct feedings.(3, 4, 15–19) Studies propose that cup feeding provides the infant positive oral, tactile, and auditory stimulation, exposure to the smell and taste of breast milk, tongue and motor skill experience, and the ability to control feeding pace.(3–5, 8, 17) Reported concerns about cup feeding include that it is too slow, prone to spillage, results in insufficient intake(19–21), or that milk poured from a cup into the infant’s mouth increases the risk of choking or aspiration.(5, 19)
Two Cochrane reviews evaluated the extent to which cup feeding and avoidance of bottle-feeding in the neonatal period influenced breastfeeding outcomes.(22, 23) These reviews included four of ten published RCTs. The outcomes in the Cochrane reviews were limited. There are 24 studies on cup feeding that have never been summarized, including a recently published RCT(24) and numerous observational studies that examine outcomes not in the reviews. Synthesizing the breadth of outcomes has important implications for understanding cup feeding and identifying gaps in knowledge. We conducted a systematic review of neonatal cup feeding to synthesize the broad body of evidence and identify gaps to facilitate research.
Methods
We broadly included all studies with original data collection on cup feeding in the neonatal period conducted in humans and written in English between 1990 and 2014. We searched the MEDLINE database and a global health database, CABdirect, for all papers that met these criteria. Search terms included cup*, palada* (paladai), suthi* AND newborn or infant AND human AND English from January 1, 1990 to December 2014. The search was last conducted February 27, 2015. The symbol * denotes the root word of the search. A paladai (also referred to as a suthi) is an infant feeding cup used in India which is a small (10ml) metal cup with a long slender pour spout (Figure 2).(14) Research on spoon feeding was excluded.
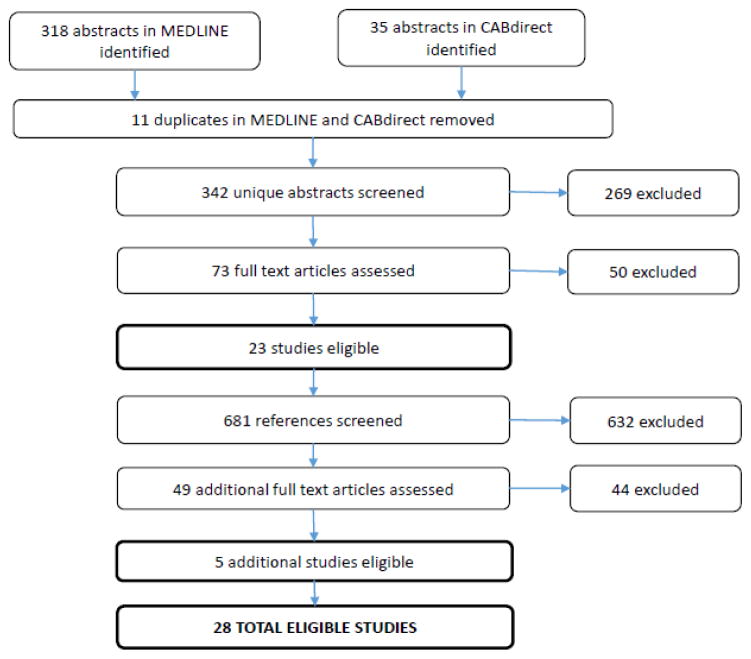
Figure 2.
Flowchart of abstracts, references and papers reviewed to identify eligible studies
We reviewed abstracts to identify original research articles. We also reviewed the reference lists of all included papers and abstracted additional articles for review. All studies including case reports, case series, observational studies, non-randomized intervention studies and RCTs were included. We abstracted study design and country, type of participants, gestational of age, number of participants, comparison, and outcomes into Excel. To the extent available, we reported original results including means, mean differences, p-values, prevalence relative risks, and 95% confidence intervals (CIs). When not calculated, we used raw frequencies to calculate these statistics to facilitate comparisons. We grouped studies according to study design because RCTs typically have less bias that observational studies and by gestational age since effects in the outcomes we report (e.g. physiologic measures, breastfeeding outcomes) may differ based on gestational age.
Results
We reviewed 342 abstracts and 681 references to identify 28 studies that meet inclusion criteria (Figure 1). There were ten RCTs, seven non-randomized intervention studies, and eleven observational studies (Table 1). Five studies employed a cross-over design where the infant acted as her own control. (21, 25–28) Four studies evaluated provider and/or caregiver preferences.(29–32) All RCTs except for one (33) were conducted in high-resource countries. Six studies were conducted in upper-middle income countries (Brazil, Turkey) and five studies were in lower-middle income countries (India, Egypt); no studies were in the least developed countries (Table 1).(34) Twenty-three studies employed a comparison group using a different feeding method. Nineteen compared a cup to a bottle, six compared breastfeeding to a cup and/or bottle, two compared Paladai to bottle-feeding, and one compared Paladai to tube feeding (Table 1). Most studies did not describe the cup or bottle used.(20, 24, 26, 28, 30, 35–39) The scope of research was broad and fell into five domains: 1) Physiologic stability and safety; 2) Intake, duration, spillage and weight gain/loss; 3) Breastfeeding outcomes; 4) Duration of hospitalization; and 5) Feeder compliance and acceptability. We also ordered studies by topic (see Online Resource 1).
Figure 1.
Table 1.
Original research studies on cup feeding ordered by study design and sample size
| Author, Year | Study design details and population | N | GA (weeks) | Country | Feeding Methods Used: Cup/Paladai | Bottle/Tube/Breast | Domains | |
|---|---|---|---|---|---|---|---|---|
| Randomized clinical trials (RCT)
| ||||||||
| 1 | Howard et al., 2003 | Healthy neonates | 700 | 36–42 | USA | Cup+early pacifier=185 Cup/late pacifier=179 |
Bottle/early pacifier=169 Bottle/late pacifier=179 |
|
| 2 | Yilmaz, et al., 2014 | Late preterm | 607 | 32–35 | Turkey* | Cup=254 | Bottle=268 |
|
| 3 | Schubinger et al., 1997 | Healthy full-term neonates | 602 | >37 | Switzerland | UNICEF protocol that included cup or spoon with no artificial teats=294 | Standard protocol of bottle post-breastfeeding, pacifier offered=308 |
|
| 4 | Collins et al., 2004 | Early preterm neonates | 303 | 23–33 | Australia | Cup, no pacifier=82 Cup + pacifier=69 |
Bottle, no dummy=70, Bottle + dummy=82 |
|
| 5 | Howard et al., 1999 | Healthy neonates | 98 | 36–42 | USA | Cup=51 | Bottle=47 Breast=25 |
|
| 6 | Rocha et al., 2002 | Preterm neonates | 78 | 32–36 | Brazil* | Cup=44 | Bottle=34 |
|
| 7 | Marinelli et al., 2001 | Early preterm neonates, cross-over study | 56 | ≤34 | USA | Cup=56 | Bottle=56 |
|
| 8 | Gilks et al., 2004 | Early preterm neonates | 54 | 25–34 | UK | Cup=27 | Bottle=27 |
|
| 9 | Aloysius et al., 2007 | Preterm neonates, cross-over study | 15 | 32–36 | UK | Paladai=15 | Bottle=15 |
|
| 10 | Mosley, et al., 2001 | Preterm neonates | 14 | 32–37 | UK | Cup=6 | Bottle=8 |
|
|
| ||||||||
| Non-randomized intervention studies
| ||||||||
| 11 | Huang, et al., 2009 | Full term singleton neonates, no congenital anomalies, vaginal birth | 205 | >37 | Taiwan | Cup=67 | Bottle=62 Breast=76 |
|
| 12 | Malhotra et al., 1999 | Cross-over study, neonates in special care nursery, also included evaluation of nurses | 100 | All | India | Cup=100 Paladai=100 |
Bottle=100 |
|
| 13 | Abouelfettoh et al., 2008 | Late preterm neonates | 60 | 34–37 | Egypt | Cup=30 | Bottle=30 |
|
| 14 | Freer, 1999 | Preterm neonates, cross-over study | 20 | 28–31 | UK | Cup=32 feedings | Bottle=32 feedings |
|
| 15 | Gomes et al., 2006 | Healthy full-term infants 2–3 months of age | 60 | >37 | Brazil | Cup + breast=20 | Bottle + breast=20 Breast=20 |
|
| 16 | Rekha et al., 1996 | Preterm neonates | 32 | 33–37 | India | Paladai= 16 | Tube= 16 |
|
| 17 | Lopez et al., 2014 | Preterm neonates, cross-over study | 19 | 34–36 | Brazil | Cup=19 | Bottle=19 |
|
|
| ||||||||
| Observational studies | ||||||||
|
| ||||||||
| 18 | Lang, 1994 | Cohort study, neonates | 475 | All | UK | Cup=85 | Not cup=372 |
|
| 19 | Al-Sahab et al., 2010 | Cross-sectional, providers | 103 | n/a | Canada | n/a | n/a |
|
| 20 | Franca, et al., 2014 | Cross-sectional, term | 81 | 37–42 | Brazil | Cup=27 | Bottle=27, Breast=27 |
|
| 21 | Brown et al. 1999 | Cross-sectional, neonatesfull term | 63 | >37 | UK | Cup= 30 | Bottle= 33 |
|
| 22 | Cloherty et al., 2005 | Ethnographic study of mothers and providers | 60 | n/a | UK |
|
||
| 23 | Gupta et al., 1999 | Case-series, neonatespreterm | 59 | <37 | India | Cup |
|
|
| 24 | Nyqvist et al., 1999 | Evalaution of nurses and parents’ use of two cups | 48 | Sweden | Medicine cup Spouted cup |
|
||
| 25 | Dalal et al., 2013 | Case series, preterm neonates | 20 | 28–32 | India | Paladai=20 |
|
|
| 26 | Dowling et al., 2002 | Case series, neonatespreterm | 8 | 30–37 | USA | Cup |
|
|
| 27 | Gomes, et al., 2014 | Case series, preterm | 5 | 28–35 | Brazil | Cup=1 | Bottle=2, Breast=2 |
|
| 28 | Thorley et al., 1997 | Case report | 1 | n/a | Australia | Cup |
|
|
Physiologic Stability and Safety
Many clinical practitioners express concern that cup feeding may increase adverse events such as aspiration, or oxygen desaturation.(5, 19, 40) Overall, compared to bottle feeding, cup feeding tended to have higher oxygen saturation levels, and a smaller fraction of infants experiencing oxygen saturation <90% and <85% and equivalent or less elevation of heart and respiratory rates. There were no consistently reported adverse physiologic events across studies. Collins, et al. reported no adverse events in early premature infants.(41) Marinelli, et al., found no differences in choking, spitting up or apnea, or bradycardia between cup and bottle fed infants (all p-values >0.05), although these were noted in both groups of neonates.(25) Aloysius, et al. (n=15) reported that 73% had stress cues when paladai feeding compared to 20% bottle feeding, but there was no difference in preterm neonate stress cues (p=0.67).(26) A case report proposes infant aspiration can occur with an improper feeding technique, but a recent RCT with 522 infants reported no apnea or aspiration.(10, 24) A case series of the paladai in very preterm infants found 12/68 feedings had desaturation but 7 feedings occurred in 2 infants.(42)
Intake, Duration of Feeding, Spillage, and Weight Gain
Clinical practitioners express concern that cup feeding may not provide sufficient intake, is time-consuming, and that spillage results in decreased intake.(19) All comparative studies that examined intake reported lower intake with a cup compared to bottle or tube. Of the five studies with hypothesis testing, only one reported a statistically significant lower intake with cup feeding. Findings on feeding duration were variable. Two studies reported cup feeding took more time(25, 26); two less time(33, 43); and one the same time(24) as bottle feeding. Cup feeding took less time than breastfeeding.(27) Cup feeding was associated with a 3-fold increase in spillage compared to a bottle(21, 26) and more spillage than the paladai.(21) All of these comparisons were statistically stable (p-values<0.01).(21, 26) The mean spillage when using a cup was high (25% to 39%).(16, 21) No studies found statistically significant differences in newborn weight loss/gain with the cup versus bottle feeding.(24, 33, 39–41, 44) Data from six studies on weight gain were not tabulated because of variability in measures reported (see Online Resource 1).(24, 33, 39–41, 44)
Breastfeeding Outcomes
The primary reason to cup feeding in high-resource settings is to optimize the likelihood the infant will successfully initiate and sustain breastfeeding. Consequently, the extent to which cup, as compared to bottle, influences breastfeeding in infancy is a primary outcome of interest. We identified eleven reports on breastfeeding outcomes; seven of these were RCTs(24, 33, 36, 38, 39, 41, 44) and four were observational studies.(3, 30, 35, 45) The most commonly reported breastfeeding outcomes were any or exclusive/full breastfeeding. Exclusive breastfeeding was similarly defined by most studies as receiving all food from the breast.(3, 35, 36, 38, 41, 44) Two studies classified infants who had taken vitamins or minerals as exclusively breastfed.(24, 41) Two studies defined ‘full breastfeeding’ and ‘almost exclusive breastfeeding’ as breastfeeding with infrequent feedings (e.g. <1 per day) of other liquids such as water or herbal drinks.(35, 44)
Breastfeeding was reported at or near the time of hospital discharge and up to 6 months of age. Any breastfeeding at hospital discharge was reported by eight studies. All of the studies that employed a comparison group reported a greater proportion of cup-fed as compared to bottle-fed infants with any breastfeeding at hospital discharge, but the differences were mostly small and only the largest RCT(24) showed statistically significant differences.(24, 30, 33, 36, 41) Findings were similar when any breastfeeding outcomes post-discharge up to six months were examined. Other statistically significant differences for cup versus bottle fed infants for any breastfeeding were in subgroups (e.g. mothers who had a Caesarean section) (Table 4).
Table 4.
Breastfeeding in cup versus bottle fed infants by study design and time point measured
| Any breastfeeding and/or breast milk | Time point | GA | Study Design | Author | Cup | Bottle | p | Estimate | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Any breastfeeding versus none (%) – At hospital discharge | Discharge | PT | RCT | Yilmaz et al., 2014 | 99 | 91 | <0.001 | 1.08 | 1.05, 1.13 |
| Discharge | PT | RCT | Gilks et al., 2004 | 52 | 44 | 0.59 | 1.2 | 0.7, 2.0 | |
| Discharge | PT | RCT | Collins et al., 2004a | 74 | 68 | 0.27 | 1.1 | ||
| Discharge | PT | RCT | Rocha et al., 2002 | 81.8 | 79.4 | 0.79 | 1.0 | 0.8, 1.3 | |
| Discharge | FT | Observ. | Brown et al., 1999 | 70 | 55 | 0.30 | 1.9 | 0.6, 6.3 | |
| Discharge | All | Observ. | Lang, 1994 | 92.1 | |||||
| Discharge | PT | Observ. | Gupta, 1999 | 89.8 | |||||
| Any breastfeeding versus none (%) – After discharge | Day 5 | FT | RCT | Schubinger et al., 1997 | 100 | 99.3 | ns | ||
| 5–19 days post discharge | PT | RCT | Rocha et al., 2002 | 43.2 | 44.1 | 0.93 | 1.0 | 0.6, 1.6 | |
| 2 months | FT | RCT | Schubinger et al., 1997 | 88.0 | 87.7 | ns | -- | -- | |
| 3 months post discharge | PT | RCT | Yilmaz et al., 2014 | 88 | 82 | 0.09 | 1.06 | 0.99, 1.14 | |
| 3 months post discharge | PT | RCT | Collins et al., 2004a | 42 | 36 | 0.33 | 1.2 | ||
| 3 months post discharge | PT | RCT | Rocha et al., 2002 | 29.5 | 14.7 | 0.12 | 2.0 | 0.8, 5.1 | |
| 4 months | FT | RCT | Schubinger et al., 1997 | 75.4 | 70.5 | ns | -- | -- | |
| 6 months | PT | RCT | Yilmaz et al., 2014 | 69 | 59 | 0.02 | 1.2 | 1.03, 1.3 | |
| 6 months | FT | RCT | Schubinger et al., 1997 | 57.0 | 55.3 | ns | -- | -- | |
| 6 months post discharge | PT | RCT | Collins et al., 2004a | 31 | 24 | 0.22 | 1.3 | ||
| Any breastfeeding (days) | Duration, in days | FT | RCT | Howard et al., 2003b | 105 | 140 | 0.50 | 0.9 | 0.8, 1.1 |
| Subgroups | |||||||||
| Any breastfeeding(days), Those who had >2 supplemental Feedings | Duration | FT | RCT | Howard et al., 2003 | ---- | ---- | ns | ---- | ---- |
| Any breastfeeding(days), Those who had a cesarean section | Duration | FT | RCT | Howard et al., 2003 | 161 | 90 | 0.04 | ---- | ---- |
| Any breastfeeding versus none(%), Those who complied with assigned method | Not specified | PT | RCT | Collins et al., 2004 | -- | -- | 0.004 | 21.1 | 2.6, 169.8 |
| Any breastfeeding versus none(%), Those breastfeeding 5–19 days post discharge | 3 months post discharge | PT | RCT | Rocha et al., 2002 | 68.4 | 33.3 | 0.04 | 2.0 | 0.9, 4.5 |
|
| |||||||||
| Exclusive or full breastfeeding or received only breast milk | |||||||||
|
| |||||||||
| Exclusively breastfeeding versus not (%) | Prior to discharge | PT | RCT | Mosley et al., 2001 | 66.7 | 75.0 | 0.88 | 0.9 | 0.4, 2.5 |
| Exclusively breastfeeding versus not (%) | Discharge | PT | RCT | Yilmaz et al., 2014 | 72 | 46 | <0.000 | 1.6 | 1.4, 1.8 |
| Exclusive breastfeeding versus not (%) | Discharge | PT | RCT | Gilks et al., 2004 | 37.0 | 14.8 | 0.06 | 2.5 | 0.9, 7.0 |
| Fully breastfed versus not (%) | Discharge | PT | RCT | Collins et al., 2004a | 61 | 47 | 0.03 | 1.3 | |
| Exclusively breastfed (%) | Discharge | All | Observ. | Lang, 1994 | 90 | -- | -- | -- | -- |
| Exclusively breastfed versus not (%) | Discharge | PT | Observ. | Gupta, 1999 | 55.9 | ||||
| Exclusive, high breastfeeding vs. medium/low breastfeeding(%) | 1-week after discharge | PT | Interv. | Abouelfettoh et al., 2008 | 67.0 | 40.0 | 0.04 | 1.7 | 1.0, 2.8 |
| Almost exclusively breastfeeding or fed with expressed breast milk versus not (%) | 1-week after discharge | PT | Interv. | Abouelfettoh et al., 2008 | 47 | 33 | 0.29 | 1.4 | 0.7, 2.6 |
| Exclusively breastfeeding versus not (%) | 3 months | PT | RCT | Yilmaz et al., 2014 | 77 | 47 | <0.001 | 1.6 | 1.4, 1.9 |
| Exclusively breastfeeding versus not (%) | 6 months | PT | RCT | Yilmaz et al., 2014 | 57 | 42 | <0.001 | 1.4 | 1.1, 1.6 |
| Exclusive breastfeeding (median, days) | Duration | FT | RCT | Howard et al., 2003b | 21 | 14 | 0.29 | 1.1 | 0.9, 1.3 |
| Full breastfeeding2 (median, days) | Duration | FT | RCT | Howard et al., 2003b | 45 | 37 | 0.74 | 1.0 | 0.8, 1.2 |
| Subgroups | |||||||||
| Exclusive breastfeeding versus not (%), Those that roomed-in | In hospital | PT | RCT | Gilks et al., 2004 | 91.0 | 36.4 | 0.01 | 2.5 | 1.1, 5.6 |
| Exclusively breastfed versus not (%), Those that intended to breastfeed | Discharge | All | Observ. | Lang, 1994c | 81 | 63 | <0.01 | 1.3 | 1.1, 1.5 |
| Exclusive breastfeeding, Those who had >2 supplemental feedings | Duration | FT | RCT | Howard et al., 2003 | ---- | ---- | <0.01 | ---- | ---- |
| Full breastfeeding, Those who had >2 supplemental feedings | Duration | FT | RCT | Howard et al., 2003 | ---- | ---- | <0.01 | ---- | ---- |
| Exclusive breastfeeding (median, days) Those who had a Cesearan section | Duration | FT | RCT | Howard et al., 2003 | 21 | 11 | 0.04 | ---- | ---- |
| Full breastfeeding (median, days), Those who had a Cesearan section | Duration | FT | RCT | Howard et al., 2003 | 56 | 21 | 0.02 | ---- | ---- |
NOTE: Bolded, italicized estimates were calculated based on data provided in the paper; GA = Gestational Age; PT = Preterm; FT = Fullterm; RCT = Randomized Clinical Trial
No confidence interval was reported nor calculated since there were a substantial number of twins that could not be accounted for in a post-hoc analysis
Hazard ratios were adjusted for predictors that were statistically significant at the p<0.10 level
Comparison was ‘not cup fed’ rather than bottle-fed
Most measures of exclusive breastfeeding were collected around the time of hospital discharge. Of the six studies that employed a comparison group(24, 35, 36, 38, 41, 44), all but one small study(38) reported a higher prevalence or longer duration of exclusive breastfeeding in cup versus bottle fed infants. The larger (24, 41) but not the smaller (36, 38) RCTs in preterm infants reported statistically significant differences in exclusive breastfeeding at discharge. The only RCT to examine exclusive breastfeeding at 3 and 6 months post discharge reported cup fed infants were more likely to exclusively breastfeed than bottle-fed infants (p<0.001).(24) Statistically significant differences were reported in subgroups (Table 4).
Length of Stay in Hospital
Four studies reported on the length of stay in hospital for cup versus bottle or tube fed infants. Four of the 5 studies on length of stay in hospital, including a large RCT, reported stays were similar or shorter for cup versus breastfeeding infants and none of the differences in length of stay were statistically significant (Table 5). One RCT in Australia reported extended hospital stays among cup fed infants relative to bottle fed infants(41), which has raised concern that cup-feeding increases cost and demand for limited resources.(22, 23, 41) However, the authors reported no difference in duration of hospital stay among those who complied with their assigned feeding method (p=0.27), and found that length of stay after supplemental feeding by cup or bottle was started was similar for infants fed by cup versus bottle (12 versus 11 days, p=0.05).(41)
Table 5.
Hospital stay according to feeding method by gestational age and study design
| Author | Measure | GA | Study Design | Duration of hospital stay | Cup | Bottle/Tube | Difference | p |
|---|---|---|---|---|---|---|---|---|
| Yilmaz et al., 2014 | Cup vs. bottle | PT | RCT | Duration of hospital stay (days, mean, SD) | 25.7 (2.2) | 25.9 (2.2) | 0.2 | 0.15 |
| Collins et al., 2004 | Cup vs. bottle | PT | RCT | Duration of hospital stay (days, median, IQR) | 59 (37–85) | 48 (33–65) | 11 | 0.01 |
| Aboulefettoh et al., 2008 | Cup vs. bottle | PT | Interv. | Duration of hospital stay (days, mean, SD) | 15.5 (8.1) | 19.4 (9.8) | −3.9 | 0.09 |
| Rekha, 1996 | Paladai vs. tube | PT | Interv. | Duration of hospital stay (days, mean) | 10.5 | 10 | 0.5 | |
| Brown, et al., 1999 | Cup vs. bottle | FT | Observ. | Median difference in maternity unit stay after supplement started (days) | 1 | 0.09 | ||
|
| ||||||||
| Subgroups | ||||||||
|
| ||||||||
| Collins et al., 2004 | Cup vs. bottle | PT | RCT | Duration of hospital stay after supplemental feeding started (days) | 12 | 11 | 1 | 0.46 |
| Duration of hospital stay among those <28 weeks gestational age (days, IQR) | 93 (86–113) | 93 (72, 100) | 0 | 0.03 | ||||
| Duration of hospital stay among those 28 to 34 weeks gestational age (days, IQR) | 45 (32–66) | 40 (32–55) | 5 | 0.01 | ||||
| Among the subgroup who complied with assigned method | ---- | ---- | ---- | 0.27 | ||||
NOTE: GA = gestational age, PT = preterm, FT=full term, Observ. = observational study, RCT = randomized clinical trial, Interv. = intervention study, SD = standard deviation, IQR = interquartile range.
Compliance and Acceptability
Non-compliance with cup feeding was the primary limitation of the RCT in Australia that examined cup versus bottle-feeding on breastfeeding outcomes; 56% of participants assigned cup feeding group were given a bottle.(41) Problems reported included spillage, taking too much time, the infant not feeding well, and staff refusing to feed an infant with a cup.(41) In the United Kingdom, mothers assigned cup feeding were 3.6 times more likely to withdraw from the trial than mothers assigned bottle feeding (p=0.01).(36) In contrast, an RCT in Switzerland found 9.5% assigned to a cup feeding protocol requested a bottle or had trouble cup feeding.(39) In an RCT in Turkey, 8.7% were non-compliant with the cup compared to 6.8% non-compliant with a bottle (p=0.39).(24) A study in the US reported similar levels of compliance with 89% and 93% using the assigned cup or bottle respectively.(44) These studies suggest >90% compliance with cup feeding in certain settings may be feasible.
Five studies reported on provider opinions about cup feeding.(21, 26, 29, 31, 32) The largest study (N=103) in Canada found beliefs about ‘nipple confusion’ and safety and utility of cup feeding varied by provider type.(32) A study in the UK found that nurses found cup feeding more difficult than bottle feeding.(26) More than 50% of nurses in Sweden reported difficulties with a new cup feeding protocol; hygiene rules were not followed and nurses thought cup feeding may not meet intake requirements.(31) An ethnographic study in the UK reported nurses thought the cup could be messy, though the majority liked the cup because it allowed the infant to ‘control the rate at which they [were] fed’. Most thought cup feeding should be given by a clinical provider - not the mother.(29) A study in India reported nurses unanimously preferred the paladai over the bottle and cup without a pour spout and thought it took less time and effort of the infant to feed than the other methods.(21) The primary disadvantage of the paladai is that, because it is made of steel, it sometimes cuts an infant’s lip.(21)
Discussion
We identified 28 original research articles on cup feeding in newborn infants. All studies were initiated after birth in a hospital setting. Neonatal cup feeding appears to be physiologically safe though intake may be less and spillage greater relative to bottle or tube feeding. Similar proportions of cup and bottle fed infants were breastfed at hospital discharge but cup fed infants appear more likely to be exclusively breastfed. Among certain subgroups, cup versus bottle feeding was statistically significantly associated with an increase in any and exclusive breastfeeding. Compliance and acceptability varied and may be problematic in certain settings.
In terms of safety, evidence on respiratory stability suggests that cup feeding is as or more stable than bottle-feeding in pre- and full term neonates that are generally healthy. A greater proportion of preterm and normal term infants with oxygen desaturation were bottle-fed suggesting infants cup feeding may be more physiologically stable.(25, 33, 43) Future studies that evaluate strategies to optimize physiologic stability while cup feeding may be informative and have particular relevance for infants with respiratory or cardiac problems. Cup size and shape may influence physiologic stability of cup feeding and may benefit from investigation.
Studies reporting on intake and spillage consistently demonstrate that cup fed infants may take in less and spill more than bottle-fed infants. Though cup fed infants may have lower intake, most studies did not report a statistically significant difference in weight loss. Differences in weight loss measures make it difficult to compare studies. Standardization of this measure would benefit future studies. Bottle fed infants having greater intake and greater oxygen desaturation is consistent with our hypothesis of a faster feeding pace with bottle-feeding. Future research could test this theory by comparing cup to bottles with different flow rates. Most studies that examined intake and spillage did not report on weight loss and vice versa. Future research that comprehensively evaluates intake, spillage, weight loss, and weight gain over time in a single RCT of cup versus bottle feeding could address whether lower intake translates into poorer weight gain.
Although most studies reported no difference in ‘any breastfeeding’ at or after hospital discharge, cup fed infants were more likely to be exclusively breastfed than bottle-fed infants. Our findings on ‘any breastfeeding’ are consistent with two Cochrane reviews.(22, 23) These reviews examined the same four RCTs on cup feeding (one also included a study on nasogastric tubes).(33, 36, 38, 41) Although not as rigorous as those in the Cochrane series, our findings included many additional studies may provide insight for future research.(3, 30, 35, 39, 44) The conclusion by one review that ‘cup feeding confers no significant benefit in maintaining breastfeeding beyond hospital discharge’ may be premature. The recent RCT from Turkey found exclusive breastfeeding was statistically significant higher at 6 months post discharge in cup fed infants.(24) Exclusive breastfeeding for the first six months of life has wide ranging, well-established benefits to mother and infant. Future RCTs should consider examining exclusive breastfeeding through 6 months post-hospital discharge. Several studies identified subgroups for whom cup feeding may be helpful (e.g. mothers who delivered via cesarean section or roomed-in). Since the cesarean rate is relatively high in high- and middle-income countries and is increasing in low-incomes countries this may be an important consideration in breastfeeding promotion globally.(46, 47) Although post-hoc findings from subgroup analyses should be viewed with caution, they do provide directions for future research.
Existing research indicates non-compliance with cup feeding is multifactorial and may involve nursing staff training and compliance, mother’s intention, and an infant’s ability to breastfeed. Nurses and parents do not always find cup feeding acceptable.(29, 36, 41) That those who complied with cup-feeding in one RCT were 21 times more likely to breastfeed than those who complied with bottle-feeding(41) suggests that cup feeding, when used as prescribed, could be a potent solution to transitioning preterm infants to breastfeeding. The mechanism by which cup feeding may enhances breastfeeding remains unclear. One explanation is that cup feeding avoids the ‘nipple confusion’ introduced by a bottle.(4) Another possibility we posit is that cup feeding is inconvenient or inefficient enough to motivate mothers to do everything possible to breastfeed. Lower intake and greater spillage with cup feeding likely affects compliance and acceptability. Clinical staff and parent acceptance may vary by context. For example, cup feeding is acceptable and even preferable to bottles in India and Kenya.(21, 48) In Europe and other high-resource settings, hospitals that routinely use and train providers on cup feeding may have greater compliance than hospitals that do not prescribe cup feeding.(8, 24, 48) In middle-income countries (e.g. Turkey) cup feeding occurs (Table 1), however there is little information on the acceptability of cup feeding in these settings. It is known that some report poor compliance even with extensive training.(41) Reasons for non-compliance and methods to improve cup feeding compliance should be investigated further.
Extended hospital stay was the primary reason the Cochrane reviews did not recommend cup feeding. The recommendation is based on a single RCT conducted in Australia.(41) Given there was no difference in length of stay in those who complied with their assigned feeding method in this study, length of hospital stay may not be due to cup feeding per se, but dissatisfaction with the method that led to a transition to another feeding method.(41) That the four other studies, including a recent large RCT from Turkey not in the Cochrane review found minimal differences in length of hospital stay suggests this recommendation may need to be reconsidered.(24, 30, 35, 40)
UNICEF and WHO programs and guidelines recommend hand expression of breast milk and cup feeding for infants unable to breastfeed in low-resource and emergency settings.(12, 49–52) Cup feeding may reduce intake and increase spillage however this needs to be carefully weighed against alternatives such as the availability of nasogastric tubes or the risks of bottle feeding in low-resource settings.(53, 54) Certain cup shapes or sizes may improve outcomes. For example, the paladai compared to a generic cup minimizes spillage.(21) A cup feeder’s training and skill may also influence intake and spillage. In low-resource settings, cup feeders are often mothers rather than nurses. Ensuring caregivers have the skill to optimally feed their infant may have a large impact on outcomes and infant survival. Current practices, compliance, and acceptability of cup feeding should be assessed in low-resource settings. Research on cup feeding is needed in low-resource settings such as Sub-Saharan Africa where cup feeding is the standard of care for infants unable to breastfeed, particularly since there is little existing research from these settings and it is the WHO and UNICEF recommendation.
There are limitations to the existing evidence. Few studies report on comparable outcomes. Within each domain, there was substantial variation in measures, making it difficult to compare studies. Several studies had methodological limitations. Some did not employ a comparison group(16, 42, 45) and many had small sample sizes.(10, 16, 26–28, 38, 42) Because most studies did not describe the cup or bottle used, it was impossible to evaluate the impact of cup design on outcomes. Shape, material, and ergonomics of feeding tools may influence intake, spillage, and feeding efficiency. Only one RCT analyzed their data using the gold standard intent-to-treat analysis.(41) Most non-randomized studies conducted unadjusted analysis.(3, 20, 30, 40) Not adjusting for confounding factors in observational studies (e.g. gestational age) could result in incorrect inference. Our search was limited to studies published in English and so we may have missed some information.
Given the wide reaching and well-established benefits of breast milk and long-term breastfeeding, perhaps the most important area of investigation is to evaluate exclusive long-term breastfeeding outcomes (e.g. 3 and 6 months), and breastfeeding outcomes in subgroups such as mothers who intend to breastfeed or had a Cesarean section. Additional research in low-resource settings is needed to optimize cup feeding in these settings. Lastly, research in infants with anomalies (cleft palate) that interfere with breastfeeding, particularly in low-resource settings, is needed to establish whether or not cup feeding is superior to other options (especially bottle) in these infants. Innovative approaches to cup feeding that optimize physiologic stability, milk intake, weight gain, and improve acceptability could potentially have a large impact on the long term health of infants with breastfeeding difficulties globally.
Supplementary Material
Table 2.
Physiologic stability and safety measures by type of comparison, gestational age and study design
| Heart Rate (beats/minute) | Respiratory Rate (breaths/minute) | Oxygen Saturation (mean %) | Proportion (%) of infants with O2 saturation <85% or 90% | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Article | Comparison | GA | Study Design | Cup | Comparison | Differ ence | P | Cup | Compar ison | Differ ence | p | Cup | Comp arison | Difference | p | % <O2 Saturation | Cup | Comparison | Difference | p |
| Marinelli et al., 2001 | Cup v. bottle | PT | RCT | 168.4 | 171.8 | −3.4 | .009 | 49.7 | 48.8 | 0.9 | 0.46 | 96.5 | 94.5 | 2.0 | 0.02 | <90% | 5.0 | 13.0 | −8.0 | 0.02 |
| Rocha et al., 2002 | Cup v. bottle | PT | RCT | ---- | ---- | ---- | ---- | ---- | ---- | ---- | 90.8 | 87.7 | 3.1 | ns | <85% | 13.6 | 35.3 | −21.7 | 0.02 | |
| Howard et al., 1999 | Cup v. bottle | FT | RCT | ---- | ---- | −3.6 | 0.11 | ---- | ---- | 0.3 | 0.74 | Diff | 0.2 | 0.78 | <85% | 5.9 | 14.9 | −9 | ns | |
| Howard et al., 1999 | Cup v. breast | FT | RCT | ---- | ---- | 6.5 | 0.22 | ---- | ---- | −2.7 | 0.01 | Diff | 1.4 | 0.04 | <85% | 5.9 | 0 | 5.9 | ---- | |
| Freer, 1999 | Cup v. breast | PT | Interv. | 155 | 159 | −4 | 0.08 | ---- | ---- | 94 | 96 | −2 | 0.05 | |||||||
| Dalal, 2013 | Paladai | PT | Observ. | 142 | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ---- | ||||||||
NOTE: GA = gestational age, PT = Preterm, FT = Fullterm, Observ. = observational study, RCT = randomized clinical trial, Interv. = intervention study, “-----“ means not reported, % = percent, Diff = difference in means only reported, not actual values by type, ns = not statistically significant; Bolded, italicized estimates were calculated based on data provided in the paper.
Table 3.
Intake, duration and spillage of feeding by study design
| Intake (ml / feed) | Duration of Feeding (min / feed) | Spillage (% / feed) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Comparison | GA | Study Design | Cup | Comparison | p | Cup | Comparison | p | Cup | Comparison | p |
| Yilmaz et al., 2014 | Cup v. bottle | PT | RCT | ---- | ---- | ---- | 13.7 | 13.6 | 0.32 | ---- | ---- | ---- |
| Marinelli et al., 2001 | Cup v. bottle | PT | RCT | 20.9 | 27.2 | 0.001 | 20.1 | 16.3 | 0.002 | ---- | ---- | ---- |
| Rocha et al., 2002 | Cup v. bottle | PT | RCT | ---- | ---- | ---- | 11.8 | 13.4 | ns | ---- | ---- | ---- |
| Aloysius et al., 2007 | Paladai v. bottle | PT | RCT | 23.1 | 29.6 | 0.20 | 17.8 | 13.1 | 0.06 | 12.1a | 3.6a | 0.004 |
| Howard et al., 1999 | Cup v. bottle | FT | RCT | 29.1 | 35.3 | >0.05 | 5.3 | 5.9 | <0.05 | ---- | ---- | ---- |
| Howard et al., 2003 | Cup v. bottle | FT | RCT | 67 | 121 | ---- | ---- | ---- | ---- | ---- | ---- | ---- |
| Huang et al., 2009 | Cup v. bottle | FT | Interv. | 363b | 438b | 0.06 | ---- | ---- | ---- | ---- | ---- | ---- |
| Malhotra et al., 1999 | Cup v. bottle | All | Interv. | ---- | ---- | ---- | ---- | ---- | ---- | 25.9 | 1.5 | <0.001 |
| Cup v. paladai | All | Interv. | ---- | ---- | ---- | ---- | ---- | ---- | 25.9 | 6.0 | <0.001 | |
| Paladai v. bottle | All | Interv. | ---- | ---- | ---- | ---- | ---- | ---- | 6.0 | 1.5 | <0.001 | |
| Freer, 1999 | Cup v. breast | PT | Interv. | ---- | ---- | ---- | 11.1 | 12.4 | ---- | ---- | ---- | ---- |
| Dowling et al., 2002 | Cup v. bottle | PT | Observ. | 4.6 | ---- | ---- | 15.2 | ---- | ---- | 38.5 | ---- | ---- |
| Brown et al., 1999 | Cup v. bottle | FT | Observ. | 28.4 | 33.9 | 0.15 | ---- | ---- | ---- | ---- | ---- | ---- |
| Dalal et al, 2013 | Paladai | PT | Observ. | 91–100 | ---- | ---- | 2.3–2.6 | ---- | ---- | ---- | ---- | ---- |
NOTE: GA = gestational age, PT = Preterm, FT=Fullterm, Observ. = observational study, RCT = randomized clinical trial, Interv. = intervention study, ml = milliliters, min = minutes, % = percentage
Calculated based on raw data provided in original source paper
Average amount consumed during the entire hospital stay (duration of hospitalization not provided)
Significance.
What is known about this subject?
Two Cochrane reviews summarized 4 randomized clinical trials and found that women who cup versus bottle or tube fed their infant were more likely to fully breastfeed at discharge. Cup feeding had no effect on any breastfeeding but extended length of stay.
What this study adds?
Twenty-four studies on cup feeding cover questions and clinical outcomes that have never been synthesized. We provide the first comprehensive review of original research on a wide range of cup feeding outcomes (physiologic stability, intake, breastfeeding, length of stay, compliance, acceptability) and propose new areas for research.
Acknowledgments
Drs. McKinney and Ms. Rue were supported by NIH NCATS grant #2KL2TR000421-06. This work was also supported by the Jean Renny Endowment for Craniofacial Research, Seattle Children’s Hospital Craniofacial Center.
Footnotes
Conflicts of Interest. The authors declare they have no conflicts of interest.
Contributor Information
Christy M. McKinney, Acting Assistant Professor, Oral Health Sciences, School of Dentistry, University of Washington, Seattle, WA.
Robin P. Glass, Occupational Therapist, Occupational Therapy, Seattle Children’s Hospital, Clinical Assistant Professor, Department of Rehabilitation Medicine, University of Washington, Seattle, WA.
Patricia Coffey, Social Scientist, Program for Appropriate Technology in Health (PATH), Seattle, WA.
Tessa Rue, Biostatistician, Institute of Translation Health Sciences, University of Washington, Seattle, WA.
Matthew G. Vaughn, Research Assistant, Oral Health Sciences, School of Dentistry, University of Washington, Seattle, WA.
Michael Cunningham, Professor, Pediatrics, School of Medicine, University of Washington, Seattle, WA.
References
- 1.World Health Organization (WHO) Beyond Survival. 2013. Pan America Health Organization (PAHO) [Google Scholar]
- 2.Horta BL, Victora CG World Health Organization (WHO) Short-term effects of breastfeeding: A systematic review on the benefits of breastfeeding on diarrhoea and pneumonia mortatlity. 2013 [Google Scholar]
- 3.Lang S, Lawrence CJ, Orme RL. Cup feeding: an alternative method of infant feeding. Archives of disease in childhood. 1994;71(4):365–9. doi: 10.1136/adc.71.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorley V. Cup-feeding. Australian Breastfeeding Association; East Malvern: 2005. [Accessed 27 April 2015]. https://www.breastfeeding.asn.au/bfinfo/cup-feeding. [Google Scholar]
- 5.Kuehl J. Cup feeding the newborn: what you should know. J Perinat Neonatal Nurs. 1997;11(2):56–60. doi: 10.1097/00005237-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Fredeen RC. Cup feeding of newborn infants. Pediatrics. 1948;2(5):544–8. [PubMed] [Google Scholar]
- 7.Davis HV, Sears RR, et al. Effects of cup, bottle and breast feeding on oral activities of newborn infants. Pediatrics. 1948;2(5):549–58. [PubMed] [Google Scholar]
- 8.Lang S. Cup-feeding: An alternative method. Midwives Chron Nurs Notes. 1994;107:171–6. [PubMed] [Google Scholar]
- 9.Neifert M, Lawrence R, Seacat J. Nipple confusion: toward a formal definition. J Pediatr. 1995;126(6):S125–9. doi: 10.1016/s0022-3476(95)90252-x. doi:a64223. [DOI] [PubMed] [Google Scholar]
- 10.Thorley V. Cup feeding: problems created by incorrect use. J Hum Lact. 1997;13(1):54–5. doi: 10.1177/089033449701300118. [DOI] [PubMed] [Google Scholar]
- 11.Edmond K, Bahl R. Optimal feeding of low-birth-weight infants: technical review. Geneva: Department of Child and Adolescent Health and Development, World Health Organization; 2006. [Google Scholar]
- 12.(WHO) UaWHO. Baby-friendly hospital initiative: Revised Updated and Expanded for Integrated Care. Section 3: Breastfeeding promotion and support in a baby-friendly hospital. A 20-hour course for maternity staff. 2009 [PubMed] [Google Scholar]
- 13.Surjono D, Ismadi SD, Suwardji, et al. Bacterial contamination and dilution of milk in infant feeding bottles. J Trop Pediatr. 1980;26(2):58–61. doi: 10.1093/tropej/26.2.58. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong H. Research in action; techniques of feeding infants-the case for cup feeding. Cajanus. 1998;31(4):178–85. [Google Scholar]
- 15.Ritchie JF. Immature sucking response in premature babies: Cup feeding as a tool in increasing maintenance of breast. J Neonatal Nursing. 1998;4(2):13–7. [Google Scholar]
- 16.Dowling DA, Meier PP, DiFiore JM, et al. Cup-feeding for preterm infants: mechanics and safety. J Hum Lact. 2002;18(1):13–20. doi: 10.1177/089033440201800103. [DOI] [PubMed] [Google Scholar]
- 17.Lanese MG. Cup feeding--a valuable tool. J Hum Lact. 2011;27(1):12–3. doi: 10.1177/0890334410396668. [DOI] [PubMed] [Google Scholar]
- 18.Jones E. Breastfeeding in the preterm infant. Modern Midwife. 1994;1:22–6. [Google Scholar]
- 19.Samuel P. Cup feeding: How and when to use it with term babies. Practising Midwife. 1998;1(12) [PubMed] [Google Scholar]
- 20.Huang YY, Gau ML, Huang CM, et al. Supplementation with cup-feeding as a substitute for bottle-feeding to promote breastfeeding. Chang Gung medical journal. 2009;32(4):423–31. doi:3204/320409. [PubMed] [Google Scholar]
- 21.Malhotra N, Vishwambaran L, Sundaram KR, et al. A controlled trial of alternative methods of oral feeding in neonates. Early Hum Dev. 1999;54(1):29–38. doi: 10.1016/s0378-3782(98)00082-6. doi:S0378378298000826. [DOI] [PubMed] [Google Scholar]
- 22.Flint A, New K, Davies MW. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane database of systematic reviews. 2007;(2) doi: 10.1002/14651858.CD005092.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Collins CT, Makrides M, Gillis J, et al. Avoidance of bottles during the establishment of breast feeds in preterm infants. Cochrane database of systematic reviews. 2008;(4) doi: 10.1002/14651858.CD005252.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz G, Caylan N, Karacan CD, et al. Effect of cup feeding and bottle feeding on breastfeeding in late preterm infants: a randomized controlled study. J Hum Lact. 2014;30(2):174–9. doi: 10.1177/0890334413517940. [DOI] [PubMed] [Google Scholar]
- 25.Marinelli KA, Burke GS, Dodd VL. A comparison of the safety of cupfeedings and bottlefeedings in premature infants whose mothers intend to breastfeed. J Perinatol. 2001;21(6):350–5. doi: 10.1038/sj.jp.7210539. [DOI] [PubMed] [Google Scholar]
- 26.Aloysius A, Hickson M. Evaluation of paladai cup feeding in breast-fed preterm infants compared with bottle feeding. Early Hum Dev. 2007;83(9):619–21. doi: 10.1016/j.earlhumdev.2006.12.004. doi:S0378-3782(06)00319-7. [DOI] [PubMed] [Google Scholar]
- 27.Freer Y. A comparison of breast and cup feeding in pre-term infants: effect on physiological parameters. J Neonatal Nursing. 1999;5(1):16–21. [Google Scholar]
- 28.Lopez CP, Chiari BM, Goulart AL, et al. Assessment of swallowing in preterm newborns fed by bottle and cup. CoDAS. 2014;26(1):81–6. [PubMed] [Google Scholar]
- 29.Cloherty M, Alexander J, Holloway I, et al. The cup-versus-bottle debate: a theme from an ethnographic study of the supplementation of breastfed infants in hospital in the United kingdom. J Hum Lact. 2005;21(2):151–62. doi: 10.1177/0890334405275447. quiz 63–6. doi:21/2/151. [DOI] [PubMed] [Google Scholar]
- 30.Brown SJ, Alexander J, Thomas P. Feeding outcome in breast-fed term babies supplemented by cup or bottle. Midwifery. 1999;15(2):92–6. doi: 10.1016/s0266-6138(99)90004-9. [DOI] [PubMed] [Google Scholar]
- 31.Nyqvist HK, Strandell E. A cup feeding protocol for neonates: Evalaution of nurses and parents’ use of two cups. J Neonatal Nursing. 1999;5(2):31–6. [Google Scholar]
- 32.Al-Sahab B, Feldman M, Macpherson A, et al. Which method of breastfeeding supplementation is best? The beliefs and practices of paediatricians and nurses. Paediatrics & child health. 2010;15(7):427–31. doi: 10.1093/pch/15.7.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha NM, Martinez FE, Jorge SM. Cup or bottle for preterm infants: effects on oxygen saturation, weight gain, and breastfeeding. J Hum Lact. 2002;18(2):132–8. doi: 10.1177/089033440201800204. [DOI] [PubMed] [Google Scholar]
- 34.World Bank. [Accessed 27 April 2015];World Econonmic Situation and Prospects: Country classification. 2015 http://www.un.org/en/development/desa/policy/wesp/wesp_archive/2015wesp_full_en.pdf.
- 35.Abouelfettoh AM, Dowling DA, Dabash SA, et al. Cup versus bottle feeding for hospitalized late preterm infants in Egypt: a quasi-experimental study. International breastfeeding journal. 2008;3:27. doi: 10.1186/1746-4358-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilks J. Improving breast feeding rates in preterm babies: cup feeding versus bottle feeding. Journal of Neonatal Nursing. 2004;10(4):118–20. [Google Scholar]
- 37.Gomes CF, Trezza EM, Murade EC, et al. Surface electromyography of facial muscles during natural and artificial feeding of infants. J Pediatr (Rio J) 2006;82(2):103–9. doi: 10.2223/JPED.1456. [DOI] [PubMed] [Google Scholar]
- 38.Mosley C, Whittle C, Hicks C. A pilot study to assess the viability of a randomised controlled trial of methods of supplementary feeding of breast-fed pre-term babies. Midwifery. 2001;17(2):150–7. doi: 10.1054/midw.2000.0244. S0266-6138(00)90244-4. [DOI] [PubMed] [Google Scholar]
- 39.Schubiger G, Schwarz U, Tonz O. UNICEF/WHO baby-friendly hospital initiative: does the use of bottles and pacifiers in the neonatal nursery prevent successful breastfeeding? Neonatal Study Group. Eur J Pediatr. 1997;156(11):874–7. doi: 10.1007/s004310050734. [DOI] [PubMed] [Google Scholar]
- 40.Rekha S, Rao SD, Fernandez M. Two different methods for feeding low birth weight babies. Indian Pediatr. 1996;33(6):501–3. [PubMed] [Google Scholar]
- 41.Collins CT, Ryan P, Crowther CA, et al. Effect of bottles, cups, and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ. 2004;329(7459):193–8. doi: 10.1136/bmj.38131.675914.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalal SS, Mishra S, Agarwal R, et al. Feeding behaviour and performance of preterm neonates on Paladai feeding. Acta Paediatr. 2013;102(4):e147–52. doi: 10.1111/apa.12148. [DOI] [PubMed] [Google Scholar]
- 43.Howard CR, de Blieck EA, ten Hoopen CB, et al. Physiologic stability of newborns during cup- and bottle-feeding. Pediatrics. 1999;104(5 Pt 2):1204–7. [PubMed] [Google Scholar]
- 44.Howard CR, Howard FM, Lanphear B, et al. Randomized clinical trial of pacifier use and bottle-feeding or cupfeeding and their effect on breastfeeding. Pediatrics. 2003;111(3):511–8. doi: 10.1542/peds.111.3.511. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Khanna K, Chattree S. Cup feeding: an alternative to bottle feeding in a neonatal intensive care unit. J Trop Pediatr. 1999;45(2):108–10. doi: 10.1093/tropej/45.2.108. [DOI] [PubMed] [Google Scholar]
- 46.Cavallaro FL, Cresswell JA, Franca CG, et al. Trends in ceasarean delivery by country and wealth quintile: cross-sectional survys in southern Asia and sub-Saharan Africa. Bulletin of the World Health Organization (WHO) 2013;91(12) doi: 10.2471/BLT.13.117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibbons L, Belizan JM, Lauer J, et al. The global numbers and costs of additionally needed and unnecessary caesarean sections performed per year: Overuse as a barrier to universal coverage. 2010 [Google Scholar]
- 48.Musoke RN. Breastfeeding promotion: feeding the low birth weight infant. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1990;31(Suppl 1):57–9. doi: 10.1016/0020-7292(90)90077-X. discussion 67–8. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Essential Newborn Care Course: Module 4, Session 11. 2010. [Google Scholar]
- 50.World Health Organization. Managing newborn problems: a guide for doctors, nurses, and midwives. Geneva: World Health Organization; 2003. [Google Scholar]
- 51.World Health Organization. Guiding principles for feeding infants and young children during emergencies. 2004. [Google Scholar]
- 52.The Partnership for Maternal NCH. A global review of the key interventions related to reproductive, maternal, newborn and child health (RMNCH) 2011 [Google Scholar]
- 53.World Health Organization. Guidelines on optimal feeding of low-birthweight infants in low-and middle-income countries. 2011 [PubMed] [Google Scholar]
- 54.World Health Organization. The international code of marketing of breast-milk substitutes: Frequently Asked Questions. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.