Abstract
Background
Women screened with digital mammography may experience false-positive and false-negative results and subsequent additional imaging and biopsies. It is unclear how these outcomes vary by age, time since last screening, and individual risk factors.
Objective
To determine factors associated with false-positive and false-negative digital mammography results, additional imaging, and biopsies among a general population of women screened for breast cancer.
Design
Analysis of registry data.
Setting
Participating facilities at five U.S. Breast Cancer Surveillance Consortium breast imaging registries with linkages to pathology databases and tumor registries.
Patients
405,191 women aged 40–89 years screened with digital mammography between 2003–2011; 2,963 were diagnosed with invasive cancer or ductal carcinoma in situ within 12 months of screening.
Measurements
Rates of false-positive and false-negative results and recommendations for additional imaging and biopsies from a single screening round, and comparisons by age, time since last screening, and risk factors.
Results
Rates of false-positive results (121.2/1,000 women; 95% CI 105.6 to 138.7) and recommendations for additional imaging (124.9/1,000; 95% CI 109.3 to 142.3) were highest among women aged 40–49 years and decreased with age; rates of false-negative results (1.0 to 1.5/1,000) and recommendations for biopsy (5.6 to 17.5/1,000) did not differ greatly by age. Results did not differ by time since last screening. All rates were higher for women with risk factors, particularly family history of breast cancer, previous benign breast biopsy, high breast density, and low body mass index for younger women.
Limitations
Additional factors were not examined, including numbers of first- and second-degree relatives with breast cancer and diagnoses of previous benign biopsies.
Conclusions
False-positive results and additional imaging are common, particularly for younger women and those with risk factors, while biopsies occur less often. Rates of false-negative results are low.
Keywords: screening, digital mammography, adverse effects, false-positive result
INTRODUCTION
Clinical guidelines recommend a personalized approach to mammography screening that considers potential benefits and harms (1). Important harms include adverse effects of the screening process including false-positive and false-negative mammography results and subsequent additional imaging and biopsies. While procedures are often necessary to evaluate findings on screening mammography, most result in benign diagnoses. Minimizing these adverse effects could improve the benefit—harm screening equation for many women.
A personalized approach to screening includes identifying individual risk factors for breast cancer. Several risk factors have been associated with breast cancer in epidemiologic studies; however, most relationships are modest or inconsistent (2). Factors associated with high risks for breast cancer include specific mutations of breast cancer susceptibility genes (3) and other hereditary genetic syndromes (4); previously diagnosed high-risk breast lesions (5, 6); previous high-dose radiation therapy to the chest (4, 7); and family history of breast cancer, particularly among first-degree relatives. The degree of risk from family history varies according to familial patterns of disease. Estimates of lifetime risk over 20% are considered high (8), although lower levels may also be clinically important (9).
Additional factors that modestly increase risk include older age; current use of menopausal hormone therapy using combined estrogen and progestin regimens (10); current use of oral contraceptives (2); high breast density (11); and high body mass index (BMI) for postmenopausal women (12). How these factors influence performance outcomes of digital mammography screening has not been extensively explored.
The purpose of this study is to estimate rates of false-positive and false-negative digital mammography results and subsequent additional imaging and biopsies among a general population of women undergoing screening, and how rates vary by age, time since last mammography screening, and individual risk factors. This analysis will be used to inform updated clinical practice recommendations in the United States (13).
METHODS
Design Overview
This study is an analysis of data collected between 2003 to 2011 from the Breast Cancer Surveillance Consortium (BCSC), a collaborative network of mammography registries across the United States, supported by the National Cancer Institute (NCI) (14, 15). Registries collected data at the time mammography was performed at participating community radiology facilities. Breast cancer diagnoses were obtained by linking BCSC data to pathology databases, regional Surveillance, Epidemiology, and End Results (SEER) programs, and state tumor registries. Data were pooled at a Statistical Coordinating Center. Registries and the Coordinating Center received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analysis. All procedures were Health Insurance Portability and Accountability Act compliant, and registries and the Coordinating Center received a federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
Setting and Participants
The analysis includes data for 405,191 women aged 40 to 89 years who had routine screening with digital mammography during 2003 to 2011 at participating facilities at five BCSC breast imaging registries (Carolina Mammography Registry, Group Health [Washington state], New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System) (Figure 1). Prior to each mammography examination, women completed questionnaires that included demographic and medical history information, including time since last mammography screening. All women with an eligible screening mammogram who completed a questionnaire providing permission to use their information for research were included.
Figure 1.
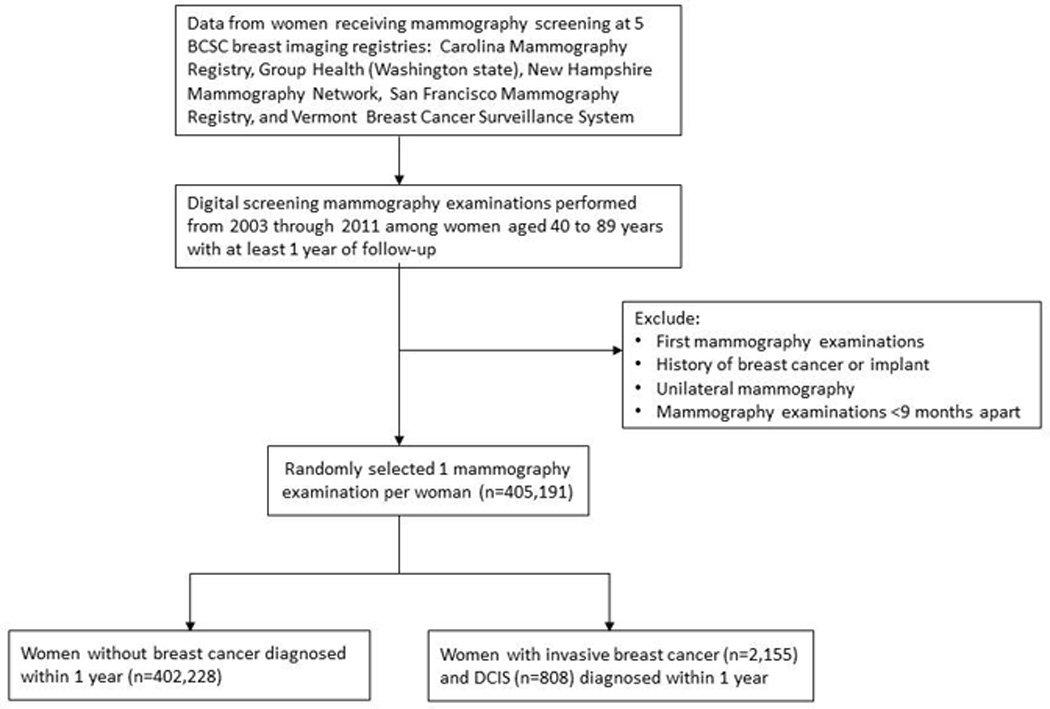
Description of Breast Cancer Surveillance Consortium data sources for the study
Abbreviations: BCSC= Breast Cancer Surveillance Consortium; DCIS=ductal carcinoma in situ.
*Screening mammography examinations were those designated by the radiologist or radiology technologist as performed for screening and occurring more than 9 months after a previous imaging examination. Routine screening required at least one mammography examination within the previous 2 years (defined as 30 months).
Screening mammography examinations were designated by the radiologist or radiology technologist as performed for screening, and occurring more than 9 months after a previous imaging examination in women without histories of breast cancer, breast augmentations, or mastectomies. Each study-eligible routine screening required at least one mammography examination within the previous 30 months. Initial and unilateral examinations were excluded. Mammography information included Breast Imaging Reporting and Data System (BI-RADS) breast density, assessment, and recommendations for further work-up. For women with more than one mammography examination during the time period of the study, one observation was randomly selected to be included in the calculations to reduce potential bias, such as preferentially choosing women with shorter or longer screening histories. These data comprise a defined subset of BCSC data intended to represent the experience of a cohort of regularly screened women without histories of breast cancer or current breast symptoms.
Outcome and Risk Factor Measures
Outcome measures included rates of false-positive and false-negative mammography results and recommendations for additional imaging and biopsies from a single screening round. False-positive and false-negative mammography results were based on follow-up data within one year of mammography screening and before the next screening examination. Positive versus negative initial and final results were defined according to BCSC standard definitions (16) which utilized standardized terminology and assessments of the American College of Radiology BI-RADS 4th edition atlas (17). Each screening mammography examination was given an initial BI-RADS assessment based on the screening views only. Positive initial results included four assessment categories: needs additional imaging evaluation (category 0), probably benign (category 3) with a recommendation for immediate work-up (these were treated as a category 0 based on the recommendation), suspicious abnormality (category 4), or highly suggestive of malignancy (category 5) (18). Negative results included assessments of negative (category 1) or benign findings (category 2), or category 3 without a recommendation for immediate work-up.
Recommendation for biopsy was defined as a positive final result after all imaging including work-up of an abnormal screening examination. Positive final results included BI-RADS assessments of 4 or 5 or 0 with a recommendation for biopsy (18). Negative final results included an assessment of 1, 2, or 3 or 0 with a recommendation for normal or short-interval follow-up or clinical exam.
We examined associations with common risk factors for breast cancer (2). These included first-degree relatives with breast cancer (none, ≥1); breast density (almost entirely fat, scattered fibroglandular densities, heterogeneously dense, extremely dense); benign breast biopsy (none, previous); race/ethnicity (white, black, Asian, Hispanic, other); menopausal status (pre, peri, postmenopausal); menopausal hormone therapy use (none, combination [estrogen with progestin], estrogen only); oral contraceptive use (no current use, current use), and body mass index (BMI) (<25, 25 to <30, ≥30 kg/m2). Since the BCSC data do not include information on types of menopausal hormone therapy, the analysis assumes that a woman with a uterus uses combination therapy, while a woman without a uterus uses estrogen-only therapy, as previously described (19, 20).
The main analysis analyzed three categories of breast density, combining almost entirely fat and scattered fibroglandular densities into one group. As a sensitivity analysis, we also analyzed density three additional ways: (1) three categories, combining heterogeneously dense and extremely dense into one group; (2) four separate BI-RADS categories; and (3) two categories that combine almost entirely fat and scattered fibroglandular densities into one group and heterogeneously dense and extremely dense into another group.
Two measures of time since last mammography screening were evaluated to represent broad and narrow estimates of one versus two years (9 to 18 versus 19 to 30 months; 11 to 14 versus 23 to 26 months).
Missing data for outcomes and risk factors are summarized in Appendix Table 1.
Statistical Analysis
From these data, we calculated age-specific rates by decade (numbers per 1,000 women per single screening round) for false-positive and false-negative mammography results, recommendations for additional imaging, and recommendations for biopsies, and determined whether outcomes differed by age, time since last mammography screening, and risk factors. To account for correlation among mammograms interpreted at the same radiology facility, we estimated robust standard errors from logistic regression using generalized estimating equations with an independence working correlation matrix (21). We then constructed 95% confidence intervals (CIs) and, to assess differences between groups, 2-sided P-values. This method provides population-averaged estimates of effects, which are not necessarily causal relationships. Analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary NC).
Role of the Funding Source
This research was funded by AHRQ under a contract to support the work of the USPSTF. AHRQ had no role in the analysis or development of conclusions. AHRQ performed a final review of the manuscript to ensure that the analysis met methodological standards. The investigators are solely responsible for the content and the decision to submit the manuscript for publication.
RESULTS
Outcomes by Age
Data for regularly screened women based on results from a single screening round using digital mammography indicated that false-positive mammography results were common in all age groups (Table 1). The rate was highest among women aged 40 to 49 years (121.2 per 1,000 women; 95% CI 105.6 to 138.7) and declined with age (p<0.001). Rates of false-negative mammography results tended to increase with age, but were not statistically significantly different across age groups, and ranged from 1.0 to 1.5 per 1,000 women.
Table 1.
Age-specific rates of false-positive and false-negative digital mammography results and recommendations for additional imaging and biopsies from a single screening round in the BCSC
| Age, y | ||||||
|---|---|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | Difference (P-value)* |
|
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | |
| Invasive breast cancer cases, n | 349 | 574 | 651 | 427 | 154 | |
| DCIS cases, n | 191 | 246 | 208 | 120 | 43 | |
| Outcomes, n per 1,000 women screened (95% CI) | ||||||
| False-positive mammography result | 121.2 (105.6, 138.7) | 93.2 (82.8, 104.7) | 80.8 (72.9, 89.4) | 69.6 (62.6, 77.3) | 65.2 (58.8, 72.2) | <0.001 |
| False-negative mammography result | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) | 1.2 (0.9, 1.5) | 1.5 (1.1, 1.9) | 1.3 (0.9, 1.9) | 0.32 |
| Additional imaging recommended† | 124.9 (109.3, 142.3) | 98.5 (88.0, 110.1) | 88.7 (80.6, 97.4) | 79.0 (71.9, 86.9) | 74.4 (67.4, 82.2) | <0.001 |
| Biopsy recommended† | 16.4 (13.2, 20.3) | 15.9 (12.7, 19.7) | 16.5 (14.3, 19.1) | 17.5 (15.2, 20.2) | 15.6 (13.4, 18.2) | 0.12 |
| Screen-detected invasive cancer | 2.2 (1.8, 2.6) | 3.5 (3.1, 4.0) | 5.8 (5.3, 6.4) | 7.2 (6.4, 8.1) | 7.1 (5.9, 8.5) | <0.001 |
| Screen-detected DCIS | 1.6 (1.3, 1.9) | 1.8 (1.5, 2.2) | 2.1 (1.7, 2.5) | 2.3 (1.7, 3.0) | 2.1 (1.5, 3.0) | 0.05 |
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
After positive mammography result.
Abbreviations: CI=confidence interval; DCIS=ductal carcinoma in situ.
For women with initially positive mammography results, rates of recommendations for additional imaging were highest among women aged 40 to 49 years (124.9 per 1,000 women; 95% CI 109.3 to 142.3) and decreased with age (p<0.001). Rates of recommendations for biopsy were not statistically significantly different across age groups, and ranged from 15.6 to 17.5 per 1,000 women.
Rates of invasive breast cancer were lowest among women aged 40 to 49 years (2.2 per 1,000 women; 95% CI 1.8 to 2.6) and increased across age groups (p<0.001). Rates of DCIS were also lowest among women aged 40 to 49 years (1.6 per 1,000 women; 95% CI 1.3 to 1.9) and increased with age (p=0.05). Women aged 70 to 79 had the highest rates of invasive cancer (7.2 per 1,000 women; 95% CI 6.4 to 8.1) and DCIS (2.3 per 1,000 women; 95% CI 1.7 to 3.0), and the yield of screening was more favorable for older women. For every case of invasive breast cancer detected by mammography screening in women aged 40 to 49 years, 464 women had mammography, 58 were recommended for additional imaging, and 10 were recommended for biopsies. In comparison, for women aged 70 to 79, for every case of invasive breast cancer detected by screening, 139 women had mammography, 11 were recommended for additional imaging, and 3 were recommended for biopsies.
Outcomes by Time since Last Mammography Examination
Rates of false-positives, false-negatives, and recommendations for additional imaging did not differ in comparisons of times since last mammography regardless of interval durations (9 to 18 versus 19 to 30 months; 11 to 14 versus 23 to 26 months) (Appendix Table 2). Biopsies were recommended at a higher rate for women aged 60 to 69 years who had their last mammogram 23 to 26 months previously compared to 11 to 14 months (18.8 versus 15.2 per 1,000 women; p=0.03).
Outcomes by Risk Factors
False-positive mammography results
Rates of false-positive mammography results were statistically significantly higher for women with specific risk factors compared with women without them (Table 2). These include women with first-degree relatives with breast cancer compared with no relatives for women aged 40 to 69 years. Women with heterogeneously dense breasts had higher false-positive rates than those with almost entirely fat and scattered fibroglandular densities, or extremely dense breasts, for all ages except 80 to 89 years. Rates were also higher among women with previous benign breast biopsies for ages 40 to 79 years. Comparisons based on race and ethnicity indicated the lowest rates among Asians for all age groups.
Table 2.
Rates of false-positive results after screening with digital mammography by risk factors*
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ||||||
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | ||||||
| False-positive, n | 13,784 | 11,923 | 7,633 | 3,494 | 1,223 | ||||||
| Number per 1,000 women screened per round (95% CI) | |||||||||||
| First-degree relatives with breast cancer |
None | 118.7 (104.3, 134.7) | 0.03 | 90.4 (81.1, 100.7) | 0.005 | 79.4 (71.8, 87.7) | 0.02 | 68.6 (61.1, 76.8) | 0.11 | 63.3 (56.8, 70.5) | 0.05 |
| One or more | 139.8 (113.9, 170.5) | 109.0 (92.3, 128.2) | 87.2 (77.2, 98.4) | 75.0 (67.6, 83.1) | 73.1 (64.1, 83.3) | ||||||
| Breast density† | Fat- Scattered |
108.4 (95.5, 122.7) | <0.001 | 80.5 (71.1, 90.9) | <0.001 | 74.1 (66.4, 82.6) | <0.001 | 67.3 (60.4, 74.9) | 0.003 | 60.3 (54.0, 67.4) | 0.001 |
| Hetero- geneous |
142.2 (120.2, 167.4) | 115.8 (100.3, 133.2) | 101.8 (91.0, 113.8) | 88.7 (78.7, 99.9) | 82.4 (72.6, 93.5) | ||||||
| Extreme | 112.1 (94.4, 132.7) | 92.7 (77.5, 110.5) | 75.2 (64.7, 87.1) | 57.7 (43.9, 75.5) | 85.1 (61.7, 116.2) | ||||||
| Benign breast biopsy | None | 114.3 (99.8, 130.5) | 0.001 | 85.9 (76.7, 96.0) | <0.001 | 74.6 (66.8, 83.1) | <0.001 | 63.4 (56.2, 71.3) | <0.001 | 63.0 (56.3, 70.6) | 0.09 |
| Previous | 167.3 (140.6, 197.9) | 122.5 (106.2, 140.7) | 98.6 (88.8, 109.3) | 88.6 (79.1, 99.2) | 71.6 (62.3, 82.3) | ||||||
| Race/ethnicity | White | 127.0 (115.5, 139.4) | 0.001 | 97.6 (89.5, 106.4) | 0.01 | 83.8 (77.4, 90.7) | 0.006 | 73.5 (67.7, 79.8) | <0.001 | 68.9 (62.6, 75.7) | 0.04 |
| Black | 92.6 (82.0, 104.5) | 78.9 (65.2, 95.3) | 64.5 (53.6, 77.3) | 58.9 (51.7, 67.0) | 52.4 (43.6, 63.0) | ||||||
| Asian | 85.2 (72.2, 100.4) | 67.6 (56.5, 80.7) | 58.0 (47.9, 70.2) | 43.6 (36.9, 51.6) | 35.8 (29.6, 43.4) | ||||||
| Hispanic | 125.4 (106.8, 146.7) | 80.9 (69.1, 94.6) | 72.9 (60.3, 87.8) | 60.7 (50.6, 72.8) | 55.7 (31.3, 97.2) | ||||||
| Other | 127.8 (105.8, 153.6) | 102.3 (88.5, 117.8) | 91.5 (76.2, 109.5) | 72.6 (53.3, 98.2) | 48.9 (29.3, 80.6) | ||||||
| Menopausal status | Pre | 131.3 (113.3, 151.8) | 0.02 | 118.3 (107.2, 130.5) | <0.001 | NA | NA | NA | |||
| Peri | 103.9 (88.5, 121.7) | 97.6 (75.7, 125.1) | |||||||||
| Post | 111.3 (100.2, 123.4) | 87.2 (78.8, 96.4) | |||||||||
| Menopausal hormone therapy |
None | 123.3 (107.4, 141.2) | 0.69 | 91.8 (81.6, 103.2) | 0.27 | 76.2 (69.2, 84.0) | 0.22 | 67.6 (61.1, 74.8) | 0.01 | 62.2 (55.5, 69.8) | 0.27 |
| Combination | 122.0 (78.8, 184.1) | 131.1 (99.5, 170.7) | 122.5 (87.3, 169.2) | 105.9 (81.8, 136.0) | 94.0 (74.0, 118.8) | ||||||
| Estrogen | 108.7 (84.4, 138.8) | 101.3 (87.1, 117.6) | 97.6 (77.3, 122.5) | 114.0 (94.8, 136.5) | 89.1 (68.5, 115.1) | ||||||
| Oral contraceptives | No current | 122.9 (107.2, 140.6) | 0.05 | 93.6 (83.1, 105.4) | 0.63 | NA | NA | NA | |||
| Current use | 106.2 (86.4, 130.0) | 97.0 (81.3, 115.2) | |||||||||
| Body mass index, kg/m2 |
<25 | 129.0 (113.8, 145.9) | 0.009 | 99.5 (89.3, 110.8) | 0.04 | 85.8 (77.9, 94.4) | 0.14 | 70.5 (62.0, 80.0) | 0.78 | 73.9 (60.6, 89.8) | 0.33 |
| 25 to <30 | 124.8 (110.1, 141.2) | 93.6 (85.0, 103.0) | 78.6 (69.5, 88.9) | 72.7 (64.8, 81.6) | 62.2 (51.4, 75.1) | ||||||
| ≥30 | 107.2 (96.0, 119.5) | 86.1 (77.7, 95.2) | 81.1 (74.1, 88.6) | 74.2 (64.1, 85.7) | 73.8 (59.1, 91.9) | ||||||
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
Categories include: almost entirely fat=fat; scattered fibroglandular densities=scattered; heterogeneously dense=heterogeneous; and extremely dense=extreme.
Abbreviations: kg=kilogram; m=meter; NA=not applicable; peri=perimenopausal; pre=premenopausal; post=postmenopausal.
Premenopausal women had the highest false-positive rates for women aged 40 to 59 years compared with perimenopausal and postmenopausal women. Women using menopausal hormone therapy had the highest rates for ages 70 to 79 years, while comparisons for other age groups were not statistically significant. Women with lower body mass (BMI <30) had higher false positive rates for ages 40 to 59 years.
False-negative mammography results
Rates of false-negative results were higher for women with first-degree relatives with breast cancer for ages 40 to 79 years, although results were of borderline statistical significance for ages 50 to 69 years (Appendix Table 3). Women with almost entirely fat and scattered fibroglandular densities had lower rates than those with other types of breast density for ages 40 to 69 years. Rates were higher among women with previous benign breast biopsies for ages 50 to 89 years, and women with lower body mass (BMI <30) for ages 50 to 59 years. Other comparisons between groups were not statistically significant.
Recommendations for additional imaging
Risk factors associated with differences in rates of recommendations for additional imaging were similar to those for false positive mammography results (Appendix Table 4). Rates were highest among women with first-degree relatives with breast cancer for all ages, heterogeneously dense breasts (ages 40 to 79), previous benign breast biopsies (ages 40 to 79), premenopausal status (ages 40 to 50), use of menopausal hormone therapy (ages 70 to 79), and lower BMI (ages 40 to 49). Comparisons based on race and ethnicity indicated the lowest rates among Asians for all age groups.
Recommendations for biopsy
Rates of recommendations for biopsy were statistically significantly higher for women aged 40 to 69 years with first-degree relatives with breast cancer, and for women aged 40 to 79 years with previous breast biopsies (Table 3). Women aged 40 to 59 years with heterogeneously dense or extremely dense breasts had higher rates than women with less dense breasts, while for women aged 60 to 79 years, rates were highest for women with heterogeneously dense breasts only. Higher rates were also associated with premenopausal status for ages 50 to 59 years; no current use of oral contraceptives for ages 40 to 49 years; lower BMI for ages 40 to 49, but higher BMI for ages 70 to 79. Other comparisons between groups were not statistically significant.
Table 3.
Rates of recommendations for biopsy after screening with digital mammography by risk factors*
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ||||||
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | ||||||
| Biopsy recommended, n | 1,863 | 2,030 | 1,562 | 880 | 293 | ||||||
| Number per 1,000 women screened per round (95% CI) | |||||||||||
| First-degree relatives with breast cancer |
None | 15.7 (12.6, 19.4) | 0.002 | 14.8 (11.8, 18.4) | <0.001 | 15.8 (13.7, 18.3) | 0.002 | 17.0 (14.7, 19.6) | 0.09 | 15.2 (12.8, 18.0) | 0.24 |
| One or more | 21.1 (16.9, 26.3) | 21.9 (17.5, 27.3) | 20.1 (17.0, 23.7) | 20.3 (16.7, 24.6) | 17.6 (14.1, 22.1) | ||||||
| Breast density† | Fat-Scattered | 12.2 (9.9, 15.0) | <0.001 | 11.8 (9.6, 14.5) | <0.001 | 15.6 (13.7, 17.7) | 0.008 | 16.2 (14.2, 18.4) | 0.007 | 14.2 (12.0, 16.8) | 0.07 |
| Heterogeneous | 18.9 (15.8, 22.5) | 20.2 (17.3, 23.7) | 19.3 (16.9, 22.2) | 21.0 (18.0, 24.5) | 19.0 (15.5, 23.2) | ||||||
| Extreme | 20.2 (16.8, 24.3) | 19.2 (14.3, 25.7) | 13.8 (10.5, 18.2) | 13.0 (7.2, 23.3) | 16.1 (8.0, 32.1) | ||||||
| Benign breast biopsy | None | 14.8 (11.8, 18.7) | <0.001 | 13.9 (11.1, 17.3) | <0.002 | 15.0 (12.7, 17.8) | <0.001 | 15.3 (13.1, 17.7) | <0.001 | 15.8 (13.4, 18.7) | 0.54 |
| Previous | 27.8 (22.8, 33.7) | 25.1 (20.1, 31.2) | 21.8 (19.1, 24.9) | 25.2 (21.4, 29.7) | 17.1 (13.7, 21.5) | ||||||
| Race/ethnicity | White | 16.7 (13.7, 20.3) | 0.21 | 16.6 (13.6, 20.2) | 0.39 | 17.6 (15.6, 20.0) | 0.05 | 18.7 (16.6, 21.2) | 0.23 | 16.2 (13.5, 19.4) | 0.12 |
| Black | 13.6 (10.4, 17.8) | 14.7 (10.4, 20.6) | 13.9 (10.6, 18.0) | 14.9 (11.4, 19.5) | 8.9 (4.4, 18.0) | ||||||
| Asian | 16.2 (10.6, 24.5) | 14.8 (9.5, 22.9) | 12.0 (6.9, 20.6) | 11.8 (6.8, 20.3) | 9.2 (5.6, 15.3) | ||||||
| Hispanic | 16.3 (10.3, 25.6) | 11.9 (8.1, 17.5) | 14.2 (11.4, 17.6) | 15.9 (10.1, 25.1) | 16.4 (8.5, 31.5) | ||||||
| Other | 19.8 (14.4, 27.3) | 17.4 (10.5, 28.6) | 16.4 (10.8, 24.8) | 16.6 (10.0, 27.6) | 5.4 (0.7, 39.2) | ||||||
| Menopausal status | Pre | 17.6 (14.0, 22.1) | 0.49 | 19.8 (15.7, 24.9) | 0.02 | NA | NA | NA | |||
| Peri | 17.8 (14.4, 22.0) | 16.4 (10.6, 25.4) | |||||||||
| Post | 15.8 (12.5, 20.0) | 15.4 (12.1, 19.4) | |||||||||
| Menopausal hormone therapy |
None | 16.3 (13.2, 20.2) | 0.34 | 15.6 (12.6, 19.2) | 0.50 | 15.9 (13.9, 18.3) | 0.37 | 17.2 (15.1, 19.4) | 0.14 | 15.2 (12.8, 17.9) | 0.13 |
| Combination | 15.2 (8.2, 28.2) | 18.3 (12.7, 26.3) | 16.9 (12.6, 22.6) | 33.0 (23.7, 45.9) | 21.9 (14.0, 34.2) | ||||||
| Estrogen only | 26.4 (14.7, 47.2) | 18.3 (12.3, 27.2) | 21.0 (14.5, 30.2) | 25.3 (17.7, 36.1) | 32.2 (22.2, 46.4) | ||||||
| Oral contraceptives | No current | 16.7 (13.6, 20.6) | 0.007 | 16.0 (13.1, 19.5) | 0.32 | NA | NA | NA | |||
| Current use | 12.5 (9.5, 16.3) | 13.0 (7.0, 24.3) | |||||||||
| Body mass index, kg/m2 |
<25 | 21.4 (17.0, 26.8) | 0.02 | 19.3 (14.7, 25.1) | 0.40 | 17.4 (14.4, 21.0) | 0.12 | 16.5 (13.5, 20.1) | 0.02 | 17.1 (13.8, 21.2) | 0.26 |
| 25 to <30 | 17.6 (13.7, 22.6) | 18.0 (13.3, 24.4) | 18.9 (15.3, 23.4) | 21.9 (18.2, 26.3) | 16.6 (12.5, 21.9) | ||||||
| ≥30 | 15.3 (12.3, 19.2) | 18.4 (14.5, 23.4) | 22.2 (18.1, 27.2) | 26.7 (21.9, 32.4) | 26.6 (18.5, 38.1) | ||||||
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
Categories include: almost entirely fat=fat; scattered fibroglandular densities=scattered; heterogeneously dense=heterogeneous; and extremely dense=extreme.
Abbreviations: kg=kilogram; m=meter; NA=not applicable; peri=perimenopausal; pre=premenopausal; post=postmenopausal.
Breast density categories
Rates of false-positives, false-negatives, recommendations for additional imaging, and recommendations for biopsies were lowest for women with almost entirely fat breasts for all ages. False-negative rates were highest for women with extremely dense breasts for all ages, except those aged 60 to 69 years (Appendix Table 5). Rates of false-positives, recommendations for additional imaging, and recommendations for biopsies were highest for women with heterogeneously dense breasts or for the combined category of heterogeneously and extremely dense breasts, except for women aged 40 to 49 years where rates of recommendations for biopsies were highest among women with extremely dense breasts.
DISCUSSION
Our analysis of BCSC data on digital mammography screening indicated that false-positive rates and recommendations for additional imaging were highest among women aged 40 to 49 years and declined with age, while false-negative rates were low across all age groups. Rates of recommendations for biopsy did not differ between ages. Results did not differ by time since last mammography screening regardless of whether broad or narrow estimates of one versus two years were used.
Several risk factors were statistically significantly associated with higher rates of false-positive and false-negative results and recommendations for additional imaging and biopsy across most age groups. These included family history of breast cancer, high breast density, and previous benign breast biopsy. Premenopausal status, use of menopausal hormone therapy, and lower BMI were associated with some of the outcomes for specific age groups only. Comparisons based on race and ethnicity indicated the lowest rates of false-positive results and additional imaging among Asians. While some risk factors reflect high exposure to estrogen and related changes in breast tissue (premenopause, menopausal hormone therapy), others may serve primarily as markers of increased breast cancer risk (family history, previous benign biopsy).
Our analysis comparing different combinations of breast density categories indicated that rates for all outcomes were lowest for women with almost entirely fat breasts, and highest for women with heterogeneously dense breasts or for those in the combined category of heterogeneous and extreme density. Women with extremely dense breasts had the highest rates of false-negative results. The high rates for women with dense breasts are likely related to their particularly complex mammography images that are more difficult to interpret, limiting the discrimination of breast cancer from normal tissue and leading to more call backs and biopsies and higher false-negative rates in clinical practice (22–24).
This analysis indicates higher rates of false-positive results and recommendations for additional imaging, and lower rates of recommendations for biopsy, than our previous analysis of BCSC data that included 600,830 women screened between 2000 and 2005 using predominantly film mammography (25). The lower rates of recommendations for biopsy may suggest more selective use of procedures by radiologists because of improvements in image quality and interpretation for digital mammography and ultrasound over time.
Our finding that results did not differ by time since last mammography screening differs from previous analyses from the BCSC that indicate higher rates for annual versus biennial screening (19, 26–30). However, our rates were based on digital mammography only and on a single round of screening that did not capture the longitudinal screening experiences of individual women that more accurately reflect clinical practice. A previous analysis of BCSC data that provided results of screening over a 10-year time period indicated that when screening began at age 40, cumulative rates of false-positive mammography and benign biopsy results were higher for annual than biennial screening (mammography 61% versus 41%; biopsy 7% versus 5%) (29).
The results of our analysis of associations with risk factors are generally consistent with previous BCSC analyses indicating that 10-year cumulative risks of false-positive results and benign biopsies were higher for women with heterogeneously dense or extremely dense breasts, family histories of breast cancer, and those who used combination menopausal hormone therapy (29, 31). While our analysis identified associations with additional risk factors, it differed from the study of 10-year cumulative risks because it was based on a single round of screening, did not adjust for other covariates, and included only digital mammography.
The strengths of this study include its use of digital mammography data and patient information from a large national collaborative of women screened in the United States, providing a comprehensive data source representing current clinical practice. This study uses the methods of the BCSC that have been standardized across the participating registries, allowing our analysis to build on prior work in this area (23, 32, 33).
To estimate screening outcomes applicable to clinical practice in the U.S., data sources must include information from U.S. practices because rates of false-positive and false-negative results and additional imaging and biopsies are substantially different elsewhere (34–36). These differences relate to how mammography screening and diagnosis are delivered and practiced in diverse areas.
This study has several limitations. The BCSC data reflect opportunistic screening in a fluctuating population of women in the U.S. whose information was collected by the participating registries. Findings may not be applicable to other populations. Limitations also include restrictions of registry data with pre-defined data elements and the inherent biases of observational data. Some outcomes, such as the effectiveness and harms of different screening intervals, would be more accurately determined by comparing outcomes between women who were randomly assigned to comparison groups. However, this question has not yet been addressed by randomized trials of screening that used intervals ranging from 12 to 33 months (13).
Our goal was to provide overall rates of the outcome measures by time since last mammography screening and risk factors and thus, our estimates are derived from population-averaged models that provide variance estimates adjusted for correlation within facilities, but do not decompose within versus between facility effects or adjust for potential confounders. For example, it is possible that women with risk factors tend to seek care at facilities with different performance characteristics (e.g., academic medical centers) than women without risk factors, present for screening more often, or have other characteristics that explain the higher rates of screening harms, such as more complex breast tissue or increased risk of benign breast disease. Understanding the mechanisms through which risk factors affect performance or variation across facilities and radiologists is beyond the scope of this paper.
Our estimates are based on digital mammography performed without supplemental imaging. Digital breast tomosynthesis (37–42) and supplemental screening tests such as screening ultrasound (43) are becoming more widely used in the United States. A similar analysis of screening outcomes of these newer technologies using a large, generalizable cohort such as the BCSC is needed.
In conclusion, our analysis of outcomes from a single round of digital mammography screening in 405,191 women indicates that false-positive results and additional imaging are common, particularly for younger women and those with risk factors, while biopsies occur less often. Rates of false-negative results are low. The results of this study may be useful for women and clinicians considering the individual benefits and harms of screening, as well as health service administrators and planners determining the implications of screening on populations.
Acknowledgments
Funding: Agency for Healthcare Research and Quality (Contract Number 290-2012-00015-I, Task Order 2), Rockville, MD; and the National Cancer Institute-funded Breast Cancer Surveillance Consortium (P01 CA154292 and HHSN261201100031C) and U54 CA163303.
The collection of Breast Cancer Surveillance Consortium data was supported by the National Cancer Institute (BCSC; P01CA154292 and HHSN261201100031C) and, in part, by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
Appendix
Appendix Table 1.
Rates of missing data for outcome and risk factor measures for 405,191 women screened. Women with missing data were excluded from the specific analysis only.
| Women with missing data | ||
|---|---|---|
| Number | % | |
| Outcome | ||
| Invasive breast cancer cases, n | 0 | 0% |
| DCIS cases, n | 0 | 0% |
| False-positive mammography result | 0 | 0% |
| False-negative mammography result | 0 | 0% |
| Additional imaging recommended | 0 | 0% |
| Biopsy recommended | 9,633 | 2.4% |
| Screen-detected invasive cancer | 0 | 0% |
| Screen-detected DCIS | 0 | 0% |
| Risk factor | ||
| First-degree relatives with breast cancer | 3,943 | 1.0% |
| Breast density | 65,919 | 16.3% |
| Benign breast biopsy | 23,588 | 5.8% |
| Race/ethnicity | 31,061 | 7.7% |
| Menopausal status | 41,288 | 17.1%* |
| Menopausal hormone therapy | 65,717 | 16.2% |
| Oral contraceptives | 37,098 | 15.3%* |
| Body mass index, kg/m2† | 190,560 | 47.0% |
| Time since last exam, mo‡ | 0 | 0% |
% of 241,728 screened women aged 40–59; women ≥60 are postmenopausal.
Most missing values are from facilities that do not collect this information.
No missing data for broad categories (9–18 vs. 19–30 months).
Appendix Table 2.
Rates of false-positive and false-negative digital mammography results and recommendations for additional imaging and biopsies based on time since last mammography examination
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Time since last exam, mo |
40–49 | 50–59 | 60–69 | 70–79 | 80–89 | |||||
| Comparing 9–18 vs. 19–30 months* | |||||||||||
| Women screened, n | 9–18 | 79,637 | 91,864 | 71,324 | 39,474 | 14,865 | |||||
| 19–30 | 34,133 | 36,094 | 23,183 | 10,730 | 3,887 | ||||||
| Invasive breast cancer cases, n | 9–18 | 240 | 391 | 474 | 322 | 119 | |||||
| 19–30 | 109 | 183 | 177 | 105 | 35 | ||||||
| DCIS cases, n | 9–18 | 126 | 185 | 156 | 94 | 32 | |||||
| 19–30 | 65 | 61 | 52 | 26 | 11 | ||||||
| Outcomes, n per 1,000 women screened (95% CI) | |||||||||||
| False-positive mammography result | 9–18 | 122.1 (105.4, 141.0) | 0.65 | 94.2 (83.3, 106.5) | 0.37 | 80.6 (72.8, 89.2) | 0.89 | 69.1 (61.9, 77.0) | 0.55 | 66.5 (60.8, 72.8) | 0.22 |
| 19–30 | 119.0 (103.0, 137.1) | 90.5 (80.4, 101.8) | 81.1 (71.4, 92.1) | 71.6 (62.2, 82.2) | 60.2 (49.3, 73.3) | ||||||
| False-negative mammography result | 9–18 | 1.1 (0.9, 1.3) | 0.14 | 1.2 (1.0, 1.4) | 0.06 | 1.3 (1.0, 1.6) | 0.26 | 1.6 (1.2, 2.1) | 0.17 | 1.4 (0.9, 2.2) | 0.27 |
| 19–30 | 0.8 (0.6, 1.1) | 0.9 (0.6, 1.2) | 0.9 (0.6, 1.5) | 1.0 (0.5, 2.0) | 0.8 (0.3, 2.3) | ||||||
| Additional imaging recommended† | 9–18 | 125.6 (109.0, 144.3) | 0.74 | 99.3 (88.2, 111.7) | 0.47 | 88.2 (80.2, 96.9) | 0.59 | 78.0 (70.7, 86.1) | 0.30 | 75.3 (68.6, 82.6) | 0.46 |
| 19–30 | 123.3 (107.0, 141.7) | 96.4 (85.9, 108.0) | 90.1 (80.1, 101.2) | 82.8 (72.5, 94.3) | 71.3 (59.8, 84.8) | ||||||
| Biopsy recommended† | 9–18 | 15.6 (12.8, 19.0) | 0.11 | 15.7 (12.7, 19.3) | 0.50 | 15.9 (14.0, 18.2) | 0.10 | 17.3 (15.2, 19.6) | 0.44 | 14.9 (12.4, 17.9) | 0.25 |
| 19–30 | 18.2 (13.7, 24.1) | 16.4 (12.5, 21.4) | 18.4 (14.7, 23.0) | 18.5 (14.6, 23.5) | 18.3 (13.9, 24.0) | ||||||
| Screen-detected invasive cancer | 9–18 | 2.0 (1.6, 2.5) | 0.12 | 3.2 (2.7, 3.7) | 0.009 | 5.5 (4.9, 6.2) | 0.07 | 6.7 (5.8, 7.7) | 0.04 | 6.8 (5.4, 8.5) | 0.39 |
| 19–30 | 2.5 (2.1, 3.0) | 4.3 (3.7, 5.1) | 6.8 (5.7, 8.1) | 8.9 (7.4, 10.8) | 8.2 (5.8, 11.6) | ||||||
| Screen-detected DCIS | 9–18 | 1.5 (1.2, 1.8) | 0.18 | 1.9 (1.5, 2.4) | 0.13 | 2.0 (1.8, 2.4) | 0.79 | 2.3 (1.7, 3.0) | 0.97 | 2.0 (1.2, 3.1) | 0.42 |
| 19–30 | 1.8 (1.4, 2.3) | 1.6 (1.2, 2.0) | 2.2 (1.4, 3.3) | 2.2 (1.4, 3.6) | 2.8 (1.5, 5.3) | ||||||
| Comparing 11–14 vs. 23–26 months* | |||||||||||
| Women screened, n | 11–14 | 55,278 | 65,219 | 53,419 | 30,497 | 11,299 | |||||
| 23–26 | 13,584 | 14,407 | 9,907 | 4,291 | 1,504 | ||||||
| Invasive breast cancer cases, n | 11–14 | 163 | 274 | 348 | 247 | 78 | |||||
| 23–26 | 42 | 70 | 76 | 41 | 15 | ||||||
| DCIS cases, n | 11–14 | 83 | 127 | 111 | 71 | 20 | |||||
| 23–26 | 26 | 22 | 23 | 12 | 3 | ||||||
| Outcomes, n per 1,000 women screened (95% CI) | |||||||||||
| False-positive mammography result | 11–14 | 119.1 (103.5, 136.8) | 0.69 | 93.3 (82.8, 105.0) | 0.46 | 79.2 (72.2, 86.8) | 0.91 | 67.6 (60.7, 75.2) | 0.70 | 63.8 (58.2, 69.9) | 0.71 |
| 23–26 | 115.8 (98.7, 135.4) | 89.9 (78.8, 102.4) | 79.6 (70.3, 90.2) | 65.7 (56.7, 76.0) | 61.2 (47.3, 78.7) | ||||||
| False-negative mammography result | 11–14 | 1.2 (1.0, 1.5) | 0.20 | 1.2 (1.0, 1.5) | 0.11 | 1.2 (0.9, 1.6) | 0.32 | 1.4 (1.1, 2.0) | 0.95 | 1.2 (0.7, 1.8) | 0.44 |
| 23–26 | 0.9 (0.5, 1.5) | 0.8 (0.4, 1.4) | 0.8 (0.4, 1.8) | 1.4 (0.6, 3.4) | 2.0 (0.7, 6.0) | ||||||
| Additional imaging recommended† | 11–14 | 122.4 (106.7, 139.9) | 0.77 | 98.3 (87.7, 109.9) | 0.57 | 86.6 (79.5, 94.3) | 0.55 | 76.6 (69.3, 84.5) | 0.98 | 71.3 (65.4, 77.7) | 0.98 |
| 23–26 | 119.9 (102.6, 139.7) | 95.5 (83.9, 108.5) | 88.8 (79.2, 99.5) | 76.7 (66.5, 88.2) | 71.1 (57.1, 88.3) | ||||||
| Biopsy recommended† | 11–14 | 14.7 (12.2, 17.8) | 0.31 | 15.1 (12.2, 18.6) | 0.66 | 15.2 (13.5, 17.2) | 0.03 | 16.6 (14.5, 18.9) | 0.85 | 13.2 (10.8, 16.0) | 0.33 |
| 23–26 | 16.9 (11.9, 24.0) | 15.8 (11.7, 21.3) | 18.8 (15.2, 23.2) | 17.0 (12.6, 23.0) | 16.6 (11.2, 24.7) | ||||||
| Screen-detected invasive cancer | 11–14 | 1.8 (1.5, 2.3) | 0.31 | 3.1 (2.6, 3.7) | 0.05 | 5.5 (4.9, 6.2) | 0.07 | 6.8 (5.8, 7.9) | 0.35 | 5.9 (4.5, 7.8) | 0.33 |
| 23–26 | 2.3 (1.6, 3.2) | 4.2 (3.3, 5.4) | 7.0 (5.7, 8.5) | 8.4 (5.7, 12.4) | 8.0 (4.9, 13.1) | ||||||
| Screen-detected DCIS | 11–14 | 1.4 (1.1, 1.8) | 0.20 | 1.9 (1.4, 2.4) | 0.22 | 1.9 (1.6, 2.3) | 0.59 | 2.2 (1.6, 3.0) | 0.69 | 1.6 (0.9, 2.8) | 0.75 |
| 23–26 | 1.8 (1.3, 2.7) | 1.4 (0.9, 2.1) | 2.2 (1.3, 3.7) | 2.6 (1.3, 5.1) | 2.0 (0.6, 6.1) | ||||||
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
After positive mammography result.
Abbreviations: CI=confidence interval; DCIS=ductal carcinoma in situ.
Appendix Table 3.
Rates of false-negative results after screening with digital mammography by risk factors*
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ||||||
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | ||||||
| False-negative mammography result, n | 115 | 139 | 112 | 73 | 24 | ||||||
| Number per 1,000 women screened per round (95% CI) | |||||||||||
| First-degree relatives with breast cancer |
None | 0.9 (0.8, 1.1) | 0.02* | 1.0 (0.8, 1.2) | 0.09 | 1.1 (0.8, 1.4) | 0.10 | 1.2 (0.9, 1.6) | 0.01 | 1.2 (0.8, 1.9) | 0.49 |
| One or more | 1.8 (1.3, 2.5) | 1.6 (1.1, 2.4) | 1.7 (1.1, 2.7) | 2.4 (1.6, 3.7) | 1.6 (0.8, 3.1) | ||||||
| Breast density | Fat-Scattered | 0.4 (0.3, 0.6) | <0.001 | 0.6 (0.4, 0.8) | 0.002 | 0.8 (0.5, 1.1) | 0.006 | 1.0 (0.6, 1.5) | 0.01 | 0.9 (0.5, 1.6) | 0.25 |
| Heterogeneous | 1.3 (1.0, 1.7) | 1.4 (1.0, 2.0) | 1.7 (1.3, 2.3) | 2.3 (1.6, 3.4) | 1.1 (0.5, 2.4) | ||||||
| Extreme | 1.7 (1.2, 2.5) | 1.6 (0.9, 2.8) | 1.2 (0.6, 2.7) | 5.6 (2.4, 12.9) | 6.9 (2.5, 18.5) | ||||||
| Benign breast biopsy† | None | 0.9 (0.8, 1.1) | 0.53 | 0.8 (0.7, 1.1) | 0.002 | 0.8 (0.6, 1.1) | 0.001 | 0.9 (0.6, 1.3) | 0.004 | 0.9 (0.5, 1.6) | 0.02 |
| Previous | 1.1 (0.7, 1.7) | 1.7 (1.3, 2.3) | 2.1 (1.6, 2.8) | 2.6 (1.8, 3.9) | 2.6 (1.6, 4.2) | ||||||
| Race/ethnicity | White | 1.2 (1.0, 1.4) | 0.31 | 1.2 (0.9, 1.4) | 0.04 | 1.3 (1.0, 1.6) | 0.36 | 1.7 (1.2, 2.4) | 0.29 | 1.4 (0.9, 2.3) | 0.77 |
| Black | 0.7 (0.3, 1.4) | 1.2 (0.6, 2.2) | 1.5 (0.8, 2.9) | 0.9 (0.3, 2.3) | 1.0 (0.2, 6.4) | ||||||
| Asian | 0.8 (0.5, 1.3) | 1.1 (0.7, 1.7) | 0.6 (0.3, 1.2) | 0.8 (0.4, 1.6) | 0‡ | ||||||
| Hispanic | 0.5 (0.2, 1.6) | 0.2 (0.0, 1.1) | 0.7 (0.2, 2.4) | 0.8 (0.1, 4.6) | 3.3 (0.4, 23.9) | ||||||
| Other | 1.1 (0.4, 3.2) | 1.6 (0.6, 4.1) | 1.2 (0.2, 7.1) | 1.5 (0.3, 8.5) | 5.4 (1.0, 27.8) | ||||||
| Menopausal status | Pre | 1.2 (1.0, 1.4) | 0.17 | 1.3 (0.9, 1.9) | 0.53 | NA | NA | NA | |||
| Peri | 0.8 (0.2, 2.5) | 1.0 (0.5, 2.1) | |||||||||
| Post | 0.7 (0.4, 1.3) | 1.0 (0.8, 1.3) | |||||||||
| Menopausal hormone therapy |
None | 1.0 (0.9, 1.2) | 0.76 | 1.0 (0.8, 1.2) | 0.37 | 1.0 (0.8, 1.3) | 0.33 | 1.3 (0.9, 1.8) | 0.58 | 1.2 (0.8, 2.0) | 0.62 |
| Combination | 0‡ | 1.9 (0.9, 3.7) | 2.3 (1.0, 5.6) | 0‡ | 3.1 (1.5, 6.6) | ||||||
| Estrogen only | 1.5 (0.2, 10.1) | 0.4 (0.1, 2.6) | 1.2 (0.4, 3.1) | 0.8 (0.1, 5.6) | 2.5 (0.4, 13.7) | ||||||
| Oral contraceptives | No current | 1.0 (0.8, 1.2) | 0.77 | 1.1 (0.9, 1.3) | 0.54 | NA | NA | NA | |||
| Current use | 1.1 (0.6, 2.1) | 1.4 (0.6, 3.5) | |||||||||
| Body mass index, kg/m2 | <25 | 1.4 (1.2, 1.7) | 0.06 | 1.3 (1.0, 1.6) | 0.008 | 1.3 (0.9, 1.8) | 0.66 | 2.4 (1.6, 3.6) | 0.09 | 1.7 (0.7, 3.8) | 0.96 |
| 25 to <30 | 0.8 (0.6, 1.3) | 1.0 (0.7, 1.6) | 1.2 (0.7, 2.1) | 1.0 (0.5, 1.8) | 1.6 (0.7, 3.7) | ||||||
| ≥30 | 0.7 (0.3, 1.4) | 0.4 (0.2, 0.8) | 1.0 (0.6, 1.8) | 1.0 (0.4, 2.4) | 0‡ | ||||||
2-sided P-value and 95% confidence intervals from logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
Categories include: almost entirely fat=fat; scattered fibroglandular densities=scattered; heterogeneously dense=heterogeneous; and extremely dense=extreme.
No false-negative outcomes. Category omitted from model used to obtain CI and P-value.
Abbreviations: kg=kilogram; m=meter; NA=not applicable; peri=perimenopausal; pre=premenopausal; post=postmenopausal.
Appendix Table 4.
Rates of recommendations for additional imaging after screening with digital mammography by risk factors*
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ||||||
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | ||||||
| Additional imaging recommended, n | 14,209 | 12,604 | 8,380 | 3,968 | 1,396 | ||||||
| Number per 1,000 women screened per round (95% CI) | |||||||||||
| First-degree relatives with breast cancer |
None | 122.1 (107.7, 138.1) | 0.02 | 95.2 (85.8, 105.6) | 0.003 | 86.7 (79.0, 95.1) | 0.002 | 77.5 (69.9, 85.7) | 0.02 | 71.7 (64.6, 79.5) | 0.01 |
| One or more | 145.6 (119.6, 176.2) | 117.1 (99.7, 137.0) | 98.3 (87.9, 109.8) | 86.9 (79.1, 95.4) | 86.0 (75.5, 97.7) | ||||||
| Breast density | Fat-Scattered | 110.8 (97.9, 125.2) | 0.001 | 84.4 (74.8, 95.1) | <0.001 | 81.0 (73.1, 89.6) | <0.001 | 75.6 (68.5, 83.4) | 0.003 | 68.9 (61.7, 76.9) | 0.002 |
| Heterogeneous | 146.0 (123.9, 171.3) | 121.6 (105.8, 139.3) | 110.6 (99.7, 122.6) | 99.0 (87.9, 111.4) | 93.6 (82.4, 106.2) | ||||||
| Extreme | 116.5 (98.4, 137.4) | 98.4 (83.1, 116.2) | 81.0 (70.3, 93.2) | 63.3 (49.7, 80.1) | 92.0 (66.6, 125.7) | ||||||
| Benign breast biopsy | None | 117.8 (103.4, 134.0) | 0.001 | 90.9 (81.7, 101.0) | <0.001 | 81.9 (74.1, 90.6) | <0.001 | 72.2 (65.1, 79.9) | <0.001 | 72.1 (64.3, 80.7) | 0.07 |
| Previous | 172.5 (145.9, 202.8) | 129.3 (112.8, 147.8) | 108.2 (98.2, 118.9) | 100.5 (90.0, 112.1) | 82.7 (72.9, 93.7) | ||||||
| Race/ethnicity | White | 131.1 (119.4, 143.8) | 0.001 | 103.2 (94.8, 112.3) | 0.01 | 92.4 (85.7, 99.4) | 0.005 | 83.3 (77.1, 90.1) | 0.004 | 78.0 (71.1, 85.5) | 0.11 |
| Black | 95.9 (85.0, 108.0) | 82.6 (68.4, 99.4) | 70.8 (59.3, 84.3) | 66.3 (59.1, 74.4) | 60.3 (49.1, 74.0) | ||||||
| Asian | 89.1 (76.0, 104.2) | 73.5 (62.1, 86.8) | 64.6 (54.0, 77.0) | 52.6 (44.9, 61.4) | 40.5 (33.4, 48.9) | ||||||
| Hispanic | 127.8 (109.2, 149.0) | 84.6 (71.9, 99.3) | 76.9 (64.1, 92.0) | 72.1 (61.6, 84.3) | 62.3 (38.7, 98.9) | ||||||
| Other | 131.6 (109.8, 157.1) | 109.8 (97.1, 123.8) | 98.8 (82.5, 117.8) | 84.7 (64.0, 111.3) | 65.2 (39.4, 106.2) | ||||||
| Menopausal status | Pre | 135.4 (117.4, 155.6) | 0.01 | 124.6 (113.6, 136.4) | <0.001 | NA | NA | NA | |||
| Peri | 109.0 (92.8, 127.7) | 101.4 (78.7, 129.8) | |||||||||
| Post | 114.2 (103.1, 126.4) | 92.7 (84.0, 102.1) | |||||||||
| Menopausal hormone therapy |
None | 127.0 (111.2, 144.8) | 0.63 | 97.0 (86.7, 108.5) | 0.28 | 83.8 (76.5, 91.7) | 0.18 | 76.5 (69.8, 83.9) | 0.01 | 71.5 (64.2, 79.6) | 0.20 |
| Combination | 125.8 (83.6, 185.0) | 137.4 (105.5, 177.1) | 129.5 (96.3, 172.0) | 120.7 (94.9, 152.4) | 106.6 (79.4, 141.6) | ||||||
| Estrogen only | 110.1 (85.6, 140.7) | 105.4 (90.9, 121.8) | 106.1 (86.0, 130.3) | 125.1 (106.4, 146.6) | 106.4 (82.6, 136.1) | ||||||
| Oral contraceptives | No current | 126.6 (110.9, 144.2) | 0.05 | 99.0 (88.3, 110.7) | 0.85 | NA | NA | NA | |||
| Current use | 110.4 (90.9, 133.6) | 100.3 (84.4, 118.9) | |||||||||
| Body mass index, kg/m2 |
<25 | 133.9 (118.1, 151.3) | 0.006 | 105.9 (95.6, 117.2) | 0.05 | 93.4 (85.4, 102.1) | 0.31 | 79.5 (70.5, 89.5) | 0.28 | 83.4 (69.8, 99.5) | 0.20 |
| 25 to <30 | 129.2 (114.7, 145.2) | 99.3 (90.4, 108.9) | 88.7 (79.1, 99.4) | 84.1 (75.5, 93.6) | 69.5 (58.5, 82.3) | ||||||
| ≥30 | 110.7 (99.4, 123.2) | 93.1 (84.2, 102.8) | 89.2 (82.1, 96.8) | 89.3 (78.5, 101.5) | 88.4 (71.5, 108.8) | ||||||
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
Categories include: almost entirely fat=fat; scattered fibroglandular densities=scattered; heterogeneously dense=heterogeneous; and extremely dense=extreme.
Abbreviations: kg=kilogram; m=meter; NA=not applicable; peri=perimenopausal; pre=premenopausal; post=postmenopausal.
Appendix Table 5.
Rates of false-positive and false-negative digital mammography results and recommendations for additional imaging and biopsies by different breast density categories*
| Age, y | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | |||||||
| Women screened, n | 113,770 | 127,958 | 94,507 | 50,204 | 18,752 | ||||||
|
False-positive mammography results Number per 1,000 women screened per round (95% CI) |
|||||||||||
| A | Fat-Scattered | 108.4 (95.5, 122.7) | <0.001 | 80.5 (71.1, 90.9) | <0.001 | 74.1 (66.4, 82.6) | <0.001 | 67.3 (60.4, 74.9) | 0.003 | 60.3 (54.0, 67.4) | 0.001 |
| Heterogeneous | 142.2 (120.2, 167.4) | 115.8 (100.3, 133.2) | 101.8 (91.0, 113.8) | 88.7 (78.7, 99.9) | 82.4 (72.6, 93.5) | ||||||
| Extreme | 112.1 (94.4, 132.7) | 92.7 (77.5, 110.5) | 75.2 (64.7, 87.1) | 57.7 (43.9, 75.5) | 85.1 (61.7, 116.2) | ||||||
| B | Fat | 63.0 (51.2, 77.4) | <0.001 | 52.1 (44.9, 60.3) | <0.001 | 48.5 (43.1, 54.4) | <0.001 | 45.4 (39.7, 51.9) | <0.001 | 39.5 (32.1, 48.5) | <0.001 |
| Scattered | 116.8 (102.9, 132.3) | 87.7 (77.1, 99.6) | 81.6 (72.7, 91.4) | 73.4 (65.4, 82.2) | 65.8 (58.4, 73.9) | ||||||
| Heterogeneous-Extreme | 135.3 (113.9, 160.0) | 112.0 (96.9, 129.2) | 98.9 (88.4, 110.4) | 86.2 (76.4, 97.1) | 82.7 (72.6, 93.9) | ||||||
| C | Fat | 63.0 (51.2, 77.4) | <0.001 | 52.1 (44.9, 60.3) | <0.001 | 48.5 (43.1, 54.4) | <0.001 | 45.4 (39.7, 51.9) | <0.001 | 39.5 (32.1, 48.5) | <0.001 |
| Scattered | 116.8 (102.9, 132.3) | 87.7 (77.1, 99.6) | 81.6 (72.7, 91.4) | 73.4 (65.4, 82.2) | 65.8 (58.4, 73.9) | ||||||
| Heterogeneous | 142.2 (120.2, 167.4) | 115.8 (100.3, 133.2) | 101.8 (91.0, 113.8) | 88.7 (78.7, 99.9) | 82.4 (72.6, 93.5) | ||||||
| Extreme | 112.1 (94.4, 132.7) | 92.7 (77.5, 110.5) | 75.2 (64.7, 87.1) | 57.7 (43.9, 75.5) | 85.1 (61.7, 116.2) | ||||||
| D | Fat-Scattered | 108.4 (95.5, 122.7) | 0.003 | 80.5 (71.1, 90.9) | <0.001 | 74.1 (66.4, 82.6) | <0.001 | 67.3 (60.4, 74.9) | <0.001 | 60.3 (54.0, 67.4) | <0.001 |
| Heterogeneous-Extreme | 135.3 (113.9, 160.0) | 112.0 (96.9, 129.2) | 98.9 (88.4, 110.4) | 86.2 (76.4, 97.1) | 82.7 (72.6, 93.9) | ||||||
|
False-negative mammography results Number per 1,000 women screened per round (95% CI) |
|||||||||||
| A | Fat-Scattered | 0.4 (0.3, 0.6) | <0.001 | 0.6 (0.4, 0.8) | 0.002 | 0.8 (0.5, 1.1) | 0.006 | 1.0 (0.6, 1.5) | 0.01 | 0.9 (0.5, 1.6) | 0.25 |
| Heterogeneous | 1.3 (1.0, 1.7) | 1.4 (1.0, 2.0) | 1.7 (1.3, 2.3) | 2.3 (1.6, 3.4) | 1.1 (0.5, 2.4) | ||||||
| Extreme | 1.7 (1.2, 2.5) | 1.6 (0.9, 2.8) | 1.2 (0.6, 2.7) | 5.6 (2.4, 12.9) | 6.9 (2.5, 18.5) | ||||||
| B | Fat | 0.2 (0.0, 0.9) | <0.001 | 0.3 (0.1, 0.7) | <0.001 | 0.6 (0.2, 1.5) | 0.007 | 0.3 (0.1, 1.1) | 0.001 | 0.4 (0.1, 3.1) | 0.14 |
| Scattered | 0.5 (0.3, 0.7) | 0.7 (0.5, 0.9) | 0.8 (0.6, 1.2) | 1.2 (0.7, 1.9) | 1.0 (0.6, 1.7) | ||||||
| Heterogeneous-Extreme | 1.4 (1.2, 1.8) | 1.5 (1.1, 1.9) | 1.6 (1.2, 2.2) | 2.6 (1.8, 3.7) | 1.7 (0.8, 3.3) | ||||||
| C | Fat | 0.2 (0.0, 0.9) | <0.001 | 0.3 (0.1, 0.7) | <0.001 | 0.6 (0.2, 1.5) | 0.02 | 0.3 (0.1, 1.1) | 0.002 | 0.4 (0.1, 3.1) | 0.17 |
| Scattered | 0.5 (0.3, 0.7) | 0.7 (0.5, 0.9) | 0.8 (0.6, 1.2) | 1.2 (0.7, 1.9) | 1.0 (0.6, 1.7) | ||||||
| Heterogeneous | 1.3 (1.0, 1.7) | 1.4 (1.0, 2.0) | 1.7(1.3, 2.3) | 2.3 (1.6, 3.4) | 1.1 (0.5, 2.4) | ||||||
| Extreme | 1.7 (1.2, 2.5) | 1.6 (0.9, 2.8) | 1.2 (0.6, 2.7) | 5.6 (2.4, 12.9) | 6.9 (2.5, 18.5) | ||||||
| D | Fat-Scattered | 0.4 (0.3, 0.6) | <0.001 | 0.6 (0.4, 0.8) | <0.001 | 0.8 (0.5, 1.1) | 0.002 | 1.0 (0.6, 1.5) | 0.003 | 0.9 (0.5, 1.6) | 0.18 |
| Heterogeneous-Extreme | 1.4 (1.2, 1.8) | 1.5 (1.1, 1.9) | 1.6 (1.2, 2.2) | 2.6 (1.8, 3.7) | 1.7 (0.8, 3.3) | ||||||
|
Recommendations for additional imaging Number per 1,000 women screened per round (95% CI) |
|||||||||||
| A | Fat-Scattered | 110.8 (97.9, 125.2) | 0.001 | 84.4 (74.8, 95.1) | <0.001 | 81.0 (73.1, 89.6) | <0.001 | 75.6 (68.5, 83.4) | 0.003 | 68.9 (61.7, 76.9) | 0.002 |
| Heterogeneous | 146.0 (123.9, 171.3) | 121.6 (105.8, 139.3) | 110.6 (99.7, 122.6) | 99.0 (87.9, 111.4) | 93.6 (82.4, 106.2) | ||||||
| Extreme | 116.5 (98.4, 137.4) | 98.4 (83.1, 116.2) | 81.0 (70.3, 93.2) | 63.3 (49.7, 80.1) | 92.0 (66.6, 125.7) | ||||||
| B | Fat | 64.4 (52.3, 79.1) | <0.001 | 54.1 (46.5, 62.8) | <0.001 | 53.4 (47.9, 59.4) | <0.001 | 52.0 (46.5, 58.2) | <0.001 | 44.8 (36.6, 54.6) | <0.001 |
| Scattered | 119.4 (105.5, 135.0) | 92.1 (81.2, 104.3) | 89.0 (79.9, 99.1) | 82.1 (73.9, 91.2) | 75.2 (66.5, 84.8) | ||||||
| Heterogeneous-Extreme | 139.3 (117.7, 164.1) | 117.8 (102.4, 135.3) | 107.3 (96.7, 118.9) | 96.1 (85.5, 107.9) | 93.5 (82.1, 106.3) | ||||||
| C | Fat | 64.4 (52.3, 79.1) | <0.001 | 54.1 (46.5, 62.8) | <0.001 | 53.4 (47.9, 59.4) | <0.001 | 52.0 (46.5, 58.2) | <0.001 | 44.8 (36.6, 54.6) | 0.001 |
| Scattered | 119.4 (105.5, 135.0) | 92.1 (81.2, 104.3) | 89.0 (79.9, 99.1) | 82.1 (73.9, 91.2) | 75.2 (66.5, 84.8) | ||||||
| Heterogeneous | 146.0 (123.9, 171.3) | 121.6 (105.8, 139.3) | 110.6 (99.7, 122.6) | 99.0 (87.9, 111.4) | 93.6 (82.4, 106.2) | ||||||
| Extreme | 116.5 (98.4, 137.4) | 98.4 (83.1, 116.2) | 81.0 (70.3, 93.2) | 63.3 (49.7, 80.1) | 92.0 (66.6, 125.7) | ||||||
| D | Fat-Scattered | 110.8 (97.9, 125.2) | 0.003 | 84.4 (74.8, 95.1) | <0.001 | 81.0 (73.1, 89.6) | <0.001 | 75.6 (68.5, 83.4) | 0.001 | 68.9 (61.7, 76.9) | <0.001 |
| Heterogeneous-Extreme | 139.3 (117.7, 164.1) | 117.8 (102.4, 135.3) | 107.3 (96.7, 118.9) | 96.1 (85.5, 107.9) | 93.5 (82.1, 106.3) | ||||||
|
Recommendations for biopsy Number per 1,000 women screened per round (95% CI) |
|||||||||||
| A | Fat-Scattered | 12.2 (9.9, 15.0) | <0.001 | 11.8 (9.6, 14.5) | <0.001 | 15.6 (13.7, 17.7) | 0.008 | 16.2 (14.2, 18.4) | 0.007 | 14.2 (12.0, 16.8) | 0.07 |
| Heterogeneous | 18.9 (15.8, 22.5) | 20.2 (17.3, 23.7) | 19.3 (16.9, 22.2) | 21.0 (18.0, 24.5) | 19.0 (15.5, 23.2) | ||||||
| Extreme | 20.2 (16.8, 24.3) | 19.2 (14.3, 25.7) | 13.8 (10.5, 18.2) | 13.0 (7.2, 23.3) | 16.1 (8.0, 32.1) | ||||||
| B | Fat | 7.5 (5.5, 10.1) | <0.001 | 8.4 (6.0, 11.7) | <0.001 | 11.7 (9.5, 14.6) | <0.001 | 12.8 (10.2, 16.1) | 0.003 | 9.7 (5.8, 16.0) | 0.04 |
| Scattered | 13.1 (10.6, 16.1) | 12.7 (10.3, 15.6) | 16.7 (14.7, 19.0) | 17.1 (14.9, 19.6) | 15.4 (12.5, 18.8) | ||||||
| Heterogeneous-Extreme | 19.2 (16.2, 22.7) | 20.1 (16.9, 23.7) | 18.7 (16.6, 21.2) | 20.4 (17.4, 23.8) | 18.7 (15.2, 23.0) | ||||||
| C | Fat | 7.5 (5.5, 10.1) | <0.001 | 8.4 (6.0, 11.7) | <0.001 | 11.7 (9.5, 14.6) | <0.001 | 12.8 (10.2, 16.1) | 0.003 | 9.7 (5.8, 16.0) | 0.06 |
| Scattered | 13.1 (10.6, 16.1) | 12.7 (10.3, 15.6) | 16.7 (14.7, 19.0) | 17.1 (14.9, 19.6) | 15.4 (12.5, 18.8) | ||||||
| Heterogeneous | 18.9 (15.8, 22.5) | 20.2 (17.3, 23.7) | 19.3 (16.9, 22.2) | 21.0 (18.0, 24.5) | 19.0 (15.5, 23.2) | ||||||
| Extreme | 20.2 (16.8, 24.3) | 19.2 (14.3, 25.7) | 13.8 (10.5, 18.2) | 13.0 (7.2, 23.3) | 16.1 (8.0, 32.1) | ||||||
| D | Fat-Scattered | 12.2 (9.9, 15.0) | <0.001 | 11.8 (9.6, 14.5) | <0.001 | 15.6 (13.7, 17.7) | 0.002 | 16.2 (14.2, 18.4) | 0.008 | 14.2 (12.0, 16.8) | 0.03 |
| Heterogeneous-Extreme | 19.2 (16.2, 22.7) | 20.1 (16.9, 23.7) | 18.7 (16.6, 21.2) | 20.4 (17.4, 23.8) | 18.7 (15.2, 23.0) | ||||||
2-sided P-values and 95% confidence intervals from a logistic regression model that accounts for clustering by radiology facility using generalized estimating equations.
Categories include: almost entirely fat=fat; scattered fibroglandular densities=scattered; heterogeneously dense=heterogeneous; and extremely dense=extreme.
Footnotes
Disclaimer: The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or the U.S. Department of Health and Human Services.
REFERENCES
- 1.U. S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. PMID: 19920272. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O'Meara ES, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Annals of Internal Medicine. 2012;156(9):635–648. doi: 10.1059/0003-4819-156-9-201205010-00006. PMID: 22547473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;160(4):255–266. doi: 10.7326/M13-1684. PMID: 24366442. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute; 2012. [on 30 July, 2012]. National Cancer Institute Screening mammograms: questions and answers. Accessed at http://www.cancer.gov/cancertopics/factsheet/detection/mammograms. [Google Scholar]
- 5.Coopey S, Mazzola E, Buckley J, Sharko J, Belli A, Kim EH, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Research and Treatment. 2012;136(3):627–633. doi: 10.1007/s10549-012-2318-8. PMID: 23117858. [DOI] [PubMed] [Google Scholar]
- 6.Tice JA, O'Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105(14):1043–1049. doi: 10.1093/jnci/djt124. PMID: 23744877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–2223. doi: 10.1200/JCO.2013.54.4601. PMID: 24752044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Fort Washington, PA: 2014. [on September 18, 2014]. Accessed at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 9.Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA. Family history and the risk of breast cancer: a systematic review and meta-analysis. International Journal of Cancer. 1997;71(5):800–809. doi: 10.1002/(sici)1097-0215(19970529)71:5<800::aid-ijc18>3.0.co;2-b. PMID: 9180149. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations. Annals of Internal Medicine. 2012;157(2):104-W-23. doi: 10.7326/0003-4819-157-2-201207170-00466. PMID: 77944506. [DOI] [PubMed] [Google Scholar]
- 11.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. PMID: 18316752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. PMID: 9355998. [PubMed] [Google Scholar]
- 13.Nelson HD, Cantor A, Humphrey L, Fu R, Pappas M, Daeges M, et al. Screening for Breast Cancer: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. [on April 22, 2014];2015 AHRQ Report No.: 14-05201-EF-1. Accessed at http://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review-screening-for-breast-cancer/breast-cancer-screening1. [PubMed] [Google Scholar]
- 14.Bethesda, MD: National Cancer Institute, National Institutes of Health U.S. Department of Health and Human Services; 2004. Apr, [on September 19, 2014]. National Cancer Institute Breast Cancer Surveillance Consortium: Evaluating Screening Performance in Practice. NIH Publication No. 04-5490. Accessed at http://breastscreening.cancer.gov/espp.pdf. [Google Scholar]
- 15.Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR. 1997;169(4):1001–1008. doi: 10.2214/ajr.169.4.9308451. PMID: 9308451. [DOI] [PubMed] [Google Scholar]
- 16.BCSC Glossary of Terms. [on October 27, 2014];2009 Accessed at http://breastscreening.cancer.gov/data/bcsc_data_definitions.pdf.
- 17.D'Orsi CJ, Bassett LW, Berg WA. Breast imaging reporting and data system: ACR BIRADS. 4th. Reston, VA: American College of Radiology; 2003. Follow-up and outcome monitoring; pp. 229–251. [Google Scholar]
- 18.Rosenberg RD, Yankaskas BC, Abraham LA, Sickles EA, Lehman CD, Geller BM, et al. Performance benchmarks for screening mammography. Radiology. 2006;241(1):55–66. doi: 10.1148/radiol.2411051504. PMID: 16990671. [DOI] [PubMed] [Google Scholar]
- 19.Kerlikowske K, Zhu W, Hubbard RA, Geller B, Dittus K, Braithwaite D, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Internal Medicine. 2013;173(9):807–816. doi: 10.1001/jamainternmed.2013.307. PMID: 23552817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, Weaver DL, Buist DSM, Barlow WE, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. Journal of Clinical Oncology. 2003;21(23):4314–4321. doi: 10.1200/JCO.2003.05.151. PMID: 14645420. [DOI] [PubMed] [Google Scholar]
- 21.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 22.Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Annals of Internal Medicine. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [Summary for patients in Ann Intern Med. 2011 Oct 18;155(8):I30; PMID: 22007060]. PMID: 22007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–681. doi: 10.7326/M14-1465. PMID: 25984843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. PMID: 17229950. [DOI] [PubMed] [Google Scholar]
- 25.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2009;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. PMID: 19920273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braithwaite D, Zhu W, Hubbard RA, O'Meara ES, Miglioretti DL, Geller B, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? Journal of the National Cancer Institute. 2013;105(5):334–341. doi: 10.1093/jnci/djs645. PMID: 23385442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dittus K, Geller B, Weaver DL, Kerlikowske K, Zhu W, Hubbard R, et al. Impact of mammography screening interval on breast cancer diagnosis by menopausal status and BMI. Journal of General Internal Medicine. 2013;28(11):1454–1462. doi: 10.1007/s11606-013-2507-0. PMID: 23760741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Meara ES, Zhu W, Hubbard RA, Braithwaite D, Kerlikowske K, Dittus KL, et al. Mammographic screening interval in relation to tumor characteristics and false-positive risk by race/ethnicity and age. Cancer. 2013;119(22):3959–3967. doi: 10.1002/cncr.28310. PMID: 24037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study.[Summary for patients in Ann Intern Med. 2011 Oct 18;155(8):I14; PMID: 22007059] Annals of Internal Medicine. 2011;155(8):481–492. doi: 10.1059/0003-4819-155-8-201110180-00004. PMID: 22007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbard RA, Miglioretti DL, Smith RA. Modelling the cumulative risk of a false-positive screening test. Statistical Methods in Medical Research. 2010;19(5):429–449. doi: 10.1177/0962280209359842. PMID: 20356857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook AJ, Elmore JG, Miglioretti DL, Sickles EA, Aiello Bowles EJ, Cutter GR, et al. Decreased accuracy in interpretation of community-based screening mammography for women with multiple clinical risk factors. Journal of Clinical Epidemiology. 2010;63(4):441–451. doi: 10.1016/j.jclinepi.2009.06.008. PMID: 19744825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. PMID: 22007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson L, Miglioretti D, Kerlikowske K, Wernli KJ, Sprague BL, Lehman C. Breast cancer characteristics associated with digital versus screen-film mammography for screen-detected and interval cancers. AJR. 2015;205 doi: 10.2214/AJR.14.13904. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen K, Abraham L, Buist D, Hubbard R, O'Meara ES, Sprague BL, et al. Comparison of cumulative false-positive risk of screening mammography in the United States and Denmark. Cancer Epidemiol. 2015;(15):103–104. doi: 10.1016/j.canep.2015.05.004. pii:S1877–7821. PMID: 26013768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemp Jacobsen K, O'Meara ES, Key D, D SMB, Kerlikowske K, Vejborg I, et al. Comparing sensitivity and specificity of screening mammography in the United States and Denmark. Int J Cancer. 2015 doi: 10.1002/ijc.29593. PMID: 25944711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofvind S, Geller BM, Skelly J, Vacek PM. Sensitivity and specificity of mammographic screening as practised in Vermont and Norway. British Journal of Radiology. 2012;85(1020):e1226–e1232. doi: 10.1259/bjr/15168178. PMID: 22993383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CI, Lehman CD. Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol. 2013;10(12):913–917. doi: 10.1016/j.jacr.2013.09.010. PMID: 24295940. [DOI] [PubMed] [Google Scholar]
- 38.Lee CI, Cevik M, Alagoz O, Sprague BL, Tosteson AN, Miglioretti DL, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology. 2015;274(3):772–780. doi: 10.1148/radiol.14141237. PMID: 25350548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56. doi: 10.1148/radiol.12121373. PMID: 23297332. [DOI] [PubMed] [Google Scholar]
- 40.Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncology. 2013;14(7):583–589. doi: 10.1016/S1470-2045(13)70134-7. PMID: 23623721. [DOI] [PubMed] [Google Scholar]
- 41.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507. doi: 10.1001/jama.2014.6095. PMID: 25058084. [DOI] [PubMed] [Google Scholar]
- 42.Brem RF, Tabar L, Duffy SW, Inciardi MF, Guingrich JA, Hashimoto BE, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology. 2015;274(3):663–673. doi: 10.1148/radiol.14132832. PMID: 25329763. [DOI] [PubMed] [Google Scholar]
- 43.Diagnostic Imaging Breast Density Notification Laws by State-Interactive Map. [on March 20, 2015]; Accessed at http://www.diagnosticimaging.com/login?referrer=http%3A//www.diagnosticimaging.com%2Fbreast-density-notification-laws-state-interactive-map. [Google Scholar]


