Abstract
A traditional vaporizer depends on flowing gas and atmospheric pressure for passive anesthetic vaporization. Newly developed direct injection vaporizers utilize a syringe pump to directly administer volatile anesthetics into a gas stream. Unlike a traditional vaporizer, it can be used at very low flow rates, making it ideal for use on mice and rats.
The equipment's capability to use low flow rates could result in a substantial cost savings due to the reduced need for anesthetic agents, compressed gas, and charcoal scavenging filters1. A lower flow rate means less waste of anesthetic gas and likely reduces the risk of anesthetic exposure to laboratory personnel. Thus, the high levels of precision and safety associated with direct injection vaporizers, along with a reduced need for anesthetic agents, compressed gas, and charcoal filters are beneficial for research requiring small animal anesthesia.
The goal of this protocol is to demonstrate the use of a syringe-driven direct injection vaporizer as part of a digital, low-flow anesthesia system. The direct injection vaporizer is capable of accurately delivering anesthesia at very low flow rates compared to a traditional vaporizer, making it a promising alternative for controlled gas anesthetic delivery to rodents.
Keywords: Medicine, Issue 115, Anesthesia, isoflurane, low-flow, vaporizer, mouse, pulse oximetry
Introduction
There are many precision vaporizers available for veterinary use that operate between flow rates of 0.5-10 L/min2. These flow rates are not ideal for rodents, as the range is high compared to their small respiratory minute volume. High flow rates are not recommended in veterinary practice due to their promotion of hypothermia and drying of the respiratory tract3,4. Furthermore, many common veterinary vaporizer manufacturer manuals warn that high flow rates may cause an increase in the occurrence of backpressure fluctuations. It has also been shown that many standard vaporizers become inaccurate below flow rates of 500 ml/min, and this rate is regarded as a minimum flow rate in the veterinary field5-7.
An animal can be maintained on a T-piece circuit or modified bain circuit using a flow rate as low as 1.5-2.2 times the animal's minute volume8-10. These flow rates are considered sufficient to prevent rebreathing of expired gases and prevent an increase in blood carbon dioxide concentrations8. Using this flow rate recommendation, a 30 g mouse could be maintained at a flow rate as low as 52 ml/min, nearly ten times less than the accepted 500 ml/min minimum of a traditional vaporizer.
While a traditional vaporizer depends on gas flow and atmospheric pressure for passive anesthetic vaporization, a direct injection vaporizer measures the total fresh gas flow and injects the vapor directly into the gas stream2. Some direct injection vaporizers utilize a syringe pump to administer anesthetic into the gas stream. Computerized controls allow these systems to automatically adjust the syringe pump speed to inject the volume of liquid agent required to reach the desired concentration of anesthetic. Syringe driven vaporizers are available and approved for clinical and pediatric use, and many similar configurations are regarded as anesthetic conserving devices in clinical practice11-16. Shortly after their approval, anesthetic conserving devices with syringe pump vaporizers were adapted for use in animal studies8,17,18. Unlike a traditional vaporizer, a direct injection system utilizing a syringe pump is not limited by a minimum flow rate in order to maintain accuracy. For this reason, this technology is ideal for use in rodent anesthesia and other instances where low flow rates are necessary. The benefits and potential cost savings associated with this vaporizer design inspired the development of new anesthesia systems designed specifically for rodents1,19,20. This new system also incorporates a built-in air pump, allowing the user to administer anesthesia without requiring a compressed gas source. As an additional benefit, the system is pre-calibrated for use with both isoflurane and sevoflurane. With the introduction of this vaporizer technology in the laboratory animal field, it is now possible to anesthetize small laboratory animals at flow rates closer to recommended levels without the need for compressed gas.
Protocol
All studies were completed in accordance with regulatory and institutional guidelines. The animal aspects of this study were evaluated by the Kent Scientific Corporation Animal Use Program, approved by the Purdue Animal Care and Use Committee (PACUC), and performed in accordance with the Guide for the Care and Use of Laboratory Animals22.
Note: The low-flow digital anesthesia system used in this protocol is equipped with an integrated pulse oximeter.
1. Set Up the Low-flow Anesthesia System with Integrated Pulse Oximeter
- For Isoflurane Delivery
- Select a carrier gas source. To utilize the internal air pump, unscrew Inlet Port on back, allowing the internal pump to intake room air.
- Connect charcoal canister to exhaust port.
- Connect Y adapter to front of the low flow, digital anesthesia system. Use the color-coded clips to connect the white branches to the nose cone, and blue branches to the induction chamber.
- Close white clip clamps and open blue clip clamps to direct airflow to the chamber.
- Select 2 ml syringe.
- For Physiological Monitoring, Using the Integrated Pulse Oximeter
- Connect the pulse oximeter paw sensor to port on the back of the low-flow anesthesia system, labeled "AUX".
2. Configure the Settings
- For Anesthesia
- Power on the anesthesia system, and access the Set Up menu. Press Set Up to access Main Menu > Anesthesia > Setup in red.
- Choose the anesthetic agent. Press Set Up to highlight Type Anest in red. Use the Up and Down arrows to select Isoflurane.
- Set the syringe size. Press Set Up to highlight Syr Size. Use the Up and Down arrows to select a 2 ml syringe.
- Set the Empty Position. Press Set Up to highlight Set Empty in red. Secure empty, fully depressed glass syringe in the Syringe Retention Block by placing the syringe so that the Syringe Holding Clamp is seated on the metal collar of the syringe. Press the Up or Down arrows to move the Pusher Block so that the Pusher Block makes light contact with the top of the syringe plunger. Press select to set empty position.
- Press Set Up to highlight Remove in red. Remove the syringe and fill the syringe with isoflurane using the bottle top adapter.
- Connect the Syringe to the anesthesia system.
- Prime the anesthetic delivery tubing. Press Set Up to highlight Prime Tube in red. Press Down until the anesthetic travels through the syringe and into the black fitting on the vaporizer block.
- Enable anesthesia. Press Set Up to highlight Enable in red. Use the Up and Down arrows to select Yes. Press Run/Back to return to the Main Menu.
- Select the air supply and minute volume. Press Set Up to highlight Air Supply in red. Use the Up and Down arrows to select Internal Pump. Press Set Up to highlight Minute Vol in red. Set flow rate to 250 ml/min.
- For Physiological Monitoring
- Set the minimum detected heart rate to Mouse (240). Press Set Up to access Main Menu > MouseStat. Use up and down arrows to set minimum heart rate.
3. Begin Anesthesia Delivery
- Induce the Mouse
- Press Run/Back twice to enter run mode and begin airflow.
- Place the mouse in the induction chamber, closing the lid tightly. Adjust the Anesthetic Agent Concentration knob to 3%.
- Monitor until the mouse has reached the desired plane of anesthesia, determined by loss of righting reflex. Adjust the Anesthetic Agent Concentration as necessary.
- Once the animal loses its righting reflex and is sufficiently anesthetized, turn the Anesthesia concentration dial to 0%. The authors have previously found that allowing the airflow to flush the induction chamber 30-60 sec is sufficient to purge the chamber without reversing the anesthetic depth1.
- Quickly open the white clamps to direct the air to the facemask, and close the blue clamps leading to the induction chamber.
- Open the chamber away from researcher, remove the mouse, and immediately fit the nose cone.
- Center the animal on an infrared warming pad, set to maintain body temperature at 37 °C via a rectal probe on a feedback loop.
- When the mouse is stable on the nose cone, adjust the concentration of isoflurane to 1.5% or as needed for maintenance by turning the Anesthetic Agent Concentration knob.
- Reduce minute volume for maintenance. The minimum flow rate to support the animal is equal to 1.5-2.2 times the animal's minute volume (for a 30 g mouse, a minimum of 52 ml/min). Refer to the manufacturer's instructions for a recommended flow rate setting specific to the nose cone style and adjust as necessary. Press Set Up to access the Main Menu, and then press Set Up until Minute Vol is highlighted in red. Use the Up and Down arrows to adjust target flow. Press Run/Back to return to the main screen.
- Confirm depth of anesthesia as determined by a lack of withdrawal reflex during an interdigital pinch. Apply ophthalmic ointment to the eyes to prevent dryness during anesthesia.
4. Begin Physiological Monitoring
Place sensor over pad of hind paw. Position the sensor so that the red light is beneath the paw and illuminates the paw. Use the up and down arrows on the screen to display the oxiwave. The animal is now safely anesthetized using a low flow, syringe-driven, digital vaporizer.
5. Remove the Animal
- Turn Off the Anesthesia Delivery.
- To stop the delivery of anesthetic, turn Anesthetic Agent Concentration knob to Min (or 0%) and remove animal from facemask.
- Monitor the mouse during anesthesia recovery. Once the mouse has become fully ambulatory, return it to the cage.
Representative Results
Animals
3 adult C57/BL6NTac female mice (Taconic, age 6-7 weeks; weight 15+/-1 g) were anesthetized and maintained with 1.3-1.5% isoflurane while heart rate, oxygen saturation, and respiration rate were monitored. All mice were Murine Pathogen Free as determined by routine vendor testing before arrival to the facility. The animals were group-housed in microisolation caging and provided free-access to standard rodent chow and water by bottle.
Isoflurane Usage
The low-flow anesthesia system measures the amount of anesthetic agent remaining in the syringe during use. The volume in the syringe, as measured by the anesthesia system, was noted as the animal was transferred to the nose cone, and again at the end of the maintenance period. The final volume was subtracted from the initial volume to quantify the amount of anesthetic consumed during the maintenance period (Figure 1).
Physiological Parameters
Heart rate, SpO2, and respiration rate were monitored during maintenance via pulse oximetry (Figures 2-4). Body temperature was maintained at 37.5 °C via an infrared warming pad. Each mouse was successfully maintained at low flow rates of 100 ml/min of room air under a surgical plane of anesthesia for 60 min, as determined by a lack of withdrawal reflex from an interdigital pinch. The mouse did not awake or respond to interdigital pinches applied intermittently during the maintenance period. The animals' heart rates (Figure 2), blood oxygen (Figure 3), and respiratory rate (Figure 4) remained relatively stable throughout the study. Due to animal and sensor positioning, the respiratory rate signal from Mouse 1 and Mouse 3 was intermittent and the measurement was interrupted. When the animal's positioning was adjusted, the signal improved and the measured respiratory rate was comparable to others at similar time points. The low-flow digital anesthesia system used an average of 0.63 ml of isoflurane during the 60 min of maintenance period (Figure 1).
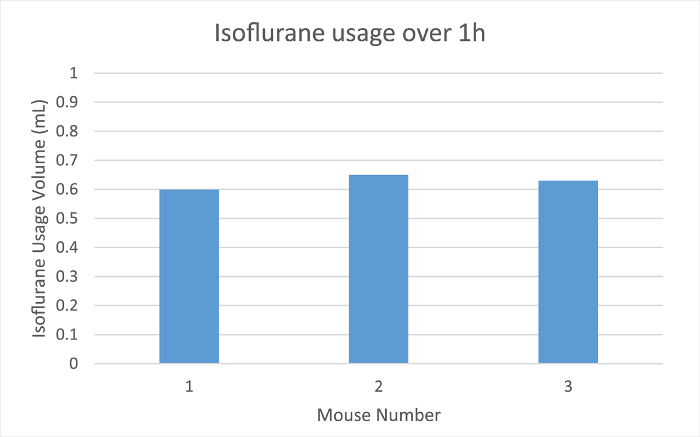 Figure 1:Isoflurane Usage. The amount of isoflurane used in ml for three different mice over 1 hr of anesthesia maintenance using the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
Figure 1:Isoflurane Usage. The amount of isoflurane used in ml for three different mice over 1 hr of anesthesia maintenance using the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
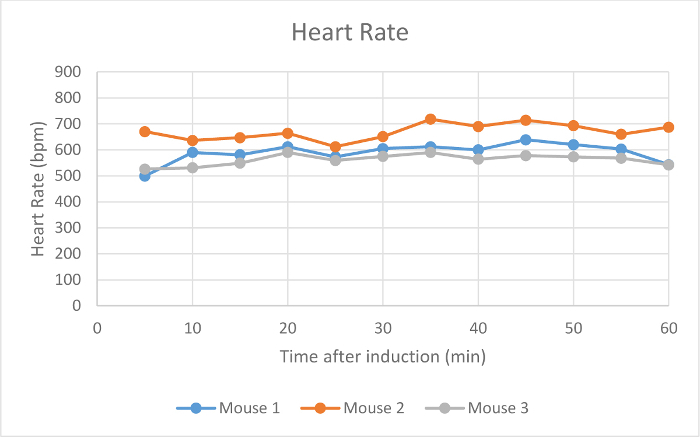 Figure 2:Heart Rate. The heart rate of three mice in beats per min (bpm) 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
Figure 2:Heart Rate. The heart rate of three mice in beats per min (bpm) 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
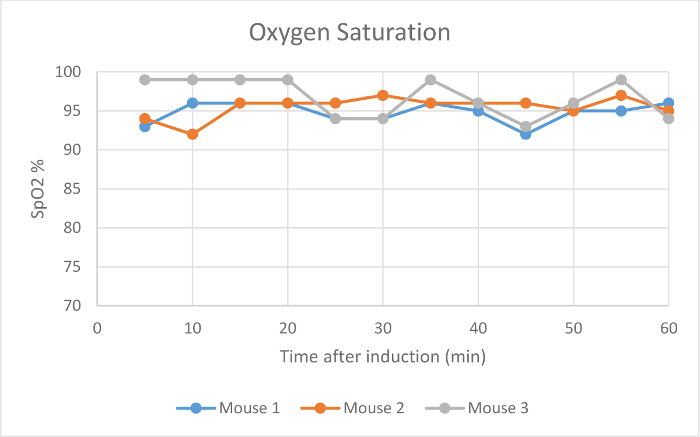 Figure 3:Oxygen Saturation. The blood oxygen saturation levels (%) of three mice 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
Figure 3:Oxygen Saturation. The blood oxygen saturation levels (%) of three mice 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
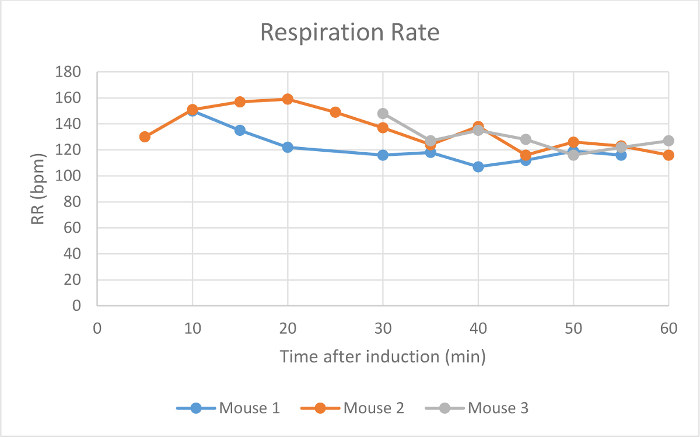 Figure 4: Respiration Rate. The respiration rate of three mice in breaths per min (bpm) 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
Figure 4: Respiration Rate. The respiration rate of three mice in breaths per min (bpm) 5-60 min after initial anesthetic induction with the digital low-flow anesthesia system. Please click here to view a larger version of this figure.
Discussion
The digital low-flow anesthesia system allows the user to effectively anesthetize mice at very low flow rates without the use of any compressed gas. This differs greatly from standard passive vaporizers, most of which require a compressed gas source at minimum flow rates of about 500 ml/min. Standard vaporizers utilize dials that lack precision between gradations, and they must be serviced annually to maintain accuracy. A syringe driven anesthetic system can provide a specific concentration of anesthetic at the set flow rate to calculate the exact necessary speed of the syringe pump. Routine calibrations are unnecessary, resulting in additional cost and time savings.
The recommended minimum flow rate to maintain an animal on a non-rebreathing circuit is 1.5-2.2 times the animal's minute volume. The flow rate of 100 ml/min used in this study exceeded this minimum to deliver sufficient anesthetic to the animals. The flow rate settings are critical for this anesthetic delivery technique, as the flow rate is directly related to the amount of isoflurane used for a given timeframe. When used at low flow rates, this technique can greatly reduce the amount of isoflurane required during use, while the animal is still anesthetized effectively1,19-21.
New equipment costs between traditional vaporizers and low-flow digital vaporizers are comparable. However, the digital low-flow anesthesia system has the ability to deliver either isoflurane or sevoflurane. This eliminates the need for designated isoflurane and sevoflurane precision vaporizers, reducing initial equipment costs for groups using both anesthetic agents. Recently published comparisons between vaporizer technologies have suggested cost savings over time when using a low-flow digital vaporizer1,19,20. The results of these comparisons could be used to approximate potential cost savings over the course of a year. Assuming typical usage settings performed in 2 hr increments, 5 days a week for 52 weeks, a traditional isoflurane vaporizer will consume 3.8 L of isoflurane, or twelve 250 ml bottles. A low-flow digital vaporizer used at the same frequency would consume just 0.32 L, or two 250 ml bottles. Charcoal canister consumption is also reduced. Assuming that each canister holds 50 g of scavenged waste gas, a traditional vaporizer will fill approximately 21 charcoal canisters over the course of a year. In comparison, a low-flow digital vaporizer will require 6 or fewer. A traditional vaporizer would require approximately 5 large gas cylinders per year, each with a capacity of 9,500 L. The internal air pump, available in some models of digital low-flow vaporizers, eliminates the requirement for compressed gas. If compressed gas were to be used, the system would use only 1 cylinder per year1.
The technique can be modified based on need. Low-flow digital vaporizers allow the user to adjust anesthetic depth quickly and precisely. If the anesthetic depth must be increased or decreased, the user can increase the anesthetic concentration in 0.1% increments using the dial on the top of the system. The flow rate can also be adjusted as needed throughout the procedure. This protocol utilizes a 2 ml syringe, though larger syringe sizes are available for longer procedures. The internal air pump offers users the option to anesthetize animals without requiring a compressed gas source. For procedures requiring compressed gas or supplemental oxygen, the user has the option to connect a gas source to the low-flow system rather than using surrounding air. The user can continue to deliver the selected air source throughout the procedure, or can switch between the internal pump and a compressed gas source as needed. For example, the user may set the system to deliver room air via the internal pump during induction and maintenance, but deliver supplemental oxygen during recovery.
Though there are many advantages to using a low-flow digital vaporizer, there are limitations as well. Because a flush valve is not included, manually flushing the chamber with clean air before opening is the only way to purge the induction chamber. This system is designed to operate at low flow rates only and does not deliver anesthesia above flow rates of 800 ml/min, where traditional vaporizers can be used with flow rates up to 10 L/min. This particular system is therefore only suitable for small animal species. Additionally, the system holds less anesthetic agent compared to a traditional vaporizer. There may be situations where the syringe must be refilled during a procedure. However, delays during refilling can be reduced by pre-filling a second syringe nearby to replace the empty syringe. Syringe sizes up to 10 ml are available to reduce the need to refill syringes mid-procedure. Finally, unlike a traditional vaporizer, the low-flow digital vaporizer requires electricity. Batteries are available for use in instances where electrical power is unavailable or in the event of a power outage.
Previous studies have shown that low-flow digital systems consume less isoflurane, carrier gas, and charcoal canisters compared to a traditional anesthesia system1,19,20. The reduction in scavenged anesthetic gas also could identify a reduction in waste anesthetic gas, though further work is needed in these areas. Infrared gas spectroscopy can be used to monitor waste isoflurane production, and dosimeter badges can be used to quantify isoflurane exposure to laboratory personnel in future comparisons.
In summary, this technique for anesthetic delivery will be beneficial to groups performing rodent anesthesia due to improved safety, efficacy, and precision over traditional systems.
Disclosures
This project was supported with equipment and funding by Kent Scientific Corporation, the American Heart Association to CJ Goergen (SDG18220010), and Purdue University. The authors Krista Bigiarelli and Irina Toore are employees of Kent Scientific Corporation that produces equipment used in this article. Open access publication of this article is sponsored by Kent Scientific Corporation.
Acknowledgments
The authors have no acknowledgements.
References
- Damen FW, Adelsperger AR, Wilson KE, Goergen CJ. Comparison of traditional and integrated digital anesthetic vaporizers. JAALAS. 2015;54(6):756–762. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Basu S. Modern Anaesthesia Vapourisers. Indian J Anaesth. 2013;57(5):464–471. doi: 10.4103/0019-5049.120142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. Small Animal Anesthesia and Analgesia. Ames, IO: Blackwell Publishing; 2008. [Google Scholar]
- Tranquilli WJ, Thurmon JC, Grimm KA, editors. Lumb and Jones' veterinary anesthesia and analgesia. Hoboken, NJ: John Wiley & Sons; 2013. pp. 23–86. [Google Scholar]
- Ambrisko TD, Klide AM. Evaluation of isoflurane and Sevoflurane vaporizers over a wide range of oxygen flow rates. Am J Vet Res. 2006;67(6):936–940. doi: 10.2460/ajvr.67.6.936. [DOI] [PubMed] [Google Scholar]
- McKelvey DH. Veterinary Anesthesia and Analgesia. St. Louis, MI: Mosby; 2003. [Google Scholar]
- Thomas J, Lerche P. Anesthesia and Analgesia for Veterinary Technicians. 4th ed. St. Louis, MI: Mosby; 2011. p. 335. [Google Scholar]
- Flecknell P. Laboratory animal anaesthesia. London, UK: Academic Press, Elsevier; 2009. [Google Scholar]
- Mapleson WW. The elimination of rebreathing in various semiclosed anaesthetic systems. Brit J Anaesth. 1954;26(5):323–332. doi: 10.1093/bja/26.5.323. [DOI] [PubMed] [Google Scholar]
- Ward CS. Anaesthetic equipment. London: W. B. Saunders; 1985. Physical principles and maintenance. [Google Scholar]
- El-Attar AM. Guided isoflurane injection in a totally closed circuit. Anaesthesia. 1991;46(12):1059–1063. doi: 10.1111/j.1365-2044.1991.tb09924.x. [DOI] [PubMed] [Google Scholar]
- Lockwood G, Chakrabarti MK, Whitwam JG. A computer-controller closed anaesthetic breathing system. Anaesthesia. 1993;48(8):690–693. doi: 10.1111/j.1365-2044.1993.tb07182.x. [DOI] [PubMed] [Google Scholar]
- Lowe HJ, Cupic M. Dose-regulated automated anesthesia (Abstract) Br. J. Clin. Pharmacol. 1971;12(2):281–282. [Google Scholar]
- Soro M, et al. The accuracy of the anesthetic conserving device (Anaconda) as an alternative to the classical vaporizer in anesthesia. Anes Analg. 2010;111(5):1176–1179. doi: 10.1213/ANE.0b013e3181f4db38. [DOI] [PubMed] [Google Scholar]
- Walker TJ, Chackrabarti MK, Lockwood GG. Uptake of desflurane during anaesthesia. Anaesthesia. 1996;51(1):33–36. doi: 10.1111/j.1365-2044.1996.tb07650.x. [DOI] [PubMed] [Google Scholar]
- Weingarten M, Lowe HJ. A new circuit injection technic for syringe-measured administration of methoxyflurane: a new dimension in anesthesia. Anes Analg. 1973;52(4):634–642. [PubMed] [Google Scholar]
- Enlund M, Wiklund L, Lambert H. A new device to reduce the consumption of a halogenated anaesthetic agent. Anaesthesia. 2001;56(5):429–432. doi: 10.1046/j.1365-2044.2001.01900.x. [DOI] [PubMed] [Google Scholar]
- Kelly JM, Kong KL. Accuracy of ten isoflurane vaporisers in current clinical use. Anaesthesia. 2011;66(8):682–688. doi: 10.1111/j.1365-2044.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, et al. NARCOBIT - A newly developed inhalational anesthesia system for mice. Exp Anim. 2007;56(2):131–137. doi: 10.1538/expanim.56.131. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, et al. Comparison of newly developed inhalation anesthesia system and intraperitoneal anesthesia on the hemodynamic state in mice. Biol Pharm Bull. 2007;30(9):1716–1720. doi: 10.1248/bpb.30.1716. [DOI] [PubMed] [Google Scholar]
- Voightsverger S, et al. Sevoflurane ameliorates gas exchange and attenuates lung damage in experimental lipopolysaccharide-induced lung injury. Anesthesiology. 2009;111(6):1238–1248. doi: 10.1097/ALN.0b013e3181bdf857. [DOI] [PubMed] [Google Scholar]
- Garber J, et al. Guide for the Care and Use of Laboratory Animals. 8th edn. Washington DC: The National Academic Press; 2011. [Google Scholar]


