Abstract
Various animal models of lung injury exist to study the complex pathomechanisms of human acute respiratory distress syndrome (ARDS) and evaluate future therapies. Severe lung injury with a reproducible deterioration of pulmonary gas exchange and hemodynamics can be induced in anesthetized pigs using repeated lung lavages with warmed 0.9% saline (50 ml/kg body weight). Including standard respiratory and hemodynamic monitoring with clinically applied devices in this model allows the evaluation of novel therapeutic strategies (drugs, modern ventilators, extracorporeal membrane oxygenators, ECMO), and bridges the gap between bench and bedside. Furthermore, induction of lung injury with lung lavages does not require the injection of pathogens/endotoxins that impact on measurements of pro- and anti-inflammatory cytokines. A disadvantage of the model is the high recruitability of atelectatic lung tissue. Standardization of the model helps to avoid pitfalls, to ensure comparability between experiments, and to reduce the number of animals needed.
Keywords: Medicine, Issue 115, Acute Respiratory Distress Syndrome, ARDS, lung injury, animal model, pig, lung lavage, surfactant washout
Introduction
The mortality of human acute respiratory distress syndrome (ARDS) remains high with values between 40 and 50% 1 despite more than 4 decades of intense research. Animal models of lung injury play a major role in investigating the complex pathomechanisms or novel therapeutical approaches to reduce mortality and limit long term disabilities.
Various models have been established to induce lung injury that simulates aspects of human ARDS in either large (e.g. pigs) or small animals (e.g. rodents). Methods differ strongly, including pulmonary arterial infusion of oleic acid, intravenous (i.v.) infusion of bacteria, and endotoxins or cecal ligation and puncture (CLP) models causing sepsis-induced ARDS. In addition, direct lung injuries due to large tidal volumes and high peak inspiratory pressures (ventilator-induced lung injury; VILI), smoke/burn injuries or lung ischemia/reperfusion (I/R) models are often used 2. One major disadvantage of CLP models, as well as models working with endotoxins, is the underlying inflammation which hinders the analysis of biotrauma caused by lung injury alone. Furthermore, it may take hours to days to result in lung injury, as is the case for VILI in large animals.
The induction of lung injury by surfactant washout with repeated lung lavages, as it was first described by Lachmann et al. in guinea pigs 3, is a time efficient method to induce lung injury with reproducible functional and mechanical compromises, as well as changes in pulmonary vascular resistance. The adaption of this model to mechanically ventilated pigs of about 30-60 kg body weight supports basic research with clinically used mechanical ventilators, catheters and monitors, while the compromises in gas exchange and hemodynamics are highly reproducible at the same time 4. In addition, induction of lung injury by lavages does not require specific equipment that is not commonly available in respiratory laboratories designed for experiments in large animals. The model presented in this article is suitable for research demanding equipment (e.g. ventilators) that is designed for use in humans, and furthermore ensures a high reproducibility in the occurring deteriorations in lung function. Standardization of this model helps to ensure comparability between experiments and reduce the number of animals needed. The potential recruitability of atelectatic lung regions with deliberate or unknown recruitment maneuvers is a severe limitation of this specific model. In the following article we give a detailed description of the lavage model for the induction of lung injury and provide representative data to characterize the stability of the compromises in lung function.
Protocol
The experiments were conducted at the Department of Experimental Medicine, Charité - Universitätsmedizin, Berlin, Germany (certified according to the EN DIN ISO 9001:2000), and were approved by the federal authorities for animal research in Berlin, Germany prior to the experiments. The principles of laboratory animal care, which were used in all experiments, were in accordance with the guidelines of the European and German Society of Laboratory Animal Sciences.
1. Animal Welfare and Laboratory Animals
All experiments were conducted in fully anesthetized male pigs (German Landrace × Large White) of 3-4 months of age, weighing 30-60 kg.
2. Anesthesia, Intubation, and Mechanical Ventilation
Withhold food for 12 hr prior to anesthesia to avoid a full stomach of the pig, but allow free access to water to minimize stress.
- For premedication, inject a combination of azaperone (3 mg/kg), atropine (0.03 mg/kg), ketamine (25 mg/kg), and xylazine (3.5 mg/kg) into the neck muscles of the pig while the animal is still kept in its box to minimize stress.
- Place the animal onto a stretcher and cover the eyes with a cloth for transportation once an adequate level of anesthesia is achieved.
- Transport the pig to the surgical theater and ensure sufficient spontaneous breathing at all times by keeping the snout unobstructed.
- Place the pig in the prone position and preoxygenate with a mask that fits the animal's snout using a high flow of oxygen (e.g. 10 L/min).
- Start monitoring the peripheral oxygen saturation (SpO2) by clipping the respective sensor of the monitor onto one of the ears. Gain venous access with a clinically used peripheral vein catheter (usually 18 or 20 G) placed into one of the ear veins after a wipe down procedure with alcohol swaps.
- Start an infusion with a balanced crystalloid solution with a 500 ml bolus followed by continuous infusion of about 4 ml/kg/hr (as deemed clinically necessary depending on the experiment) and ensure correct placement of the catheter for the subsequent infusion of anesthetics. NOTE: The infusion of larger volumes of normal saline instead of a balanced crystalloid solution can result in hyperchloremic acidosis, whereas infusion of a solution containing lactate can result in increased serum lactate concentrations, and thus interfere with the interpretation of the blood gas analysis or the results of subsequent experiments.
After sufficient preoxygenation (preoxygenation during the complete time for peripheral vein access, measured SpO2 of 95-100%) inject propofol (about 5-10 mg/kg body weight - the exact dose depends on the effect of the premedication and differs from animal to animal) using the peripheral vein catheter.
- Intubate the pig in the prone position using an endotracheal tube for clinical application (7.5-8.5 ID) and a laryngoscope designed for large animals (straight blade of about 25 cm length).
- Strap two bandages through the snout of the animal (first investigator). Pull one bandage upwards to move the head into the correct position and straighten the oropharyngeal structures, pull the other bandage down to open the snout. Pull the tongue to one side (second investigator).
- Press the tongue down with the blade of the laryngoscope and advance the blade towards the epiglottis. Note, in this position the epiglottis is often wedged behind the soft palate of the pigs.
- Mobilize the epiglottis with the tube, press it down with the blade of the laryngoscope and visualize the vocal cords for intubation.
- Advance the tube through the vocal cords while turning the tube upwards and block the cuff as described in detail by Theisen et al. 5. NOTE: Intubation may also be possible in the supine position, depending on the training of the investigator as well as the standard procedures of a certain institution. A large tube diameter supports the surfactant washout due to faster inflow and outflow of the lavage fluid.
- Check the correct placement of the tube using capnography and auscultation. For this, ensure the capnogram is 'normally' shaped and auscultate both lungs for equal breath sounds as done in the clinic. NOTE: Mechanically ventilate the pig with chest compressions in case of failed or delayed intubation. This requires manual compression of the rib cage from both sides while supplying oxygen with a high flow via a tight fitting face mask.
- Start mechanical ventilation, setting the fraction of inspired oxygen (FIO2) to 1, respirator frequency to 15-20/min, tidal volume of 8-9 ml/kg body weight, inspiration to expiration ratio (I:E) of 1:1.5, and apply a positive end expiratory pressure (PEEP) of 5 cm H2O. Adjust the settings to target an end expiratory partial pressure of carbon dioxide (PetCO2) of 35-40 mmHg and a SpO2 above 95%.
- Maintain anesthesia with continuous i.v. infusion of thiopentone (20 mg/kg/h) and fentanyl (7 µg/kg/h). NOTE: The necessary dosage may vary from animal to animal. Do not leave the animal unattended. Ensure a sufficient anesthesia at all times during the experiment for animal welfare and scientific reasons.
- Check for absence of corneal reflexes and monitor the animal closely for stress/pain reactions during instrumentation. Instrumentation should be possible without administering a muscle relaxant if anesthesia is sufficient. Administer pancuronium bromide (0.15 mg/kg BW i.v. bolus followed by a continuous infusion of 0.15 mg/kgBW/hr) if muscle relaxation is necessary for the experiment (e.g. lung compliance measurements).
3. Instrumentation Techniques
Place the animal into the supine position and retract the legs using bandages to stretch the skin above the planned incision sites. Sterilize the operating areas using a pre-operative skin disinfectant like an alcohol/1% iodine solution. NOTE: We use a wipe down procedure to sterilize the operating area but do not use complete aseptical techniques, since this is a nonsurvival model. The level of surgical asepsis depends upon the investigation following the induction of lung injury.
Make a 10 cm incision on the line connecting the mandible and the sternum (left or right side possible) cutting through the skin using a scalpel for placement of the central venous line and the introducer sheath of the pulmonary artery catheter. Reassess the depth of anesthesia and increase the dosage if necessary.
Separate the subcutaneous tissue and the platysma using tissue forceps and surgical scissors. Once the brachiocephalic and the sternocephalic muscles are visible perform a blunt cut down procedure separating the fascia between the muscles until the external jugular vein is visible using instruments like forceps or the fingers.
- Cannulate the external jugular vein with the central venous catheter and the introducer sheath by means of a modified Seldinger technique. Flush all catheters with normal saline before introducing them.
- For this, advance the respective needle from the introducer set into the vein until venous blood (dark, not pulsating) can be aspirated. Advance the guide wire through the cannula into the vein for about 15 cm. Remove the needle and advance the introducer sheath into the vein. Remove the guide wire. Repeat the same procedure for placement of a central venous catheter if necessary for the experiment.
Control the correct placement of the catheters by aspiration of venous blood. Close with standard sutures. NOTE: Do not dilate the vein with a vasodilator as you would in case of a percutaneous approach, because this will tear the vein (Figure 1). An ultrasound-guided approach is also possible if the investigator is trained in ultrasound-guided cannulization techniques in pigs.
Identify the fold between the gracilis and sartorius muscle of the hind leg (left or right is possible) for placement of the arterial catheter. This is the fold where the pulsation of the femoral artery can be palpated.
Make a 5 cm incision along the fold cutting through the skin using a scalpel.
Separate the subcutaneous tissue using tissue forceps and surgical scissors. Use a blunt cut down procedure separating the fascia between the muscles to the level of the femoral artery. Note, avoid cutting the saphenous vessels by performing the cut down procedure cranial of them.
Cannulate the femoral artery by means of a modified Seldinger technique as described in 3.2. A ligature can be looped around the artery and closed in case of bleeding at the site of puncture. This step should be avoided, if possible, as it compromises blood flow to the hind leg. Close with standard sutures.
Connect the arterial catheter and the central venous line to the transducer system and calibrate both against the atmosphere (zero) and either 200 mmHg (arterial line) or 50 mmHg (central venous line) to start monitoring.
Place all pressure transducers at the height of the right atrium (in pigs in the supine position about half the height of the thorax).
Perform a small (4-5 cm) incision cutting through the skin above the bladder using a scalpel for catherization of the urinary bladder. Again, separate the subcutaneous tissue using blunt instruments.
Perform a purse-string suture (1-2 cm in diameter) in the wall of the bladder once it is visualized. NOTE: The sutures should not penetrate through all layers of the bladder wall since this would result in the loss of urine through the punctures.
Do a minimal incision in the middle of the suture, introduce the urinary catheter, block the balloon with 10 ml of aqua dest, pull the catheter back until a light resistance is felt, and close the purse-string suture around the catheter. Close the skin using standard sutures.
4. Introduction of the Pulmonary Artery Catheter
Inject 0.5-1 ml of air into the balloon of the pulmonary artery catheter (depending on the size of the catheter) and check for possible damage of the balloon. Deflate the balloon again.
- Connect the pulmonary artery catheter to the pressure transducer system and calibrate the PAC against the atmosphere (zero) and 100 mmHg (Figure 2 and 3).
- Introduce the pulmonary artery catheter through the introducer sheath (deflated balloon) for 10 to 15 cm, depending on the sheath length.
- Inflate the balloon (the balloon has to have left the sheath for this) and advance the pulmonary artery catheter further while monitoring the pressure and the typical wave forms on the hemodynamic monitor.
- Advance the PAC while the wave forms which are typical for the right atrium, right ventricle, and pulmonary artery appear and stop advancing when the pulmonary capillary wedge pressure (PCWP) curve appears (Figure 4). Deflate the balloon. NOTE: Once the balloon is deflated the PCWP-waveform must disappear and the pulmonary arterial pressure waveform must be visible. Otherwise the catheter is most likely inserted too far into a pulmonary artery resulting in permanent occlusion of the artery (auto wedge position). In this case, pull the catheter back until the pulmonary arterial pressure waveform reappears to avoid serious complications (e.g. rupture of the blood vessel) 6.
- Make sure the balloon is deflated whenever the catheter is pulled back to avoid serious complications. NOTE: Pulmonary artery catheters are often accidentally advanced into liver veins via the inferior caval vein in pigs. Thus, pull back the catheter and start all over again, if the right ventricle is not reached after about 30 cm.
5. Pulmonary Artery Thermodilution Technique and Hemodynamic Measurements
Copy all hemodynamic values like heart rate, systolic, diastolic, and mean arterial pressure (MAP), pulmonary arterial pressures, and central venous pressure (CVP) from the hemodynamic monitor.
- Measure the PCWP promptly. For this, inflate the balloon of the pulmonary artery catheter and ensure that a correct PCWP curve is displayed (Figure 4). Copy the pulmonary capillary wedge pressure (PCWP) at end-expiration from the monitor. Immediately deflate the balloon afterwards (see 4.2.4). Deflate the balloon, pull the catheter back and reposition it if you are unable to see a correct PCWP curve as described in 4.2.2.
- Connect the thermistor and an appropriate flow through housing to the central venous lumen of the pulmonary artery catheter and to the monitor for measuring cardiac output (CO). Next, connect the distal temperature port of the catheter (red cap) with the monitor.
- Start the monitor and select 'bolus CO' to monitor time-temperature curves and thus measure cardiac output (CO) with the pulmonary artery thermodilution technique 7.
- Press 'Inj Vol' and select the volume of cooled saline (5 ml in the experiments presented here). Return to the previous screen. Press 'catheter' and select the size of the pulmonary artery catheter that is used. Return to the previous screen.
- Select 'start bolus' and inject 5 ml of normal saline of a temperature of 4 °C as quickly as possible using the flow through housing. Wait until the measurement is completed and the respective time-temperature curve appears on the monitor. Copy the CO value from the monitor.
- Perform 5 measurements in quick succession in a randomized order over the respiratory cycle of the ventilator as described in 5.3.4. Ignore the highest and the lowest value and use the remaining three to calculate the mean value of the cardiac output. NOTE: This monitoring setup is described for an Edwards Vigilance monitor, model VGS1. The setup may differ depending on the monitor. Nevertheless, it is essential to select the correct injection volume of saline as well as size of the catheter. Some monitors require the selection of a computation constant that codes the respective amount of saline and catheter size. The constants are usually found in a leaflet inside the packaging of the catheter. Keep the saline at the same temperature throughout the experiment (<5 °C) to assure correct measurements. Use 5% glucose solutions instead of saline for studies involving exact measurements of electrolyte intake and homeostasis.
Make sure that all parameters have been recorded and that arterial and mixed venous blood samples were taken to enable calculation of the intra-pulmonary right-to-left shunt.
Record all needed respiratory data promptly like peak and plateau inspiratory pressure from the respirator, or perform additional measurements like measuring transpulmonary pressure to complete the data at any given time point of the experiment.
6. Lung Lavages to Induce Lung Injury
Ensure that the animal is ventilated with a FIO2 of 1.0 and set the PEEP to 2-4 cm H2O for the lavage procedure. Disconnect the animal from the respirator.
Fill the lungs with warmed normal sterile saline (37 °C, 50 ml/kg body weight). For this, prefill a funnel and connect it to the endotracheal tube with a fitting elastic tube. Raise the funnel 1 m above the animal and pour the saline into the lungs as quickly as possible. The hydrostatic pressure will allocate the saline into all pulmonary sections. NOTE: Sterile normal saline is used to avoid the pulmonary wash in of pathogens and possible, septic decompensation of the animal. The use of 0.9% saline is crucial, since hypotonic fluids will result in immediate pulmonary edema, electrolyte imbalance and death of the animal. Do not reuse the saline after a lavage to maximize surfactant wash out.
Stop filling when MAP falls below 50 mmHg. NOTE: Only hemodynamic parameters and SpO2 can be used to monitor the animal for decompensation, since the animal is disconnected from the ventilator during lung lavages.
Lower the funnel manually to ground level, drain the lavage fluid passively and reconnect the animal to the ventilator for oxygenation.
Wait until the animal compensates (increase in MAP and SpO2) and repeat the lavage as soon as possible. The time frame for re-lavage should not exceed 5 min. NOTE: Stabilization of the animal between two lavages in the case of hemodynamic decompensation serves to avoid the use of vasopressors to treat systemic hypotension, and to further avoid recruitment maneuvers to encounter hypoxemia. The animals are ventilated with a FIO2 of 1.0 during the lavages and the following experiments to maintain oxygenation despite reduced PaO2/FIO2 ratios. Setting the PEEP at 2-4 cm H2O during the lavages will promote rapid formation of atelectasis. But, PEEP has to be set at or above 5 cm H2O after the induction of lung injury to fulfill the Berlin definition of ARDS. During the experiment no recruitment maneuver or change in PEEP is allowed, to prevent any investigator-induced bias with regards to the severity of lung injury.
Take an arterial blood gas sample after the second or third lavage depending on the hemodynamic deterioration and compromise in SpO2.
Repeat lavages until the PaO2/FIO2 ratio (Horowitz index) is persistently measured below 100 mmHg for at least 60 min at FIO2 1.0 and PEEP ≥ 5 cmH2O.
Adjust the ventilator rate during the period of lavages to keep the arterial pH above 7.25 in order to prevent hemodynamic decompensation.
Start the experiment/treatment based on the surfactant washout model once the PaO2/FIO2 ratio (Horowitz index) is persistently measured below 100 mmHg for 60 min. NOTE: After the induction of the lung injury as described, the changes in lung function will remain stable for hours, deteriorate, or even improve depending on the ventilator settings. NOTE: This animal model is based on surfactant washout and consequent formation of atelectasis. Hence, any deviation from the specified ventilator settings, which may lead to recruitment of atelectatic lung regions (increase in PIP or PEEP), will partially reverse the deleterious effect of the lavages and hinder standardization of this model.
7. End of Experiment and Euthanasia
Ensure that all measurements are performed and data is secured before the end of the experiment.
Inject 0.5 mg of fentanyl additionally to the continuous anesthesia and wait at least 5 min. Inject an overdose of thiopental (at least 1,000 mg) quickly followed by at least 60 mmol of potassium using the central line.
Representative Results
PaO2/FIO2-ratio decreases during lung lavages, but the exact impact of a single lavage is difficult to predict. We start to take arterial blood gas samples from the third lavage onwards to detect a decrease in PaO2/FIO2-ratio below 100 mmHg. Once a decrease in PaO2/FIO2-ratio below 100 mmHg is achieved, we require this ratio to remain below 100 mmHg for one hour at a PEEP ≥ 5 cm H2O. This ensures the induction of lung injury, which will formally meet the Berlin definition of ARDS. The concomitant changes in blood gases and hemodynamics will remain 'stable' for hr, deteriorate further, or even improve depending on the ventilator settings (Figure 5). In the case that the PaO2/FIO2-ratio does increase above 100 mmHg during the one hour baseline period, further lavages are performed as described above to prevent spontaneous recovery of the animal during the time course of the experiment (Figure 5). PAP increases with each lavage due to increasing atelectatic regions of the lungs, hypercapnia and hypoxemia (Figure 5). PAP values usually increase two- to triple-fold, but can increase above 60-70 mmHg during a single lavage. This may result in sudden hemodynamic decompensation and death of the animal. Overall death rate of this model averages 10-15%.
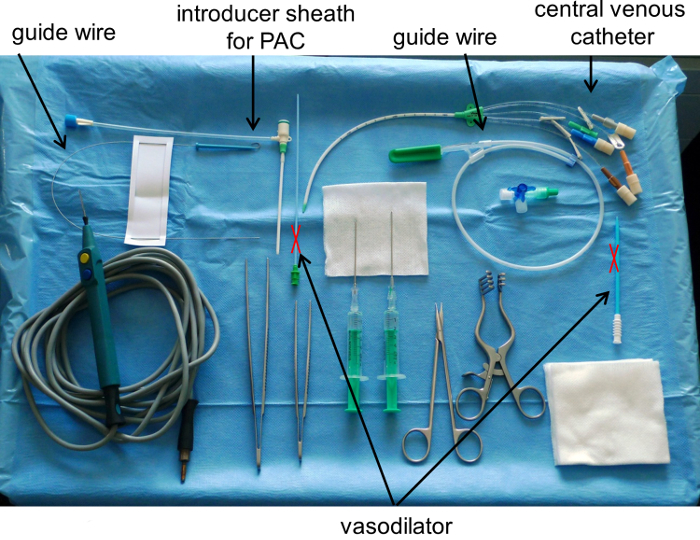 Figure 1: Instrument Table for Introducing a Central Venous Catheter and an Introducer Sheath by Seldinger Technique after a Cut Down Procedure. Note, do not use a vasodilator for direct cannulization of a blood vessel. PAC means pulmonary artery catheter. Please click here to view a larger version of this figure.
Figure 1: Instrument Table for Introducing a Central Venous Catheter and an Introducer Sheath by Seldinger Technique after a Cut Down Procedure. Note, do not use a vasodilator for direct cannulization of a blood vessel. PAC means pulmonary artery catheter. Please click here to view a larger version of this figure.
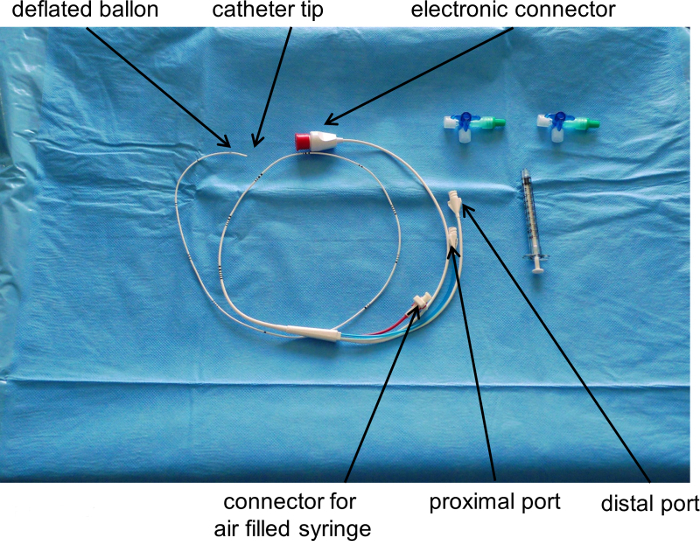 Figure 2
: Pulmonary Artery Catheter.
Please click here to view a larger version of this figure.
Figure 2
: Pulmonary Artery Catheter.
Please click here to view a larger version of this figure.
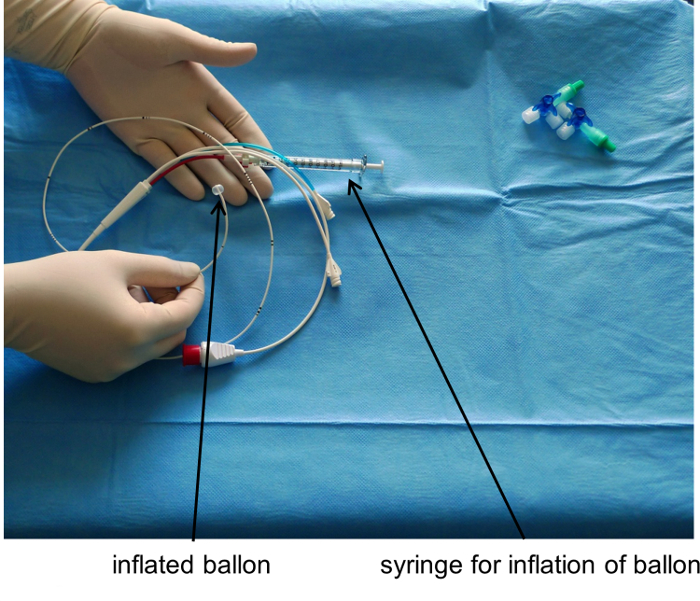 Figure 3: Pulmonary Artery Catheter with Inflated Balloon.Please click here to view a larger version of this figure.
Figure 3: Pulmonary Artery Catheter with Inflated Balloon.Please click here to view a larger version of this figure.
 Figure 4: Schematic Sketch of the Waveforms Visible while Advancing a Pulmonary Artery Catheter. The sketch depicts which waveform can be usually seen at which insertion depth of the catheter in pigs of about 40 kg body weight. PCWP means pulmonary capillary wedge pressure. Please click here to view a larger version of this figure.
Figure 4: Schematic Sketch of the Waveforms Visible while Advancing a Pulmonary Artery Catheter. The sketch depicts which waveform can be usually seen at which insertion depth of the catheter in pigs of about 40 kg body weight. PCWP means pulmonary capillary wedge pressure. Please click here to view a larger version of this figure.
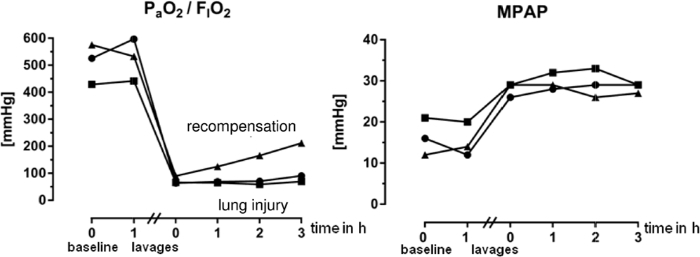 Figure 5: Individual Measured Values for PaO2/FIO2 Ratio and Mean Pulmonary Arterial Pressure (MPAP) of Three Pigs. PaO2 means partial arterial pressure of oxygen, FIO2 means fraction of inspired oxygen. The data was recorded during workshops at our institution. Note, that the PaO2/FIO2 ratio increases after lung lavages in one animal, whereas it remains below 100 mmHg in the other two. Thus, this animal should have received further lavages as described in the article. Please click here to view a larger version of this figure.
Figure 5: Individual Measured Values for PaO2/FIO2 Ratio and Mean Pulmonary Arterial Pressure (MPAP) of Three Pigs. PaO2 means partial arterial pressure of oxygen, FIO2 means fraction of inspired oxygen. The data was recorded during workshops at our institution. Note, that the PaO2/FIO2 ratio increases after lung lavages in one animal, whereas it remains below 100 mmHg in the other two. Thus, this animal should have received further lavages as described in the article. Please click here to view a larger version of this figure.
Discussion
This article describes a step by step instruction to induce severe lung injury in pigs due to surfactant washout by repeated lung lavages. This specific method enables a reproducible and comparable deterioration in lung function and pulmonary vascular resistance. It is imperative to lavage the pigs until the PaO2/FIO2 ratio decreased below 100 mmHg and stays below 100 mmHg for one hr. Once, this is achieved the animals usually do not recover from the lung injury for at least 4 to 8 hr as long as no recruitment maneuvers are conducted 4,8. Adherence to this protocol helps to increase the comparability between the findings from different experiments using the same animal model.
The induction of lung injury with lavages has several limitations. First, repeated lavages result in some of the histopathological properties of human ARDS including the formation of major atelectasis, perivascular edema formation and an increase of the alveolar-capillary membrane thickness. Yet, some important features like severe epithelial damage or the formation of hyaline membranes are not found in this model 2,9.
Second, the recruitment effect of high inspiratory pressures and increased PEEP seem to be higher in lavage-induced lung injury in dogs than in lung injury induced by the infusion of oleic acid or intratracheal installation of E. coli (pneumonia model) 10. Thus, lavage models may be a quick, suitable method to test e.g. the effect of different ventilation regimes, but the investigator has to be careful to avoid any alveolar recruitment whenever it is not desired. In our experience, the compromises in lung function and pulmonary vascular resistance remain stable for hours, as long as no accidental recruitment maneuvers are performed. But, the animal can deteriorate or even improve depending on the ventilator settings.
Third, the inflammatory response to lung injury greatly differs between models and furthermore between species. The role of e.g. inflammatory mediators like TNFα in pig lavage models are still controversial 9.
Fourth, this model requires complex instrumentation and monitoring procedures typically used in critical care medicine. In addition, the maintenance of anesthesia in hypoxic large animals exposed to sudden hemodynamic changes is necessary. Thus, only experienced investigators trained in large animal research and intensive care medicine should work with this model.
Finally, the induction of lung injury with lung lavages may result in a sudden hemodynamic decompensation and ultimately death of the animal. Up to 10-15 % of the animals may die during the induction period. In our experience this is usually the case, when the MAP decreases below 50 mmHg or the SpO2 falls below 70% resulting in sudden ischemic heart failure. Monitoring mean pulmonary arterial pressure (MPAP) during the lavage is also possible to reduce mortality because a rise of MPAP above 50-60 mmHg will result in right ventricular failure and death of the animal. In our experience right and left ventricular failure may occur simultaneously during lavages and monitoring hemodynamics during the procedure is essential to reduce mortality. We stop an ongoing lavage, drain the lavage fluid, and ventilate the animal whenever we record a decrease in MAP below 50 mmHg. Nevertheless, the lavages should be performed in a quick succession to washout a significant amount of surfactant. When the PaO2/FIO2 ratio decreases below 100 mmHg it should not increase above this threshold for at least one hour. This practical approach enables a time efficient induction of lung injury.
The advantage of this model is reproducibility with respect to lung function and pulmonary vascular resistance while allowing their precise quantification in the evaluation of therapeutic strategies. Furthermore, the size of the animals supports the use of clinically used catheters, endotracheal tubes, ventilators and monitors that are not fully available in smaller mammals (e.g. rodents). In addition, the acquired data format (e.g. cardiac output measurements with the thermodilution technique) is comparable to the bedside situation known to intensive care physicians.
Disclosures
We gratefully acknowledge the help of Prof. Burkhard Lachmann when contemplating the intricacies of PEEP settings during surfactant washout and the excellent technical assistance of Birgit Brandt and Sabine Molling.
Acknowledgments
All authors disclose no financial or any other conflicts of interests.
GRANTS:
This study was supported by a grant from the Deutsche Forschungsgemeinschaft to P. Pickerodt and W. Boemke (Pi795/2-2).
References
- Rubenfeld GD, et al. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Ballard-Croft C, Wang D, Sumpter LR, Zhou X, Zwischenberger JB. Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg. 2012;93(4):1331–1339. doi: 10.1016/j.athoracsur.2011.06.107. [DOI] [PubMed] [Google Scholar]
- Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24(3):231–236. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Donaubauer B, et al. Low-dose inhalation of an endothelin-A receptor antagonist in experimental acute lung injury: ET-1 plasma concentration and pulmonary inflammation. Exp Biol Med (Maywood) 2006;231(6):960–969. [PubMed] [Google Scholar]
- Theisen MM, et al. Ventral recumbency is crucial for fast and safe orotracheal intubation in laboratory swine. Lab Anim. 2009;43(1):96–101. doi: 10.1258/la.2008.008044. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Rabbani LE. Videos in clinical medicine. Pulmonary-artery catheterization. N Engl J Med. 2013;369(25):35. doi: 10.1056/NEJMvcm1212416. [DOI] [PubMed] [Google Scholar]
- Forrester JS, et al. Thermodilution cardiac output determination with a single flow-directed catheter. Am Heart J. 1972;83(3):306–311. doi: 10.1016/0002-8703(72)90429-2. [DOI] [PubMed] [Google Scholar]
- Deja M, et al. The inhaled ET(A) receptor antagonist LU-135252 acts as a selective pulmonary vasodilator. Clin Sci (Lond) 2002;103:21–24. doi: 10.1042/CS103S021S. Suppl 48. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloot TE, et al. Recruitment maneuvers in three experimental models of acute lung injury. Effect on lung volume and gas exchange. Am J Respir Crit Care Med. 2000;161(5):1485–1494. doi: 10.1164/ajrccm.161.5.9809014. [DOI] [PubMed] [Google Scholar]


