Abstract
Although adsorption-induced conformational changes of proteins play an essential role during protein adsorption on interfaces, detailed information about these changes is lacking. To further the current understanding of protein adsorption, in this study, the orientation, conformation, and local stability of bovine α-lactalbumin (BLA) adsorbed on polystyrene nanospheres is characterized at the residue level by hydrogen/deuterium exchange and 2D NMR spectroscopy. Most of the adsorbed BLA molecules have conformational properties similar to BLA molecules in the acid-induced molten globule state (A state). A folding intermediate of BLA is thus induced and trapped by adsorption of the protein on the hydrophobic interface. Several residues, clustered on one side of the adsorbed folding intermediate of BLA, have altered amide proton exchange protection factors compared to those of the A state of BLA. This side preferentially interacts with the interface and includes residues in helix C, the calcium binding site, and part of the β–domain. Local unfolding of this interacting part of the adsorbed protein seems to initiate the adsorption-induced unfolding of BLA. Adsorption-induced protein unfolding apparently resembles more the mechanical unfolding of a protein than the global unfolding of a protein as induced by denaturant, pH, or pressure. 2D macromolecular crowding prevented the minority of adsorbed BLA molecules, which arrived late at the interface, to unfold to the A state. Protein adsorption is a novel and challenging approach to probe features of the free energy landscapes accessible to unfolding proteins.
Keywords: bovine α-lactalbumin, hydrogen/deuterium exchange, NMR
Adsorption of protein molecules on solid interfaces is important in fields like biomedical materials engineering (1), chromatography (2), and nanotechnology (3, 4). To understand protein adsorption, knowledge of adsorption-induced conformational changes of proteins is essential (5, 6). Unfortunately, because of considerable experimental difficulties, detailed information at the submolecular level about these conformational changes is sparse (7–12), which hampers the further development of the theory of protein adsorption. To stimulate this development, we characterize here at the residue level, the orientation, conformation, and local stability of bovine α-lactalbumin (BLA) adsorbed on polystyrene nanospheres by hydrogen/deuterium (H/D) exchange and 2D NMR spectroscopy. BLA, a 14-kDa protein from milk, is chosen to study protein adsorption because its structure, stability, and folding behavior have been thoroughly investigated. In addition, the adsorption of BLA on a variety of interfaces has been studied.
Information about the stability and dynamics of a protein at the level of single amino acids can be obtained by investigating its H/D exchange characteristics (13). In this study, BLA molecules in the native state are added to solid polystyrene nanospheres in 2H2O. All added protein molecules adsorb spontaneously and rapidly [i.e., within 15 ms (14)] on the nanosphere surface. The result is a monolayer of adsorbed BLA molecules with no free BLA molecules remaining in solution. The interface induces partial unfolding of these adsorbed protein molecules with an unfolding rate of 74 s–1, as determined by stopped-flow fluorescence spectroscopy (14). Amide protons of the adsorbed, partially unfolded BLA molecules are allowed to exchange with deuterated solvent for a certain period. Subsequently, a procedure (15) is applied that uses the surfactant 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) to displace and refold the adsorbed protein under quenched exchange conditions. This process allows the removal of the nanospheres and enables the measurement of the H/D exchange in the previously adsorbed BLA molecules by NMR spectroscopy. As a result, the detailed characterization of adsorbed BLA is made possible.
Materials and Methods
NMR Spectroscopy and Sample Preparation. All NMR experiments were recorded at 35°C on a Bruker AMX500. Samples were prepared from a filtered stock solution of ≈8.3 mM BLA in a sodium acetate buffer at pH 5.7. The sample used for resonance assignments contained 0.60 mM BLA (Sigma, measured by using a molar extinction coefficient of 28,540 M–1·cm–1 at 280 nm) in 19 mM sodium phosphate at pH 7.0 in 90% H2O/10% 2H2O. The pH readings in 2H2O are uncorrected for isotope effects (pD). Cross peaks were assigned by using conventional procedures and reported assignments (16). Total correlation spectroscopy (TOCSY) H/D exchange NMR spectra were acquired by using a mixing time of 40 ms with 2,048 complex points in t2 (eight scans) and 350 complex points in t1. The spectral widths were 8,064 Hz in both dimensions. All samples contained 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt as a reference.
H/D Exchange of Adsorbed BLA. In a typical exchange experiment, 21.50 ml of 9.1 nM polystyrene nanospheres (radius 60 nm, Interfacial Dynamics, Portland, OR) in 20 mM sodium acetate buffer in 2H2O at pD 5.5 was incubated at 20°C. Exchange was initiated by pipeting 45 μl of 8.3 mM BLA (Sigma) in 20 mM acetate in H2O at pH 5.7 into the stirred nanosphere suspension. After 5 s to 8 min, H/D exchange was quenched by adding 1.38 ml of 5 M CaCl2 in 2H2O, and by the subsequent immediate addition of 112 μl of 0.64 M CHAPS in 2H2O. This process results in a pD of 5.2, a CHAPS concentration of 3.1 mM, a CaCl2 concentration of 300 mM, and a H2O content of ≈6%. CHAPS causes the immediate irreversible displacement of >90% of the adsorbed BLA molecules and the subsequent refolding of these displaced molecules (15). The refolding of BLA significantly quenches H/D exchange of many of the displaced BLA backbone amide protons compared to their exchange in the partially unfolded adsorbed state of BLA. Additional quenching is obtained by the decrease of the pD from 5.5 to 5.2 and by the increase of the CaCl2 concentration from 30 μM to 300 mM. The displaced BLA molecules were separated from the polystyrene nanospheres by using a 100-kDa ultrafiltration filter and concentrated to ≈0.3 mM by using a 5-kDa ultrafiltration filter. Reference experiments were performed with the same procedure, except that CHAPS was added to the nanosphere suspension before addition of BLA. This procedure ensures identical experimental conditions (pH, time of H/D exchange, presence of particles, concentration of reagents) but without protein adsorption taking place. The period between quenching of H/D exchange, which includes protein displacement, and the acquisition of TOCSY spectra ranged from 90 to 140 min. Surfactant-free stable 60-nm radius nanosphere suspensions, instead of 23-nm radius nanosphere suspensions (14, 15), were used throughout the H/D exchange experiments on adsorbed BLA because the surfactant required to keep the suspension of the 23-nm radius nanospheres stable would affect the H/D exchange behavior of BLA.
Hydrogen Exchange Data Analysis. Maximum peak intensities were measured of each observed NH-CαH TOCSY cross peak or of the corresponding diagonal peak (where required) by using xwin-nmr version 2.1 (Bruker Analytik, Rheinstetten, Germany). Relative peak intensities were obtained by dividing the maximum peak intensity by the average of the intensities of five cross peaks that result from nonexchangeable aromatic protons. A single exponential decay function was fitted to the time-dependent relative peak intensities:
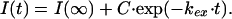 |
[1] |
In Eq. 1, t is the time between initiation of H/D exchange and quenching of this exchange, I(∞) is the peak intensity at infinite time, C is the preexponential factor, and kex is the amide proton exchange rate. Protection factors were calculated by dividing the intrinsic amide proton exchange rates (kint) by the measured amide proton exchange rates. The kint values were determined by using free peptide exchange rates, which were corrected for the effects of the local amino acid sequence and calibrated for the pH and temperature of the exchange experiment according to Bai et al. (17) by using the program sphere (www.fccc.edu/research/labs/roder/sphere/sphere.html).
CD Spectroscopy. Near-UV CD measurements were performed as described (14). The samples of native BLA and adsorbed BLA contained 10 mM Tris·HCl at pH 7.5 and 1 mM CaCl2. BLA in the A state was made by titrating the protein in nanopure water with HCl to pH 2.1. The sample of adsorbed BLA contained 4.5 μM BLA and 10 nM polystyrene nanospheres (radius 23 nm, Polymer Laboratories, Heerlen, The Netherlands), which results in the same BLA coverage of the nanospheres as compared to the samples used in the H/D exchange experiments. Polystyrene nanospheres with a radius of 23 nm instead of 60 nm were required to significantly reduce light scattering and light absorption in the CD measurements. Eight scans were averaged in the case of native BLA and of BLA in the A state, and 128 scans were averaged in the case of adsorbed BLA. The response time was 1 s; the spectral bandwidth was 1.0 nm. A CD spectrum of a blank, which contained all components except BLA, was subtracted.
Results and Discussion
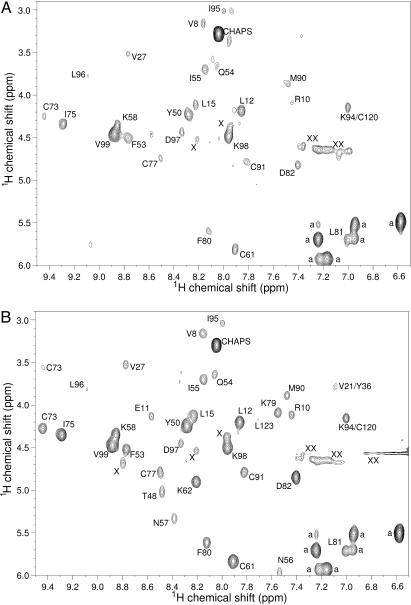
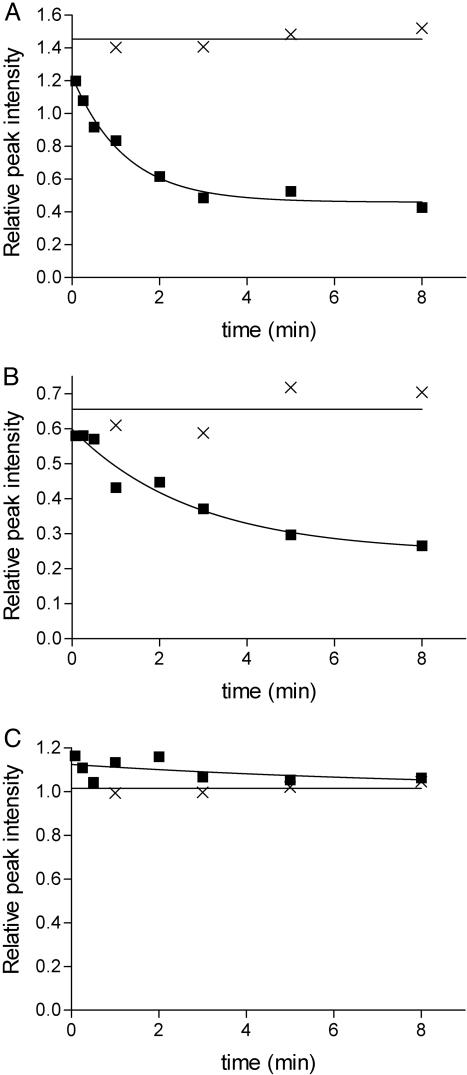
The H/D exchange behavior of adsorbed BLA was studied by recording TOCSY NMR spectra of displaced BLA molecules that were previously adsorbed on polystyrene nanospheres during H/D exchange periods ranging from 5 s to 8 min. Analysis of these TOCSY spectra (Fig. 1) shows that the observable amide protons of adsorbed BLA that exchange during the experiment exchange faster than those of native, nonadsorbed BLA. The time-dependent amide proton exchange curves and corresponding exchange rates of 28 amide hydrogens of BLA adsorbed on a solid surface could be determined. Examples of three such H/D exchange curves are shown in Fig. 2. The backbone amide protons of residues D88 and L52 show significant exchange within 8 min, whereas the one of L96 does not exchange at all within this period. No detectable amide proton exchange is observed during an 8-min exchange period for the 28 corresponding residues of nonadsorbed, native BLA (Fig. 2).
Fig. 1.
1H-TOCSY NMR spectra (500 MHz) show that H/D exchange in adsorbed BLA is faster than in nonadsorbed BLA. (A) Displaced BLA, the amide protons of which were allowed to exchange with deuterons for a 3-min period during which BLA was adsorbed on polystyrene nanospheres. (B) Reference sample of BLA prepared under identical H/D exchange conditions except that adsorption is avoided. The cross peak assignments are indicated with the one-letter amino acid code and corresponding sequence number. Cross peaks from nonexchangeable aromatic protons are labeled a, unassigned cross peaks are labeled X, and peaks caused by baseline distortions are labeled XX.
Fig. 2.
H/D exchange curves of three residues of adsorbed and nonadsorbed BLA. Time-dependent decrease of the backbone amide proton NMR signal of D88 (A), L52 (B), and L96 (C) of BLA adsorbed on polystyrene nanospheres in deuterium oxide as detected after displacement of the protein by CHAPS (squares) and native BLA in deuterium oxide (crosses). The data are extracted from 1H-TOCSY NMR spectra like the ones shown in Fig. 1.
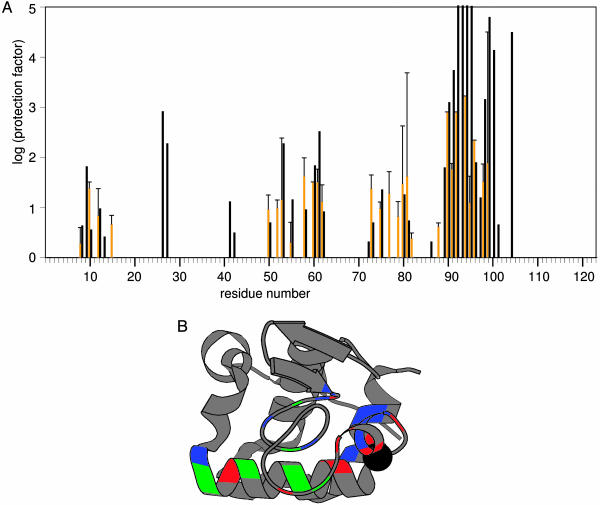
CD and fluorescence spectroscopy show that adsorbed BLA has molten globule properties, i.e., it has preserved secondary structure and disordered tertiary structure compared to native BLA (14). The protection against H/D exchange determined here for the backbone amide protons of 28 residues of adsorbed BLA have the same order of magnitude as those of the corresponding 28 residues of the acid-induced state (A state) of BLA (Fig. 3A). Nine residues of adsorbed BLA (i.e., V8, L12, Y50, F53, K58, K62, F80, L81, and V99) have, within a 95% confidence interval, protection factors identical to those of the corresponding residues of the A state of BLA. Adsorbed BLA is much less protected against H/D exchange than native BLA, the backbone amide protection factors of which are all >3,000 (16). The distribution of protection factors within adsorbed BLA has a similar pattern as the one found for the A state of BLA. Consequently, the conformational characteristics of these adsorbed BLA molecules are similar at the residue level to those of the A state of BLA. A folding intermediate of BLA is trapped by adsorption on polystyrene nanospheres.
Fig. 3.
Protection against H/D exchange of adsorbed BLA and BLA in the A state. (A) Histograms showing the distribution of amide proton exchange protection factors of adsorbed BLA determined here (orange bars) and the A state of BLA (16) (black bars). In case of residues 60, 90, 92, 94, and 96 of adsorbed BLA the lower limit of the protection factors is shown (standard errors not available). The protection factors of residues 11, 26, 27, 41, 42, 48, 51, 54, 56, 72, 86, 89, 93, 97, 100, 101, and 104 of adsorbed BLA are not determined because either the corresponding resonances could not be assigned, spectral overlap exists, or the corresponding cross peaks have a too low intensity. Residues 52, 77, 79, 82, and 88 are not protected against amide proton H/D exchange in the nonadsorbed A state of BLA (16). The error bars show the standard errors of the protection factors of adsorbed BLA, and only the positive error is shown. (B) molscript (28) cartoon representation of the x-ray structure of HOLO-BLA (Protein Data Bank code 1F6S) (29) in which residues that have identical (blue), or significantly higher (red) or significantly lower (green) backbone amide proton exchange protection factors in adsorbed BLA compared to in the A state of BLA are highlighted. The sphere shows the calcium ion in HOLO-BLA.
Although adsorbed BLA and the A state of BLA have approximately similar H/D exchange features, differences in the exchange behavior (considering a 95% confidence interval) are also observed between both states. Eight residues of adsorbed BLA (i.e., R10, L52, C73, C77, K79, D82, D88, and L96) have a significantly higher protection factor than the corresponding ones in the A state. Six residues of adsorbed BLA (i.e., I55, C61, I75, C91, I95, and K98) have a significantly lower protection factor than the corresponding ones in the A state. Remarkably, these 14 residues (except R10) are clustered on one side of the BLA molecule, which comprises helix C, the calcium-binding site, and part of its β-domain (Fig. 3B). This cluster of residues indicates a region of the adsorbed BLA folding intermediate that most likely interacts with the polystyrene interface. Besides the adsorption-induced conformational change of the native protein to an intermediate state, adsorption also has a local effect on the protection against H/D exchange of the adsorbed protein folding intermediate. Favorable interactions between the adsorbed protein and the polystyrene interface may be increased by rotation of helix C in the adsorbed BLA folding intermediate compared to native BLA. Such conformational adaptations are not shown in Fig. 3B.
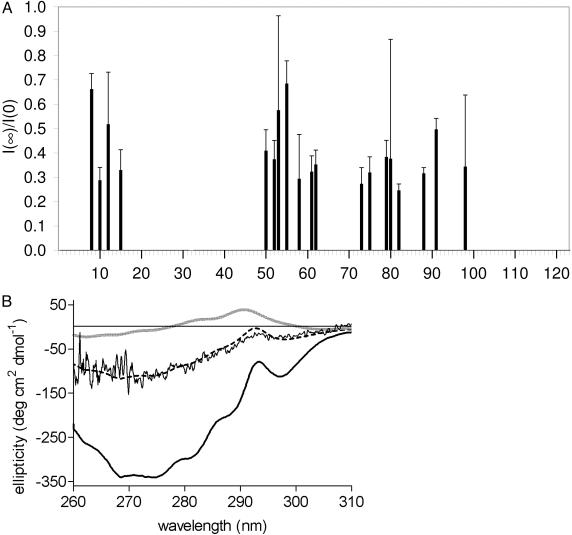
The NMR signals of the exchanging amide protons of adsorbed BLA do not reach zero intensity after 8 min of exchange, but reach a plateau value with intensity I(∞) as can be seen for residues D88 and L52 in Fig. 2. The plateau value normalized with respect to the initial amide NMR signal I(0) [I(0) is extracted from reference exchange data of nonadsorbed BLA] could be determined for 19 residues (Fig. 4A). The average of I(∞)/I(0) is 0.40 ± 0.13. This fraction of the amide proton cross peak intensity that remains during the 8-min H/D exchange experiment is caused by a population of adsorbed BLA molecules that has significantly higher protection factors than the majority of the adsorbed BLA molecules. Thus, ≈40% of the total amount of adsorbed BLA experiences nondetectable H/D exchange on the time scale of our H/D exchange experiments.
Fig. 4.
Approximately 31–40% of adsorbed BLA molecules are native-like. (A) Fraction I(∞)/I(0) of the amide proton cross peak intensity of adsorbed BLA that remains during an 8-min H/D exchange experiment. I(0) is extracted from reference exchange data of nonadsorbed BLA. The average of I(∞)/I(0) is 0.40 ± 0.13. Error bars show the standard errors. (B) Near-UV CD spectra of native BLA (thick black line), BLA in the A state (thick gray line), and adsorbed BLA (noisy black line). The simulated near-UV CD spectrum (dashed black line), as obtained by least-squares analysis, is composed of 31% of the intensity of the near-UV CD spectrum of native BLA and 69% of the intensity of the near-UV CD spectrum of BLA in the A state.
Further insight into the conformations of the two populations of adsorbed BLA molecules is obtained by the acquisition of a near-UV CD spectrum of BLA adsorbed on polystyrene nanospheres (Fig. 4B). Although 128 scans are acquired, the presence of nanospheres causes a rather noisy CD spectrum. The spectrum is similar to the near-UV CD spectrum of native BLA, although with significantly reduced absolute ellipticities. The spectrum differs from the one of the A state of BLA, which has ellipticities around zero due to the absence of persistent tertiary structure. Based on the results presented, we propose that the near-UV CD spectrum of adsorbed BLA represents the combined CD spectra of two populations of adsorbed molecules, each with a different conformation. The main population consists of adsorbed BLA molecules in the A state (with A state-like H/D exchange rates) and the minor population consists of adsorbed BLA molecules with a native-like near-UV CD spectrum (and with slow H/D exchange rates). Indeed, a simulated spectrum composed of 31% of the intensity of the near-UV CD spectrum of native BLA and 69% of the intensity of the near-UV CD spectrum of the A state of BLA coincides with the near-UV CD spectrum of BLA adsorbed on polystyrene nanospheres (Fig. 4B).
The H/D exchange and CD data presented are detailed support for the phenomenon of structural heterogeneity expected to occur in a monolayer of adsorbed protein molecules. This structural heterogeneity is explained by the altered conditions experienced by proteins that arrive late at the interface during the adsorption process (18–21). Protein molecules that arrive first at the interface have sufficient space to adapt to the interface, allowing conformational changes like expansion to a molten globule to occur. In contrast, proteins that arrive late at the now crowded interface have no space to optimize their interactions with the interface. Crowding favors native protein structure at the expense of less compact nonnative structures (22).
Is the mechanism of adsorption-induced protein unfolding similar to the one causing global protein unfolding, which is traditionally induced by using a denaturant, pressure, or pH? We show here, on the basis of protection factors, that the adsorbed BLA folding intermediate is oriented with a specific side toward the interface. It seems likely that the adsorption-induced protein unfolding is initiated through local interactions of this side of the protein with the interface. Consequently, the mechanism of adsorption-induced protein unfolding needs not be similar to the one causing global protein unfolding. Another indication that adsorption-induced protein unfolding differs from the one causing global protein unfolding comes from our recent finding that the adsorption-induced partial unfolding of BLA to the molten globule state is surprisingly fast, i.e., up to 74 s–1 (14). This rate is much faster than the rate of unfolding of native BLA to its A state as induced by a pH jump (23), by removing the calcium ion (24), or by guanidine hydrochloride (25). Adsorption-induced protein unfolding apparently resembles more the mechanical unfolding of a protein, during which local interactions also play an important role (26, 27), than the global unfolding of a protein.
Hydrophobic interfaces, like the polystyrene nanospheres used here, can cause unfolding of an adsorbing protein as demonstrated for BLA. H/D exchange detected by NMR spectroscopy is a powerful method for studying adsorbed proteins and enables the characterization of conformationally heterogeneous adsorbed states. Denaturation induced by a surface has similarities with the reverse of the protein folding problem. Protein adsorption is a novel and challenging approach to probing the features of the free energy landscapes accessible to unfolding proteins. The unfolding routes to be discovered and the influence of, for instance, 2D macromolecular crowding on their population will further current insight into the conformational space accessible to unfolding proteins. Studies that reveal fundamental features of adsorbed proteins, like the one presented here, aid in the rational manipulation of protein–surface interactions that benefits biomedical materials engineering, chromatography, and nanotechnology.
Acknowledgments
We thank Jos Buijs, Willem Norde, Elles Steensma, and the members of our research group for critically reading the manuscript. This research was financially supported by Senter (The Hague, The Netherlands) (IOP-IE 98004).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BLA, bovine α-lactalbumin; CHAPS, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; H/D, hydrogen/deuterium; TOCSY, total correlation spectroscopy; pD, glass-electrode reading of the pH meter, uncorrected for isotope effects.
References
- 1.Andrade, J. D. & Hlady, V. (1986) Adv. Polym. Sci. 79, 1–63. [Google Scholar]
- 2.Regnier, F. E. (1987) Science 238, 319–323. [DOI] [PubMed] [Google Scholar]
- 3.Huber, D. L., Manginell, R. P., Samara, M. A., Kim, B.-I. & Bunker, B. C. (2003) Science 301, 352–354. [DOI] [PubMed] [Google Scholar]
- 4.Lee, K.-B., Park, S.-J., Mirkin, C. A., Smith, J. C. & Mrksich, M. (2002) Science 295, 1702–1705. [DOI] [PubMed] [Google Scholar]
- 5.Haynes, C. A. & Norde, W. (1995) J. Colloid Interface Sci. 169, 313–328. [Google Scholar]
- 6.Norde, W. & Lyklema, J. (1991) J. Biomat. Sci. Polym. Ed. 2, 183–202. [DOI] [PubMed] [Google Scholar]
- 7.Gray, J. J. (2004) Curr. Opin. Struct. Biol. 14, 110–115. [DOI] [PubMed] [Google Scholar]
- 8.Nagadome, H., Kawano, K. & Terada, Y. (1993) FEBS Lett. 317, 128–130. [DOI] [PubMed] [Google Scholar]
- 9.Keire, D. A. & Gorenstein, D. G. (1992) Bull. Magn. Reson. 14, 57–63. [Google Scholar]
- 10.McNay, J. L. & Fernandez, E. J. (1999) J. Chromatogr. A 849, 135–148. [Google Scholar]
- 11.Buijs, J., Ramström, M., Danfelter, M., Larsericsdotter, H., Håkansson, P. & Oscarsson, S. (2003) J. Colloid Interface Sci. 263, 441–448. [DOI] [PubMed] [Google Scholar]
- 12.Halskau, Ø., Frøystein, N. Å., Muga, A. & Martínez, A. (2002) J. Mol. Biol. 321, 99–110. [DOI] [PubMed] [Google Scholar]
- 13.Englander, S. W. & Krishna, M. M. (2001) Nat. Struct. Biol. 8, 741–742. [DOI] [PubMed] [Google Scholar]
- 14.Engel, M. F. M., van Mierlo, C. P. M. & Visser, A. J. W. G. (2002) J. Biol. Chem. 277, 10922–10930. [DOI] [PubMed] [Google Scholar]
- 15.Engel, M. F. M., Visser, A. J. W. G. & van Mierlo, C. P. M. (2003) Langmuir 19, 2929–2937. [Google Scholar]
- 16.Forge, V., Wijesinha, R. T., Balbach, J., Brew, K., Robinson, C. V., Redfield, C. & Dobson, C. M. (1999) J. Mol. Biol. 288, 673–688. [DOI] [PubMed] [Google Scholar]
- 17.Bai, Y., Milne, J. S., Mayne, L. & Englander, S. W. (1993) Proteins Struct. Funct. Genet. 17, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrissey, B. W. (1977) Ann. N.Y. Acad. Sci. 283, 50–64. [Google Scholar]
- 19.Van Tassel, P. R., Viot, P. & Tarjus, G. (1997) J. Chem. Phys. 106, 761–771. [Google Scholar]
- 20.Norde, W. (1999) in Physical Chemistry of Biological Interfaces, eds. Baszkin, A. & Norde, W. (Dekker, New York), pp. 115–135.
- 21.Zoungrana, T., Findenegg, G. H. & Norde, W. (1997) J. Colloid Interface Sci. 190, 437–448. [DOI] [PubMed] [Google Scholar]
- 22.Minton, A. P. (2000) Curr. Opin. Struct. Biol. 10, 34–39. [DOI] [PubMed] [Google Scholar]
- 23.Kuwajima, K., Nitta, K. & Sugai, S. (1975) J. Biochem. (Tokyo) 78, 205–211. [PubMed] [Google Scholar]
- 24.Aramini, J. M., Hiraoki, T., Grace, M. R., Swaddle, T. W., Chiancone, E. & Vogel, H. J. (1996) Biochim. Biophys. Acta 1293, 72–82. [DOI] [PubMed] [Google Scholar]
- 25.Kuwajima, K., Mitani, M. & Sugai, S. (1989) J. Mol. Biol. 206, 547–561. [DOI] [PubMed] [Google Scholar]
- 26.Smith, D. A., Brockwell, D. J., Zinober, R. C., Blake, A. W., Beddard, G. S., Olmsted, P. D. & Radford, S. E. (2003) Philos. Trans. R. Soc. London A 361, 713–730. [DOI] [PubMed] [Google Scholar]
- 27.Matouschek, A. (2003) Curr. Opin. Struct. Biol. 13, 98–109. [DOI] [PubMed] [Google Scholar]
- 28.Kraulis, P. J. (1991) J. Appl. Crystallogr. 24, 946–950. [Google Scholar]
- 29.Chrysina, E. D., Brew, K. & Acharya, K. R. (2000) J. Biol. Chem. 275, 37021–37029. [DOI] [PubMed] [Google Scholar]