Abstract
Endocrine Disrupting Compounds pose a substantial risk to the aquatic environment. Ethinylestradiol (EE2) and estrone (E1) have recently been included in a watch list of environmental pollutants under the European Water Framework Directive. Municipal wastewater treatment plants are major contributors to the estrogenic potency of surface waters. Much of the estrogenic potency of wastewater treatment plant (WWTP) effluents can be attributed to the discharge of steroid estrogens including estradiol (E2), EE2 and E1 due to incomplete removal of these substances at the treatment plant. An evaluation of the efficacy of wastewater treatment processes requires the quantitative determination of individual substances most often undertaken using chemical analysis methods. Most frequently used methods include Gas Chromatography-Mass Spectrometry (GCMS/MS) or Liquid Chromatography-Mass Spectrometry (LCMS/MS) using multiple reaction monitoring (MRM). Although very useful for regulatory purposes, targeted chemical analysis can only provide data on the compounds (and specific metabolites) monitored. Ecotoxicology methods additionally ensure that any by-products produced or unknown estrogenic compounds present are also assessed via measurement of their biological activity. A number of in vitro bioassays including the Yeast Estrogen Screen (YES) are available to measure the estrogenic activity of wastewater samples. Chemical analysis in conjunction with in vivo and in vitro bioassays provides a useful toolbox for assessment of the efficacy and suitability of wastewater treatment processes with respect to estrogenic endocrine disrupting compounds. This paper utilizes a battery of chemical and ecotoxicology tests to assess conventional, advanced and emerging wastewater treatment processes in laboratory and field studies.
Keywords: Environmental Sciences, Issue 115, Ecotoxicology, YES Screen, LCMS/MS, SPE, GPC, Estrogenic, Endocrine Disrupting Compound, Green Chemistry, Wastewater Treatment, Fathead minnow, Vitellogenin
Introduction
Concerns regarding the adverse effects of endocrine disrupting compounds on wildlife reproductive health has led the European Union to place two estrogenic substances (estradiol and ethinylestradiol) on a "watch list" under the Water Framework Directive (WFD). EDC encompass a variety of chemical classes, including natural and synthetic steroid estrogens, drugs, pesticides, and industrial chemicals and constituents of consumer products with known adverse effects on wildlife. Some of these compounds may potentially impact human health1.
Research has shown that effluents from WWTP are estrogenic to fish2 and as a consequence many receiving waters are also estrogenic to fish3. This was first demonstrated through national surveys in the United Kingdom that showed increased vitellogenin concentrations (a female specific yolk protein precursor4) in the blood of wild male fish and a high prevalence of intersex (developing eggs and/or female reproductive ducts in the testis of male fish) in normally gonochoristic fish species5,6.
Conventional sewage treatment is typically a three stage process consisting of a preliminary screening followed by primary and secondary treatment which removes both dissolved and suspended organic matter. The efficacy of removal of individual EDC is dependent on the physicochemical properties of the substances and on the effectiveness of the treatment process applied. For many EDC removal via adsorption and biological degradation can be significant but incomplete. Tertiary treatment, such as sand filtration, can be effective at increasing EDC removal7 whereas advanced treatment using advanced oxidation (for example ozone) or activated carbon can be effective in achieving near complete removal7.
The assessment of any new technology for wastewater treatment needs to determine the efficacy of the proposed process in EDC removal. A battery of tests, including targeted chemical analysis alongside ecotoxicology testing, using in vivo and in vitro bioassays, provides comprehensive data for this purpose. Although very useful for regulatory purposes, targeted chemical analysis can only provide data on the compounds (and specific metabolites) monitored. Bioassays additionally allow the "detection" of adverse effects of metabolites and treatment-generated wastewater transformation by-products that would otherwise be undetected8,9. This paper describes the use of a battery of chemical and ecotoxicity laboratory assays to assess the efficacy of a number of advanced and emerging wastewater treatment processes in removing the estrogenic potency of crude and treated sewage and receiving waters.
Protocol
Ethics statement: Protocols for assessing endocrine disrupting activity of chemicals/mixtures in fish have been approved by Brunel University London's Animal Welfare and Ethical Review Body (AWERB) and by the UK Home Office under the Animals (Scientific Procedures) Act 1986.
1. Water Sample Collection, Preservation and Extraction
- Sample collection and preservation
- Prior to use, clean the bottles with a suitable surface active cleaning agent. After cleaning, rinse the bottles with water, drain and dry.
- Collect samples in glass bottles of 2-L capacity containing a preservative consisting of 0.5 g of copper(II) nitrate and 6 ml of 3.6 M hydrochloric acid solution. Store samples below 10 °C. Extract and analyze as soon as possible following collection and preservation.
- Sample extraction and clean-up (for steroid estrogen analysis)10
- Solid Phase Extraction (SPE)
- Prior to extraction, to reduce suspended solids, filter water samples using 1 µm pore size filter paper.
- Once filtered, spike samples with internal standard by adding 100 µl of spiking deuterated internal standard stock solution containing 2,4,16,16-d4-estrone: 2,4,16,16-d4-17β-estradiol (E2): and 2,4,16,16-d4-17α-ethinylestradiol (EE2) (all at 40 µg/L in methanol) to 1,000 ml effluent (or 100 ml influent) resulting in internal spike of 2 ng/L for sewage effluent samples, and 20 ng/L for sewage influent samples.
- Attach disposable valve liners, styrene divinyl benzene SPE cartridges, and cartridge reservoirs to the SPE apparatus. Switch on vacuum pump to test the equipment is adequately sealed.
- Pipette 5 ml of ethyl acetate into each reservoir, to condition the cartridges. Switch on the vacuum pump (to below 10 inHg to allow a flow rate of less than 10 ml per minute) and pull through the liquid. Do not let the SPE cartridges dry out. Repeat process with 5 ml of methanol followed by 5 ml of water.
- Top up each cartridge reservoir with water and connect 1/8" PTFE tubes between the cartridge reservoirs and glass sample bottles. Switch on the vacuum at a flow rate of less than 10 ml per minute and allow the whole sample to pass through the cartridge. Empty the waste flask as necessary.
- Thoroughly dry the SPE cartridges under vacuum (or by using air or nitrogen) until the cartridge contents change color (e.g., from dark brown to light brown).
- Put clean dry 10 ml glass collection vials into rack and place inside the extraction manifold. Check that each liner is above a vial. Pipette 8 ml of dichloromethane into each sample reservoir, switch on the vacuum pump (flow rate of less than 10 ml per minute) and pull the liquid through into the collection vials.
- Remove 10 ml vials from the SPE manifold and use a concentrator to reduce the volume to 1 ml. Transfer each sample into auto-sampler vial and further concentrate to 100 µl using nitrogen blow down equipment.
- Gel Permeation Chromatography (GPC) clean-up
- Inject 95 µl of the eluted sample extract into the GPC equipped HPLC using the conditions described in Table 1. Concentrate the GPC extract down to 200 µl using a concentrator and nitrogen blow down apparatus and then make up to 2.0 ml with hexane.
- SPE clean-up
- Attach disposable valve liners, aminopropyl cartridges, and cartridge reservoirs to the SPE manifold. Put clean dry 10 ml glass collection vials into rack and place inside the extraction manifold. Pipette 2 ml of hexane into each reservoir, to condition the cartridges. Allow the liquid to pass through the cartridges.
- Pipette the GPC sample extract into the reservoir and again allow the liquid to pass through the cartridge. Collect the sample eluate in the vial and remove from the manifold. Do not discard eluate.
- Put a new clean dry 10 ml collection vial into rack and place inside extraction manifold. To wash the cartridge, add 2 ml of ethyl acetate in hexane (30% v/v) to the reservoir and pull the liquid through the cartridge. Repeat with a further 2 ml of ethyl acetate in hexane, discarding all washings.
- Place the original 10 ml collection vial (step 1.2.3.2) back in rack inside the extraction manifold. Pipette 2 ml of ethyl acetate in acetone (50% v/v) into the reservoir, switch on the vacuum pump (to below 2 inHg to allow a flow rate of less than 2 ml per minute) and pull the liquid through into the vial. Repeat the process with another 2 ml of ethyl acetate.
- Remove the sample vial and use a concentrator to reduce the extract volume to 1 ml. Transfer the sample into a smaller glass vial and use nitrogen blow down equipment, to evaporate the extract to incipient dryness.
- Add 100 µl of methanol and mix well. Transfer the extract to an auto-sampler vial (with 0.3 ml insert) and cap the vial. Analyze the samples using LCMS/MS (see section 2).
| Column: | PL gel, 50 A, 300 x 7.5 mm, 5 µm |
| Guard Column: | PL gel, 50 x 7.5 mm, 5 µm |
| Mobile phase: | Dichloromethane |
| Flow rate: | 1 ml per min |
| Column temperature: | 25 °C |
| UV detector: | 210 nm |
| Injection volume: | 95 µl |
| Injection mode: | Standard |
| Draw speed: | 500 ml per min |
| Eject speed: | 500 ml per min |
| Draw position: | 3 mm |
| Fraction collected: | 3 ml fraction (7 - 10 min) in 10 ml vials |
Table 1. Conditions and parameters for Gel Permeation Chromatography (GPC) clean-up of extracted wastewater samples. Table details GPC column, mobile phase, temperature, injection volume, and detector wavelength.
- Sample extraction (for Yeast Estrogen Screen)
- Filter samples as detailed in section 1.2.1.1. Attach disposable valve liners, C18 SPE cartridges, and cartridge reservoirs to the SPE apparatus. Switch on the vacuum pump to test the equipment is adequately sealed.
- Pipette 5 ml methanol into each reservoir, to condition the C18 cartridges. Turn on vacuum (to about 5 inHg to allow a flow of about 5 ml per minute) and let the liquid run through, pausing for 1 min half way through. Do not let the cartridges run dry. Repeat process with either HPLC grade or double distilled water (ddH2O). Again do not run dry.
- Extract water samples as described in section 1.2.1.4. Continue the vacuum for at least 30 min to thoroughly dry the cartridges.
- Place clean dry 10 ml collection vials into a rack in the extraction manifold. Check that each liner is above a vial. Add 5 ml of methanol to each reservoir. Turn on the vacuum (maximum flow of 5 ml/min) and allow the liquid to pass through the cartridge into the vial, pausing for 2 min half way through.
- Prior to analysis using the YES screen, reduce the extract to incipient dryness using nitrogen and reconstitute with 500-1,000 µl ethanol. Seal the lids of sample vials to avoid evaporation and keep at 4 °C (in a spark-free fridge).
2. Chemical Analysis Using LCMS/MS
Optimize the operating conditions of the LCMS/MS system using manufacturer's instructions.
Analyze calibration standard solutions, the sample extracts, blank and analytical quality control (AQC) samples using the liquid chromatography and mass spectrometry conditions, and monitor the ion transitions as detailed in Table 2. Determine the concentration of steroid estrogens in the sample extract using internal standards10.
| LCMS | |||||
| Liquid Chromatography | |||||
| Column: | C18(2), 150 x 4.6 mm, 5 μm. | ||||
| Injection volume: | 20 µl | ||||
| Flow: | 0.5 ml per minute. | ||||
| Mobile phase: | Solvent A: water containing 0.1% ammonia. | ||||
| Solvent B: acetonitrile. | |||||
| Gradient program: | |||||
| Time (min) | 0 | 10 | 18 | 24 | 28 |
| A:B solvent ratio | 90:10:00 | 50:50:00 | 0.479167 | 0.479167 | 90:10:00 |
| Mass Spectrometry | |||||
| Source: | Electrospray (negative ion) | ||||
| Gas and source: | CUR: 20 psi, GS1: 70 psi, GS2: 30 psi | ||||
| TEM: 600 °C, CAD gas 5 and IonSpray voltage -900 | |||||
| MRM transitions: | |||||
| E1: | 269/145 & 269/143 | ||||
| E2: | 271/145 & 271/143 | ||||
| EE2: | 295/145 & 295/143 | ||||
| E1-D4 : | 273/147 | ||||
| E2-D4: | 275/147 | ||||
| EE2-D4: | 299/145 |
Table 2. Details parameters and conditions for LCMS/MS analysis of steroid estrogens in wastewater extracts. Table gives sample injection volume and flow rate, mobile phase conditions and gradient.
3. Estrogenic Activity Using In Vitro Yeast Estrogen Screen (YES) Assay8
Prepare and store minimal medium and medium components as per Table 3 (a) to (g).
| (a) Minimal Medium (pH 7.1): |
| Prepare a Fe2(SO4)3 solution by adding 40 mg of Fe2(SO4)3 to 50 ml of double-distilled water (ddH2O) |
| Add 1 L ddH2O to a 2 L glass beaker |
| Add the following components to the beaker: |
| 13.61 g KH2PO4 |
| 1.98 g (NH4)2SO4 |
| 4.2 g KOH |
| 0.2 g MgSO4 |
| 1 ml of the Fe2(SO4)3 solution |
| 50 mg L-leucine |
| 50 mg L-histidine |
| 50 mg adenine |
| 20 mg L-arginine-HCl |
| 20 mg L-methionine |
| 30 mg L-tyrosine |
| 30 mg L-isoleucine |
| 30 mg L-lysine-HCl |
| 25 mg L-phenylalanine |
| 100 mg L-glutamic acid |
| 150 mg L-valine |
| 375 mg L-serine |
| Put the beaker on heated stirrer with a magnetic flea and stir until it is all dissolved |
| Check that the pH is 7.1 and adjust if necessary |
| Using a 50 ml sterile syringe dispense 45 ml aliquots into glass bottles with metal screw top lids |
| Sterilize the Minimal Medium at 121 °C for 10 min in an autoclave |
| Store at room temperature |
| (b) D-(+)-Glucose: |
| Prepare a 20% w/v solution in ddH2O |
| Dispense 20 ml aliquots in to glass vials with metal screw top lids |
| Sterilize the Glucose solution at 121 °C for 10 min in an autoclave |
| Store at room temperature |
| (c) L-Aspartic Acid: |
| Make a stock solution of 4 mg/ml in ddH2O |
| Dispense 20 ml aliquots in to glass vials with metal screw top lids |
| Sterilize the L-Aspartic Acid solution at 121 °C for 10 min in an autoclave |
| Store at room temperature |
| (d) Vitamin Solution: |
| Prepare a biotin solution by adding 2 mg of biotin to 100 ml of ddH2O |
| Weigh out 8 mg thiamine, 8 mg pyridoxine, 8 mg pantothenic acid, 40 mg inositol. Add all the dry components and 20 ml of the biotin solution to 180 ml ddH2O |
| Make 10 ml sterile aliquots by filtering through a 0.2-µm pore size disposable filter into sterile glass bottles, in a laminar air flow cabinet |
| Store at 4 °C |
| (e) L-Threonine: |
| Prepare 100 ml of 24 mg/ml L-threonine in ddH2O |
| Dispense 10 ml aliquots in to glass vials with metal screw top lids |
| Sterilize the L-threonine solution at 121 °C for 10 min in an autoclave |
| Store at room temperature |
| (f) Copper(II) Sulfate: |
| Prepare 25 ml of a 20 mM Copper(II) Sulfate solution in ddH2O |
| Make 5 ml sterile aliquots by filtering through a 0.2-µm pore size filter into sterile glass bottles, in a laminar flow cabinet |
| Store at room temperature |
| (g) Chlorophenol red-β-D-galactopyranoside (CPRG): |
| Prepare 25 ml of a 10 mg/ml solution of CPRG in ddH2O |
| Make 5 ml sterile aliquots by filtering through a 0.2-µm pore size filter into sterile glass bottles, in a laminar flow cabinet |
| Store at 4 °C |
Table 3. Yeast Estrogen Screen assay; preparation and storage of minimal medium and medium components.
- Preparation and storage of 10x concentrated yeast culture
- On day 1, prepare growth medium (as detailed in 3.4.1) and pour into a sterile conical flask. Add 125 µl of 10x concentrated yeast from cryogenic vial stored at -20 °C. Incubate the inoculated media at 28 °C for approximately 24 hr on an orbital shaker.
- On day 2, make two bottles of growth medium (~ 50 ml) and pour into separate sterile conical flasks. Add 1 ml of 24-hr cultured yeast into each flask of growth media. Incubate the inoculated media at 28 °C for approximately 24 hr on an orbital shaker.
- On day 3, pour each 24-hr culture into a sterile 50-ml centrifuge tube. Centrifuge the 50 ml tubes at 4 °C for 10 min at 2,000 x g. Pour off the supernatant, and re-suspend each pellet in 5 ml of minimal medium with 15% glycerol. Make 0.5 ml aliquots of the 10x concentrated yeast culture in 1.2-ml sterile cryovials and store at -20 °C for a maximum of 4 months.
- Preparation and storage of chemical stocks for standard curves
- Rinse all glassware and spatulas twice with absolute ethanol and leave to dry before use to remove any traces of contaminates.
- Weigh E2 directly into a glass vial in a powder weighing cabinet and adjust concentration by volume in absolute ethanol. Dilute to a concentration of 2x10-7 M (54.48 µg/L). Seal lid and store at 4 °C (in a spark-free fridge).
- Assay producer
- On day 0, prepare growth medium. To the 45 ml bottle of minimal medium add 5 ml glucose solution, 1.25 ml L-aspartic acid solution, 0.5 ml vitamin solution, 0.4 ml L-threonine solution, and 125 µl copper(II) sulfate solution. Pour growth medium into a sterile conical flask.
- Add 125 µl of 10x concentrated stock (defrosted from -20 °C storage) to the flask and incubate the inoculated media at 28 °C for approximately 24 hr on an orbital shaker.
- On day 1, label a sterile 96-well 'dilution plate' and make serial dilutions (100 µl volumes in ethanol) of E2 standard curve and test chemical(s)/effluent extract(s) (e.g., EE2).
- Label sterile 96-well optically flat bottom microtiter 'assay plate(s)'. On every plate include blanks (plus/minus solvent) and an E2 standard curve, in addition to rows of test chemical(s)/effluent extract(s).
- Pipette 10 µl of each concentration (E2, chemical or extract) into the appropriate well of the assay plate. Pipette 10 µl of ethanol into each 'solvent blank' well of the assay plate. Leave assay plate with the lid off to evaporate to dryness.
- Make a bottle of growth medium (~ 50 ml) and add 0.5 ml of chlorophenol red-β-D-galactopyranoside (CPRG) solution per bottle. Determine the density of yeast cells in the 24-hr culture by measuring turbidity of the culture at 620 nm in a plate reader. Inoculate the assay medium with 4x107 yeast cells from the 24 hr culture.
- Pour inoculated assay medium into a sterile trough. Using a multi-channel pipette add 200 µl of inoculated assay medium to each well of the 96-well assay plate.
- Put the lid on the 96-well assay plate and seal the edges with tape. Shake the assay plate(s) vigorously for 2 min on a titer plate shaker and incubate at 32 °C in a naturally ventilated heating cabinet.
- On day 2, shake the assay plate(s) vigorously on a titer plate shaker for 2 min. Return to 32 °C incubator.
- On day 4, shake the assay plate(s) vigorously for 2 min on a titer plate shaker. Leave the plate(s) to stand for approximately 1 hr then read the assay plate(s) at an absorbance of 540 nm (optimum absorbance for CPRG ~575 nm) and 620 nm (for turbidity) using a plate reader. Leave plate(s) at room temperature and read later if necessary.
- Estrogenic activity calculations
- Correct assay readings for turbidity using the following equation: Corrected value = sample or standard (E2) absorbance at 540 nm - [sample or standard (E2) absorbance at 620 nm - blank absorbance at 620 nm]. Plot E2 standard curve with appropriate blanks (to check for contamination)8.
- Use the corrected sample (extract or chemical) to calculate 'Estradiol equivalents'. Use the plotted E2 standard curve values and regression equation (3-parameter polynomial or linear depending on fit) to interpolate the sample/extract absorbance values to E2 equivalent values. Use concentration factor (i.e., water extracted and volume of ethanol the extract is re-suspended in) to calculate estrogenic activity in pre-extracted sample.
4. Laboratory Based Assessment of Estrogenic Activity Using In Vivo Vitellogenin Induction in Male Fathead Minnows
- Use male fathead minnows (Pimephales promelas) >4 months old, which display secondary sexual characteristics (i.e., the development of nuptial tubercles and a dorsal fatpad) indicative of male sexual determination.
- To prevent spawning activity, separate mature males three weeks (± 3 days) prior to the initiation of the test. Set up at least two 45 L glass aquaria with a maximum loading of 3 g/L. To ensure adequate numbers of healthy fish for the test, use a minimum of 100 males.
- Keep the male fish under identical environmental conditions as will be experienced in the test (i.e., water temperature of 25 ± 1 °C and a 16:8 hr light:dark photoperiod). Maintain the dilution water flow rate to each tank to ensure a 95% replacement time of at least every 6 to 8 hr, i.e., 330 ml/min for a 45 L tank.
- Test apparatus and experimental design
- Use large glass tanks to accommodate a loading of up to 3 g of fish per liter of water. For 8 adult males (nominally 4.5 g each), use a 10-20 L tank.
- Use two replicate tanks for each treatment and shield each tank from any unnecessary visual disturbances (i.e., use laminated card screens between the tanks). Identify each tank with a study number, an exposure concentration and a vessel identification number.
- For wastewater effluent studies
- On arrival, immediately transfer effluent into a storage tank at 10 ± 1 °C. Start dosing effluent within two hr of receipt of the effluent.
- Feed the effluent, via peristaltic pump, from the 10 °C storage tank to an acclimation vessel. Heat the effluent in the acclimation vessel to 18 ± 2 °C. Pump the heated effluent from the acclimation vessel to the dosing/mixing vessels and then to the test aquaria (which holds the fish). Heat the aquaria to a temperature of 25 ± 1 °C.
- For chemical dosing studies
- Weigh test chemicals in a powder weighing cabinet. Prepare concentrated chemical stocks in ddH2O preferably without the use of solvents. Note: If solvents are required to solubilize test compounds, use solvent controls in addition to the dilution water control.
- Gravity feed or pump dilution water from a temperature controlled header tank via flow control device(s) to dosing/mixing vessel. Pump concentrated chemical stock(s) using a peristaltic pumping system to the dosing/mixing vessels. Control the pump rate (of stock chemical) and water flow rate (dilution water) to achieve the desired exposure concentration. Note: Use silicone tubing to feed water/test chemical to each test vessel from the dosing/mixing vessel. Use a flow rate that is sufficient to provide a 75% vessel replacement in at least 24 hr, i.e., 20 ml/min for a 20 L tank. Maintain the flow rate at ± 10% of the specified nominal value.
- Maintain exposure tank water temperature at 25 ± 1 °C, dissolved oxygen above 70% of the air saturation value (5.8 mg L-1 at 25 °C) and ± 0.5 pH units (starting pH between 6.5 and 8.5) throughout study. Set lighting conditions to photoperiod of 16 hr light: 8 hr dark, with dawn/dusk transition periods of 20 min.
- Feed the fish twice a day with freshly defrosted frozen adult brine shrimp (Artemia) at 2.5 (± 0.1) g per tank per feed. Also feed the fish once a day with a small quantity (two finger pinch) of a tropical flake fish food. Leave a minimum of 3 hr between each feed.
- Keep a daily feeding record to monitor the feeding response/behavior (good, moderate or poor, compared with the controls). Siphon the tanks at least twice a week (preferably daily) to remove any uneaten food and feces. Clean the sides and bottoms of the test vessels at least once per week.
- Take weekly water samples from each tank to confirm estrogenic activity (via Yeast screen) and chemical composition (via analytical chemistry). See sections 1-3 for details of water sampling, extraction and analysis. Note: For each experiment, use dilution water control (negative control), positive control (E2 or EE2) plus at least three dilutions of effluent (100, 50 and 25%) or test chemical to monitor dose response. If novel technologies are tested, use compound/effluent with or without treatment (e.g., EE2 without treatment, EE2 with TAML/hydrogen peroxide (H2O2) treatment12; Figure 1).
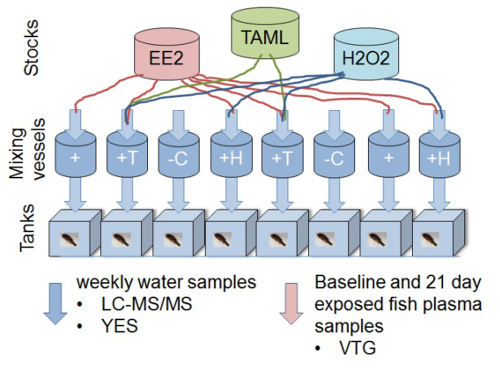 Figure 1. Diagram representing experimental design of an in vivo fathead minnow vitellogenin bioassay to determine removal of ecotoxicity of 17α-ethinylestradiol using TAML/peroxide water treatment. The experimental set up consists of eight 11 L glass aquaria each fed with continuous flow of water. Individual chemical stock solutions and water (filtered de-chlorinated) are delivered to the mixing chambers. Nominal concentrations (without reaction) in the mixing vessels are 2 ng/L EE2, 80 nM TAML and 0.16 µg/L H2O2. Chemical stock solutions (EE2, H2O2 and TAML) are prepared and dosed separately so that the reactions commence in the mixing vessels. Fish (8 male fathead minnows per tank) are exposed to the mixture(s) after a reaction contact time of approximately 45 minutes. Water samples are taken from the exposure tanks weekly. Plasma samples are taken from fathead minnows to measure the estrogenic biomarker vitellogenin (VTG) from a baseline group, at the start of the study, and all other fish after 21 days of exposure. The specific treatments are: '-C'; negative control (dilution water only), '+'; positive control of EE2, '+H'; EE2 plus H2O2, '+T'; EE2 plus H2O2 plus TAML. This figure has been modified from Mills et al. 201512. Please click here to view a larger version of this figure.
Figure 1. Diagram representing experimental design of an in vivo fathead minnow vitellogenin bioassay to determine removal of ecotoxicity of 17α-ethinylestradiol using TAML/peroxide water treatment. The experimental set up consists of eight 11 L glass aquaria each fed with continuous flow of water. Individual chemical stock solutions and water (filtered de-chlorinated) are delivered to the mixing chambers. Nominal concentrations (without reaction) in the mixing vessels are 2 ng/L EE2, 80 nM TAML and 0.16 µg/L H2O2. Chemical stock solutions (EE2, H2O2 and TAML) are prepared and dosed separately so that the reactions commence in the mixing vessels. Fish (8 male fathead minnows per tank) are exposed to the mixture(s) after a reaction contact time of approximately 45 minutes. Water samples are taken from the exposure tanks weekly. Plasma samples are taken from fathead minnows to measure the estrogenic biomarker vitellogenin (VTG) from a baseline group, at the start of the study, and all other fish after 21 days of exposure. The specific treatments are: '-C'; negative control (dilution water only), '+'; positive control of EE2, '+H'; EE2 plus H2O2, '+T'; EE2 plus H2O2 plus TAML. This figure has been modified from Mills et al. 201512. Please click here to view a larger version of this figure.
- Test Procedure
- Acclimation period
- Measure wet weight (g) of each sexually mature male fish, and allocate at random to each tank (8 males per tank).
- Keep unallocated fish from the same batch and maintain them under the same test conditions. Use these fish to replace any individuals that show signs of physical damage or a lack of condition during the 7-day acclimation period. Ensure at the end of the acclimation period each treatment tank has 8 males that have fully acclimated to the test conditions.
- Take 'baseline' plasma samples from an additional 8 males from the same batch of male fish. Follow the blood sampling method in section 4.4 and the Vitellogenin (VTG) analysis method in 4.5.
- After the acclimation period, deliver effluent or chemical dosing stocks to the exposure tanks for 21 days as described in 4.2.2 or 4.2.3. Monitor mortality, behavior and physical appearance of the fish in each replicate tank daily prior to the first feed of the day. Record any abnormal behavior or incidents. Note: Ensure environmental conditions and feeding rates maintain the health of the fish (sections 4.2.4 and 4.2.5).
- Fish Sampling after 21-day exposure period
- 12 hr prior to sampling, stop feeding the fish.
- Label micro-centrifuge tubes for the collection of blood samples, add ~5 µl of Aprotinin (a protease inhibitor) and place them on ice.
- Make a 500 mg/L solution of MS222 (anesthetic) by dissolving 500 mg of MS222 per 1 L of de-chlorinated water (previously acclimated to 25 ± 1 °C). Neutralize the MS222 to pH 7.4 ± 0.4 using 1 M NaOH.
- Move each fish from the tank into the buffered MS222. Keep in the solution until the cessation of all operculum movement (typically 5 ± 1 min).
- Measure and record the fork length (mm) under the terminal anesthetic. Use a disposable scalpel to amputate the tail, and use a heparinized hemocrit tube to collect the blood from the caudal artery (Figure 2). Carefully dispense the blood into the pre-labeled microcentrifuge tube and keep on ice.
- Kill the fish immediately after drawing the blood. Confirm death by permanent cessation of the circulation and/or the destruction of the brain. Measure and record total fish weight (to nearest 0.01 g), and dissect tissues as required.
- Centrifuge the blood (7,000 x g for 5 min at 4 °C) within 2 hr of collection. Transfer the plasma supernatant using a pipette into new labeled 0.4 ml microcentrifuge tubes and store on ice. Freeze the tubes containing plasma on dry ice within 30 min of centrifugation and store at -80 °C before analysis of plasma VTG concentrations.
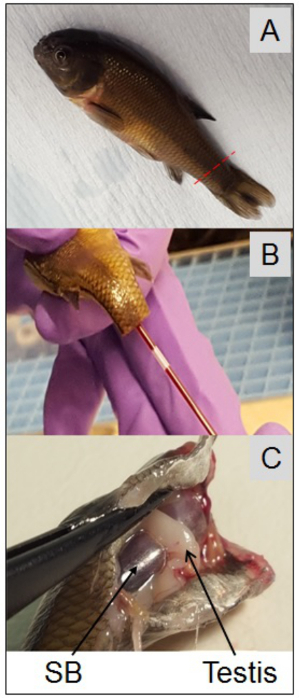 Figure 2. Photos depicting male fathead minnow (Pimephales promelas), plasma collection and location of testis. At the end of the 21-day exposure all fish should be killed to collect blood samples. Once under this terminal anesthetic fish length (fork length, mm) should be measured, quickly followed by blood collection from the caudal artery. Photo-A: red dotted line indicates location for tail amputation (a disposable scalpel should be used to amputate the tail). Photo-B shows a heparinized hemocrit tube used to collect the blood. Each fish should then be killed immediately after its blood sample has been taken, in this case the whole head has been severed from the body (Photo-C). Once the fish has been killed the body cavity can be opened up to reveal the internal organs. Photo-C shows the location of the testis (gonad) in relation to the swim bladder (SB) in cyprinid fish, e.g., fathead minnow, roach, carp, etc.
Please click here to view a larger version of this figure.
Figure 2. Photos depicting male fathead minnow (Pimephales promelas), plasma collection and location of testis. At the end of the 21-day exposure all fish should be killed to collect blood samples. Once under this terminal anesthetic fish length (fork length, mm) should be measured, quickly followed by blood collection from the caudal artery. Photo-A: red dotted line indicates location for tail amputation (a disposable scalpel should be used to amputate the tail). Photo-B shows a heparinized hemocrit tube used to collect the blood. Each fish should then be killed immediately after its blood sample has been taken, in this case the whole head has been severed from the body (Photo-C). Once the fish has been killed the body cavity can be opened up to reveal the internal organs. Photo-C shows the location of the testis (gonad) in relation to the swim bladder (SB) in cyprinid fish, e.g., fathead minnow, roach, carp, etc.
Please click here to view a larger version of this figure.
- Measure plasma VTG concentrations with a homologous VTG enzyme-linked immunosorbent assay (ELISA) kit designed specifically for fathead minnows.
- Prepare VTG standards and buffers as outlined in the manufacturer's protocol. Dilute plasma sample 1:50, 1:5,000, and 1:500,000 and assay them in duplicate to obtain readings within the VTG standard curve range as per manufacturer's instructions. Follow manufacturer's guidelines to calculate vitellogenin concentrations.
5. Field Assessments of Advanced/Novel Wastewater Treatment Technologies to Mitigate Estrogenic Activity Using In Vivo Vitellogenin and Intersex Induction in Roach (Rutilus rutilus)
- Capture of wild roach living down stream of Wastewater Treatment Plant outfalls Note: Use roach (Rutilus rutilus) or other abundant freshwater or brackish fish species, which are gonochoristic and known to be sensitive to estrogenic endocrine disruption.
- Catch fish using electrofishing, netting, trapping or other recognized fishing methods depending on the situation13. Transport the fish in aerated tanks back to laboratory for sampling.
- Prepare microcentrifuge tubes as detailed in 4.4.2. Prepare buffered MS222 as described in 4.4.3. Anesthetize fish as detailed in 4.4.4.
- Measure fish length and weight under terminal anesthetic.
- Collect blood from the caudal artery using disposable heparinized syringe. Kill each fish immediately after blood sample collection. Carefully dispense the blood into pre-labeled microcentrifuge tubes and keep on ice. Prepare plasma as described in step 4.4.7 and measure VTG by ELISA (4.5).
- Using forceps remove 2-3 fish scales from each fish and place in individually labeled small paper envelopes. Store envelopes at room temperature in dry conditions for later fish age determination and growth analysis.
- Open body cavity with a scalpel to reveal the internal organs. Take out the gut to reveal the swim bladder with a gonad located on either side (Figure 2). Carefully remove the paired gonads with fine nosed forceps and place into a glass vial. Cover the gonads with Bouin's fixative at a ratio of 1:10 tissue: fixative.
- Leave the tissue in Bouin's for 6-24 hr depending on the size of the tissue (fixative penetrates at 1 mm per hr). Once fixed, pour off the Bouin's fixative and replace with 70% industrial methylated spirits (IMS). Store the fixed tissue at room temperature until tissues are processed for histopathology (section 5.3).
- Field based assessment of estrogenic activity using in vivo vitellogenin and intersex induction in roach
- Experimental design and set up Note: Set up the tanks and pilot plant well in advance of the in vivo work starting. Start the tanks flowing 3-4 weeks prior to any addition of fish to the system. Monitor water flow rates, water quality and conditions (pH, temperature, dissolved oxygen, etc.) regularly to make sure parameters can be maintained.
- Construct large tanks (e.g., 300-1,000 L) on site at the pilot plant, which can receive 'control' de-chlorinated tap water, standard treated effluent(s) and advanced treated effluent(s) in parallel. Attach air pump/aerators to the tanks. Set water flow rates to achieve at least 6 tank volume exchanges per day. Note: Make sure tanks are well insulated and shaded to prevent excessive daily temperature fluctuations. Design tanks to allow observation of the fish, for behavioral alterations (lack of feeding, etc.), signs of disease and mortality.
- Start the exposure by floating the bags of roach (from the fish farm) in the respective tanks for 1 hr. Add tank water to the bags gradually until water temperature and conditions are ambient. Release the fish into their tanks.
- Feed adult roach daily on pelleted fish food (size 0.5−0.8). Feed juvenile roach daily on small pelleted (pellet sizes 100−300) non-estrogenic feed14 ad libitum, adjusted on the basis of uneaten feed. Keep a daily feeding record documenting feeding response/behavior (good, moderate or poor, compared with the controls).
- Take weekly water samples from each tank to confirm estrogenic activity and chemical composition. See sections 1-3 for details of water sampling and analysis methods.
- Histopathology of fish gonads15
- Carefully remove the dissected and fixed gonads (described in steps 5.1.6 and 5.1.7) from the container with tweezers and place on a cutting board.
- Use a microtome blade to cut each gonad into 3 parts (anterior, mid and posterior) and from each part cut a 3-5 mm thick cross section. Carefully place all six pieces into a labeled plastic biopsy cassette, and place into tissue processor using the timings listed in Table 4.
- Wax embed tissues and section on rotary microtome (3-5 μm). Transfer sections to labeled bio-adhesive coated glass slides and place slides on a heated plate (set at 45 °C) to dry for 24 hr.
- Stain the slides, either manually or using an automated stainer, using the timings detailed in Table 5. Place a drop of mounting agent on the stained tissue, and lay a glass coverslip over the mounting agent to protect the tissue.
- First, examine each slide at low magnification (20X, i.e. 2X objective, with 10X eye lines magnification) to determine sex and the number of points of attachments to the body cavity15. Note any abnormalities for each fish.
- At a higher magnification (100X or 400X), examine the tissue to assess gametogenesis stages, abnormalities and the presence of oocytes in testicular tissue. Record the severity of intersex using the following grading system ranging from 0 (normal male tissue) to 7 (100% ovarian tissue)6 (see Table 6).
| Step Number | Treatment | Purpose | Time (hr) |
| 1 | 70% IMS | Dehydration | 3 |
| 2 | 90% IMS | Dehydration | 2.5 |
| 3 | 95% IMS | Dehydration | 1.5 |
| 4 | 100% IMS | Dehydration | 1.5 |
| 5 | 100% IMS | Dehydration | 1.5 |
| 6 | 100% IMS | Dehydration | 1.5 |
| 7 | 100% IMS | Dehydration | 1.5 |
| 8 | Histology clearing agent | Clearing | 1.5 |
| 9 | Histology clearing agent | Clearing | 1.5 |
| 10 | Histology clearing agent | Clearing | 1.5 |
| 11 | WAX | Wax infiltration | 1.25 |
| 12 | WAX | Wax infiltration | 1.25 |
| 20 hr TOTAL |
Table 4. Processing regime for wax impregnating tissues for histopathology. Tissues should be processed in an automatic tissue processor. Tissues should be immersed in the solutions detailed for the period of time specified.
| Stain no. | Stain | Purpose | Time (min) |
| 1 | Histology Clearing agent | Dissolves wax | 15 |
| 2 | 100% IMS | Hydration | 2 |
| 3 | 90% IMS | Hydration | 2 |
| 4 | 70% IMS | Hydration | 2 |
| 5 | TAP WATER (RUNNING) | Rinse | 2 |
| 6 | HAEMOTOXYLIN | Stains cell nuclei blue | 10 |
| 7 | TAP WATER (RUNNING) | Remove excess | 10 |
| 8 | Acidified IMS | Dechlorination | 20 sec |
| 9 | TAP WATER (RUNNING) | Rinse | 20 sec |
| 10 | LiCO3 | Salt | 20 sec |
| 11 | TAP WATER (RUNNING) | Rinse | 20 sec |
| 12 | 1% EOSIN (AQUEOUS) | Stains cytoplasm pink | 20 sec |
| 13 | TAP WATER (RUNNING) | Remove excess | 5 |
| 14 | 70% IMS | Dehydration | 2 |
| 15 | 90% IMS | Dehydration | 2 |
| 16 | 100% IMS | Dehydration | 5 |
| 17 | Histology Clearing agent | Remove IMS, binding agent | 5 |
Table 5. Solutions and immersion times for Haematoxylin and Eosin (H & E) staining of fish gonadal tissues. Slides should be placed in each bath for the allocated time in sequence. H & E staining of tissues is required to determine developmental or organizational impacts of estrogenic wastewater effluents on fish gonads.
| Score | Section Description |
| 0 | Normal male testis |
| 1 | Multifocal ovotestis with 1–5 oocytes (usually singly) scattered among the testicular tissue |
| 2 | Multifocal ovotestis, 6–20 oocytes often in small clusters scattered among the testicular tissue |
| 3 | Multifocal ovotestis, 21–50 oocytes in clusters |
| 4 | >50 and <100 oocytes. Section is usually multifocal and has the appearance of a mosaic of testicular and ovarian tissue. |
| 5 | >100 oocytes, usually multifocal but could also be focal with clearly identifiable zones of ovarian and testicular tissue separated from the testicular tissue. |
| 6 | >50 per cent of the gonadal tissue on the section is ovarian and is clearly separated from the testicular tissue by epithelial cells and phagocytic tissues. |
| 7 | 100 per cent of gonadal tissue on the section is ovarian. |
Table 6. Scoring system to assess severity of intersex condition in roach. Histologically prepared slides of gonadal tissue should be examined under light microscope, at 20X, 100X and 400X magnification, to assess any abnormalities and the presence of oocytes in testicular tissue. This table is modified from Jobling et al. 20066.
Representative Results
Attempts to understand the impact of improvements to wastewater treatment processes or to determine the most appropriate technology to retrofit equipment as tertiary treatment at existing WWTP with respect to the efficacy of removal of endocrine disrupting activity of discharged effluents, requires not only the measurement of key chemical components that enter the works but requires the analysis of the breakdown products which may also have endocrine disrupting activity. In domestic sewage effluents, the most estrogenic substances present are the steroid hormones, estrone (E1), 17β-estradiol (E2) and 17α-ethinylestradiol (EE2)5,8. Steroid estrogens are primarily excreted from the body as a mixture of inactive conjugates16,17. These conjugated estrogens are substantially deconjugated in the sewerage system by bacterial activity and further degradation occurs in the WWTP. The deconjugated steroids are removed from the wastewater stream by adsorption to sludge or biodegraded during secondary treatment resulting in the formation, firstly of transformation byproducts and ultimately complete mineralization can occur of the initial active component. The chemical analysis of all individual compounds in the effluent stream would be difficult, time consuming and costly and would not cover unknown active components present in a sample. Furthermore, a sum of the estrogenic contribution of each component will only provide an indication of the cumulative estrogenic potency of a sample of the compounds analyzed. This is a risk where transformation processes generate unknown estrogenic substances or where the influent is of industrial origin. Combining chemical analysis with in vivo and in vitro ecotoxicology bioassays provides a solution to the presence of unknown estrogenic components in mixtures such as treated sewage. In vitro assays such as the Yeast Estrogen Screen (YES) have been used extensively to determine the estrogenic activity of sewage effluents and to help identify the active components in treated samples8,18,19. However, comparisons between in vivo and in vitro testing can be significant11 and a comprehensive assessment of new processes with respect to treatment of endocrine disrupting potency requires a battery of chemical and ecotoxicology tests.
A determination of whether individual treatment plants or processes remove active compounds from the wastewater stream can be achieved using chemical analysis which follows sample extraction, concentration and clean-up of the extract prior to analysis, most often undertaken using LCMS(/MS) or GCMS(/MS) methods. The data obtained from chemical analysis can be used to determine compliance with individual predicted no effect concentrations (PNEC)20 or environmental quality standards (EQS)21 of specific individual compounds and therefore such methods are vital for regulatory compliance data. Furthermore, targeted or non-targeted chemical analysis methods allow the identification and quantitation of individual compounds or isomers compared with biological methods, which provide a total response. Chemical analysis methods therefore allow the assessment of discrete compounds to be made to meet and to address these wastewater treatment challenges on an individual treatment plant basis. Studies have shown that conventional wastewater treatment (e.g., activated sludge plants) can be highly effective in the removal of natural steroid hormones although removal of the synthetic hormone EE2 tends to be less effective. Field studies using advanced treatment utilizing techniques such as ozone, granulated activated carbon (GAC) and membranes have demonstrated, albeit at high cost, that they can be used as an end-of-pipe solution to remove EE2 to below predicted effect levels and to below the detection limits. Figure 3 shows the removal of EE2 using GAC at a pilot scale municipal wastewater treatment plant. Studies undertaken at pilot scale at municipal wastewater treatment plants using end of pipe GAC treatment also show the reduction in estrogenic potency following GAC measured using the Yeast Estrogen Screen (YES) as seen in Figure 4.
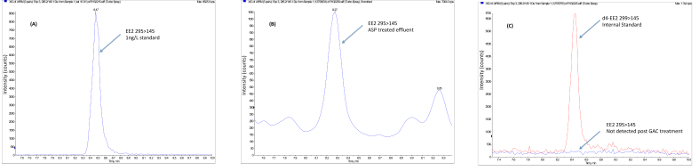 Figure 3. Example field data showing the removal of ethinylestradiol following advanced tertiary treatment. (A) Samples are collected from the WWTP following conventional (activated sludge plant) treatment following the procedures described for sample preservation. (B) Samples are extracted using solid phase extraction, cleaned-up to remove interfering substances using normal phase SPE and gel permeation chromatography. (C) The clean concentrated extract is concentrated to a low volume and analyzed using negative ion electrospray LCMS/MS in MRM mode. Results are calculated using internal standardization using isotopically labeled internal standards. In the example shown, EE2 is present in the final ASP effluent at a concentration above the predicted no effect level (PNEC) of 0.1 ng/L and is removed using GAC and ozone (O3) to an environmentally safe concentration. Please click here to view a larger version of this figure.
Figure 3. Example field data showing the removal of ethinylestradiol following advanced tertiary treatment. (A) Samples are collected from the WWTP following conventional (activated sludge plant) treatment following the procedures described for sample preservation. (B) Samples are extracted using solid phase extraction, cleaned-up to remove interfering substances using normal phase SPE and gel permeation chromatography. (C) The clean concentrated extract is concentrated to a low volume and analyzed using negative ion electrospray LCMS/MS in MRM mode. Results are calculated using internal standardization using isotopically labeled internal standards. In the example shown, EE2 is present in the final ASP effluent at a concentration above the predicted no effect level (PNEC) of 0.1 ng/L and is removed using GAC and ozone (O3) to an environmentally safe concentration. Please click here to view a larger version of this figure.
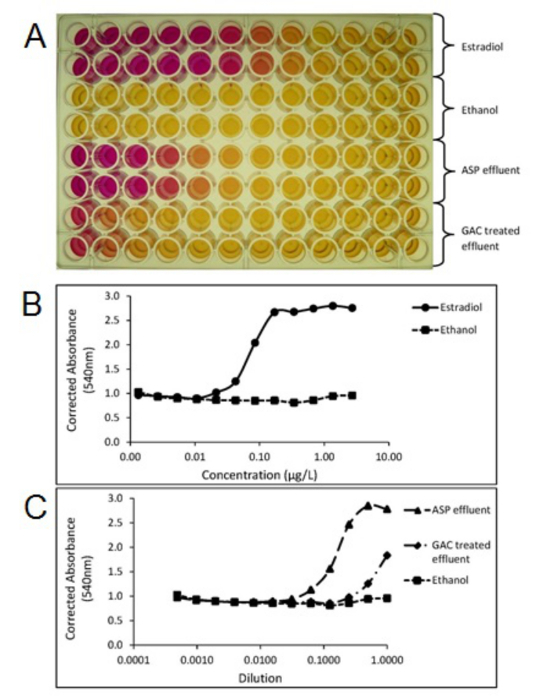 Figure 4. Photo of a Yeast Estrogen Screen (YES) assay plate (A) showing color change from yellow to red, relating to estrogenic activity of the samples. Plots created from the YES assay plate showing corrected absorbance (540 nm) of the estradiol standard (B), activated sludge process effluent (ASP) and Granular activated carbon (GAC) treated wastewater effluent samples (C). Each sample was tested in duplicate. ASP and GAC effluents were extracted and concentrated using the SPE methods outlined in section 1. Please click here to view a larger version of this figure.
Figure 4. Photo of a Yeast Estrogen Screen (YES) assay plate (A) showing color change from yellow to red, relating to estrogenic activity of the samples. Plots created from the YES assay plate showing corrected absorbance (540 nm) of the estradiol standard (B), activated sludge process effluent (ASP) and Granular activated carbon (GAC) treated wastewater effluent samples (C). Each sample was tested in duplicate. ASP and GAC effluents were extracted and concentrated using the SPE methods outlined in section 1. Please click here to view a larger version of this figure.
Ozonation is also efficient in removing steroid estrogens and estrogenic activity from conventionally treated wastewater treatment plants. Ozone is able to oxidize a wide range of organic contaminants and dissolved organic matter in wastewater samples and provides disinfection properties. The effectiveness of ozonation depends on water characteristics such as pH, amount of organic matter and the applied dose of ozone. Estrogens that are poorly removed by conventional treatment can be removed from wastewater with doses between 0.8 and 2 mg O3/mg DOC. Ozone is a selective oxidizing agent, which reacts with electron rich sites (unsaturated carbon-carbon bonds, aromatic compounds including aromatic alcohols), which makes ozone applicable for the breakdown of a number of EDC. However, the elimination of individual compounds does not necessarily lead to the complete mineralization of the original compound. Organic substances following ozonation may be transformed generating intermediates or transformation oxidation by-products which include a number of low molecular weight, polar classes of compounds such as aldehydes, ketones, carboxylic acids, keto acids, and brominated compounds. Examples include, bromate, formaldehyde, acetaldehyde and carboxylic acids. Using in vivo and in vitro bioassays it has been shown that although ozone only partially oxidizes some chemical substances, the resulting major transformation products have a lower estrogenic potency and hence the application of ozone at an appropriate dose results in a high removal of estrogenic activity.
One of the major benefits of additional treatment of wastewaters is the reduction in the feminization of male fish in receiving waters; an adverse effect that can lead to reduced fertility3. In vivo studies using fish (e.g., roach or fathead minnow) exposed to wastewater show female germ cells or oocytes in the testis of male fish (e.g., as seen in Figure 5). Intersex or male VTG is absent or significantly reduced in fish following advanced treatment such as GAC7 or ozone22. These studies show that transformation products produced during ozonation are not estrogenic, however this does not address the toxicity of the effluent produced. This issue has been addressed in other studies, for example a study by Magdeburg et al.23 which shows that ozone oxidation by-products are toxic to rainbow trout but this toxicity can be removed by downstream sand filtration following ozonation.
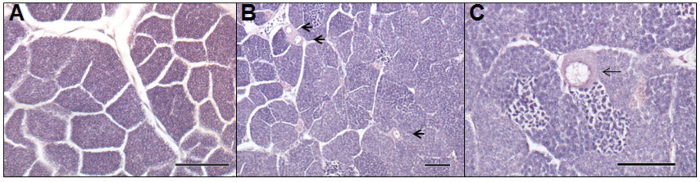 Figure 5. Photomicrographs of a normal male (A) and intersex (B, C) gonads from adult roach (Rutilus rutilus) exposed to wastewaters in a field based assessment. Photomicrograph-A, depicts a histological section of normal male testis. Photomicrograph-B and -C, depicts histological sections of an intersex male fish, having been exposed to activated sludge process wastewater effluent for six months. Arrows indicate oocytes present in the testicular tissue. Scale bar represents 100 µm in each photomicrograph. Please click here to view a larger version of this figure.
Figure 5. Photomicrographs of a normal male (A) and intersex (B, C) gonads from adult roach (Rutilus rutilus) exposed to wastewaters in a field based assessment. Photomicrograph-A, depicts a histological section of normal male testis. Photomicrograph-B and -C, depicts histological sections of an intersex male fish, having been exposed to activated sludge process wastewater effluent for six months. Arrows indicate oocytes present in the testicular tissue. Scale bar represents 100 µm in each photomicrograph. Please click here to view a larger version of this figure.
The high cost of end of pipe treatment using ozone, GAC or membrane technology necessitates the development of alternative lower cost, sustainable methods for endocrine disrupting chemical (EDC) removal. Furthermore, adsorption and separation methods simply separate EDC from one phase to another rather than eliminating them via degradation. TAML activators have been developed to catalyze hydrogen peroxide oxidation of organic micropollutants in wastewater12,24-26. TAML activators with H2O2 effectively degrade EE2 and other steroid estrogens in pure laboratory water as well as in effluents from municipal wastewater treatment plants and in spiked urine samples12. Laboratory studies, shows TAML/H2O2 treatment provides high steroid estrogen removal including EE2 removal and substantially reduces estrogenic activity measured in vitro using the YES bioassay and substantially diminishes fish feminization in vivo measured using the VTG bioassay (Figure 1 and Figure 6).
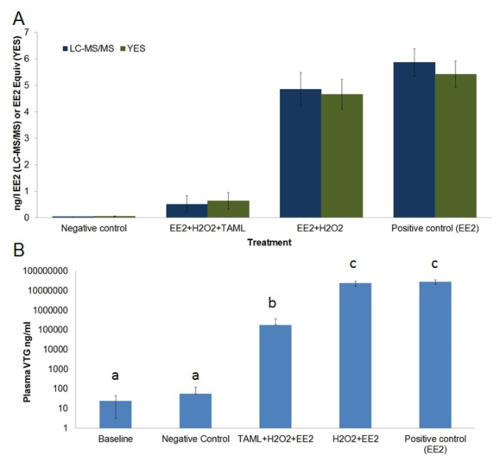 Figure 6. Average EE2 concentration and estrogenic activity in treated and untreated tank waters (A) and plasma vitellogenin in baseline and exposed male fish (B).A) EE2 concentration (ng/L, dark blue bars) was measured by LCMS/MS, estrogenic activity (EE2 equivalent ng/L, dark green bars) was measured via in vitro Yeast Estrogen Screen (YES). B) Plasma vitellogenin (ng/ml, light blue bars) concentration in male fathead minnows were measured via a quantitative enzyme-linked immunosorbent assay (ELISA). EE2 chemical analysis results reported as < 0.03 ng/L EE2 (i.e., lower than detection limit (LOD)) were treated as having half LOD (i.e., 0.015 ng/L EE2) for use in calculations of averages, standard error and statistical analysis. EE2 and estrogenic activity are average measured concentrations sampled over the 21 day exposure. Plasma VTG was measured prior to exposure (baseline) and after 21 days exposure. The treatment regime consisted of; negative control (dilution water only), EE2+H2O2+TAML, EE2+H2O2, and EE2-only. Error bars in graph-A represent standard error of the mean, error bars in graph-B represent standard deviation. Letters above bars in graph-B represent statistical similarity. This figure has been modified from Mills et al.12
Please click here to view a larger version of this figure.
Figure 6. Average EE2 concentration and estrogenic activity in treated and untreated tank waters (A) and plasma vitellogenin in baseline and exposed male fish (B).A) EE2 concentration (ng/L, dark blue bars) was measured by LCMS/MS, estrogenic activity (EE2 equivalent ng/L, dark green bars) was measured via in vitro Yeast Estrogen Screen (YES). B) Plasma vitellogenin (ng/ml, light blue bars) concentration in male fathead minnows were measured via a quantitative enzyme-linked immunosorbent assay (ELISA). EE2 chemical analysis results reported as < 0.03 ng/L EE2 (i.e., lower than detection limit (LOD)) were treated as having half LOD (i.e., 0.015 ng/L EE2) for use in calculations of averages, standard error and statistical analysis. EE2 and estrogenic activity are average measured concentrations sampled over the 21 day exposure. Plasma VTG was measured prior to exposure (baseline) and after 21 days exposure. The treatment regime consisted of; negative control (dilution water only), EE2+H2O2+TAML, EE2+H2O2, and EE2-only. Error bars in graph-A represent standard error of the mean, error bars in graph-B represent standard deviation. Letters above bars in graph-B represent statistical similarity. This figure has been modified from Mills et al.12
Please click here to view a larger version of this figure.
Discussion
Wastewater treatment plants are the major route of surface water contamination with EDC. An evaluation of the efficacy of removal of endocrine activity of conventional, advanced or emerging treatment processes requires the use of a variety of chemical and biological assays. Chemical analysis using non-targeted and targeted analysis provides qualitative or quantitative data on the efficacy of removal of individual components and therefore allows an assessment to be made against environmental quality standards or predicted no effect concentrations for the compounds or mixtures of compounds analyzed.
The generation of transformation products resulting from incomplete mineralization of substances following treatment and the presence of unknown biologically active components in wastewater limits the usefulness of chemical testing alone. A combination of in vivo and in vitro bioassays in combination with analytical chemistry screening provides a useful toolbox to determine the efficacy of EDC removal by emerging wastewater treatment processes. These tests, when conducted alongside traditional water quality parameters and other toxicological and microbiological end-points allow a critical evaluation of current and emerging wastewater treatment technologies.
It is important to note that Yeast based estrogen screens (e.g., YES) are not the only in vitro assays to determine estrogenic potency of chemicals and wastewaters. A number of stably transfected mammalian cell based assays have been developed too, for example, the ER-CALUX27 and hERα-HeLa-990328 with human breast cancer cells or cervical tumor cells respectively. The YES has been compared to similar mammalian cell based assays and has been found to have a comparable high level of reproducibility, true positive and true negative estrogenic identification rates29, although it is sometimes considered to be slightly less sensitive27. One benefit of Yeast based reporter assays is that in labs without significant experience with mammalian cell culture the YES can be more easily adopted, as it requires less stringent bio-control measures and sterile techniques (YES can be performed on the bench top if necessary). The human cell based assays also require CO2 incubators and luminometers compared to the standard incubator and microplate readers used in the YES. Two yeast based estrogen reporter assays (YES, Saccharomyces cerevisiae and A-YES, Arxula adeninivorans) are currently undergoing inter-laboratory trails for the validation of ISO 19040 "Water quality - Determination of the estrogenic potential of water and waste water" highlighting the industries interest in these techniques.
There are a number of limitations of the methods described which include the potential contamination of samples during sampling, sample storage and analysis with estrogenic substances originating from the field or laboratory environment or by human contamination (e.g., plasticizers, surfactants, personal care products). This type of contamination in the YES assay (or other cell based reporter assays) will elevate the background and impact the use of the assay. Water samples or solvents stored in plastic bottles can easily cause false positives. False negatives are also of concern as both LCMS/MS and the YES assay require SPE to concentrate estrogens to detectable levels. The matrix, the choice of SPE sorbent and elution solvent can affect the extraction efficiency and the types of compounds eluted. Using C18 SPE cartridges for extraction using the conditions described in this protocol may generate a negative bias, as highly polar and basic compounds would be poorly retained by the sorbent. Furthermore, this protocol requires reconstitution of the eluted YES eluent from methanol to ethanol via evaporation to dryness funder nitrogen resulting in the loss of volatile compounds. As a result the protocol could provide underestimated estrogenic activity of tested samples. These limitations are especially important when considering the YES assay as unknown or unexpected compounds might be missed, because they have not been extracted or they are lost due to evaporation. Furthermore, the LCMS/MS technique makes use of labeled internal standards to correct for recovery; this approach cannot be used with the YES assay.
Significant limitations of in vivo testing of effluents include high cost and time required for assessment compared to in vitro methods. Currently the use of fish embryo tests to detect estrogenic activity is limited. However, there has been some success with producing estrogen responsive transgenic glowing fish embryos30, which could have future applications. Fathead minnows (used in this protocol) are a common laboratory species and VTG induction in male fish is a well-documented bio-marker of estrogenic exposure and a quantifiable measure of wastewater effluent estrogenicity22 or other estrogenic compounds or mixtures31. OECD test guidelines for endocrine disrupting chemicals have been validated using adult fathead minnow, Japanese medaka and zebrafish32,33, with VTG being a sensitive biomarker of estrogen exposure in all three species. However, VTG induction does not directly correlate to reproductive impairment and therefore the ecological consequences of wastewater exposure, as seen in severely intersex roach3. On the other hand, roach are not a classic 'laboratory species' for ecotoxicology research due to their large size, long generation time (2-3 years to reach sexual maturity), reproductive style; group spawning (breeding) takes place once a year, and the difficulty to identify males from females (other than during the spawning season). However, this normally gonochoristic species has been very well studied in the UK, due to the discovery that downstream of estrogenic wastewater effluents, male fish exhibited perturbations to their endocrinology (e.g., presence of female-specific vitellogenin in their blood) and histopathology (ovotestes - developing eggs in the testis and/or female reproductive ducts)5,6. Therefore, as a future application of these protocols, roach (or similar species) could be a useful wild sentinel species to show if real improvements to wastewater quality (and reduced estrogenicity) are seen in rivers receiving advanced treated effluents. They can also be employed in end of pipe systems to monitor technologically improved effluents from pilot scale plants7. When considering which species to use in in vivo wastewater assessments there is a tradeoff between relatively quick and controlled testing using laboratory species compared to the longer field based, but more environmentally relevant, testing using native species. However, such in vivo assessments are high cost and should only be considered as the final set of tests following assessments using chemical analysis and in vitro assays.
Critical steps within the protocols described include the preparation and handling of samples and glassware (i.e., bottles and sampling equipment should be pre-treated with suitable surface active cleaning agent) to avoid contamination of samples from environmental contaminants including limiting contact of samples with plastics and other materials that can produce false positives. This is equally important when designing and building aquaria and fish exposure systems. Ideally aquaria (housing stocks and during exposures) should be built from materials with low adsorption32 with minimal contamination risk. Stainless steel can be used for effluent or water holding tanks. Whereas tanks of a glass construction are preferred for fish tanks (as this also provides easy observation of the fish). The use of low grade plastic pipes or tubing should be avoided32, PVC34 and ABS can be used if 'properly seasoned', i.e., left to leach out any contaminants in running dilution water for at least 12 hr prior to use. Medical grade silicon tubing has been employed successfully in our facility for peristaltic pump delivery of chemicals and wastewater/dilution water to tanks. As well as considering estrogenic contamination in construction and running of the aquatics system, it is also important to think about the diet of the fish; many propriety fish foods have been found to be estrogenic to fish. Therefore it is important to test any foods for activity (e.g., in the Yeast Estrogen Screen, See Beresford et al.14) prior to using them in these types of studies.
Troubleshooting of the chemical analysis or YES assay protocols described is simplified if quality assurance samples including multiple travel, laboratory and solvent blanks are analyzed alongside positive controls and real samples to eliminate false positive and false negative results. Positive (e.g., EE2) and negative (dilution water only) control should also always be used in the in vivo assays to confirm sensitivity of expected biological biomarker or endpoint (i.e., VTG or histopathology), and allow any unexpected contamination to be detected (e.g., from experimental set up, diet, or dilution waters). Any modifications in the protocol should be validated prior to conducting any study.
With stricter regulation of estrogenic compounds entering the environment via WWTP effluents it is envisage that more effective wastewater treatment technologies will need to be developed. The battery of tests described in this manuscript compliment the ecotoxicological and chemical evaluation tests normally applied to wastewater treatment plant effluent discharges. Therefore, future application of this type of holistic battery of test should enable wastewater technology developers, and plant operators, to implement the most ecologically safe designs considering the best methods to remove both specific regulated estrogenic chemicals and overall biological activity.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Projects presented in this paper were funded by Severn Trent Water and Brunel University London. The authors would like to thank Alan Henshaw and John Churchley for providing field and laboratory assistance. T.J.C. thanks the Heinz Endowments for support. M.R.M. thanks the Steinbrenner Institute for a Steinbrenner Doctoral Fellowship and Carnegie Mellon University for a Presidential Fellowship.
References
- Bergman Å, Heindel J, Jobling S, Kidd K, Zoeller RT. State-of-the-science of endocrine disrupting chemicals. Toxicol. Lett. 2012;211 [Google Scholar]
- Rodgers-Gray TP, et al. Exposure of juvenile roach (Rutilus rutilus) to treated sewage effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ. Sci. Technol. 2001;35(3):462–470. doi: 10.1021/es001225c. [DOI] [PubMed] [Google Scholar]
- Jobling S, et al. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol. reprod. 2002;66(2):272–281. doi: 10.1095/biolreprod66.2.272. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Der Eerden BVan, Jobling S, Panter G, Sumpter JP. Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 1996;166(7):418–426. [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998;32(17):2498–2506. [Google Scholar]
- Jobling S, et al. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006;114(Suppl 1):32–39. doi: 10.1289/ehp.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes A, et al. Additional treatment of wastewater reduces endocrine disruption in wild fish - a comparative study of tertiary and advanced treatments. Environ. Sci. Technol. 2012;46(10):5565–5573. doi: 10.1021/es204590d. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, Sumpter JP. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 1996;15(3):241–248. [Google Scholar]
- Grover DP, Balaam J, Pacitto S, Readman JW, White S, Zhou JL. Endocrine disrupting activities in sewage effluent and river water determined by chemical analysis and in vitro assay in the context of granular activated carbon upgrade. Chemosphere. 2011;84(10):1512–1520. doi: 10.1016/j.chemosphere.2011.04.032. [DOI] [PubMed] [Google Scholar]
- The determination of steroid oestrogens in waters using chromatography and mass spectrometry (2008) Methods for the Examination of Waters and Associated Materials. 2008. Bristol, UK: Environment Agency; 2008. [Google Scholar]
- Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. Comparative study on the in vitro/in vivo estrogenic potencies of 17beta-estradiol, estrone, 17alpha-ethynylestradiol and of 17beta-estradiol, estrone, 17alpha-ethynylestradiol and nonylphenol. Aquat. toxicol. 2004;66(2):183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mills MR, et al. Removal of ecotoxicity of 17α-ethinylestradiol using TAML/peroxide water treatment. Sci. Rep. 2015;5:10511. doi: 10.1038/srep10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EN 14962:2006 Water quality - Guidance on the scope and selection of fish sampling methods. London, UK: British Standards Institute; 2006. [Google Scholar]
- Beresford N, Brian JV, Runnalls TJ, Sumpter JP, Jobling S. Estrogenic activity of tropical fish food can alter baseline vitellogenin concentrations in male fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 2011;30(5):1139–1145. doi: 10.1002/etc.479. [DOI] [PubMed] [Google Scholar]
- Nolan M, Jobling S, Brighty G, Sumpter JP, Tyler CR. A histological description of intersexuality in the roach. J. Fish Biol. 2001;58(1):160–176. [Google Scholar]
- Dascenzo G, et al. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 2003;302(1-3):199–209. doi: 10.1016/s0048-9697(02)00342-x. [DOI] [PubMed] [Google Scholar]
- Gomes RL, Birkett JW, Scrimshaw MD, Lester JN. Simultaneous determination of natural and synthetic steroid estrogens and their conjugates in aqueous matrices by liquid chromatography/mass spectrometry. Int. J. Environ. Anal. Chem. 2005;85(1):1–14. [Google Scholar]
- Metcalfe CD, et al. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) Environ. Toxicol. Chem. 2001;20(2):297–308. [PubMed] [Google Scholar]
- Stalter D, Magdeburg A, Wagner M, Oehlmann J. Ozonation and activated carbon treatment of sewage effluents: Removal of endocrine activity and cytotoxicity. Water Res. 2011;45(3):1015–1024. doi: 10.1016/j.watres.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Caldwell DJ, Mastrocco F, Anderson PD, Länge R, Sumpter JP. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 2012;31(6):1396–1406. doi: 10.1002/etc.1825. [DOI] [PubMed] [Google Scholar]
- Gardner M, Comber S, Scrimshaw MD, Cartmell E, Lester J, Ellor B. The significance of hazardous chemicals in wastewater treatment works effluents. Sci. Total Environ. 2012;437:363–372. doi: 10.1016/j.scitotenv.2012.07.086. [DOI] [PubMed] [Google Scholar]
- Filby AL, Shears JA, Drage BE, Churchley JH, Tyler CR. Effects of advanced treatments of wastewater effluents on estrogenic and reproductive health impacts in fish. Environ. Sci. Technol. 2010;44(11):4348–4354. doi: 10.1021/es100602e. [DOI] [PubMed] [Google Scholar]
- Magdeburg A, Stalter D, Oehlmann J. Whole effluent toxicity assessment at a wastewater treatment plant upgraded with a full-scale post-ozonation using aquatic key species. Chemosphere. 2012;88(8):1008–1014. doi: 10.1016/j.chemosphere.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Collins TJ. TAML oxidant activators: a new approach to the activation of hydrogen peroxide for environmentally significant problems. Acc. Chem. Res. 2002;35(9):782–790. doi: 10.1021/ar010079s. [DOI] [PubMed] [Google Scholar]
- Collins TJ. Designing Ligands for Oxidizing Complexes. Acc. Chem. Res. 1994;27(9):279–285. [Google Scholar]
- Truong L, Denardo MA, Kundu S, Collins TJ, Tanguay RL. Zebrafish Assays as Developmental Toxicity Indicators in The Design of TAML Oxidation Catalysts. Green Chem. 2013;15(9):2339–2343. doi: 10.1039/C3GC40376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk AJ, et al. Detection of estrogenic potency in wastewater and surface water with three in vitro bioassays. Environ. Toxicol. Chem. 2002;21(1):16–23. [PubMed] [Google Scholar]
- OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing; 2009. Test No 455: The Stably Transfected Human Estrogen Receptor-alpha Transcriptional Activation Assay for Detection of Estrogenic Agonist-Activity of Chemicals. [Google Scholar]
- Kolle SN, et al. In house validation of recombinant yeast estrogen and androgen receptor agonist and antagonist screening assays. Toxicol in Vitro. 2010;24(7):2030–2040. doi: 10.1016/j.tiv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Lee O, Tyler CR, Kudoh T. Development of a transient expression assay for detecting environmental oestrogens in zebrafish and medaka embryos. BMC Biotechnology. 2012;12:32. doi: 10.1186/1472-6750-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian JV, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113(6):721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Guidelines for the Testing of Chemicals, Section 2. Paris: OECD Publishing; 2009. Test No 229: Fish Short Term Reproduction Assay. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals, Section 2. Paris: OECD Publishing; 2009. Test No 230: 21-day Fish Assay: A Short-Term Screening for Oestrogenic and Androgenic Activity and Aromatase Inhibition. [Google Scholar]


