Abstract
Pancreatic cancer remains one of the cancers for which survival has not improved substantially in the last few decades. Only 7% of diagnosed patients will survive longer than five years. In order to understand and mimic the microenvironment of pancreatic tumors, we utilized a murine orthotopic model of pancreatic cancer that allows non-invasive imaging of tumor progression in real time. Pancreatic cancer cells expressing green fluorescent protein (PANC-1 GFP) were suspended in basement membrane matrix, high concentration, (e.g., Matrigel HC) with serum-free media and then injected into the tail of the pancreas via laparotomy. The cell suspension in the high concentration basement membrane matrix becomes a gel-like substance once it reaches room temperature; therefore, it gels when it comes in contact with the pancreas, creating a seal at the injection site and preventing any cell leakage. Tumor growth and metastasis to other organs are monitored in live animals by using fluorescence. It is critical to use the appropriate filters for excitation and emission of GFP. The steps for the orthotopic implantation are detailed in this article so researchers can easily replicate the procedure in nude mice. The main steps of this protocol are preparation of the cell suspension, surgical implantation, and whole body fluorescent in vivo imaging. This orthotopic model is designed to investigate the efficacy of novel therapeutics on primary and metastatic tumors.
Keywords: Cancer Research, Issue 115, Cancer Biology, Orthotopic model, Nude Mice, Pancreatic Cancer, in vivo fluorescence imaging, Cancer, animal model, cell culture, laparotomy, surgery
Introduction
Pancreatic cancer is diagnosed with increased frequency compared to other cancers and is the 4th leading cause of cancer-related deaths in the United States. From the time of diagnosis, over 90% of patients die within five years 1,2. Currently, surgical tumor removal is the only cure for pancreatic cancer, but less than 20% of patients are eligible to undergo surgery mainly because at the time of diagnosis the disease is at an advanced stage and has metastasized 3,4. The lack of specific symptoms makes pancreatic cancer a silent disease; some of the symptoms include abdominal pain, back pain, loss of appetite, jaundice and nausea; which can be easily interpreted as common digestive illnesses 4. For this reason, it is important to develop new pharmacological tools to aid in the diagnosis and treatment of pancreatic cancer.
The use of animal models allows us to understand the biology of pancreatic cancer and provides an insight into applying this knowledge to humans. Xenograft orthotopic models of pancreatic cancer are realistic, because tumors grow in the organ of origin 5. In contrast to heterotopic models, where cell lines or tumor fragments are implanted subcutaneously, orthotopic modeling allows for the recreation of the tumor microenvironment and mimics the interaction of tumor cells with its surroundings 6. The xenograft model described here derives tumors from the human pancreatic cancer cell line PANC-1 GFP, which is genetically engineered to express the green fluorescent protein (GFP). GFP detection enables for a non-invasive imaging and monitoring of tumor growth and metastasis 7. Tumor development occurs rapidly, spontaneously, and closely resembles that of primary tumors of human pancreatic cancer patients 8. Orthotopic models provide a more accurate prediction of drug efficacy in response to therapeutic agents, while mimicking the tumor microenvironment.
As mentioned above, this animal model allows fluorescent detection of tumor growth and metastasis in real time. Fluorescent detection allows for a more direct/live imaging compared to luminescence. With fluorescence the emitted light is a result of an excitation by another light of a shorter wavelength; whereas in luminescence, the emitted light is the result of a chemical reaction and may not have strong emission9. Furthermore, whole body in vivo fluorescent imaging is not detrimental to the animal and allows researchers to monitor tumor growth over time in response to therapeutic treatments.
Protocol
The protocol described below is executed under guidance and approval of Western University's Animal Care and Use Committee. All experiments are performed in compliance with all relevant guidelines, regulation and regulatory agencies.
1. Cell Culture
- Preparation of Complete Medium
- Using a Class II biological safety cabinet, prepare complete medium by aseptically adding fetal bovine serum (FBS) and penicillin streptomycin (P/S) to a 500 ml bottle of RPMI medium. Mix gently by swirling. The final concentration of each supplement is 10% FBS and 1% P/S (v/v). For example, supplement 500 ml of media with 56 ml FBS and 6 ml P/S.
- Place complete medium in a water bath at 37 °C.
- Thawing and Propagating Cells
- Retrieve PANC-1 GFP cells from liquid nitrogen. Thaw the vial by gentle agitation in a water bath at 37 °C. Note: Thawing should be rapid (approximately 2 - 3 min).
- Label T-75 flasks to be used with (a) cell-line name, (b) passage number, (c) date, and (d) researcher's initials.
- Wipe the vial with 70% ethanol and transfer vial to a biosafety cabinet.
- To propagate the cells, add 9.0 ml of complete medium to a 15 ml conical tube. Using a 1.0 ml pipette, carefully transfer the cell suspension and suspend it in 9 ml of complete medium.
- Centrifuge at 125 x g for 8 min at RT. Transfer the conical vial to a biosafety cabinet and aspirate the supernatant without disturbing the pellet.
- Suspend the pellet in 10 ml of fresh complete medium by gently pipetting the suspension up and down.
- Count the cells with a hemocytometer and plate them in T-75 flasks at a density of 2.1 x 106 cells/flask. Place flask in an incubator at 37 °C and 5% CO2.
- Monitor the cells daily by eye and under the microscope. If fluid renewal is needed, aseptically aspirate the complete growth medium from the flask and discard. Add equal volume of fresh complete medium.
- Propagating Cells
- Aseptically remove medium from the flask. Add 5 ml of Dulbecco's phosphate buffer saline (DPBS) to rinse cells and discard.
- Detach the cells from the flask by adding 3 ml of 0.25% trypsin. Incubate flask containing trypsin at 37 °C and 5% CO2 for 5 to 10 min.
- Observe cells under the microscope (10X magnification) and ensure they are round and dislodged.
- Neutralize trypsin by adding an equal volume of complete medium and break up clumps by gently pipetting up and down.
- Count the cells using a hemocytometer and transfer the appropriate cell suspension volume to new flasks at the cell density mentioned in 1.2.7; add enough fresh media so the final volume is 10 ml per flask.
- Cell Suspension Preparation for Injection Note: Since the basement membrane matrix, high concentration, solidifies at RT, keep all materials on ice. Place all sterile pipette tips and vials in the freezer overnight, and keep on ice while working on the suspension.
- Keep a bottle of RPMI media without any additives on ice. Thaw the basement membrane matrix, high concentration, by following manufacturer's instructions. Preferably, aliquot into smaller vials to avoid multiple freeze-thaw cycles 10.
- Aspirate media from all flasks, and rinse with 5 ml of DPBS. Repeat all steps on the previous section up to step 1.3.4.
- Transfer contents to a 15 ml conical tube and centrifuge at 125 x g for 8 min at RT. Transfer conical vial to biosafety cabinet and re-suspend pellet using 10 ml of DPBS.
- Gently pipette the suspension up and down to break up the pellet. Count cells using a hemocytometer.
- Centrifuge again as outlined in step 1.4.3. Aspirate supernatant and depending on the number of cells, add appropriate diluent to yield 3 x 106 cells into a volume of 50 µl. The diluent will be a mixture of serum free media and high concentration basement matrix membrane in a 1:1 (v/v) ratio.
- Vortex the suspension gently and keep on ice at all times. The suspension is now ready for injection.
- Surgical Implantation Note: Ensure that all surgical materials and instruments are sterile. Practice aseptic techniques at all times.
- Place a heating pad on the table and cover with a sterile drape. Set up the anesthesia machine and ensure all the supplies are within arm's reach.
- Place the animal's snout into the anesthesia mask and set up the anesthesia to 1 L/min of oxygen and 2.5% isoflurane.
- Gently scrub the flank of the animal with iodine, and rinse with 70% ethanol. Repeat three times.
- Confirm the animal is fully anesthetized by pinching the hind paw. The animal is fully anesthetized and ready for surgery if no response is observed. However, if the animal flinches, ensure there is sufficient anesthesia in the vaporizer and allow the animal more time to go completely under anesthesia.
- Load approximately 200 µl of the cell suspension into a 1.0 ml TB syringe with an 18 G needle (previously cooled). Replace the needle with a 27 G needle and return to ice until ready to use.
- Locate the general area of the spleen (left upper quadrant of the abdomen) and using forceps pinch the skin on top of that region. Using surgical scissors make an incision of approximately 1.0 cm to create a pocket. Similarly, pinch the smooth muscle on top of the spleen and cut through in order to access the peritoneal cavity.
- Gently grab the caudal end of the spleen and pull it out of the body. The pancreas will be attached to the spleen. Spread the pancreas using a wet sterile Q-tip and locate the tail of the pancreas.
- Deliver the 50 µl injection into the tail of pancreas, leave the needle inside for 10 sec and slowly rotate the needle out of the pancreas. A successful implantation will look like a superficial bubble without any leaks.
- Return the pancreas and spleen to the peritoneal cavity. First enclose the muscle and then enclose the skin separately. Use a 6-0 suture or staple to close the incision. To avoid pain in the animal, administer ketoprofen subcutaneously (SC) (5 mg/kg) over 24 hr. Alternatively, administer buprenorphine sc (0.05 - 0.1 mg/kg) every 12 hr over a 36 hr period.
- Recover the animal from anesthesia and return it to its cage. Also monitor for pain. Animals must be provided with pain relief at the time of (or even prior to — pre-emptive) surgery. Additional pain relief must be provided to animals that experience pain according to IACCUC-protocol or as prescribed by the attending veterinarian or designee.
- In vivo Imaging Note: Image capture was performed using a commercial imaging system equipped with a dark chamber and the appropriate filters for GFP imaging. Image acquisition was accomplished using a CCD camera and an optical system consisting of interchangeable excitation/emission lenses (excitation: 455 - 495 nm; emission: 513 - 557 nm). Bright field images were captured without a filter at 1 x 1 binning for each time point. Excitation of GFP utilized a xenon multispectral light source. Animals were maintained under anesthesia throughout the imaging process by connecting the anesthesia machine to a gas anesthesia manifold integrated into the dark chamber of the imaging system.
- Anesthetize the animal as outlined in 1.5.2. An anesthesia mask is in the interior of the commercial imaging system's dark chamber in order to keep the animal under anesthesia during the imaging process.
- Set up the correct excitation and emission filters.
- Begin by obtaining an initial image using white light only. Keep the same position of the animal throughout the imaging session and switch to GFP filters to take a second image.
- For best results superimpose both images. Analyze the image for fluorescent area and intensity.
Representative Results
This method describes a surgical orthotopic implantation of fluorescent human pancreatic cancer cells, focusing on the preparation of the cell suspension for injection, proper anesthesia for rodents, delivery of cell suspension via laparotomy, and the use of fluorescent in vivo small animal imaging. The detection of a green fluorescence signal (GFP signal) between two and three weeks post-implantation, provides researchers a visual cue to confirm the presence of a developing pancreatic cancer tumor (Figure 1). Figure 1 consists of three images of a mouse with PANC-1 GFP fluorescent tumor. The first is taken under white light (Figure 1A); the second image is taken under blue fluorescent light (excitation: 455 - 495 nm; emission: 513 - 557 nm) to image the green fluorescence emitted from the PANC-1 pancreatic tumor (Figure 1B); the third is a composite of the first two and shows the location of the tumor within the body of the mouse (Figure 1C). Animals which do not develop tumors do not show GFP signal (Figure 2). Furthermore, representative images of tumor progression over time may be non-invasively monitored, by recording the GFP signal at different time points (Figure 3). Figure 3 shows several composites of GFP signals over time. As the time progresses and the tumor size increases, the GFP signal increases. Twenty days after the implantation, the tumor appears as a small green dot, and 50 days after implantation, the tumor size increases significantly. Figure 4A shows metastases to the spleen, liver, and gastrointestinal tract, which can be confirmed after the animal has been euthanized and the organs are removed for ex vivo fluorescent imaging (Figure 4B).
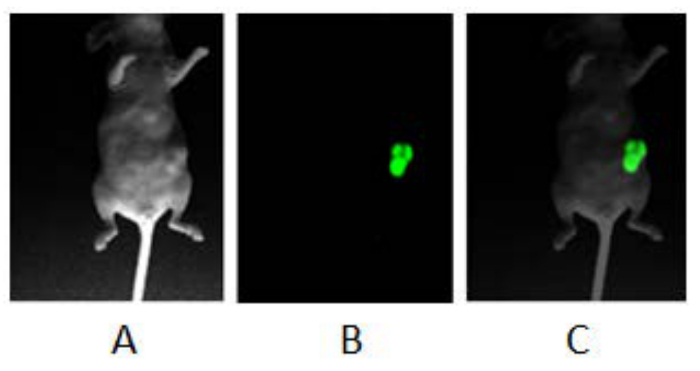 Figure 1: Fluorescent Imaging of Orthotopic Pancreatic Tumor. Balb/c nude mouse was anesthetized with isoflurane and a series of images were obtained using a fluorescent in vivo imaging system: (A) Image was obtained with white light and no filter. (B) Image was obtained using blue light and specific filters for GFP. (C) A composite image of A and B. Please click here to view a larger version of this figure.
Figure 1: Fluorescent Imaging of Orthotopic Pancreatic Tumor. Balb/c nude mouse was anesthetized with isoflurane and a series of images were obtained using a fluorescent in vivo imaging system: (A) Image was obtained with white light and no filter. (B) Image was obtained using blue light and specific filters for GFP. (C) A composite image of A and B. Please click here to view a larger version of this figure.
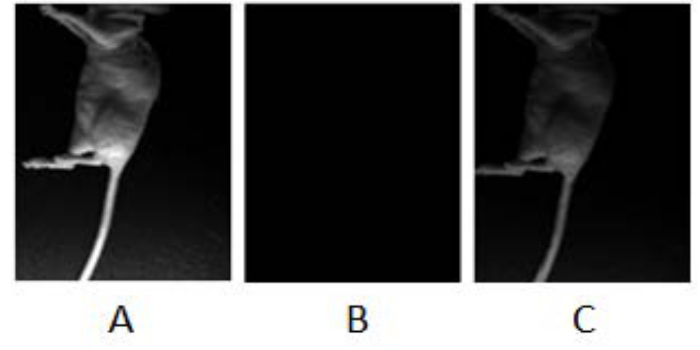 Figure 2: Implantation Troubleshooting, Lack of Fluorescence. Representation of a mouse at a time where the tumor had not yet developed: (A) Image was obtained with white light and no filter. (B) Image was obtained using blue light and specific filters for GFP. (C) A composite image of A and B. Please click here to view a larger version of this figure.
Figure 2: Implantation Troubleshooting, Lack of Fluorescence. Representation of a mouse at a time where the tumor had not yet developed: (A) Image was obtained with white light and no filter. (B) Image was obtained using blue light and specific filters for GFP. (C) A composite image of A and B. Please click here to view a larger version of this figure.
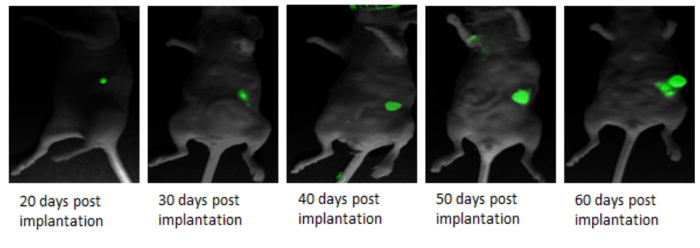 Figure 3:In vivo Real Time Fluorescent Imaging to Track Primary Tumor Growth.In vivo imaging of PANC-1 GFP pancreatic cancer progression at various time points post-implantation. Please click here to view a larger version of this figure.
Figure 3:In vivo Real Time Fluorescent Imaging to Track Primary Tumor Growth.In vivo imaging of PANC-1 GFP pancreatic cancer progression at various time points post-implantation. Please click here to view a larger version of this figure.
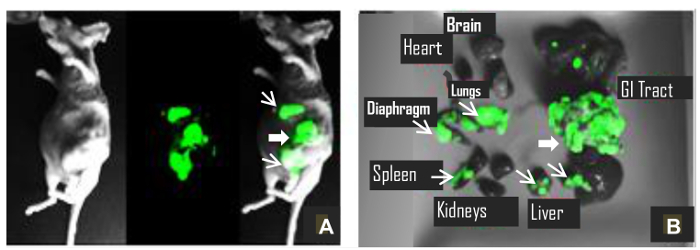 Figure 4:Evidence of Metastasis.(A) A mouse under anesthesia with metastatic tumors; and (B) its primary tumor and metastases ex vivo 100 days post-implantation. Thick arrow shows primary tumor, and thin arrows indicate metastatic tumors. Please click here to view a larger version of this figure.
Figure 4:Evidence of Metastasis.(A) A mouse under anesthesia with metastatic tumors; and (B) its primary tumor and metastases ex vivo 100 days post-implantation. Thick arrow shows primary tumor, and thin arrows indicate metastatic tumors. Please click here to view a larger version of this figure.
Discussion
We describe an orthotopic murine model of pancreatic cancer which expresses GFP, thus allowing non-invasive monitoring of tumor growth using whole body in vivo fluorescent imaging (Figure 1). This technique allows us to monitor the tumor development in real time (Figure 3); it can be an important tool for researchers to study the therapeutic efficacy of novel agents against pancreatic cancer. Another important aspect of this model is that GFP fluorescence provides a visual cue indicating successful implantation and growth of pancreatic cancer; which would otherwise be difficult to gauge in live animals. Consistent with the clinical setting, this model provides an insight on metastasis. It shows metastasis to the surrounding organs: spleen, mesenteric lymph nodes, liver, and gastrointestinal tract (Figure 4). We observed metastases as early as 7 weeks post transplantation. PANC-1 metastases have been reported in the range of 10 - 18 weeks11. The time at which metastasis is observed may depend on the number of implanted cells, implantation and visualization techniques. In the current study, we chose a green fluorescent cell line, PANC-1 GFP, which is commercially available. The model described here is reproducible and the metastases can be easily visualized because they have bright fluorescence. A limitation of xenograft nude mouse models produced from established cancer cell lines is the possibility of reduced tumor heterogeneity compared to an original human tumor12; nevertheless the model is reproducible, easy to develop and follow its progress in live animals. Furthermore, xenograft mice models continue to be used widely in cancer research.
The fluorescently labeled cells must be pelletized and re-suspended in ice cold serum-free medium mixed with equal volume of ice-cold basement membrane 8. The cell suspension must be maintained on ice at all times to prevent gelling or solidification. It is important to load the syringes with an 18 gauge needle in order to avoid lysing the cells. If the cells are lysed, the cell suspension is rendered useless, and no tumor growth will be attained (Figure 2). Immediately post-injection, allow approximately 10 sec for the cell suspension to solidify prior to removing the needle from the injection site. The solidification properties of the suspension allowed us to inject the cells without leakage from the injection site. Leakage of cells can lead to metastasis as an artifact rather than from cell dissemination. We have optimized this orthotopic implantation of PANC-1 GFP cell line using 3 x 106 cells per 50 μl injection; other cell line implantations must be determined empirically. Higher volumes of injection up to 100 µl containing 500,000 cells have been reported in the literature12. The main optimization parameters taken into account were successful orthotopic tumor growth and positive tumor imaging via GFP fluorescence within three weeks post-implantation.
The tumor growth and development is not only affected by the number of cells injected, but also by the age of the mice used. We have used athymic nude mice and determined that using young mice between the ages of 6 to 8 weeks yields more reproducible results when compared to older mice. As these mice age, they begin to regain some immunity which may result in rejection of the human pancreatic cells. The imaging techniques described here are not limited to orthotopic use; they can also be used for heterotopic implantation of tumors. Care must be taken to avoid auto-fluorescence which may obscure the tumor GFP signal. Certain plastics used for anesthesia tubing may produce auto-fluorescent artifacts.
Fluorescent whole-body imaging enables rapid analysis of tumor growth and progression 13. A major advantage of using fluorescence is the ability to track cancer without the traditional cumbersome procedures of histopathological examination or immunohistochemistry 14. This model has bright fluorescence and enables image acquisition without the need of skin flaps or other manipulations. Fluorescence differs from bioluminescence in that it does not create light based on a chemical reaction; it simply absorbs light and reemits it at a lower frequency. Orthotopic xenograft models provide invaluable knowledge and understanding of pancreatic tumor biology which can be translated into novel therapies for human use.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank the Western University of Health Sciences for the Intramural Grant.
References
- Siegel R. Pancreatic Cancer Stats: American Cancer Society: Cancer Facts & Figures. 2014. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf.
- Smyth E, Cunningham D. In: Harrison's Principles of Internal Medicine. 19th edn. Kasper D, et al., editors. McGraw-Hill Education; 2015. [Google Scholar]
- Mahipal A, Frakes J, Hoffe S, Kim R. Management of borderline resectable pancreatic cancer. World J Gastrointest Oncol. 2015;7:241–249. doi: 10.4251/wjgo.v7.i10.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89:626–632. [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- Jiang YJ. Establishment of an orthotopic pancreatic cancer mouse model: cells suspended and injected in Matrigel. World J Gastroenterol. 2014;20:9476–9485. doi: 10.3748/wjg.v20.i28.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz A, Ripoll J. Advances in optical imaging for pharmacological studies. Front Pharmacol. 2015;6:189. doi: 10.3389/fphar.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corning. Corning Matrigel Matrix: Frequently Asked Questions. 2013. Available from: http://csmedia2.corning.com/LifeSciences/media/pdf/faq_DL_026_Corning_Matrigel_Matrix.pdf.
- Metildi CA, Kaushal S, Hoffman RM, Bouvet M. In vivo serial selection of human pancreatic cancer cells in orthotopic mouse models produces high metastatic variants irrespective of Kras status. J Surg Res. 2013;184:290–298. doi: 10.1016/j.jss.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MP. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MH. Survival efficacy of adjuvant cytosine-analogue CS-682 in a fluorescent orthotopic model of human pancreatic cancer. Cancer Res. 2004;64:1828–1833. doi: 10.1158/0008-5472.can-03-3350. [DOI] [PubMed] [Google Scholar]
- Bouvet M. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–1540. [PubMed] [Google Scholar]


