Abstract
Voltage Dependent Anion-selective Channel 2 (VDAC2) contributes to oxidative metabolism by sharing a role in solute transport across the outer mitochondrial membrane (OMM) with other isoforms of the VDAC family, VDAC1 and VDAC3. Recent studies revealed that VDAC2 also has a distinctive role in mediating sarcoplasmic reticulum to mitochondria local Ca2+ transport at least in cardiomyocytes, which is unlikely to be explained simply by the expression level of VDAC2. Furthermore, a strictly isoform-dependent VDAC2 function was revealed in the mitochondrial import and OMM-permeabilizing function of pro-apoptotic Bcl-2 family proteins, primarily Bak in many cell types. In addition, emerging evidence indicates a variety of other isoform-specific engagements for VDAC2. Since VDAC isoforms display 75% sequence similarity, the distinctive structure underlying VDAC2-specific functions is an intriguing problem. In this paper we summarize studies of VDAC2 structure and functions, which suggest a fundamental and exclusive role for VDAC2 in health and disease. This article is part of a Special Issue entitled: Mitochondrial Channels edited by Pierre Sonveaux, Pierre Maechler and Jean-Claude Martinou.
Keywords: VDAC2, Mitochondria, Sarcoplasmic reticulum, Calcium, Apoptosis, Bid, Bak, Bax
1. Introduction
To exchange solutes with the cytosol, mitochondria use the most abundant proteins of the outer membrane (OMM) called Voltage Dependent Anion-selective Channels (VDACs). These channels have a relatively wide pore with an internal diameter estimated at 2–4 nm [1–5] and capable of supporting the membrane transport of various substrates, metabolites such as ADP/ATP, NADH [6–9], both negatively and positively charged ions and even larger molecules up to 4–6 kDa [5,10].
VDACs represent a protein family consisting of three isoforms (VDAC1–3) with around 75% sequence similarity (Fig. 1A) and 32–34 kDa molecular weights [11]. VDACs are expressed in almost all mammalian tissues with VDAC3 as the oldest and least expressed which VDAC1/2 derived from it by duplication in around 350 million years (MY) ago and VDAC1 as the youngest and dominant isoform which diverged from VDAC2 in 300 MY ago [12–15]. The 3 isoforms can substitute for each other in the metabolite and ion flux functions and have long been recognized as central to oxidative metabolism [16], but recent studies indicate their broad and non-redundant engagement in a variety of cell functions.
Fig. 1.
Protein sequence of different VDAC isoforms. A) Protein sequence comparison using Clustal Omega [144] among different VDAC isoforms including VDAC1 (NP_003365.1), VDAC2 (1): VDAC2 isoform1 (NP_001171712.1), VDAC2 (2): VDAC2 isoform2 (NP_003366.2), VDAC3 (1): VDAC3 isoform1 (NP_005653.3). VDAC3 isoform 2 (NP_001129166.1, not shown) is similar to VDAC3 isoform 1 with an extra methionine in the position of amino acid 40 (see arrow). B) Protein sequence comparison of VDAC2 in different species including human (Homo sapiens): NP_003366.2, monkey (Macaca mulatta): NP_ 001244353.1, cow (Bos taurus): NP_776911.2, pig (Sus scrofa): NP_999534.1, rat (Rattus norvegicous): NP_112644.1, rabbit (Oryctolagus cuniculus): NP_001076187.1, mouse (Mus musculus): NP_035825.1, frog (Xenopus laevis): NP_001089399.1, fish (Danio rerio): NP_955879.1, and chicken (Gallus gallus): NP_990072.1. NP code protein: Protein sequences that have been curated from Refseq database (an open access database from NCBI: national center for biotechnology information). The sequence has been colored by conservation using Jalview software and 33 was the assigned number for threshold.
In 2003, VDAC2 came to attention because of its role in apoptosis and the lethal consequences of Vdac2 knockout in mouse embryos [17]. Since then, new clues to the isoform-specific dependence of mitochondrial apoptosis on VDAC2 have been presented and several apparently unrelated processes have been specifically linked to VDAC2. In this review, we summarize these findings and the isoform-specific structure that underlines the functional versatility of VDAC2.
2. Gene expression, protein abundance and distribution
Genes encoding various VDAC isoforms are located in different chromosomes in each species (Table 1). Human VDAC2 was first isolated by Blachly-Dyson et al. [18]. hVdac2 has 10 exons with 1 alternative exon that is located between exon 2 and 3, whereas both hvdac1 and 3 have nine exons. In hVDAC1 there are two alternative exons before and after exon 1 [19–21] (Table 1). Vdac3 has an internal 3 base ATG mini exon which results in insertion of methionine in position of 40 (Fig. 1A) [22]. The start codon in each isoform is located in exon 2 [20, 23]. A similar organization applies to the mVdac isoforms [24,25] although the longer product of alternative splicing for Vdac3 had been only reported only in human [26]. The more expressed form of hVdac2 is also without the alternative exon. The alternative exon in hVdac2 results in a 26-residue long extension in the N terminal of the protein (vs 11–12 residues without the alternative exon) (Fig. 1A). The cDNA of VDAC2 with a long N-terminal extension has been isolated from lymphocytes but the function of this variant is not clear [26]. Transcript prediction studies using expressed sequence tags (ESTs) and cDNA libraries have described 13–14 splice variants for hVdac2 (e.g. Aceview, http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/index.html [27] and Ensembl 83 database [28], Table 1), but the presence of these variants has not been validated. Furthermore, Vdac2 has multiple polyadenylation sites in the 3′-UTR region that enables the transcription machinery to generate transcripts with different length [11,23] that might also be a potential target for regulatory elements like miRNA [11]. Again, while 3 polyadenylation sites have been documented for Vdac2 and only 1 for other isoforms [11,25], multiple polyadenylation has been predicted for all 3 isoforms in Aceview, http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/index.html [27].
Table 1.
Genetic features of VDAC1-3 based on Aceview (a) and Ensemble (e) databases. Superscript a or e letters refer to Aceview or Ensemble databases. Numbers in columns 1 and 3 without any superscription had been annotated by both databases. Paralogues and orthologues had been annotated only in Ensemble and polyadenylation prediction had been predicted only by Aceview.
| Protein | Chromosome | Reconstructed splice variants (#) |
Predicted length (aa #) | Length of NP-coded protein (aa#) |
Paralogues (Ensemble) |
Orthologues (Ensemble) |
Polyadenylation prediction (AceView) |
|---|---|---|---|---|---|---|---|
| hVDACl | 5 | 18a, 7e | 283, 184, 351a, 320a, 315a, 258a, 250a, 155a,... | 283 | 2 | 79 | 11 |
| → hVDAC2 | 10 | 13a, 14e | 309, 294, 322a, 255a, 245a, 209a, 164a, 119a, 104a, 106a, 90a... 204e, 195e, 109e,... | 309, 294 | 2 | 80 | 10 |
| hVDAC3 | 8 | 10a, 12e | 184a, 194a, 152a, 95a, 63a, 58a, 52a, 283e, 134e, 100e, 284e, 108e, 57e, 165e, 159e, 63e | 283, 284 | 2 | 85 | 6 |
| mVDACl | 11 | 3a, 5e | 381a, 121a, 68a, 283e, 254e, 296e | 283 | 3 | 84 | 3 |
| → mVDAC2 | 14 | 11a, 9e | 295, 280a, 223a, 124a, 117a, 86a, 75a, 71a, 77a, 53a, 283e, 75e, 201e | 295 | 3 | 82 | 4 |
| mVDAC3 | 8 | 6a, 2e | 381a, 277a, 236a, 108a, 100a, 99a, 63a, 55a, 44a, 32a, 283e, 284e | 283 | 3 | 85 | 4 |
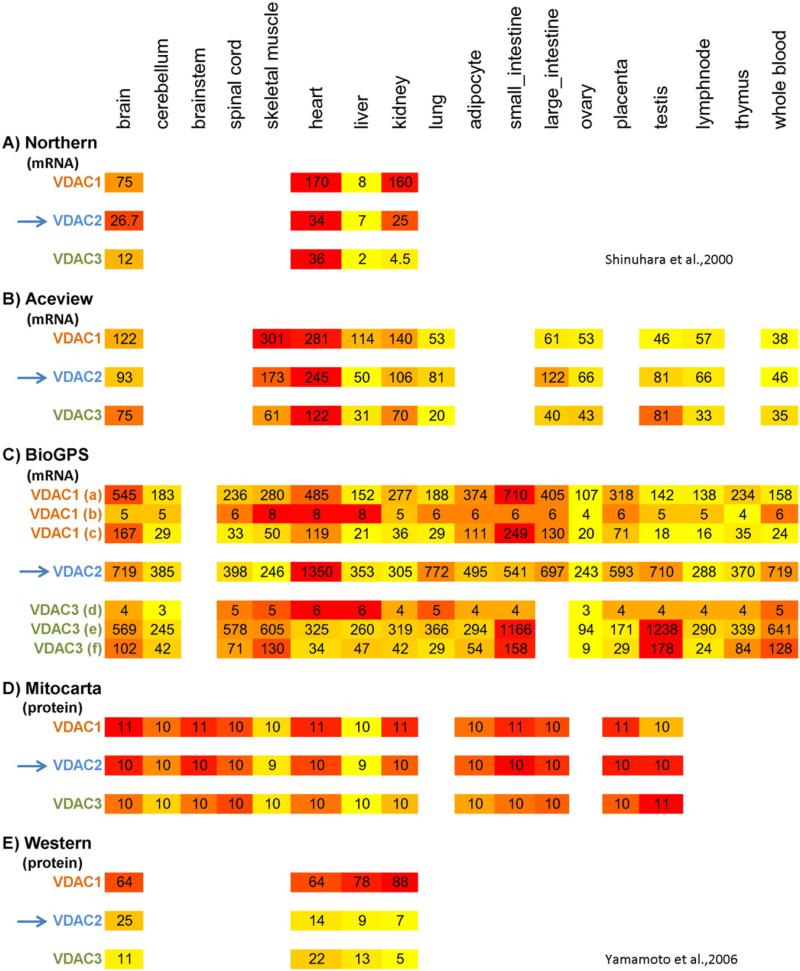
To check the tissue specific expression of VDACs, RT-PCR [18], Northern [24,29], Southern blot [13,30] and real time PCR have been used in different tissues (Fig. 2). Furthermore, high throughput data using microarray or mass spectrometry is available (Fig. 2). Northern blot analysis [24,29] shows that mVDAC2 is highly expressed in heart, kidney, skeletal muscle and brain and is the least expressed in the liver and lung. In addition, the mVdac2 mRNA level is high in the testis, where mVdac1 appears to be low [17].
Fig. 2.
High throughput data for hVDAC expression pattern in various tissues using different methods. A) RNA quantification from the brain, heart, liver and kidney using northern blot. The values are amol·μg−1/total RNA. B) Aceview: RNA-seq based gene expression profile in different tissues. Values are sFPKM, which stands for significant fragment per kilobase in one million read. C) BioGPS: tissue specific pattern of mRNA expression using customized Affymetrix high-density oligonucleotide biochip (dataset: GeneAtlas U133A). The probes used are 211662_s_at for VDAC2, 212038_s_at (a), 217139_at (b), 217140_s_at (c) for VDAC1 and 208845_ at (d), 208844_at (e), 208846_s_at (f) for VDAC3. The expression values are based on the fluorescence intensity and have been summarized using gcrma algorithms (https://www.bioconductor.org). Since each probe has different background characteristics, comparing the values from different probe sets is not recommended. In addition, it needs to be considered that one single probe might result in no similar result from study-to-study (comparing presented BioGPS data with information from coxpresdb database. (http://coxpresdb.jp). D) MitoCarta: Relative abundance of proteins reported in the MitoCarta Human database from different tissues. The values were determined by MS total peak intensity and can only be used to compare between tissues for a given protein. E) Western blot quantification from an antibody that recognizes all VDAC isoforms similarly. Therefore the levels of the different isoforms are comparable with each other. Values are expressed as the percentage of total VDACs in the mitochondria isolated from each tissue. Color intensity is set to darker color as higher number.
VDAC protein levels were predicted from analysis of mRNA in the Aceview [27] and BioGPS databases [31,32], and have also been estimated by analysis of mass spectrometry data (in human MitoCarta 2.0 [33]) (Fig. 2). These studies confirmed that the VDAC family is expressed in all tested tissues. VDAC1 and 2 proteins are highly enriched in heart and skeletal muscle and low in liver but in the MitoCarta skeletal muscle also showed low level of VDACs. VDAC1 and 2 are enriched in the small/large intestine (Fig. 2 B, C, D). Within rat small intestine, the duodenum shows the highest level of VDAC2 comparing with the level of this protein in ileum or jejunum [34]. VDAC2 and 3 are at high abundance in testis [13]. Immunoblotting confirmed the abundance of VDAC1/2 protein in the heart and skeletal muscle and their low amount in the liver [13,30].
In summary, the mRNA and protein measurements converge to support that VDAC2 is abundant in the heart and testis and low in the liver. Although the mitochondrial density is 2-fold higher in the heart than in liver, the difference in VDAC2 abundance also applies when it is measured in and normalized to isolated mitochondria [13]. In terms of the other tissues, the picture is less clear and some potentially important tissues are not included in the tissue surveys. For example, recent evidence indicated that VDAC2 might be important for the mitochondrial steps of steroid hormone production [35,36] and VDAC2 protein seems to be enriched in the testis and placenta but the steroid producing cells of the adrenal cortex have not been evaluated.
The intracellular residence of VDAC protein has been generally accepted to be in the OMM, but VDAC has also been reported in other cellular membranes like the plasma membrane [37–39]. However, a difficulty with the localization of the endogenous VDAC2 is that based on our experience, none of the commercial anti-VDAC2 antibodies are applicable for immunohistochemistry and pure membrane fractions are difficult to obtain by cell fractionation. In terms of distribution of VDAC2 in the mitochondrial population we have shown that protein import alone produces heterogeneity among individual mitochondria, and that mitochondrial fusion might be an essential mechanism for transferring VDAC2 protein among different mitochondria [40]. Superresolution microscopy of overexpressed VDACs indicated that VDAC1 and 2 form clusters, whereas VDAC3 distributes more homogenously [41]. A site of special interest in the distribution of VDAC2 is the OMM domain that is coupled to the sarco/endoplasmic reticulum (SR/ER) membrane, the mitochondria associated membrane (MAM) where the presence or enrichment of all VDAC isoforms has also been shown [42,43].
3. Structure
The 3 VDAC isoforms show approximately 75% sequence similarity in mammals (Fig. 1A). When the protein sequence of mammalian VDAC2 was compared to VDAC1 and VDAC3 isoforms, the comparison showed a unique 11–12 amino acid extension at the N terminal end of VDAC2 (Fig. 1A). This extension was a prime candidate to encode VDAC2-specific functions but it is not conserved in other chordates (Fig. 1B). Thus, the N terminal extension can only be important for some functions confined to mammals [44,45].
All amino acid residues are similarly frequent in VDAC2 and the other VDAC isoforms except cysteine (C), which is exclusively higher in mammalian VDAC2 (9 in hVDAC2 vs 2 in hVDAC1 and 6 in hVDAC3). Four of the C′s (47, 76, 103, 133) in VDAC2 are in the linkers between the membrane spanning domains, seemingly at the same side of the membrane. They might play a role in redox regulation, protein–protein interactions or might have an effect on the stability of β-barrels and on VDAC2 interaction with the lipid environment [46–48]. In line with these findings, C′s in VDAC3 have shown to be in different oxidation states and were implicated in the stability of the channel and in controlling reactive oxygen species (ROS) levels close to the OMM [49,50].
The membrane topology and 3D structure of the VDAC family, primarily VDAC1 have been studied using biochemical and biophysical methods (i.e. nuclear magnetic resonance and crystallography method) [4,51–55]. These studies uniformly suggested that VDACs are β-barrel forming transmembrane channels. Based on the biochemical studies, the β-barrel is composed of one-helix and 13 β-sheets with some relatively long loops between β-sheets. In this model, the α-helix is part of the wall of the channel. It had been proposed that the long loops might play a role in protein–protein interactions [56]. Differently, the biophysical studies showed a β-barrel formed by 19 β-sheets with the N-terminal α-helix lying inside the pore. The α-helix is highly flexible and is likely to be associated with VDAC channeling activity for the transfer of metabolites like ATP [55,57–60]. The literature about the orientation of the VDACs is divergent, however a recent work by De Pinto et al. showed that the C-terminal faces the intermembrane space [61]. The only crystallography study of VDAC2 used zebrafish VDAC2 (zfVDAC2) that has>90% sequence similarity to mammalian VDAC2 [62]. This study showed that there is significant similarity between the published structure of m/hVDAC1 and zfVDAC2. Specifically, zfVDAC2 is also recognized as a β-barrel consisting of 19 β-strands and one α-helix in the N-terminal. However there were some differences between VDAC1 and zfVDAC2 in rearrangement of the loops between β-sheets 1/2 and 5/6 [62]. In addition, in this study, a homodimer structure for zfVDAC2 was suggested [62]. Similarity between hVDAC2 and hVDAC1 was supported by solid-state NMR studies, though more conformational heterogeneity was shown by the VDAC2 structure [63].
The recently solved structure of zfVDAC2 provided a platform for modeling mammalian VDAC2, including the effect of specific mutations. Furthermore, the alignment of the crystal structure of VDAC2 with the crystal structure of mVDAC1 (2.3 Å) [52] or hVDAC1 (4.1 Å) [51] offered an opportunity to evaluate the isoform-specific structural differences. We have used this approach recently to predict how the essential residues identified by systematic mutagenesis contribute to a potential protein–protein interaction of VDAC2 involved in the recruitment of Bak to the OMM [64]. Comparison of the superimposed structures of zfVDAC2 and mVDAC1 showed that N156 and E158 in VDAC1 form a closed area, whereas S156 and D158 in zfVDAC2 form the edge of an open “cup” (Fig. 3). The wider space created by the side chains of S156 and D158 might favor protein–protein interaction (Fig. 3). We assume these spatial differences between the VDAC1 and VDAC2 isoforms could contribute to efficient Bak recruitment and Bak-dependent cell death through exclusively the VDAC2 isoform.
Fig. 3. VDAC2 specific 3D structure potentially relevant for Bak recruitment.
A,B) Side and top view of zfV2 (PDB code: 4bum) illustrated using Pymol. Pink is the sufficient segment and yellow (sticks) is the necessary residues including S156 and D158. C) Upper Right: Side view of the segment containing amino acids S156 and D158 (yellow sticks) in zfV2 versus the relevant residues in mV1 (N156 and E158 orange sticks). Residues 111-167 in zfV2 (blue) and mV1 (PDB code: 3emn, orange) were superimposed. Upper Left: Interior view of the channels showing the residues,which are within 4Å distance from either S156 or D158 in zfV2 (sticks with carboncolored blue) or N156 or E158 in V1 (sticks with carbon colored orange). In sticks, oxygen is red and nitrogen is green. Lower panel: The adjacent residues have also been marked in the sequences of zfV2 (blue) and mV1 (orange). Reproduced with from Ref 60, by permission of PNAS.
4. Interaction partners
In addition to IP3R/RyR, several other interaction partners have been reported for VDACs, which imply their effect in multiple cellular functions (Fig. 4). Specifically VDAC2 has been linked to many cellular proteins, including Bak [17,64,65], stAR [35], Metaxin2 [66], Bcl-xS [67], RyR2 [68], eNOS (nitric oxide synthesize) [69], GSK3β [70], tubulin [71,72], Bax [73], BECN1-BCL2L1 [74] and Mcl1 [75]. Also, VP5, a viral protein from infectious bursal disease virus, has been demonstrated to interact with VDAC2 and Rack1 (receptor of activated protein kinase C1) in host cells [76,77]. Lastly, VDAC2 was identified as a target of chemicals, Erastin [78], an anti-tumor reagent and Efsevin [79]. Several partners are promiscuous and interact with multiple VDAC isoforms but some like Bak seem to be exclusive for VDAC2 and thus, assign new isoform-specific functions to VDAC2 [65].
Fig. 4.
Scheme of VDAC2 various functions/interaction partners. mtCU: Mitochondrial Calcium Uniporter, PTP: permeability transition pore, mtx: Metaxin.
5. Function
The primary attention to VDAC was because of its role in transferring solutes across the OMM. This function is central to mitochondrial bioenergetics and cell metabolism and is shared among the 3 different VDAC isoforms. Later on, studies showed that in spite of their high similarity, each isoform is involved in different functions. While Vdac1, Vdac3 or double knockout of Vdac1/Vdac3 mice displayed mild phenotypes [80–82], whole-body knockout of Vdac2 is not compatible with life, because embryos with homozygous Vdac2 die during development [17]. However, conditional heart and thymus Vdac2 knockout mice had been generated [11,30]. The heart-specific Vdac2 knockout displayed cardiomyopathy and dysfunction of apoptosis pathways, which culminated in early death [11]. Thus, VDAC2 has non-redundant fundamental functions in development and survival.
6. VDAC2, channeling and Ca2+ homeostasis
Channel properties of VDAC have been investigated by extraction and purification of overexpressed or endogenous protein and insertion into phospholipid bilayer [9,56,83,84]. VDAC commonly appears as a large channel that at low potentials (<30 mV) is mostly fully open (~4.5 nS conductance in 1 M KCl) and weakly anion selective, whereas at high potentials, switches to cationic selectivity and is closed to approximately half of the original conductance (classical “closed” states) [85]. In the case of VDAC2, several species including human, mouse and bovine have been studied [18,44,63,86–88]. H/mVDAC2 have been expressed in yeast, purified and characterized by insertion into lipid bilayers [18,86]. Like VDAC1, h/mVDAC2 easily inserted into the lipid bilayer and showed voltage dependency but in the study of hVDAC2, the voltage dependency was lost soon after insertion [18]. In these studies, h/mVDAC2 also showed a lower conductance with lower ion selectivity [18,86]. Based on a broader histogram of conductance for insertion events, two separate VDAC2 channel populations were hypothesized, which are not switchable to each other [86]. Bovine VDAC2 isolated from spermatozoa and inserted in a lipid bilayer showed similar voltage dependency and conductance that likely reflects two different populations [87]. However, various VDAC isoforms isolated from double knockdown human hepatoblastoma HepG2 cells and reconstituted into lipid bilayers showed similar conductance and voltage gating properties for each isoform [88]. In hVDAC2 as in VDAC1, the N-terminal helix is necessary for voltage gating and it doesn't affect ion selectivity of the channel [63]. Furthermore, based on studies of recombinant hVdac2(Δ1–12) a role for the N-terminal in sensitization of the channel to voltage gating was proposed [44]. Overall, these studies support that VDAC2 displays fundamentally similar channel forming properties to VDAC1 in terms of conductance and voltage dependence though some differences appear at least upon insertion of the channel into lipid bilayers [63].
Ca2+ is one of the cations that engage VDACs to traverse the OMM. Mitochondria play a major role in Ca2+ homeostasis and buffering in eukaryotic cells and mitochondrial Ca2+ uptake contributes to the control of oxidative metabolism and cell death induction. While a lot of attention has been paid to the molecular and functional mechanism of the machinery of mitochondrial Ca2+ uptake through the inner mitochondrial membrane (IMM), the OMM was not thought to limit Ca2+ transfer due to the abundance of VDAC. However, during IP3 receptor (IP3R)-mediated [Ca2+] transients, the mitochondrial Ca2+ uptake appeared to be limited at the OMM level, by the availability of VDAC [89,90]. In addition to providing a Ca2+ permeation pathway [85,91] VDAC seems to form a complex with the endoplasmic reticulum (ER) Ca2+ release channel IP3Rs, which might provide a local “highway” for Ca2+ transfer from endoplasmic reticulum (ER) to the mitochondria [92]. Indeed, a recent publication in Hela cells showed that Ca2+ transfer to the mitochondria stimulated by an IP3-linked agonist, histamine was attenuated when either VDAC2 or other VDAC isoforms were silenced but only VDAC1 was found in co-immunoprecipitates with IP3R receptors and was found to be relevant for H2O2-induced (1) Ca2+ transfer to the mitochondria, and (2) cell death [93]. Differently, in cardiac cells a distinctive role for VDAC2 in transfer of Ca2+ from sarcoplasmic reticulum (SR) to the mitochondria was indicated by several lines of evidence (Fig 4). In the HL1 autorythmic adult mouse atrial cell line, VDAC2 is highly expressed and upon its silencing, the ryanodine receptor 2 (RyR2)-mediated cytoplasmic [Ca2+] spikes became larger and prolonged, whereas the mitochondrial [Ca2+] rise was delayed [94]. In cardiomyocytes, co-localization and co-immunoprecipitation studies indicated an interaction between RyR2 and VDAC2, and VDAC2 knockdown impaired mitochondrial Ca2+ uptake upon RyR2-mediated Ca2+ release from SR without suppressing SR Ca2+ release [68]. In addition, activation of VDAC2 through overexpression or Efsevin treatment was found to restore rhythmic contractions in Na+/Ca2+ exchanger (NCX) deficient zebrafish hearts and to effectively suppress Ca2+ overload-induced arrhythmogenic Ca2+ events and irregular contractions in mouse and human cardiomyocytes. Potentiating VDAC2 activity by Efsevin caused enhancement of mitochondrial Ca2+ uptake, acceleration of Ca2+ transfer from intracellular stores into mitochondria and spatial and temporal restriction of Ca2+ sparks in cardiomyocytes [79]. While the specific relevance of VDAC2 these results impressively come together to support that VDAC2 is required to locally couple mitochondrial Ca2+ uptake with RyR2-mediated Ca2+ release in the heart, providing a mechanism that protects the regular Ca2+ cycling and contractile activity.
7. VDAC2 and apoptosis
Outer Mitochondrial Membrane Permeabilization (OMMP) is a critical step in intrinsic and most extrinsic pathways of cell death. Following OMMP, mitochondrial intermembrane space resident proteins like cytochrome c, AIF, SMAC/DIABLO are released into the cytosol where they activate downstream apoptosis pathways like caspase 3/9 and endonucleases to execute the cell [95,96].
The OMMP and other components of apoptosis are governed by Bcl-2 family proteins [96,97]. The family has been classified on the base of conserved BH1–4 domains and includes pro-and anti-apoptotic members. The anti-apoptotic members interact with pro-apoptotic ones and keep them in an inactive status. Activation and oligomerization in the OMM of pro-apoptotic proteins which contain BH1–3, i.e. Bak (Bcl-2 associated killer protein) [98,99] and/or Bax (Bcl-2 associated × protein) result in OMMP [100]. While Bak resides constantly in the OMM, Bax primarily distributes in the cytosol in resting conditions and translocates to the OMM upon activation [101,102]. Members of the third group (like Bid) have only the BH3 domain (BH3 only proteins) and act as “second messengers” for external/internal death stimuli [103,104]. They usually migrate to the OMM after generation/activation and promote cell death by directly activating Bak/Bax proteins and/or relieving the inhibitory effect of anti-apoptotic ones [103]. In both cases Bak/Bax undergo a conformational change and oligomerization followed by OMMP. The stoichiometry of anti- versus pro-apoptotic Bcl-2 family members [105] and the interaction of this group of proteins with other proteins like VDAC1/2 decide on survival versus mitochondrial dependent cell death. For instance, Bcl-xL interacts with VDAC1 to inhibit apoptosis promoted by mitochondrial Ca2+ uptake [106,107]. Bcl-2 has also been shown to interact with VDAC1 through its N- terminal and inhibit cytochrome c release and apoptosis [108, 109]. Other studies showed that VDAC1 interacts with Bax to exert a pro-apoptotic effect [110,111]. Notably, the apoptotic involvements of VDACs are also affected by other interactors. For example, Hexokinase 1/2 has been established to exert an anti-apoptotic effect via interaction with VDAC [109]. Some literature has specified VDAC1 as the target for hexokinase [112] but recently hexokinase 2 antiapoptotic activity was also linked to VDAC2/3 [113].
As to VDAC2, an unexpected connection with Bak and Bax has emerged in the past decade (Fig. 4). Like many other Bcl-2 family proteins, Bak and Bax have a C-terminal targeting sequence but their OMM receptor (if there is any) and insertion mechanism was unclear [114]. Studies from the group of Mihara provided evidence that import of Bak to the OMM was dependent on the presence of VDAC2, while importing only the targeting sequence of Bak was independent of VDAC2 [115]. They hypothesized that the targeting sequence of Bak was embedded inside of the molecule and interaction with VDAC2 exposed it to potentiate Bak integration into the OMM [115]. Subsequently, we showed that Bak inserted to the OMM only in cells containing Vdac2, and Vdac1 and/or Vdac3 were dispensable. Rescuing of Vdac2-KO cells with Ad-Vdac2 increased the expression and recruitment of Bak to OMM [64,65]. Thus, VDAC2 seems to be responsible for the permanent localization of Bak in the OMM. The role of VDAC2 in Bak recruitment to the OMM was confirmed by the Dewson and Ryan labs [73,116]. These groups also reported that VDAC2 is involved in Bax recruitment but is not critical for either Bak or Bax recruitment [73].
While the effect of VDAC2 in recruitment of Bak seems to be widely accepted, VDAC2's role as a promoter or inhibitor of death is under debate [17,65,73,115–117]. In 2003, Cheng and coworkers [17] found that VDAC2 was associated with monomeric Bak protein in a large complex in the OMM and hampered Bak activation. In resting condition, VDAC2 and Bak were in the same complex but in the presence of death stimuli, this complex was not detectable. Using TNFα and Staurosporine, they showed higher cell death rate in mouse embryonic fibroblasts (MEFs) lacking Vdac2 [17]. Furthermore, in a conditional model of Vdac2-KO thymocytes, a higher level of death was recorded upon T cell receptor-dependent death stimulators. This phenotype was rescued by knocking out Bak [30]. By contrast, we found that truncated Bid (tBid; or FAS activation)-induced OMMP and cell death occurred in wild type, Vdac1-KO,Vdac3-KO or Vdac1/Vdac3-DKO MEFs but was absent (or at least greatly delayed) in Vdac2-KO MEFs [65]. Rescuing of Vdac2-KO MEFs with Ad-Vdac2 increased Bak in the OMM and sensitized the cells to tBid (FAS)-induced cell death [64,65]. Thus, VDAC2 was central to FAS/tBid-mediated mitochondrial apoptosis in these paradigms. Also, the Dewson and Tsujimoto labs described that VDAC2 or Bak is required for Bax oligomerization to support apoptosis [73,118]. Notably, based on studies of Vdac1/Vdac3-DKO MEFS, in which Vdac2 was silenced, independence of mitochondrial cells death from the VDAC family was also reported [119]. However, in this model, VDAC2 was not completely eliminated and the residual fraction could be involved in control of the machinery of OMMP. To reconcile the apparently conflicting reports, one might speculate that VDAC2 is a pro-apoptotic factor due to its role in Bak (and Bax) insertion to the OMM but it might also suppress apoptosis by maintaining the inserted Bak in a protein complex that hinders Bak (Bax) oligomerization and OMMP. In the different paradigms used in the different studies, the relative importance of the opposing effects might be different and this might give rise to different outcomes [120].
To explore how VDAC2 supports Bak-mediated cell death we considered the unique amino acid motifs in the sequence of VDAC2 [64]. Our efforts were focused first on the unique N-terminus and high C content of VDAC2 but these candidates were proven unnecessary for Bak insertion to the OMM and tBid-induced OMMP. Then systematically, chimeric constructs of VDAC2 and VDAC1 were created and a 57 amino acids sequence of VDAC2 (123–179) was found to be sufficient (Fig. 3). Further studies showed that 2 residues, Thr168 and Asp170, were necessary for the recruitment of Bak to the OMM and cell death. Although the introduced amino acids were not strikingly different from the corresponding residues in VDAC1 isoform, as we discussed in the “structure” segment in this review, these two residues have distinct spatial positioning and this might increase the competency for interaction with either Bak or a third partner needed for Bak insertion [64].
In addition to the tBid-Bak pathway, VDAC2 has been implicated in another mitochondrial death mechanism triggered by hyper O-GlcNAcylation. Pallaniapan et al. showed that hyperglycosylation targets VDAC2 and causes cell death that is alleviated in the absence of VDAC2. However the exact role of VDAC2 and the possible involvement of Bak in this pathway is not known [121].
Other proteins have also been implicated in the VDAC2-Bak complex. For example, involvement of Metaxins (Mtx1 and 2), proteins form the mitochondrial sorting and assembly machinery (SAM) [66] and Bcl-xS [67], were implicated in the formation and/or dissociation of the Bak-VDAC2 complex. The interaction among VDAC2, Bak and Bid has been also studied recently by a computational approach [122], however the VDAC2 topology that was considered seems to be incorrect.
8. VDAC2 and mPTP
VDAC was originally proposed as the pore-forming component of the mitochondrial permeability transition pores (mPTP) but subsequent studies validated VDACs as a regulator of the pore formed by IMM proteins [123]. Opening of the mPTP and subsequent corruption of ATP production and permeabilization/disruption of the OMM have been involved in different forms of cell death including necrosis and apoptosis. Mitochondrial matrix Ca2+ and ROS are among the most effective inducers of the mPTP. VDAC2 is central to mitochondrial Ca2+ uptake at least in the heart [68,79,94] but little is known about its isoform-specific involvement in mPTP opening. Under oxidative stress, VDAC2 was reported to mediate glycogen synthase kinase 3β (GSK3β) translocation to OMM and to promote mPTP formation to result in cell death [70]. GSK3β is a multi-task Ser/Thr kinase involved in the regulation of a various cell function. It promotes oxidative stress-dependent mPTP formation by manipulating mitochondrial bioenergetics, ROS production and VDAC2 function [70,124].
9. VDAC2 and steroidogenesis
Cholesterol is the substrate for steroid hormones and it needs to be transported from ER to IMM for the first step of steroidogenesis that takes place in the IMM. The transport of cholesterol in a hydrophilic environment is facilitated by the steroidogenic acute regulatory protein (stAR). Recent evidence suggests that stAR that is originally synthesized in the cytosol interacts with VDAC2 in the MAM. For this interaction to occur amino acid residues 221–229 and 277–282 in VDAC2 seem to be essential. VDAC2 regulates stAR processing to a mature protein that is needed to escort cholesterol from the OMM to the mitochondrial matrix [35]. An earlier study showed that sigma-1 receptor which is involved in cholesterol transfer in MAM also associated with VDAC2 [36].
10. VDAC2's other functions
Scattered studies indicate additional roles for VDAC2. Positive regulation of cilia/flagella assembly in centrosomes/basal bodies and centriolar satellites has been ascribed to VDAC2 [125]. In pulmonary artery endothelial cells (PAEC), both VDAC1 and 2 were shown to interact with eNOS (nitric oxide synthase), VDAC2 had a higher expression level than other isoforms, and upon VDAC2 silencing eNOS could not be stimulated by calcium [69].
In Pink/Parkin dependent mitophagy VDAC1–3 have been implicated in recruiting Parkin to the OMM [126]. Furthermore, folliculogenesis in the mammalian ovary depends on autophagy that was shown to be inhibited by VDAC2. Specifically, VDAC2 interacts with and stabilizes the complex of BECN1 and Bcl2L1 to suppress BECN1-dependent autophagy [74].
VP5, a viral protein from infectious bursal disease virus, has been reported to interact with VDAC2 and Rack1 (receptor of activated protein kinase C1) in host cells. This protein was suggested to support apoptosis induction in host cells by restraining Rack1, which is an antiapoptotic protein, and VDAC2. However, the role of VDAC2 in this context is not clear [76,77].
In addition, there are additional publications that show changes in the VDAC2 level under patho/physiological conditions (Table 2), although the exact role of VDAC2 remains unclear. Alteration of this protein's abundance might be an indication of some specific involvements of VDAC2.
Table 2.
Physiological and pathophysiological conditions associated with altered VDAC2 abundance or posttranslational modification.
| Condition | Isoform ↓↑ or modified | Model/tissue (species) | Reference |
|---|---|---|---|
| Adipogenesis | VDAC2↑ | 3T3-L1 fibroblast (mouse) | [130] |
| Exercise after myocardial infarction | VDAC2↓ | Heart (rat) | [144] |
| Hyperglycemia | VDAC2↓,VDAC1↑ | INS1E pancreatic β cells (rat) | [145] |
| Iron deprivation | VDAC2↑ | K562 erythroleukemia cell (human) | [131] |
| Aging | VDAC2↑ | Skeletal muscle mitochondria (aged rat) | [132] |
| Hypoxia | Phosphorylation of VDAC1 and 2 | Brain fraction (guinea pig) | [133] |
| Hypoxia | Vdac2 gene↑ | Cerebral cortical neurons (mouse) | [146] |
| Temporal lobe epilepsy (TLE) | VDAC2↑ | Hippocampus (rat) | [143] |
| Cell model for ALS | VDAC2↓,VDAC1↓ | NSC34 motorneuron-like cells (mouse) | [136] |
| Alzheimer | VDAC2↑ in the temporal cortex,VDAC1↓ in the frontal cortex and thalamus | Brain (human) | [135] |
| Alzheimer | Hypernitrosylation in VDAC2 | Brain (human) | [134] |
| Carcinogen (2,3,7,8-tetrachlorodibenzo-p-dioxin) | VDAC2↑ and modified | 5 L hepatoma cell line (rat) | [139] |
| Thyroid cancer | VDAC2↑ | Thyroid tumors and cell lines (human) | [137] |
| Human follicular adenoma | VDAC2↑ | Tumor tissue (human) | [138] |
| Meningioma | VDAC2↑ | Meningioma tissues (rat) | [140] |
11. Regulation of VDAC2 function
Since VDAC2 is involved in the regulation of cell survival/death balance, it would be sensible for this protein to be a target for modification/regulation in both normal and pathological circumstances. However, there is scarce literature about the regulation of VDAC2. At the level of gene expression, GATA1 and MYBL2 have been reported to bind to the Vdac2 promoter and to activate its transcription in the developing mammalian ovary [74].
At the level of the protein, Erastin [78], Efsevin [79] and free tubulin [88,127] have been proposed to bind and modulate VDAC2 activity mainly by interfering in the role of this protein as a gate for metabolites. Erastin is an anti-tumor agent with lethal activity on some cancer cells by perturbing metabolite transfer (e.g.NADH) across the OMM. The investigations show that the main substrate of Erastin is VDAC2 [78]. Free tubulin has also been described as a channel blocker for VDAC1 and 2 [88,127]. It has been postulated that in cancerous cells the level of free tubulin is high and thereby it keeps the VDAC in closed status favoring glycolytic metabolism [88,128]. Efsevin has been demonstrated to interact with VDAC2, activate this protein and enhance mitochondrial Ca2+ uptake [79].
Post-translational modification also might play a role in regulating VDAC2. Glycosylation (O-GlcNAc) of VDAC2 has been shown in MEFs [121]. In addition, many post-translational modifications have been reported based on proteomics databases such as phosphositeplus (http://www.phosphosite.org/) [129] but the relevance of these modifications in the regulation of VDAC2 function is unclear.
12. VDAC2 and diseases
In spite of increasing data about the contribution of VDAC2 in different signaling pathways there is still no record of any clinical case specific to this isoform. Nevertheless, many studies indicate that VDAC2 abundance alters in various pathological/physiological cases (Table 3). For instance in adipogenesis [130], iron deprivation [131] and aging [132] VDAC2 level increased and in hypoxia it decreased. Not only changes in the level of the protein but also post-translational modification like phosphorylation can be affected under various pathological conditions [133]. Interestingly VDAC seems to be associated to neurodegenerative diseases as well. In samples from Alzheimer patients, hypernitrosylation of VDAC2 [134] and alteration of both VDAC1 and VDAC2 [135] was described. ALS cell models show a reduction in both VDAC1 and VDAC2 level [136].
Table 3.
VDAC isoform mutations from cBioPortal (black color) and COSMIC (blue color) databases with the relevant tissues. A) VDAC1, B) VDAC2 and C) VDAC3. Numbers are on the base of NP_003366.2 for VDAC2, NP_003365.1 for VDAC1 and NP_005653.3 for VDAC3.
| A)VDAC1 | ||
|---|---|---|
| Sample/mutation ID | Cancer study | Amino acid change |
| TCGA-CG-5733-01 | Stomach | P5S |
| 587376 | Colorectal | E36D |
| B82 | Bladder | S44* |
| TCGA-GC-A3BM-01 | Bladder | S46L |
| TCGA-EK-A2PM-01 | Cervical | E50Q |
| TCGA-D8-A1XK-01 | Breast | Y67H |
| COSM4392060 | Prostate | T70M |
| TCGA-IB-7651-01 | Pancreas | T72A |
| TCGA-AP-A056-01 | Uterine | D78N |
| TCGA-97-7553-01 | Lung adeno | N111fs |
| TCGA-AP-A056-01 | Uterine | D132N |
| LUAD-NYU315 | Lung adeno | A141S |
| TCGA-66-2768-01 | Lung squ | E147K |
| TCGA-2L-AAQE-01 | Pancreas | A151V |
| 12-P616 | Ewing Sarcoma | R163Q |
| TCGA-EE-A29N-06 | Melanoma | Q179* |
| COSM4502395 | Skin | T204I |
| TCGA-BR-8284-01 | Stomach | W210R |
| TCGA-BR-8284-01 | Stomach | W210R |
| TCGA-FY-A3I5-01 | Thyroid | R218C |
| TCGA-FY-A3I5-01 | Thyroid | R218C |
| COSM1433129 | Large intestine | R218H |
| COSM1433129 | Pancreas | R218H |
| TCGA-AA-3977-01 | Colorectal | G220R |
| TCGA-AA-3977-01 | Colorectal | G220R |
| TCGA-A8-A0A6-01 | Breast | D228A |
| TCGA-CD-8527-01 | Stomach | V237M |
| TCGA-A2-A4S0-01 | Breast | L262H |
| B)VDAC2 | ||
|---|---|---|
| Sample/mutation ID | Cancer study | Amino acid change |
| COSM3397256 | Glioma | H4Y |
| COSM1603747 | Liver | R10L |
| TCGA-EE-A29S-06 | Melanoma | Y18C |
| TCGA-46-3767-01 | Lung squ | G36W |
| TCGA-09-2049-01 | Ovarian | D41G |
| COSM1349183 | Large intestine | K45del |
| Upper | ||
| COSM4887963 | aerodigestive | S53L |
| track | ||
| COSM5188427 | Large intestine | T60A |
| COSM3726959 | Skin | G93V |
| TCGA-DR-A0ZM-01 | Cervical | Q104* |
| TCGA-B6-A0RT-01 | Breast | L108P |
| COSM1349184 | Large intestine | S115del |
| TCGA-BH-A1ES-01 | Breast | S115T |
| MM18T | UCS | K120T |
| TCGA-AP-A054-01 | Uterine | S128del |
| SA103 | Breast | P147L |
| TCGA-DW-7838-01 | pRCC | A148V |
| TCGA-BL-A13I-01 | Bladder | N179T |
| COSM920566 | Endometrium | T186N |
| TCGA-B5-A0JY-01 | Uterine | E200* |
| BR-V-002 | Breast | S204* |
| TCGA-GD-A3OP-01 | Bladder | L213F |
| COSM4409230 | Esophagus | D214N |
| TCGA-AN-A0FD-01 | Breast | N218I |
| COSM1965440 | Large intestine | R229C |
| TCGA-AN-A046-01 | Breast | R229H |
| COSM465989 | Kidney | P264A |
| COSM238414 | Prostate | K267K |
| TCGA-BH-A0B6-01 | Breast | L273V |
| COSM1506139 | Lung | A281S |
| 587234 | Colorectal | G283C |
| COSM920568 | Endometrium | A289T |
| COSM920568 | Large intestine | A289T |
| TCGA-B5-A0JY-01 | Uterine | A289T |
| C)VDAC3 | ||
|---|---|---|
| Sample/mutation ID | Cancer study | Amino acid change |
| TCGA-BR-8361-01 | Stomach | V26I |
| COSM402989 | Lung | A47S |
| LUAD-5V8LT | Lung adeno | A48S |
| COSM5145633 | Large intestine | E59* |
| COSM5063216 | Stomach | A92T |
| TCGA-HT-7472-01 | Glioma | P105L |
| TCGA-P4-A5E7-01 | pRCC | K109N |
| 587222 | Colorectal | K110N |
| TCGA-AR-A5QP-01 | Breast | S117F |
| TCGA-18-3419-01 | Lung squ | R120L |
| COSM4740267 | Large intestine | G126D |
| COSM1100027 | Large intestine | E147K |
| TCGA-B5-A11N-01 | Uterine | E147K |
| ME011 | Melanoma | G148W |
| GC_307T-GC_307N | Stomach | W149G |
| COSM4151089 | Ovary | M155I |
| COSM4950650 | Liver | F157C |
| 9266942 | nccRCC | K163Nfs |
| TCGA-D1-A15X-01 | Uterine | Q166* |
| TCGA-HW-A5KK-01 | Glioma | A170V |
| ESCC-191T | Esophagus sq | A176S |
| TCGA-DJ-A2PY-01 | Thyroid | I202T |
| TCGA-EE-A181-06 | Melanoma | T204R |
| H072969 | Liver | R218S |
| TCGA-AA-3941-01 | Colorectal | K224E |
| TCGA-HU-A4H8-01 | Stomach | Y247H |
| 139 | Head & neck | A270V |
| pfg043T | Stomach | E280Dfs |
Del: deletion
nonsense mutation
fs: frameshift.
The changes in the VDAC2 quantity with mostly enhancement were observed in cancer samples or cell lines [29] (Table 2). Epithelial thyroid [137], follicular adenoma (in thyroid gland) [138], hepatocellular carcinoma [139] and some advanced grades of meningioma [140] are some examples in which VDAC2 shows higher abundance than normal tissue. Information from cancer databases like Oncomine (https://www.oncomine.org/resource/login.html), Cosmic (http://cancer.sanger.ac.uk/cosmic) [141] and cBioportal (http://www.cbioportal.org) [142] were in line with this concept. Moreover, these databases show that there are large numbers of cases where VDACs have been mutated in various tissues (Table 3). Whether these alterations are cause or effect of a pathological condition is an interesting dilemma that remains to be answered.
VDAC2 showed upregulation in a pilocarpine induced temporal lobe epilepsy (TLE) model in rats, suggesting VDAC2 as a biomarker of epilepsy [143]. Interaction of VDAC2 with endothelium-dependent nitric oxide synthase (eNOS) in pulmonary endothelium is important for eNOS activity. Insufficient function of this enzyme might cause a syndrome called pulmonary hypertension of the newborn (PPHN) [69]. Therefore, VDAC2 might be relevant to pathophysiology of pulmonary hypertension.
13. Concluding remarks
At the first sight, VDAC2 seems to be just another channel with a rigid structure that has been underestimated because of high similarity with other isoforms and lower expression level than VDAC1 protein. However, gradually using genetic targeting technology and in silico analyses, researchers have had the opportunity to investigate each isoform separately. Here we showed emerging evidence indicating that specifically VDAC2 has enormous potential to be involved in a variety of cellular processes. The specific roles of VDAC2 cannot be attributed to tissue specific enrichment and cannot be taken over by VDAC1 or VDAC3 isoforms. Interestingly, at least some specific roles of VDAC2 like Bak recruitment do not result from the most striking structural heterogeneity among the VDAC isoforms like the VDAC2-specific N-terminal tail or the distinctively high C content. Rather, subtle differences in the structure seem to be functionally relevant.
In many of the functions the exact role and mechanism of operation of the VDAC2 protein is not known. Thus, numerous questions remain to be addressed. For instance, how does VDAC2 orchestrate different functions such as channeling, recruiting, or promotion/inhibition of other proteins? Does each VDAC2 in a mitochondrion play specialized role or it can get involved in multiple functions? Is there any priority order for VDAC2 in contributing to different functions in each tissue? Does the interaction with one protein have an impact on the other roles of this protein? Does the interaction of VDAC2 with Bak affect the recruitment rate of other Bak proteins or other proteins to the OMM? Does VDAC2 enable the targeting of proteins to the mitochondria beyond Bak and stAR? In addition, since expression of VDAC2 protein is different in each tissue, it is interesting to know whether alteration in the stoichiometry of VDAC2 versus other interaction partners changes their function. The tissue-specific difference in VDAC2 expression is striking and stimulates thinking about its functional significance. Along this line, VDAC2 abundance is altered and VDAC2 mutations manifest in a variety of diseases. Therefore, the possible disease-causing or progression modifying roles of VDAC2 and its role as a potential drug target are expected to attract growing attention.
Supplementary Material
Acknowledgment
We would like to thank Dr. Erin Seifert for comments on the manuscript. This work was supported by NIH grant GM59419 to G.H.
Footnotes
This article is part of a Special Issue entitled: Mitochondrial Channels edited by Pierre Sonveaux, Pierre Maechler and Jean-Claude Martinou.
Transparency document
This Transparency document associated with this article can be found, in online version.
References
- 1.Dolder M, Zeth K, Tittmann P, Gross H, Welte W, Wallimann T. Crystallization of the human, mitochondrial voltage-dependent anion-selective channel in the presence of phospholipids. J. Struct. Biol. 1999;127:64–71. doi: 10.1006/jsbi.1999.4141. [DOI] [PubMed] [Google Scholar]
- 2.Guo XW, Mannella CA. Conformational change in the mitochondrial channel, VDAC, detected by electron cryo-microscopy. Biophys. J. 1993;64:545–549. doi: 10.1016/S0006-3495(93)81399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannella CA, Kinnally KW. Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator. J. Bioenerg. Biomembr. 2008;40:149–155. doi: 10.1007/s10863-008-9143-0. [DOI] [PubMed] [Google Scholar]
- 4.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombini M. Pore size and properties of channels from mitochondria isolated from Neurospora crassa. J. Membr. Biol. 1980;53:79–84. [Google Scholar]
- 6.Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. VDAC channels differentiate between natural metabolites and synthetic molecules. J. Membr. Biol. 2002;187:147–156. doi: 10.1007/s00232-001-0159-1. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien RL, Brierley G. Compartmentation of heart mitochondria. I. Permeability characteristics of isolated beef heart mitochondria. J. Biol. Chem. 1965;240:4527–4531. [PubMed] [Google Scholar]
- 8.Gellerich FN, Wagner M, Kapischke M, Wicker U, Brdiczka D. Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter-membrane space. Biochim. Biophys. Acta. 1993;1142:217–227. doi: 10.1016/0005-2728(93)90150-e. [DOI] [PubMed] [Google Scholar]
- 9.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim. Biophys. Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 10.Zalman LS, Nikaido H, Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J. Biol. Chem. 1980;255:1771–1774. [PubMed] [Google Scholar]
- 11.Raghavan A, Sheiko T, Graham BH, Craigen WJ. Voltage-dependant anion channels: novel insights into isoform function through genetic models. Biochim. Biophys. Acta. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young MJ, Bay DC, Hausner G, Court DA. The evolutionary history of mitochondrial porins. BMC Evol. Biol. 2007;7:31. doi: 10.1186/1471-2148-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yamauchi T, Kataoka M, Nagata T, Terada H, Shinohara Y. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J. Proteome Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- 14.De Pinto V, Guarino F, Guarnera A, Messina A, Reina S, Tomasello FM, Palermo V, Mazzoni C. Characterization of human VDAC isoforms: a peculiar function for VDAC3? Biochim. Biophys. Acta. 2010;1797:1268–1275. doi: 10.1016/j.bbabio.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Saccone C, Caggese C, D'Erchia AM, Lanave C, Oliva M, Pesole G. Molecular clock and gene function. J. Mol. Evol. 2003;57(Suppl. 1):S277–S285. doi: 10.1007/s00239-003-0037-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu MY, Colombini M. Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim. Biophys. Acta. 1992;1098:255–260. doi: 10.1016/s0005-2728(05)80344-5. [DOI] [PubMed] [Google Scholar]
- 17.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 18.Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, Colombini M, Forte M. Cloning and functional expression in yeast of two human iso-forms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J. Biol. Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- 19.Messina A, Guarino F, Oliva M, van den Heuvel LP, Smeitink J, De Pinto V. Characterization of the human porin isoform 1 (HVDAC1) gene by amplification on the whole human genome: a tool for porin deficiency analysis. Biochem. Biophys. Res. Commun. 2000;270:787–792. doi: 10.1006/bbrc.2000.2487. [DOI] [PubMed] [Google Scholar]
- 20.Messina A, Oliva M, Rosato C, Huizing M, Ruitenbeek W, van den Heuvel LP, Forte M, Rocchi M, De Pinto V. Mapping of the human voltage-dependent anion channel isoforms 1 and 2 reconsidered. Biochem. Biophys. Res. Commun. 1999;255:707–710. doi: 10.1006/bbrc.1998.0136. [DOI] [PubMed] [Google Scholar]
- 21.Rahmani Z, Maunoury C, Siddiqui A. Isolation of a novel human voltage-dependent anion channel gene. Eur. J. Hum. Genet. 1998;6:337–340. doi: 10.1038/sj.ejhg.5200198. [DOI] [PubMed] [Google Scholar]
- 22.Anflous K, Blondel O, Bernard A, Khrestchatisky M, Ventura-Clapier R. Characterization of rat porin isoforms: cloning of a cardiac type-3 variant encoding an additional methionine at its putative N-terminal region. Biochim. Biophys. Acta. 1998;1399:47–50. doi: 10.1016/s0167-4781(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 23.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Sampson MJ, Lovell RS, Craigen WJ. Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. Genomics. 1996;33:283–288. doi: 10.1006/geno.1996.0193. [DOI] [PubMed] [Google Scholar]
- 25.Sampson MJ, Lovell RS, Craigen WJ. The murine voltage-dependent anion channel gene family. Conserved structure and function. J. Biol. Chem. 1997;272:18966–18973. doi: 10.1074/jbc.272.30.18966. [DOI] [PubMed] [Google Scholar]
- 26.Ha H, Hajek P, Bedwell DM, Burrows PD. A mitochondrial porin cDNA predicts the existence of multiple human porins. J. Biol. Chem. 1993;268:12143–12149. [PubMed] [Google Scholar]
- 27.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7(Suppl. 1):11–14 (S12). doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Fitzgerald S, Gil L, Giron CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Keenan S, Lavidas I, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Nuhn M, Parker A, Patricio M, Pignatelli M, Rahtz M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Birney E, Harrow J, Muffato M, Perry E, Ruffier M, Spudich G, Trevanion SJ, Cunningham F, Aken BL, Zerbino DR, Flicek P. Ensembl 2016. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinohara Y, Ishida T, Hino M, Yamazaki N, Baba Y, Terada H. Characterization of porin isoforms expressed in tumor cells. Eur. J. Biochem. 2000;267:6067–6073. doi: 10.1046/j.1432-1327.2000.01687.x. [DOI] [PubMed] [Google Scholar]
- 30.Ren D, Kim H, Tu HC, Westergard TD, Fisher JK, Rubens JA, Korsmeyer SJ, Hsieh JJ, Cheng EH. The VDAC2-BAK rheostat controls thymocyte survival. Sci. Signal. 2009;2:ra48. doi: 10.1126/scisignal.2000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iiizumi G, Sadoya Y, Hino S, Shibuya N, Kawabata H. Proteomic characterization of the site-dependent functional difference in the rat small intestine. Biochim. Biophys. Acta. 2007;1774:1289–1298. doi: 10.1016/j.bbapap.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Prasad M, Kaur J, Pawlak KJ, Bose M, Whittal RM, Bose HS. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J. Biol. Chem. 2015;290:2604–2616. doi: 10.1074/jbc.M114.605808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marriott KS, Prasad M, Thapliyal V, Bose HS. Sigma-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. J. Pharmacol. Exp. Ther. 2012;343:578–586. doi: 10.1124/jpet.112.198168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bathori G, Parolini I, Tombola F, Szabo I, Messina A, Oliva M, De Pinto V, Lisanti M, Sargiacomo M, Zoratti M. Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae-related domains. J. Biol. Chem. 1999;274:29607–29612. doi: 10.1074/jbc.274.42.29607. [DOI] [PubMed] [Google Scholar]
- 38.Jakob C, Gotz H, Hellmann T, Hellmann KP, Reymann S, Florke H, Thinnes FP, Hilschmann N. Studies on human porin: XIII. The type-1 VDAC ‘porin 31HL’ biotinylated at the plasmalemma of trypan blue excluding human B lymphocytes. FEBS Lett. 1995;368:5–9. doi: 10.1016/0014-5793(95)00465-l. [DOI] [PubMed] [Google Scholar]
- 39.De Pinto V, Messina A, Lane DJ, Lawen A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010;584:1793–1799. doi: 10.1016/j.febslet.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 40.Weaver D, Eisner V, Liu X, Varnai P, Hunyady L, Gross A, Hajnoczky G. Distribution and apoptotic function of outer membrane proteins depend on mitochondrial fusion. Mol. Cell. 2014;54:870–878. doi: 10.1016/j.molcel.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann D, Buckers J, Kastrup L, Hell SW, Jakobs S. Two-color STED microscopy reveals different degrees of colocalization between hexokinase-I and the three human VDAC isoforms. PMC Biophys. 2010;3:4. doi: 10.1186/1757-5036-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoshan-Barmatz V, Zalk R, Gincel D, Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Du X, Deng J, Gu M, Hu H, Gui M, Yin CC, Chang Z. The interactions between mitochondria and sarcoplasmic reticulum and the proteome characterization of mitochondrion-associated membrane from rabbit skeletal muscle. Proteomics. 2015;15:2701–2704. doi: 10.1002/pmic.201400493. [DOI] [PubMed] [Google Scholar]
- 44.Maurya SR, Mahalakshmi R. N-helix and cysteines inter-regulate human mitochondrial VDAC-2 function and biochemistry. J. Biol. Chem. 2015;290:30240–30252. doi: 10.1074/jbc.M115.693978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reina S, Palermo V, Guarnera A, Guarino F, Messina A, Mazzoni C, De Pinto V. Swapping of the N-terminus of VDAC1 with VDAC3 restores full activity of the channel and confers anti-aging features to the cell. FEBS Lett. 2010;584:2837–2844. doi: 10.1016/j.febslet.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 46.Maurya SR, Mahalakshmi R. Modulation of human mitochondrial voltage-dependent anion channel 2 (hVDAC-2) structural stability by cysteine-assisted barrel-lipid interactions. J. Biol. Chem. 2013;288:25584–25592. doi: 10.1074/jbc.M113.493692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maurya SR, Mahalakshmi R. Cysteine residues impact the stability and micelle interaction dynamics of the human mitochondrial beta-barrel anion channel hVDAC-2. PLoS One. 2014;9:e92183. doi: 10.1371/journal.pone.0092183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maurya SR, Mahalakshmi R. VDAC-2: mitochondrial outer membrane regulator masquerading as a channel? FEBS J. 2015 doi: 10.1111/febs.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okazaki M, Kurabayashi K, Asanuma M, Saito Y, Dodo K, Sodeoka M. VDAC3 gating is activated by suppression of disulfide-bond formation between the N-terminal region and the bottom of the pore. Biochim. Biophys. Acta. 2015;1848:3188–3196. doi: 10.1016/j.bbamem.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Reina S, Checchetto V, Saletti R, Gupta A, Chaturvedi D, Guardiani C, Guarino F, Scorciapino MA, Magri A, Foti S, Ceccarelli M, Messina AA, Mahalakshmi R, Szabo I, De Pinto V. VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: the putative role of cysteine residue modifications. Oncotarget. 2016;7:2249–2268. doi: 10.18632/oncotarget.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blachly-Dyson E, Peng S, Colombini M, Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990;247:1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- 54.De Pinto V, Prezioso G, Thinnes F, Link TA, Palmieri F. Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry. 1991;30:10191–10200. doi: 10.1021/bi00106a017. [DOI] [PubMed] [Google Scholar]
- 55.Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. The topology of VDAC as probed by biotin modification. J. Biol. Chem. 1998;273:24406–24413. doi: 10.1074/jbc.273.38.24406. [DOI] [PubMed] [Google Scholar]
- 56.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004;256-257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 57.Noskov SY, Rostovtseva TK, Bezrukov SM. ATP transport through VDAC and the VDAC-tubulin complex probed by equilibrium and nonequilibrium MD simulations. Biochemistry. 2013;52:9246–9256. doi: 10.1021/bi4011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J. Affixing N-terminal alpha-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J. Biol. Chem. 2012;287:11437–11445. doi: 10.1074/jbc.M111.314229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popp B, Court DA, Benz R, Neupert W, Lill R. The role of the N and C termini of recombinant Neurospora mitochondrial porin in channel formation and voltage-dependent gating. J. Biol. Chem. 1996;271:13593–13599. doi: 10.1074/jbc.271.23.13593. [DOI] [PubMed] [Google Scholar]
- 60.Geula S, Ben-Hail D, Shoshan-Barmatz V. Structure-based analysis of VDAC1: N-terminus location, translocation, channel gating and association with anti-apoptotic proteins. Biochem. J. 2012;444:475–485. doi: 10.1042/BJ20112079. [DOI] [PubMed] [Google Scholar]
- 61.Tomasello MF, Guarino F, Reina S, Messina A, De Pinto V. The voltage-dependent anion selective channel 1 (VDAC1) topography in the mitochondrial outer membrane as detected in intact cell. PLoS One. 2013;8:e81522. doi: 10.1371/journal.pone.0081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, Chen JN, Hubbell WL, Abramson J. High resolution structure and double electron–electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J. Biol. Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gattin Z, Schneider R, Laukat Y, Giller K, Maier E, Zweckstetter M, Griesinger C, Benz R, Becker S, Lange A. Solid-state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J. Biomol. NMR. 2015;61:311–320. doi: 10.1007/s10858-014-9876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naghdi S, Varnai P, Hajnoczky G. Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E5590–E5599. doi: 10.1073/pnas.1510574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cartron PF, Petit E, Bellot G, Oliver L, Vallette FM. Metaxins 1 and 2, two proteins of the mitochondrial protein sorting and assembly machinery, are essential for Bak activation during TNF alpha triggered apoptosis. Cell. Signal. 2014;26:1928–1934. doi: 10.1016/j.cellsig.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 67.Plotz M, Gillissen B, Hossini AM, Daniel PT, Eberle J. Disruption of the VDAC2-Bak interaction by Bcl-x(S) mediates efficient induction of apoptosis in melanoma cells. Cell Death Differ. 2012;19:1928–1938. doi: 10.1038/cdd.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min CK, Yeom DR, Lee KE, Kwon HK, Kang M, Kim YS, Park ZY, Jeon H, Kim do H. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012;447:371–379. doi: 10.1042/BJ20120705. [DOI] [PubMed] [Google Scholar]
- 69.Alvira CM, Umesh A, Husted C, Ying L, Hou Y, Lyu SC, Nowak J, Cornfield DN. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am. J. Respir. Cell Mol. Biol. 2012;47:669–678. doi: 10.1165/rcmb.2011-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanno M, Kuno A, Ishikawa S, Miki T, Kouzu H, Yano T, Murase H, Tobisawa T, Ogasawara M, Horio Y, Miura T. Translocation of glycogen synthase kinase-3beta (GSK-3beta), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2) J. Biol. Chem. 2014;289:29285–29296. doi: 10.1074/jbc.M114.563924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rostovtseva TK, Gurnev PA, Chen MY, Bezrukov SM. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2012;287:29589–29598. doi: 10.1074/jbc.M112.378778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- 73.Ma SB, Nguyen TN, Tan I, Ninnis R, Iyer S, Stroud DA, Menard M, Kluck RM, Ryan MT, Dewson G. Bax targets mitochondria by distinct mechanisms before or during apoptotic cell death: a requirement for VDAC2 or Bak for efficient Bax apoptotic function. Cell Death Differ. 2014;21:1925–1935. doi: 10.1038/cdd.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan J, Zhang Y, Sheng Y, Fu X, Cheng H, Zhou R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy. 2015;11:1081–1098. doi: 10.1080/15548627.2015.1040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang H, Shah K, Bradbury NA, Li C, White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin W, Zhang Z, Xu Z, Wang B, Li X, Cao H, Wang Y, Zheng SJ. The association of receptor of activated protein kinase C 1(RACK1) with infectious bursal disease virus viral protein VP5 and voltage-dependent anion channel 2 (VDAC2) inhibits apoptosis and enhances viral replication. J. Biol. Chem. 2015;290:8500–8510. doi: 10.1074/jbc.M114.585687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Z, Wang Y, Xue Y, Li X, Cao H, Zheng SJ. Critical role for voltage-dependent anion channel 2 in infectious bursal disease virus-induced apoptosis in host cells via interaction with VP5. J. Virol. 2012;86:1328–1338. doi: 10.1128/JVI.06104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS–RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimizu H, Schredelseker J, Huang J, Lu K, Naghdi S, Lu F, Franklin S, Fiji HD, Wang K, Zhu H, Tian C, Lin B, Nakano H, Ehrlich A, Nakai J, Stieg AZ, Gimzewski JK, Nakano A, Goldhaber JI, Vondriska TM, Hajnoczky G, Kwon O, Chen JN. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. eLife. 2015;4 doi: 10.7554/eLife.04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 81.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, Craigen WJ. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 82.Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, Sweatt JD, Craigen WJ. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J. Biol. Chem. 2002;277:18891–18897. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- 83.Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit. Rev. Biochem. 1985;19:145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- 84.Colombini M. Regulation of the mitochondrial outer membrane channel, VDAC. J. Bioenerg. Biomembr. 1987;19:309–320. doi: 10.1007/BF00768534. [DOI] [PubMed] [Google Scholar]
- 85.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J. Biol. Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 86.Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J. Membr. Biol. 1999;170:89–102. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- 87.Menzel VA, Cassara MC, Benz R, de Pinto V, Messina A, Cunsolo V, Saletti R, Hinsch KD, Hinsch E. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci. Rep. 2009;29:351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- 88.Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J. Biol. Chem. 2013;288:11920–11929. doi: 10.1074/jbc.M112.433847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Csordas G, Madesh M, Antonsson B, Hajnoczky G. tcBid promotes Ca2+ signal propagation to the mitochondria: control of Ca2+ permeation through the outer mitochondrial membrane. EMBO J. 2002;21:2198–2206. doi: 10.1093/emboj/21.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szabadkai G, Bianchi K, Varnai P, MMOMMo D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subedi KP, Kim JC, Kang M, Son MJ, Kim YS, Woo SH. Voltage-dependent anion channel 2 modulates resting Ca(2)+ sparks, but not action potential-induced Ca(2)+ signaling in cardiac myocytes. Cell Calcium. 2011;49:136–143. doi: 10.1016/j.ceca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 96.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 97.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 98.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 99.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, Foster F, Tanianis-Hughes J, Brennan K, Streuli CH, Gilmore AP. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 103.Huang DC, Strasser A. BH3-only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 104.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chi X, Kale J, Leber B, Andrews DW. Regulating cell death at, on, and in membranes. Biochim. Biophys. Acta. 2014;1843:2100–2113. doi: 10.1016/j.bbamcr.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol. Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, Bultynck G. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J. Biol. Chem. 2015;290:9150–9161. doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arbel N, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J. Biol. Chem. 2010;285:6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 110.Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- 111.Shi Y, Chen J, Weng C, Chen R, Zheng Y, Chen Q, Tang H. Identification of the protein–protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003;305:989–996. doi: 10.1016/s0006-291x(03)00871-4. [DOI] [PubMed] [Google Scholar]
- 112.Anflous-Pharayra K, Cai ZJ, Craigen WJ. VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim. Biophys. Acta. 2007;1767:136–142. doi: 10.1016/j.bbabio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 113.McCommis KS, Baines CP. Abstract P060: VDAC2 and 3, but not VDAC1, are required for HK2-mediated protection against cell death. Circ. Res. 2011;109:AP060–AP060. [Google Scholar]
- 114.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria — spec-ificity in membrane targeting for death. Biochim. Biophys. Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Setoguchi K, Otera H, Mihara K. Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. The EMBO journal. 2006;25:5635–5647. doi: 10.1038/sj.emboj.7601438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, Kluck RM, Vaux DL, Ryan MT. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J. Biol. Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, Ryan MT, Kluck RM, Dewson G. Assembly of the Bak apoptotic pore: a critical role for the Bak protein alpha6 helix in the multimerization of homodimers during apoptosis. J. Biol. Chem. 2013;288:26027–26038. doi: 10.1074/jbc.M113.490094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamagata H, Shimizu S, Nishida Y, Watanabe Y, Craigen WJ, Tsujimoto Y. Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of Bax. Oncogene. 2009;28:3563–3572. doi: 10.1038/onc.2009.213. [DOI] [PubMed] [Google Scholar]
- 119.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shore GC. Apoptosis: it's BAK to VDAC. EMBO Rep. 2009;10:1311–1313. doi: 10.1038/embor.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palaniappan KK, Hangauer MJ, Smith TJ, Smart BP, Pitcher AA, Cheng EH, Bertozzi CR, Boyce M. A chemical glycoproteomics platform reveals O-GlcNAcylation of mitochondrial voltage-dependent anion channel 2. Cell Rep. 2013;5:546–552. doi: 10.1016/j.celrep.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Veresov VG, Davidovskii AI. Structural insights into proapoptotic signaling mediated by MTCH2, VDAC2, TOM40 and TOM22. Cell. Signal. 2014;26:370–382. doi: 10.1016/j.cellsig.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 123.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 124.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ. Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Majumder S, Cash A, Fisk HA. Non-overlapping distributions and functions of the VDAC family in ciliogenesis. Cells. 2015;4:331–353. doi: 10.3390/cells4030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L. Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 2012;287:40652–40660. doi: 10.1074/jbc.M112.419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maldonado EN, Lemasters JJ. Warburg revisited: regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J. Pharmacol. Exp. Ther. 2012;342:637–641. doi: 10.1124/jpet.112.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: Mutations, PTMs and Recalibrations. Nucleic Acids Research. 432015:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ye F, Zhang H, Yang YX, Hu HD, Sze SK, Meng W, Qian J, Ren H, Yang BL, Luo MY, Wu X, Zhu W, Cai WJ, Tong JB. Comparative proteome analysis of 3T3-L1 adipocyte differentiation using iTRAQ-coupled 2D LC–MS/MS. J. Cell. Biochem. 2011;112:3002–3014. doi: 10.1002/jcb.23223. [DOI] [PubMed] [Google Scholar]
- 131.Valis K, Neubauerova J, Man P, Pompach P, Vohradsky J, Kovar J. VDAC2 and aldolase A identified as membrane proteins of K562 cells with increased expression under iron deprivation. Mol. Cell. Biochem. 2008;311:225–231. doi: 10.1007/s11010-008-9712-x. [DOI] [PubMed] [Google Scholar]
- 132.O'Connell K, Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9:5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- 133.Liberatori S, Canas B, Tani C, Bini L, Buonocore G, Godovac-Zimmermann J, Mishra OP, Delivoria-Papadopoulos M, Bracci R, Pallini V. Proteomic approach to the identification of voltage-dependent anion channel protein isoforms in guinea pig brain synaptosomes. Proteomics. 2004;4:1335–1340. doi: 10.1002/pmic.200300734. [DOI] [PubMed] [Google Scholar]
- 134.Zahid S, Khan R, Oellerich M, Ahmed N, Asif AR. Differential S-nitrosylation of proteins in Alzheimer's disease. Neuroscience. 2014;256:126–136. doi: 10.1016/j.neuroscience.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 135.Yoo BC, Fountoulakis M, Cairns N, Lubec G. Changes of voltage-dependent anion-selective channel proteins VDAC1 and VDAC2 brain levels in patients with Alzheimer's disease and Down syndrome. Electrophoresis. 2001;22:172–179. doi: 10.1002/1522-2683(200101)22:1<172::AID-ELPS172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 136.Fukada K, Zhang F, Vien A, Cashman NR, Zhu H. Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis. Mol. Cell. Proteomics. 2004;3:1211–1223. doi: 10.1074/mcp.M400094-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mato E, Barcelo-Batllori S, Orera I, Selva L, Corra M, Gonzalez C, Bell O, Lerma E, Moral A, Perez JI, de Leiva A. The proteomic 2D-DIGE approach reveals the protein voltage-dependent anion channel 2 as a potential therapeutic target in epithelial thyroid tumours. Mol. Cell. Endocrinol. 2015;404:37–45. doi: 10.1016/j.mce.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 138.Lai X, Chen S. Identification of novel biomarker and therapeutic target candidates for diagnosis and treatment of follicular adenoma. Cancer Genomics Proteomics. 2015;12:271–281. [PubMed] [Google Scholar]
- 139.Sarioglu H, Brandner S, Haberger M, Jacobsen C, Lichtmannegger J, Wormke M, Andrae U. Analysis of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced proteome changes in 5 L rat hepatoma cells reveals novel targets of dioxin action including the mitochondrial apoptosis regulator VDAC2. Mol. Cell. Proteomics. 2008;7:394–410. doi: 10.1074/mcp.M700258-MCP200. [DOI] [PubMed] [Google Scholar]
- 140.Herrmann A, Ooi J, Launay S, Searcy JL, Deighton RF, McCulloch J, Whittle IR. Proteomic data in meningiomas: post-proteomic analysis can reveal novel patho-physiological pathways. J. Neuro-Oncol. 2011;104:401–410. doi: 10.1007/s11060-010-0526-9. [DOI] [PubMed] [Google Scholar]
- 141.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu XY, Yang JL, Chen LJ, Zhang Y, Yang ML, Wu YY, Li FQ, Tang MH, Liang SF, Wei YQ. Comparative proteomics and correlated signaling network of rat hippocampus in the pilocarpine model of temporal lobe epilepsy. Proteomics. 2008;8:582–603. doi: 10.1002/pmic.200700514. [DOI] [PubMed] [Google Scholar]
- 144.Bansal A, Dai Q, Chiao YA, Hakala KW, Zhang JQ, Weintraub ST, Lindsey ML. Proteomic analysis reveals late exercise effects on cardiac remodeling following myocardial infarction. J. Proteome. 2010;73:2041–2049. doi: 10.1016/j.jprot.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed M, Muhammed SJ, Kessler B, Salehi A. Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic beta-cells exposed to high glucose. Islets. 2010;2:283–292. doi: 10.4161/isl.2.5.12639. [DOI] [PubMed] [Google Scholar]
- 146.Jin K, Mao XO, Eshoo MW, del Rio G, Rao R, Chen D, Simon RP, Greenberg DA. cDNA microarray analysis of changes in gene expression induced by neuronal hypoxia in vitro. Neurochem. Res. 2002;27:1105–1112. doi: 10.1023/a:1020913123054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.