Abstract
Biosafety level 4 (BSL-4) suit laboratories are specifically designed to study high-consequence pathogens for which neither infection prophylaxes nor treatment options exist. The hallmarks of these laboratories are: custom-designed airtight doors, dedicated supply and exhaust airflow systems, a negative-pressure environment, and mandatory use of positive-pressure (“space”) suits. The risk for laboratory specialists working with highly pathogenic agents is minimized through rigorous training and adherence to stringent safety protocols and standard operating procedures. Researchers perform the majority of their work in BSL-2 laboratories and switch to BSL-4 suit laboratories when work with a high-consequence pathogen is required. Collaborators and scientists considering BSL-4 projects should be aware of the challenges associated with BSL-4 research both in terms of experimental technical limitations in BSL-4 laboratory space and the increased duration of such experiments. Tasks such as entering and exiting the BSL-4 suit laboratories are considerably more complex and time-consuming compared to BSL-2 and BSL-3 laboratories. The focus of this particular article is to address basic biosafety concerns and describe the entrance and exit procedures for the BSL-4 laboratory at the NIH/NIAID Integrated Research Facility at Fort Detrick. Such procedures include checking external systems that support the BSL-4 laboratory, and inspecting and donning positive-pressure suits, entering the laboratory, moving through air pressure-resistant doors, and connecting to air-supply hoses. We will also discuss moving within and exiting the BSL-4 suit laboratories, including using the chemical shower and removing and storing positive-pressure suits.
Keywords: Infection, Issue 116, Biosafety, biosafety level 4 suit laboratory, biosecurity, BSL4, BSL-4, positive pressure suit, maximum containment, high containment, personal protective equipment, PPE, basic protocol
Introduction
Scientific interest in exotic, high-consequence pathogens has steadily increased in recent years. Of particular interest are pathogens considered emerging or re-emerging agents that are potential bioweapons. In terms of research priority and risk, these pathogens are classified by the Centers for Disease Control and Prevention (CDC) as Bioterrorism Category A-C Agents 1. In addition, high-consequence pathogens are classified as Select Agents [and Toxins] in regard to import, export, and access regulations. In the US, the biosafety rules and procedures that must be followed for work with special pathogens that require BSL-2, BSL-3, or BSL-4 containment are outlined in Biosafety in Microbiological and Biomedical Laboratories (BMBL) 2. Pathogens that are perceived to present the most significant health risk to humans and/or animals are considered BSL-4 pathogens. Consequently, BSL-4 research requires particular caution, highly specialized training, and a robust and redundant facility infrastructure 3. To improve general awareness of the challenges associated with BSL-4 research, understanding the requirements for high containment building operations, systems management, and routine validation testing of engineering controls is necessary. We aim to advance this understanding by visually presenting the increased standards of operation and biosafety, and the resulting increased difficulty of hands-on research in BSL-4 containment.
Facilities housing BSL-4 suit laboratories must meet stringent requirements including, but not limited to, dedicated nonrecirculating ventilation systems, rigorous waste handling system and processes 4,5, and building automation systems (BAS) 2,6,7. Laboratory supply air is filtered once and exhaust air is double-filtered through high efficiency particle air (HEPA) filters, which are recertified annually. In addition, the BMBL imposes strict requirements for decontamination of solid waste and collection and decontamination of all effluent materials before release into the general waste system. Multiple redundancies are built into the system to prevent release of any type of BSL-4 pathogen. The BAS monitor facility operations remotely and can pinpoint the problem area. Facility support systems are checked daily by staff for optimal operation and notification of problems in real time. All systems are tested on a recurring basis to comply with CDC/Division of Select Agents and Toxins requirements for facility operation.
In addition to these standards for the physical facility, laboratory staff working with Select Agents and Toxins must submit to a Security Risk Assessment (SRA) by the Department of Justice prior to working with or around Select Agents. In addition, staff working with Tier 1 Select Agents (e.g., Ebola virus, Bacillus anthracis) must be enrolled in a Personnel Reliability Program (PRP) that continually evaluates the physical and mental health of individual researchers 8. The health screenings assess whether individuals are physically capable of performing the work in BSL-4 containment in a safe manner. Mental health screenings assess general well-being, psychological welfare and resiliency, and safety awareness of the staff. At the NIAID, scientists working with Select Agents undergo additional scrutiny wherein each individual completes an Access National Agency Check and Inquiries (ANACI) background check that examines educational and professional credentials, criminal history, financial history, and risk of foreign influence.
Compared to BSL-2 laboratory entry, maximum containment entry requires a considerably greater investment of resources, time, and training. After SRA approval and registration with the Division of Select Agents and Toxins, staff must undergo stringent hands-on training before access to the BSL-4 suit laboratories. Laboratory staff receives training on operation of the facility, including daily checks of critical functions and entry/exit procedures 9. Staff is also trained on laboratory biosafety and care and use of positive-pressure suits. White suits made of polyester fabric with polyvinyl chloride coating are used at the NIH/NIAID Integrated Research Facility at Fort Detrick. Facilities that use other types/commercial brands of positive-pressure suits may require different operational procedures for entering and exiting the BSL-4 laboratory than those outlined here. Researchers in facilities using this article should account for these differences prior to training. Suit training at the NIH/NIAID Integrated Research Facility at Fort Detrick includes proper procedures for donning the suit, ensuring the suit is functioning properly, repairing and maintaining suits (within and outside the laboratory), and moving within the laboratory. Once this training is complete, the laboratory staff member can begin working within the BSL-4 suit laboratories. Initially, another experienced staff member mentors one-on-one the newly trained staff member during the first five visits to the BSL-4 suit laboratories. To work independently at the NIH/NIAID Integrated Research Facility at Fort Detrick, the newly trained staff member completes a minimum of 40 supervised visits into BSL-4 suit laboratories with at least 100 hr of practical working time inside the laboratories.
Protocol
1. Daily External Checklist
Verify safety systems. Check breathing air systems, back up breaking air systems, effluent decontamination system (EDS) systems, chemical shower system, directional airflow and autoclave functionality (Figure 1).
2. Laboratory Entry Procedures
- Buffer Corridor Entrance Procedures by Laboratory Staff
- Enter staff person’s name, time of entrance, laboratory location, and pathogens under study in the Personnel Entry/Exit Logbook. Indicate the laboratory location on a magnetic board.
- To ensure the BSL-4 suit laboratories’ systems are functioning properly, verify that external systems have been checked and the laboratories have been cleared for entry on the Daily External Systems Safety Checklist (Figure 1).
- To assure that laboratory room pressure is within negative pressure set points that maintains proper directional air flow, check the building automation system (BAS) monitor outside of the outer change room entrance. If room pressure is not in the normal range, notify biosafety or facilities staff and delay laboratory entry until such pressure returns to the normal range.
- Ensure the availability of an autoclave for sterilization of biological waste and/or laundry following experimental work.
- Obtain scrubs, socks, and towels from the storage room containing the facility’s supplies located near the Personnel Entry/Exit Logbook.
- Outer Change Room Entrance Procedures by Laboratory Staff:
- Complete security requirements that regulate and document access to the biocontainment laboratory. Enter the outer change room.
- Remove all street clothing, undergarments, jewelry and watches, don scrubs, and proceed to the suit room.
- Positive-pressure Protective Suit Inspection and Integrity Testing by Laboratory Staff in the Suit Room:
- Upon obtaining a positive-pressure protective suit of an appropriate size, perform a visual inspection of the suit looking for any holes, tears, puncture points, seam rips, or weak spots. Inspect the outer suit gloves thoroughly, since gloves are the most likely place to be damaged.
- Change gloves at least every 7 calendar days or if damage to the gloves is identified.
- To replace the outer suit gloves, remove the duct tape from the existing suit glove, roll the O-ring onto the sleeve, and remove the old suit glove.
- Put a new glove onto the cuff of the suit and ensure that the glove covers the entire cuff and the thumb is in the proper position. Place the O-ring over the glove, seat the O-ring into the groove on the cuff, and use duct tape to secure outer suit glove.
- Fold the glove cuff over the duct tape and O-ring, seal the glove with one final piece of duct tape, and re-inspect the newly attached glove.
- Apply a thin layer of zipper lubricant to the zipper at least once a week.
- Cover each of the exhaust valves, located inside the suit behind the head cover and on the left side of the back with either duct tape or an aluminum pressure test cap, and zip the suit closed.
- Connect the quick disconnect/connect attachment at the end of each breathing line to the hose connection on the suit. Inflate the suit until the arms and legs are firm and place the suit into an upright position. Disconnect the air supply.
- The suit should not be over-pressurized as excessive pressure can cause serious damage to the suit. Thoroughly inspect the suit for any indication of an air leak for approximately 5 min and visually inspect the suit for signs of deflation. Ensure that the suit is not over-pressurized as excess pressure can cause serious damage to the suit.
- If the suit loses firmness, check the suit for leaks by re-inflating the suit. Listen and feel for leaks in the suit. If needed, spray all surfaces of the protection suit with a soap solution. Some common areas for leaks are the seams of the suit, the zipper area, and the junction of the visor material with the suit material. Monitor for the formation of bubbles that indicate a leak, paying particular attention to seams and the visor. Check to ensure that the exhaust valves are fully sealed.
- Repair small fabric leaks temporarily with duct tape. Complete permanent repairs using material from a suit repair kit. If the suit cannot be repaired easily, decontaminate the suit with an alcohol-based disinfectant and remove from the suit-room. If the suit is beyond repair, retire suit from use and incinerate.
- Once the integrity test is completed, unzip the suit and remove the covers from the exhaust valves. Failure to remove the exhaust covers will clog filtration and severely damage the suit. Enter testing and repair information in the Positive-pressure Suit Integrity Test Log.
- Donning Positive-pressure Suit in the Suit Room and Entering into BSL-4 Suit Laboratories:
- Tape socks to legs of scrubs. Don inner nitrile gloves and duct tape them to the cuffs of the scrubs. Don a second pair of nitrile gloves (not taped) over the inner gloves. If necessary, clean the inner face shield with glass cleaner for increased visibility.
- Don the positive-pressure suit, making sure the suit is zipped completely closed, and connect the suit to a breathing-air line. Enter into the BSL-4 suit laboratories when the BAS monitors are free of alarms.
- To enter the BSL-4 suit laboratories, disconnect from the breathing air line in the suit room and push the request to access button for the chemical shower room.
- Once the air pressure-resistant (APR) door is activated, the seal around the door deflates and the door magnet disengages. The APR doors within the chemical shower room are interlocking, meaning one APR door must be closed when the other is open to maintain containment at all times. To pass through the chemical shower room, close the APR door to the suit room, wait for the bladder to re-inflate and fully seal the door frame, and activate the second APR door leading to the laboratory.
- Upon entry to the BSL-4 suit laboratories, connect the suit to an available breathing air line.
- Before proceeding further, close the APR door leading to the laboratory and ensure that chemical shower is automatically activated to disinfect the chemical shower area and the suit. Once disinfected, the APR door in chemical shower room can be opened again from the suit room. If a cycle does not start, contact facility management personnel.
- If the suit does not have integral boots, don a pair of overshoes which are located on the shelves adjacent to exit door, the chemical shower APR door. To avoid additional risk/hazards, check that the overshoes are form fitting and snug.
3. Movement within the BSL-4 Suit Laboratories
Move freely around the laboratories by disconnecting and reconnecting to air lines throughout the laboratories. Stay connected to an air line to maintain positive pressure when attempting to bend over or retrieve items close to the ground. NOTE: Such movements while disconnected from the air line will exhaust the breathing air from within the suit and create negative pressure that can allow laboratory air to enter any breaches in the suit.
Observe the monitors throughout the laboratories for various alarms and current suite status.
4. Laboratory Exit Procedures from BSL-4 Suit Laboratories
Decontaminate gloved hands in detergent disinfectant cleaner in plastic dunk tanks by the sink or in interior hallways. If no dunk tank is present, spray gloved hands thoroughly with disinfectant.
Inspect the bottom of overshoes or boots for visible material. If overshoes are clean, remove overshoes and place them on the shelves adjacent to the chemical shower APR door. Close all interior doors of the laboratory, disconnect from the breathing air line, and proceed to the chemical shower.
If the overshoes are dirty, wash them thoroughly in the sink with detergent disinfectant cleaner.
5. BSL-4 Chemical Shower Procedures
Enter the chemical shower by pushing the request to access button to open the APR door, and close the door. Reconnect suit to a breathing air line inside the shower.
Once the APR door is closed, the chemical shower cycle will automatically activate to release an adequate mixture of detergent disinfectant cleaner to properly disinfect the suit and the chemical shower area. If the cycle does not start automatically, pull on the deluge handle. Scrub the suit for approximately 1 min, push the deluge handle back into place to prevent fully draining the chemical disinfectant tanks. A manual shower cycle is not available.
Check for glove and foot leaks by placing gloved hands and feet in the plastic tub containing detergent disinfectant cleaner solution kept inside the chemical shower. Look for liquid or wetness underneath the outer glove and check for tears, rips or weak portions on the outer glove. If a leak is found in one of the outer gloves, wait until the shower cycle is complete, remove outer suit glove and the outermost inner glove, and leave the outer glove in the plastic tub.
Scrub the suit and integrated suit boots with a long-handled scrub brush or hands to distribute the chemical disinfectant to all surfaces of the suit. Thoroughly rinse suit of detergent disinfectant cleaner during the water cycle of the shower.
Disconnect the breathing air line, previously exposed to laboratory air, both during the chemical and the water cycle to disinfect the connection on the suit. Rinse the air line connection of any detergent disinfectant cleaner. Without disconnection, the detergent disinfectant cleaner cannot otherwise clean the air line connection.
Open the door leading to the suit room and close it after exiting.
In the event of an emergency, such as a nonlife-threatening cut or fire, follow the same procedure outlined in 5.2.
6. Suit Room Procedures after Exiting the Chemical Shower
Dry the exterior of the suit with a large towel. Unzip the positive-pressure suit, remove one hand from the outer suit glove, and examine the outside of the outer nitrile glove carefully for moisture.
If moisture has leaked through the outer suit glove, remove the outer suit glove and outer nitrile glove and dispose in biohazard waste trashcan. Remove the other hand and repeat the check.
Remove suit and continue to dry exterior of suit to prevent dry rot damage. Spray the inside of face shield with glass cleaner and wipe dry. Hang the suit up to dry. Remove duct tape and inner nitrile gloves from scrubs and dispose of these items into designated biohazard waste trash bin.
Document any leaks or repairs to the suit in the suit log and report them to the facility management.
Proceed to inner change room, remove dirty scrubs and socks, and place them in the designated laundry bin. Proceed and enter into the Personal Shower.
7. Personal Shower and Outer Change Room Exit Procedures
Activate shower and fully wash hair and body for a minimum of 3 min with soap and water. Exit the Personal Shower and proceed to the Outer Change Room.
Dry off and don street clothing, exit the Outer Change Room, place used towels in the designated laundry bin, and proceed to the Buffer Corridor.
Indicate time of exit in the Personnel Entry/Exit Logbook and indicate laboratory exit on the locator board.
Representative Results
Staff has been carefully and thoroughly trained in these techniques to ensure safe and consistent practices inside a BSL-4 facility. By checking that the facility is functioning properly, as indicated on the daily checklist (Figure 1), we are able to ensure that all of the necessary administrative and engineering controls are in place and functioning to maintain a safe and properly functioning environment. The positive-pressure suit provides an additional layer of protection for the staff member. Proper maintenance and use of these suits is integral to personal protection for the staff member. Through strict adherence to these procedures, no laboratory-acquired infections have been recorded at the NIH/NIAID Integrated Research Facility at Fort Detrick.
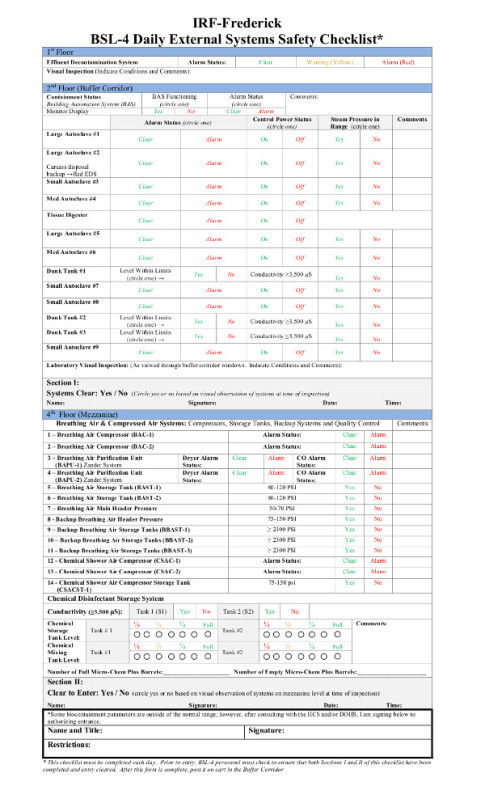 Figure 1:
Daily visual checklist for the support and backup systems for the BSL-4.
Please click here to view a larger version of this figure.
Figure 1:
Daily visual checklist for the support and backup systems for the BSL-4.
Please click here to view a larger version of this figure.
Discussion
We outlined the BSL-4 entrance and exit procedures used at the NIH/NIAID Integrated Research Facility at Fort Detrick for working with highly hazardous (Risk Group 4) pathogens. One purpose of visualizing the BSL-4 entrance and exit procedures is to emphasize the importance of safety of laboratory staff during work with such pathogens to avoid laboratory-acquired infections. Negative-pressure, BSL-4 laboratories maintain an inward directional airflow to ensure that pathogens will be contained within the laboratory. Positive-pressure suits with dedicated breathing air systems worn by laboratory staff mitigate contact of airborne pathogens with the laboratory staff. After laboratory staff leave the BSL-4 laboratory, a chemical shower disinfects the surface of the potentially contaminated suit and therefore prevents potential contamination of the rest of the building and the person changing out of the suit.
As the integrity of the positive-pressure suit is one of several important primary barriers for preventing potential pathogen exposure, staff is required to check for suit leaks before entrance and after exiting from BSL-4 laboratories. If a leak occurs, staff identifies the location of the leaks and alerts facility management. These practices ensure a swift response to any exposure to high consequence pathogens. Although every effort is taken to eliminate risk to laboratory staff, breaches of the suit may occur through the use of glassware, sharps, animal aggression, or continuous use.
While the procedures presented here generally follow the BMBL specifications outlined by CDC 2, these procedures are specific to the IRF-Frederick. Each BSL-4 facility has different building design specifications that impact the exact methods of laboratory operation. Alternative procedures for entering and exiting BSL-4 laboratories depend in part on the design and operation of these laboratories. In addition, government regulations in different countries may also have an effect on BSL-4 laboratory procedures in each country. Nevertheless, a general understanding of BSL-4 procedures and the building monitoring systems that support the safety of laboratory staff will help health administrators who are contemplating the design of similar buildings and outside collaborators involved in studies of high risk pathogens.
Productivity will increase as more laboratory staff is trained in BSL-4 laboratory entrance and exit procedures and in conducting experiments under BSL-4 conditions. However, when designing protocols with outside collaborators, sufficient time should be allotted to perform even basic laboratory operations, and expectations of time frames for delivering results have to be adjusted by accepting the difficulties inherent with work in BSL-4 laboratories. A generalized assumption is that any experiment performed at BSL-2 (e.g., 2 hr) will require twice the amount of time to perform in BSL-4 (e.g., 4 hr).
Disclosures
The authors have nothing to disclose.
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors. KJ, MRH, and LB performed this work as employees of Battelle Government Services, Inc.; JHK as an employee of Tunnell Government Services, Inc.; and MGL as an employee of Lovelace Respiratory Research Institute, Inc. Tunnell Government Services and Lovelace Respiratory Research Institute are subcontractors of Battelle Memorial Institute under its prime contract with NIAID, under Contract No. HHSN272200700016I.
References
- NIAID Category A, B, and C Priority Pathogens. National Institute of Allergy and Infectious Diseases. Bethesda, MD,: National Institute; 2013. of Allergy and Infectious Diseases. http://www.niaid.nih.gov/topics/biodefenserelated/biodefense/pages/cata.aspx. [Google Scholar]
- Chosewood LC, Wilson DE. Biosafety in Microbiological and Biomedical Laboratories. 5th. Washington, D.C: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health; 2009. http://www.cdc.gov/biosafety/publications/bmbl5. [Google Scholar]
- Shurtleff AC, et al. The impact of regulations, safety considerations and physical limitations on research progress at maximum biocontainment. Viruses. 2012;4:3932–3951. doi: 10.3390/v4123932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Stern L. A Guide to the Biosolids Risk Assessments for the EPA Part 503 rule. Washington D.C: US Environmental Protection Agency, Office of Wastewater Management; 1995. http://water.epa.gov/scitech/wastetech/biosolids/503rule_index.cfm. [Google Scholar]
- Salkin IF, Krisiunas E, Turnberg WL. Medical and infectious waste management. Appl. Biosaf. 2000;5(2):54–69. [Google Scholar]
- de Kok-Mercado F, Kutlak F, Jahrling PB. The NIAID integrated research facility at Fort Detrick. Appl. Biosaf. 2011;16(2):58–66. [Google Scholar]
- Jahrling PB, et al. The NIAID Integrated Research Facility at Frederick, Maryland: a unique international resource to facilitate medical countermeasure development for BSL-4 pathogens. Pathog. Dis. 2014. [DOI] [PMC free article] [PubMed]
- Skvorc C, Wilson DE. Developing a behavioral health screening program for BSL-4 laboratory workers at the National Institutes of Health. Biosecur. Bioterror. 2011;9:23–29. doi: 10.1089/bsp.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duc JW, et al. Framework for leadership and training of Biosafety Level 4 laboratory workers. Emerg. Infect. Dis. 2008;14(11):1685–1688. doi: 10.3201/eid1411.080741. [DOI] [PMC free article] [PubMed] [Google Scholar]


