Abstract
Granulocyte colony-stimulating factor (G-CSF) is a well known regulator of granulopoiesis, but the role of endogenous G-CSF in inflammatory joint disease has not been explored. We studied the response of G-CSF-deficient mice in experimental models of joint inflammation. We show that G-CSF deficiency protects mice from acute and chronic arthritis. Reduced severity was associated with blunted mobilization of granulocytic cells from the bone marrow and less cellular infiltrate and cellular activation in inflamed joints. We also demonstrate that G-CSF blockade in established collagen-induced arthritis in WT mice markedly reduces disease manifestations and is as effective as tumor necrosis factor blockade. Our results reveal a critical role for G-CSF in driving joint inflammation and highlight G-CSF as a potential therapeutic target in inflammatory joint diseases, such as rheumatoid arthritis.
Rheumatoid arthritis (RA) is a chronic autoimmune disease of unknown etiology in which synovial joints bear the brunt of an inflammatory process. The preclinical stages of RA are still poorly understood, but, in established RA, there is sustained local overproduction of proinflammatory cytokines, with perhaps a central role for tumor necrosis factor (TNF) (1).
Granulocyte colony-stimulating factor (G-CSF) is the major regulator of granulocyte production. G-CSF is produced by bone marrow stromal cells, endothelial cells, macrophages, and fibroblasts, and production is induced by inflammatory stimuli. G-CSF acts through the G-CSF receptor (G-CSFR), which is expressed on early myeloid progenitors, mature neutrophils, and monocytes/macrophages, as well as endothelial cells (2). Recently, G-CSFRs have also been described on human T and B lymphocytes (3, 4). Mice deficient in G-CSF or the G-CSFR exhibit marked neutropenia, demonstrating the importance of G-CSF in steady-state granulopoiesis (5, 6). However, G-CSF seems to be dispensable for emergency granulopoiesis (7). G-CSF increases the production and release of neutrophils, mobilizes hematopoietic stem and progenitor cells (8, 9), and modulates the differentiation, lifespan, and effector functions of mature neutrophils (10–13). G-CSF may also exert effects on macrophages, including expansion of monocyte/macrophage numbers, enhancement of phagocytic function (14, 15), and regulation of inflammatory cytokine and chemokine production (16, 17). G-CSF is currently used to treat neutropenia and to mobilize hematopoietic stem cells for transplantation.
Administration of G-CSF can exacerbate RA (18), murine collagen-induced arthritis (CIA) (19) and a passive transfer model of CIA in rats (20). G-CSF has been found in the serum and synovial fluid of RA patients (21). IL-1 and TNF induce the production of G-CSF by human synovial fibroblasts and chondrocytes in vitro (22, 23). Transgenic mice expressing human G-CSF develop severe osteopenia (24). This evidence indicates that G-CSF could be proinflammatory in inflammatory joint disease. However, administration of G-CSF also elicits regulatory CD4+ T cells and dendritic cells (DC) that dampen allogeneic and mitogenic responses (25–27). These effects of exogenous G-CSF have been correlated with reductions in murine graft vs. host disease (27), experimental autoimmune encephalomyelitis (17), and adjuvant arthritis in rats (28).
We examined the absolute dependence of two models of inflammatory arthritis on endogenous G-CSF and evaluated whether endogenous G-CSF influences immune cell function. We show that G-CSF-deficient (G-CSF–/–) mice are resistant to the induction of acute and chronic inflammatory arthritis. Furthermore, we demonstrate that G-CSF blockade in established CIA in WT mice markedly reduces the progression of disease. Protection against joint inflammation was associated with impaired granulopoiesis and reduced leukocyte trafficking and activation within inflamed joints. Examination of DC and T and B cell function failed to show abnormalities that would explain the marked protection of G-CSF–/– mice in these experimental models. Our results suggest that G-CSF drives inflammatory joint disease by increasing the production and mobilization of myeloid lineage cells from the bone marrow and inducing the trafficking and local activation of these cells in peripheral tissues. These data raise the possibility that G-CSF antagonists may be of therapeutic value in diseases such as RA.
Methods
Mice. C57BL/6 (B6; Ly5.2) and DBA/1 mice, as well as ovalbumin (OVA)-specific MHC II-restricted T cell receptor transgenic mice (OT-II) and OVA-specific MHC I T cell receptor transgenic/recombinase activating gene-1-deficient mice (OT-I) (29, 30), were obtained from The Walter and Eliza Hall Institute (WEHI). G-CSF–/– mice (provided by A. Dunn, Ludwig Institute for Cancer Research, Parkville, Australia) were derived by targeted disruption of the csf3 gene in 129/OLA-derived embryonic stem cells and B6-derived blastocysts, backcrossed >20 generations onto B6 mice (5). Mice (>8 weeks old) were housed under standard conditions in the WEHI animal facility. All experiments were approved by the institute ethics committee.
Acute Methylated BSA (mBSA)/IL-1-Induced Arthritis. mBSA/IL-1-induced arthritis was induced as described (31). Briefly, mice were injected intraarticularly with mBSA (200 μg; Sigma) and s.c. with IL-1 (250 ng; National Cancer Institute, Rockville, MD) or saline vehicle on days 0, 1, and 2. At day 7, knee joints were removed and processed for histology. Sections were assessed blinded to experimental groups and graded from 0 (normal) to 5 for the severity of five histological features of arthritis (exudate, synovitis, pannus, and cartilage and bone degradation) on hematoxylin/eosin (H&E)-stained sections (n = 4 sections per joint; maximum total arthritis score 25) (31). Proteoglycan loss was assessed (from 0 to 5) on safranin O-stained sections (n = 2 sections per joint).
Peripheral Blood and Bone Marrow Leukocyte Counts. Retroorbital bleeds and bone marrow were analyzed on an Advia 120 automatic cell analyzer (Bayer Diagnostics, Tarrytown, NY). Bone marrow differential cell counts were performed manually on cytocentrifuged cells stained with May–Grünwald Giemsa.
Flow Cytometry. Synovial tissue was dissected from mouse knee joints and enzymatically dissociated as described (31). Leukocytes were stained by using biotinylated and phycoerythrin- and FITC-conjugated Abs: anti-mouse CD45.2 (clone 104, BD Pharmingen), CD11b (Mac-1, clone M1/70, Caltag, Burlingame, CA), GR-1 (clone RB6.8C5, American Type Culture Collection), CD44 (clone IM7, BD Pharmingen), and CD4 (clone CT-CD4, Caltag). Biotinylated Abs were detected with streptavidin-TriColor (Caltag). The level of nonspecific staining was determined by using irrelevant isotype-matched Abs. Cells were analyzed on a FACScan by using cellquest software (Becton Dickinson).
CIA. CIA was induced as described (32). Chick type II collagen (CII, 2 mg/ml; Sigma) was dissolved in 10 mM acetic acid overnight at 4°C and emulsified in an equal volume of complete Freund's adjuvant (CFA), containing 5 mg/ml heat-killed Mycobacterium tuberculosis H37RA (Difco). In specified experiments incomplete Freund's adjuvant was used. Mice were injected intradermally at the base of the tail with 100 μl of the emulsion, and this was repeated 21 days later. Animals were regularly monitored blinded to experimental groups for erythema and swelling of limbs, and a clinical score (0–3) was given for each limb. At killing, paws of the four most clinically severe B6, plus affected and nonaffected G-CSF–/– mice, were processed for blinded histological assessment. Each paw provided more than seven joints for analyses, and each joint was graded from 0 to 3 in severity (33).
Therapeutic administration of neutralizing mAb in CIA was performed as described (19). WT DBA/1 mice were immunized, and, once arthritis was clinically evident, mice were matched for initial disease severity and then randomly allocated to treatment with 250 μg of rat anti-mouse G-CSF (clone 67604, R & D Systems), TNF (clone XT22), or isotype control (GL113, rat IgG1) mAb on days 1, 2, 3, 4, 5, 7, 9, and 12. A clinical score was given to each limb of arthritic mice every day for 14 days. Paws were also assessed histologically (≥6 joints per paw).
CII-Specific Lymph Node (LN) Responses. Inguinal LNs were harvested (50–62 days post-primary CII immunization), and single-cell suspensions prepared in RPMI medium 1640 containing 5% (vol/vol) FCS and 50 μM 2-mercaptoethanol. LN cells (4 × 105 cells per well in a 96-well round-bottom plate) were cultured with denatured CII (0–100 μg/ml) for 72 h at 37°C (5% CO2). [3H]Thymidine [1 μCi (1 Ci = 37 GBq) per well; Amersham Pharmacia] incorporation in the last8hof culture was measured to assess T cell proliferation (32). Supernatants were taken for measurement of IL-2 and IFN-γ production by ELISA by using paired Ab according to the manufacturer's instructions (BD Pharmingen).
T Cell Purification. Spleen and LN CD4+ and CD8+ T cells were isolated by positive selection, by using FITC-conjugated anti-CD4 (Caltag) or anti-CD8 (clone CT-CD8a; Caltag) Abs and anti-FITC microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were separated by using an Automacs Magnetic Cell Sorter (>95% purity) (Miltenyi Biotec).
DC Cultures. Splenic DC (purity 50–70% CD11c+) were isolated by negative selection as described (34). DC phenotypic markers, examined by flow cytometry on purified naive DC, included CD11c (clone N4/18), MHC II (clone M5/114), MHC I (clone M1/42), CD80 (B7-1, clone 16-10A1, BD Pharmingen), CD86 (B7-2, clone G1, BD Pharmingen), and CD11b. For OVA-presentation experiments, mice were injected i.v. with whole OVA protein (3 mg per mouse; Sigma) or PBS vehicle. DC were isolated 16 h later, and 0–8 × 104 irradiated (2,000 rad) DC were cultured with OVA-specific OT-II CD4+ and OT-I CD8+ T cells (1 × 104 cells per well). T cell proliferation was measured after 3–4 days by [3H]thymidine incorporation for 18 h.
Total Ig- and CII-Specific Antibodies. Serum total IgG, IgM, IgG2c, IgG2b, IgG1, IgG3, and IgA were measured by using paired capture and HRP-conjugated Abs (Southern Biotechnology Associates). ELISAs for Abs to CII were performed as described (32) by using HRP-conjugated Ab to IgG (Sigma) and IgM, IgG2c, IgG2b, IgG1, and IgG3 (Southern Biotechnology Associates).
Statistics. The χ2 test was used for CIA incidence (clinical and histological). The Mann–Whitney two-sample rank test was used to compare clinical severity scores, histological scores, and anti-CII Ab levels. Student's t test for the difference of two means was used to analyze differential cell counts, proliferation assays, ELISAs, and flow cytometric analyses. P < 0.05 was considered statistically significant.
Results
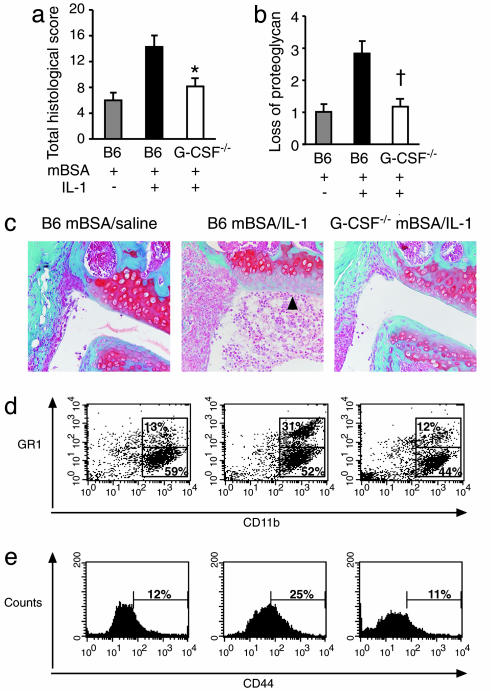
G-CSF Deficiency Reduces Acute Inflammatory Arthritis. IL-1 is a major mediator of RA (35), and, when given systemically in mice, it transforms the weak inflammatory reaction to intraarticular mBSA into a florid monoarthritis. The severity of arthritis peaks on day 7 (36). We assessed the absolute requirement for endogenous G-CSF in acute mBSA/IL-1-induced arthritis by using G-CSF–/– mice. Compared with B6 mice, G-CSF–/– mice had significantly reduced acute arthritis histological scores (Fig. 1a), and marked protection against cartilage proteoglycan depletion (Fig. 1 b and c). Disease in G-CSF–/– mice was more comparable with the mild inflammatory reaction in B6 mice treated with mBSA alone (mBSA/saline). These results demonstrate that IL-1-driven joint inflammation and destruction in this disease model is G-CSF-dependent.
Fig. 1.
G-CSF–/– mice develop less acute mBSA/IL-1-induced arthritis and have reduced leukocyte infiltration. B6 and G-CSF–/– mice were injected intraarticularly with mBSA and s.c. with IL-1 or saline vehicle on days 0, 1, and 2. (a–c) At day 7, mice knee joints were processed for histological assessment. Total histological scores (out of maximum 25) (a) and loss of proteoglycan (b) in B6 mBSA/saline (▒), B6 mBSA/IL-1 (▪), and G-CSF–/– mBSA/IL-1 (□) treated mice are shown. Results show the mean ± SEM of 10 joints from one of two experiments. (c) Representative frontal safranin O-stained knee joint sections in B6 mBSA/saline (Left), B6 mBSA/IL-1 (Center, arrow indicates proteoglycan loss), and G-CSF–/– mBSA/IL-1-treated mice (Right) (×200 magnification). (d and e) Synovium was dissected from B6 mBSA/saline, B6 mBSA/IL-1, and G-CSF–/– mBSA/IL-1 mice on day 7 of arthritis and enzymatically dissociated. Flow cytometric analysis of CD45+ synovial leukocytes stained for CD11b (Mac1) and GR1 (d) and CD44 (e) expression in synovial digests of B6 mBSA/saline (Left), B6 mBSA/IL-1 (Center), and G-CSF–/– mBSA/IL-1 (Right) treated mice is shown. Data are the mean percentages. n = 6 pooled synovial tissue samples per group from two to three experiments. *, P < 0.05; †, P < 0.01.
Reduced Granulopoiesis and Joint Infiltration in G-CSF–/– Mice in Response to IL-1. We next investigated the hematopoietic response during acute inflammatory arthritis. mBSA and IL-1 administration enhanced granulopoiesis and increased peripheral blood neutrophil numbers in B6 mice by day 7 (Table 1). In contrast, G-CSF–/– mice displayed no rise in circulating neutrophil numbers, and blunted bone marrow production of myeloid lineage cells in response to IL-1 (Table 1).
Table 1. Impaired granulopoiesis in G-CSF-/- mice during acute inflammatory arthritis.
| Day 0
|
Day 3
|
Day 7
|
||||
|---|---|---|---|---|---|---|
| B6 | G-CSF-/- | B6 | G-CSF-/- | B6 | G-CSF-/- | |
| Blood (10-4/ml) | ||||||
| Neutrophils | 33 ± 6 | 19 ± 3 | 40 ± 5 | 12 ± 3* | 63 ± 11 | 20 ± 4* |
| Monocytes | 4 ± 2 | 3 ± 1 | 2 ± 0 | 3 ± 1 | 6 ± 2 | 3 ± 0 |
| Bone marrow | ||||||
| Cells (10-6/femur) | 21.3 ± 1.0 | 19.0 ± 4.0 | 14.6 ± 0.8 | 12.2 ± 1.0 | 17.1 ± 2.3 | 14.6 ± 1.5 |
| Blasts, % | 3.5 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.7 | 3.8 ± 0.9 | 1.7 ± 0.7 | 2.7 ± 0.2† |
| Promyelocytes, Myelocytes, % | 5.0 ± 1.0 | 5.5 ± 0.5 | 10.4 ± 1.0† | 7.4 ± 1.0 | 3.8 ± 0.8 | 2.7 ± 0.2† |
| Metamyelocytes, Neutrophils, % | 38.0 ± 1.0 | 13.5 ± 3.5* | 41.8 ± 6.4 | 21.2 ± 3.2* | 58.2 ± 2.8† | 26.8 ± 3.5* |
| Monocytes, % | 4.5 ± 0.5 | 9.5 ± 1.5 | 10.6 ± 1.2† | 10.2 ± 1.9 | 7.7 ± 1.5 | 9.5 ± 1.0 |
Peripheral blood and bone marrow from mBSA/IL-1-treated B6 and G-CSF-/- mice were analyzed on days 0 (baseline), 3, and 7. Data are representative of absolute numbers or % ± SEM; n > 4 mice per group. *, P < 0.05 between groups at time point examined. †, P < 0.05 between mice of same genotype compared with day 0.
To determine whether G-CSF deficiency affected the composition of the infiltrating cells in the joint synovium, we dissociated synovial tissue and examined the expression of a range of hematopoietic markers on CD45+ leukocytes. G-CSF–/– mBSA/IL-1-treated mice had markedly less infiltrating CD45+ leukocytes at day 7 compared with B6 mBSA/IL-1-treated mice, and had similar numbers to B6 mBSA/saline control mice (CD45+ synovial leukocytes × 10–5 per joint: B6 mBSA/saline 0.88 ± 0.03; B6 mBSA/IL-1 1.85 ± 0.05; G-CSF–/– mBSA/IL-1 0.81 ± 0.03). In particular, there were marked reductions in the numbers of infiltrating neutrophils (GR1+ CD11b+), as well as fewer monocyte/macrophages (CD11b+ GR1–) (Fig. 1d) and CD4+ cells (data not shown). CD45+ synovial leukocytes in G-CSF–/– mice had decreased expression of CD44 (Fig. 1e). Thus, as well as inhibiting IL-1-induced granulopoiesis, the absence of G-CSF also impaired neutrophil, monocyte/macrophage, and CD4+ T cell recruitment into the synovium and local activation of infiltrating leukocytes.
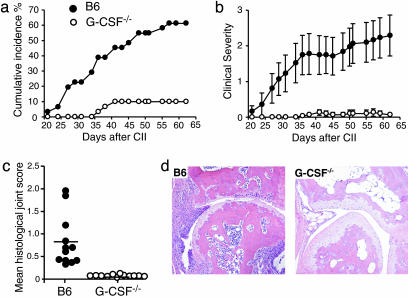
G-CSF–/– Mice Are Protected from Chronic Autoimmune Arthritis. CIA is a chronic autoimmune polyarthritis and is the most widely used model of RA. We immunized B6 and G-CSF–/– mice for CIA as described (32). The onset of CIA was slightly delayed in G-CSF–/– mice, and the cumulative incidence of disease was dramatically reduced (P < 0.001) compared with B6 mice (Fig. 2a). In affected G-CSF–/– mice, clinical severity was much lower than in B6 mice (P < 0.0001) (Fig. 2b), and joint swelling was restricted to single digits.
Fig. 2.
G-CSF–/– mice are protected from CIA. Cumulative incidence percentage (a) and clinical severity (b) of CIA in B6 (•) and G-CSF–/– (○) mice are shown. n ≥ 30 mice per group from three experiments. (c and d) At 62 days after primary CII and CFA immunization, B6 (•) and G-CSF–/– (○) mice were killed, and paws were processed for histological assessment of joints. (c) Mean histological joint score per mouse (horizontal lines show group means). Data are from three pooled experiments; n > 370 joints scored. (d) Frontal sections of a severely arthritic B6 (Left) and typical CIA-immunized G-CSF–/– (Right) metacarpophalangeal joints (×200 magnification).
CIA can also be assessed histologically. From each of the three experiments performed, four of the most severely clinically affected B6 mice, plus clinically affected and nonaffected G-CSF–/– mice, were examined for histological features of CIA. G-CSF–/– mice had features of inflammatory arthritis in only 4% of joints, compared with 48% of B6 joints (P < 0.001). Of the small proportion of joints affected in G-CSF–/– mice, none was severely inflamed (Fig. 2 c and d). G-CSF deficiency therefore affords marked protection against the clinical and histological features of CIA.
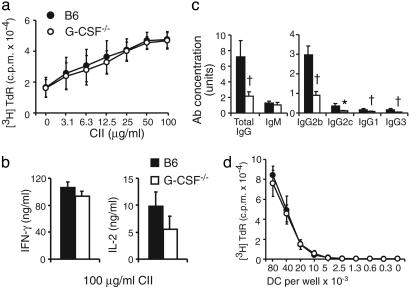
Cellular Responses in G-CSF–/– Mice. Induction of CIA depends on humoral and cellular immune responses to CII (32), and acute mBSA/IL-1-induced arthritis depends on CD4+ T cells (31). It was therefore important to carefully examine relevant immunological parameters in G-CSF–/– mice. There were no significant differences in draining LN T cell proliferative responses (Fig. 3a), or in IFN-γ or IL-2 production (Fig. 3b) to CII between CIA-immunized G-CSF–/– and B6 mice. Thus, defects in T cell function are unlikely to explain reduced CIA in G-CSF–/– mice.
Fig. 3.
Defective anti-CII Ab isotype switching but normal T cell responses and antigen presentation by DCs in immunized G-CSF–/– mice. Shown are T cell proliferation (a) and IFN-γ and IL-2 production (b) from inguinal LN cells of CIA-immunized B6 (▪ and •) and G-CSF–/– (□ and ○) mice stimulated with denatured CII in vitro.(c) Serum anti-CII IgG, IgM, IgG2b, IgG2c, IgG1, and IgG3 levels in CIA-immunized B6 (▪) and G-CSF–/– (□) mice at day 62 of CIA; n ≥ 30 mice per group. (d) Proliferation of OVA-specific OT-II CD4+ T cells in response to B6 (•) and G-CSF–/– (□) OVA-pulsed DC. Proliferation data are the mean [3H]thymidine uptake cpm × 10–4 ± SD, and ELISA data are the mean ± SEM; n = 3 experiments. *, P < 0.05; †, P < 0.01.
Impaired Humoral Responses to CII and CFA in G-CSF–/– Mice. We first determined that basal Ig levels in naive G-CSF–/– mice were not reduced compared with naive B6 mice (data not shown). To examine B cell function in CIA, mice were bled for serum anti-CII-specific Abs at day 62 post-primary immunization. In comparison with B6 mice, G-CSF–/– mice had comparable levels of anti-CII IgM but had significantly reduced total anti-CII IgG and a generalized impairment of anti-CII IgG2b, IgG2c, IgG1, and IgG3 Ab production (Fig. 3c). These results demonstrate a defect in isotype switching in G-CSF–/– mice under these immunization conditions. However, G-CSF–/– immunized with CII in incomplete Freund's adjuvant had equivalent anti-CII Ab responses compared with B6 mice (data not shown). Endogenous G-CSF therefore influences the B cell response to mycobacteria in CFA. The reduced IgG Ab response to CII in CFA could contribute to reduced CIA in G-CSF–/– mice.
G-CSF–/– DC Have Normal Antigen Presentation Function. There was no difference in the expression of DC activation and costimulation molecules between naive B6 and G-CSF–/– DCs (data not shown). DC antigen presentation was assessed by comparing the ability of G-CSF–/– and B6 DCs to present OVA to OVA-specific T cells. OVA-pulsed G-CSF–/– DC caused equivalent proliferation of OT-II CD4+ T cells (Fig. 3d) and OT-I CD8+ T cells (data not shown) compared with OVA-pulsed B6 DC. These data, along with the normal LN T cell responses to CII in G-CSF–/– mice, suggest that G-CSF is not required for DC antigen presentation, and that abnormal DC function is not responsible for protection of G-CSF–/– mice against immune-mediated inflammatory arthritis.
Reduced Granulopoiesis in G-CSF–/– Mice in Chronic CIA. Given the relatively normal immune responses in G-CSF–/– mice, we next examined granulopoiesis during chronic CIA. B6 mice had enhanced granulopoiesis in the bone marrow (data not shown) and developed a marked peripheral blood neutrophilia by the onset of CIA around day 30 (neutrophils × 10–4/ml blood ± SEM in B6 mice: day 0, 34 ± 5 vs. day 30 of CIA, 491 ± 137). In contrast, G-CSF–/– mice maintained a neutropenia throughout the course of CIA (neutrophils × 10–4/ml blood ± SEM in G-CSF–/– mice: day 0, 18 ± 2 vs. day 30 of CIA, 30 ± 5). Therefore, the autoimmune response that elicits CIA in peripheral tissues also acts on the bone marrow to promote granulopoiesis, and this response is mediated by G-CSF.
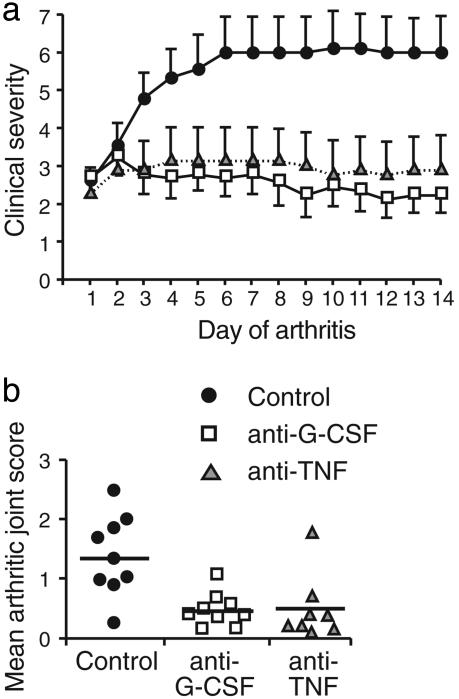
G-CSF Blockade Reduces the Progression of Established CIA. In view of the protection from acute and chronic inflammatory arthritis in G-CSF–/– mice, we posed the most clinically relevant question: does G-CSF blockade have therapeutic potential in established CIA? For therapeutic studies, DBA/1 mice (the most CIA-responsive strain) were immunized with CII in CFA and boosted at day 21. Mice were examined clinically from day 21 and, at the onset of arthritis, were matched for initial disease severity and then randomly allocated to treatment with isotype control, anti-G-CSF, or anti-TNF mAb. Inhibition of G-CSF impaired disease progression and reduced clinical severity compared with control mAb-treated mice (Fig. 4a). Anti-G-CSF mAb treatment also reduced peripheral blood neutrophils (percentage of metamyelocytes/neutrophils in peripheral blood at day 14: control mAb 41%; anti-G-CSF mAb 13%; anti-TNF mAb 42%). Importantly, the beneficial clinical response to anti-G-CSF mAb was comparable to anti-TNF mAb (mean clinical score ± SEM over 14 days of arthritis: control mAb 5.4 ± 0.8; anti-G-CSF mAb 2.5 ± 0.5, P < 0.01; anti-TNF mAb 3.3 ± 0.9, P < 0.05). Histological examination of paws confirmed a reduction in the number (percentage of arthritic joints: control mAb 54%; anti-G-CSF mAb 23%, P < 0.001; anti-TNF mAb 22%, P < 0.001) and disease severity of affected joints (Fig. 4b). Anti-G-CSF mAb did not significantly alter the serum levels of anti-CII IgM or anti-CII IgG Abs (data not shown). Inhibition of G-CSF in established CIA therefore blocks the progression of inflammatory joint disease.
Fig. 4.
Anti-G-CSF mAb impairs the progression and reduces the severity of established CIA in WT mice. DBA/1 mice were immunized for CIA. At the clinical onset of CIA, mice were matched for initial disease severity, and assigned to cohorts receiving control (•), anti-G-CSF (□), or anti-TNF () mAb. (a) Clinical severity of established CIA in treatment groups. (b) Histological examination of CIA in mAb-treated arthritic mice after 14 days. Data show the mean arthritic joint score per mouse ± SEM; n = 8–9 mice per group.
Discussion
We demonstrate that endogenous G-CSF is a critical mediator of acute and chronic inflammatory arthritis in mice and that inhibition of endogenous G-CSF in established autoimmune inflammatory arthritis reduces the progression and severity of disease. Our studies raise the following questions: why are mice lacking endogenous G-CSF protected from acute and chronic arthritis and how does anti-G-CSF mAb therapy impair the progression of CIA in WT mice? In both the acute and chronic arthritis models, we observed reduced production and mobilization of granulocytic cells from the bone marrow to the blood, and there was reduced myeloid cell activation in the joint infiltrate of G-CSF–/– mice with acute arthritis. Thus, we hypothesize that antagonizing G-CSF impairs myelopoiesis, thereby reducing the supply of inflammatory cells available to contribute to joint inflammation. The reduction in inflammatory cells in the acute IL-1-dependent arthritis model was associated with marked protection against cartilage proteoglycan depletion, highlighting the potential role of G-CSF-induced leukocyte recruitment and activation in mediating cartilage damage. Collectively, these data are consistent with the idea that G-CSF-regulated myeloid lineage cells are crucial effector cells in the expression of acute and chronic inflammatory joint disease.
The reduced inflammatory cell infiltrate and lower expression of CD44 in the absence of G-CSF also raise the possibility of local proinflammatory effects for G-CSF. CD44 is a leukocyte activation marker and adhesion receptor for hyaluronan, which may contribute to leukocyte migration within the synovium (37). G-CSF has been shown to activate endothelial cells (38), modulate the expression of leukocyte adhesion molecules (39, 40), and enhance angiogenesis (41), and may induce chemokine production (39). G-CSF also enhances neutrophil and macrophage phagocytosis (14, 15) and prolongs neutrophil survival (12). Local tissue production of G-CSF might therefore mediate adhesion and trafficking of myeloid cells through the endothelium, as well as promote local cellular activation, function, and survival within inflamed tissues.
Administration of G-CSF can induce a shift from Th1 to Th2 cytokine production (27), mobilize type 2 regulatory DC (26), and dampen mitogenic and allogenic responses in T cells (25, 27). G-CSF can also induce Ig production from human B cells (42). It was therefore important to examine immune parameters in G-CSF–/– mice. We demonstrate normal CD4+ T cell and DC responses in G-CSF–/– mice. Although reduced anti-CII Ab isotype switching in G-CSF–/– mice could partly explain reduced CIA, TNF-deficient mice had an even greater impairment in anti-CII isotype switching but could still develop severe CIA (33). The acute mBSA/IL-1 arthritis model is also B cell independent (31). Furthermore, therapeutic administration of anti-G-CSF mAb to WT mice ameliorated CIA but did not depress the anti-CII Ab response.
Our observations provide a rationale for the development of G-CSF antagonists for the treatment of RA. However, although we know a great deal about the effects of administering G-CSF, what might be the downside of inhibiting endogenous G-CSF? On the whole, cytokine antagonists have been remarkably well tolerated in RA but clearly increase the risk of infection (43). Importantly, we have shown that the adaptive immune response in G-CSF–/– mice seems to be relatively intact, apart from a CFA-specific defect in isotype switching. G-CSF–/– mice, which lack G-CSF throughout life, have an increased rate of infections, and, as a result, some older mice develop amyloidosis (5). However, G-CSF–/– mice can still mount an emergency granulopoiesis response to infection (7). How the G-CSF–/– phenotype in experimental arthritis might translate to therapeutic blockade of G-CSF in inflammatory diseases, such as RA, awaits further investigation.
Acknowledgments
We thank A. Dunn for mice, A. Milligan and staff, S. Mihajlovic and the histology department, and J. Wilkins, S. Mifsud, L. Di Rago, D. Tarlinton, A. Light, K. Davern, J. Corbin, and R. Steptoe for help. This work was funded by the Reid Charitable Trusts and the National Health and Medical Research Council of Australia (NHMRC). D.M. is supported by National Institutes of Health Grant CA22556 and is the recipient of the Cancer Council of Victoria Carden Fellowship. I.P.W. is the recipient of an NHMRC Clinical Practitioner Fellowship.
Abbreviations: B6, C57BL/6; CIA, collagen-induced arthritis; CFA, complete Freund's adjuvant; DC, dendritic cells; G-CSF, granulocyte colony–stimulating factor; LN, lymph node; mBSA, methylated BSA; OVA, ovalbumin; RA, rheumatoid arthritis; TNF, tumor necrosis factor; CII, type II collagen.
References
- 1.Feldmann, M., Brennan, F. M. & Maini, R. V. (1996) Cell 85, 307–310. [DOI] [PubMed] [Google Scholar]
- 2.Demetri, G. D. & Griffin, J. D. (1991) Blood 78, 2791–2808. [PubMed] [Google Scholar]
- 3.Franzke, A., Piao, W., Lauber, J., Gatzlaff, P., Konecke, C., Hansen, W., Schmitt-Thomsen, A., Hertenstein, B., Buer, J. & Ganser, A. (2003) Blood 102, 734–739. [DOI] [PubMed] [Google Scholar]
- 4.Morikawa, K., Morikawa, S., Nakamura, M. & Miyawaki, T. (2002) Br. J. Haematol. 118, 296–304. [DOI] [PubMed] [Google Scholar]
- 5.Lieschke, G. J., Grail, D., Hodgson, G., Metcalf, D., Stanley, E., Cheers, C., Fowler, K. J., Basu, S., Zhan, Y. F. & Dunn, A. R. (1994) Blood 84, 1737–1746. [PubMed] [Google Scholar]
- 6.Liu, F., Wu, H., Wesselschmidt, R., Kornaga, T. & Link, D. (1996) Immunity 5, 491–501. [DOI] [PubMed] [Google Scholar]
- 7.Basu, S., Hodgson, G., Zhang, H.-H., Katz, M., Quilici, C. & Dunn, A. R. (2000) Blood 95, 3725–3733. [PubMed] [Google Scholar]
- 8.Roberts, A. W., Foote, S., Alexander, W. S., Scott, C., Robb, L. & Metcalf, D. (1997) Blood 89, 2736–2744. [PubMed] [Google Scholar]
- 9.Levesque, J.-P., Hendy, J., Takamatsu, Y., Simmons, P. J. & Bendall, L. J. (2003) J. Clin. Invest. 111, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu, S., Hogland, M. & Venge, P. (1996) Br. J. Haematol. 93, 558–568. [DOI] [PubMed] [Google Scholar]
- 11.Yong, K. L. (1996) Br. J. Haematol. 94, 40–47. [DOI] [PubMed] [Google Scholar]
- 12.Villunger, A., Scott, C., Bouillet, P. & Strasser, A. (2003) Blood 101, 2393–2400. [DOI] [PubMed] [Google Scholar]
- 13.Jacob, J., Haug, J. S., Raptis, S. & Link, D. C. (1998) Blood 92, 353–361. [PubMed] [Google Scholar]
- 14.Fattorossi, A., Battaglia, A., Pierelli, L., Malinconico, P., Andreocci, L., Perillo, A., Ferrandina, G., Martelli, O., Rhughetti, A., Nuti, M., et al. (2001) Cancer Immunol. Immunother. 49, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bermudez, L. E., Petrofsky, M. & Stevens, P. (1998) Immunology 94, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu, S., Hoglund, M., Hakansson, L. & Venge, P. (2000) Br. J. Haematol. 108, 848–853. [DOI] [PubMed] [Google Scholar]
- 17.Zavala, F., Abad, S., Ezine, S., Taupin, V., Masson, A. & Bach, J.-F. (2002) J. Immunol. 168, 2011–2019. [DOI] [PubMed] [Google Scholar]
- 18.Snowden, J. A., Biggs, J. C., Milliken, S. T., Fuller, A., Staniforth, D., Passuello, F., Renwick, J. & Brooks, P. M. (1998) Bone Marrow Transplant. 22, 1035–1041. [DOI] [PubMed] [Google Scholar]
- 19.Campbell, I. K., Rich, M. J., Bischof, R. J. & Hamilton, J. A. (2000) J. Leukocyte Biol. 68, 144–150. [PubMed] [Google Scholar]
- 20.Miyahara, H., Hotokebuchi, T., Saikawa, I., Arita, C., Takagishi, K. & Sugioka, Y. (1993) Clin. Immunol. Immunopathol. 69, 69–76. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, H., Ueki, Y., Sakito, S., Matsumoto, K., Yano, M., Miyake, S., Tominaga, T., Tominaga, M. & Eguchi, K. (2000) Clin. Exp. Rheumatol. 18, 713–718. [PubMed] [Google Scholar]
- 22.Leizer, T., Cebon, J., Layton, J. E. & Hamilton, J. A. (1990) Blood 76, 1989–1996. [PubMed] [Google Scholar]
- 23.Campbell, I. K., Novak, U., Cebon, J., Layton, J. E. & Hamilton, J. A. (1991) J. Immunol. 147, 1238–1246. [PubMed] [Google Scholar]
- 24.Takahashi, T., Wada, T., Mori, M., Kokai, Y. & Ishii, S. (1996) Lab. Invest. 74, 827–834. [PubMed] [Google Scholar]
- 25.Reyes, E., Garcia-Castro, I., Esquivel, F., Hornedo, J., Cortes-Funes, H. & Alvarez-Mon, M. (1999) Br. J. Cancer 80, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arpinati, M., Green, C. L., Heimfeld, S., Heiser, J. E. & Anasetti, C. (2000) Blood 95, 2484–2490. [PubMed] [Google Scholar]
- 27.MacDonald, K. P., Rowe, V., Filippich, C., Thomas, R., Clouston, A. D., Welply, J. K., Hart, D. N., Ferrara, J. L. & Hill, G. R. (2003) Blood 101, 2033–2042. [DOI] [PubMed] [Google Scholar]
- 28.Brendolan, A., Higuchi, M., Sibley, R. & Strober, S. (2003) Cell. Immunol. 221, 6–14. [DOI] [PubMed] [Google Scholar]
- 29.Hogquist, K. A., Jameson, S. C., Heath, W. R., Howard, J. L., Bevan, M. J. & Carbone, F. R. (1994) Cell 76, 17–27. [DOI] [PubMed] [Google Scholar]
- 30.Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. (1998) Immunol. Cell Biol. 76, 34–40. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor, K. E., Campbell, I. K., O'Donnell, K., Wu, L. & Wicks, I. P. (2001) Arthritis Rheum. 44, 442–450. [DOI] [PubMed] [Google Scholar]
- 32.Campbell, I. K., Hamilton, J. A. & Wicks, I. P. (2000) Eur. J. Immunol. 30, 1568–1575. [DOI] [PubMed] [Google Scholar]
- 33.Campbell, I. K., O'Donnell, K., Lawlor, K. E. & Wicks, I. P. (2001) J. Clin. Invest. 107, 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vremec, D., Pooley, J., Hochrein, H., Wu, L. & Shortman, K. (2000) J. Immunol. 164, 2978–2986. [DOI] [PubMed] [Google Scholar]
- 35.Arend, W. P. (2002) Cytokine Growth Factor Rev. 13, 323–340. [DOI] [PubMed] [Google Scholar]
- 36.Staite, N. D., Richard, K. A., Aspar, D. G., Franz, K. A., Galinet, L. A. & Dunn, C. J. (1990) Arthritis Rheum. 33, 253–260. [DOI] [PubMed] [Google Scholar]
- 37.Brennan, F. R., Mikecz, K., Glant, T. T., Jobanputra, P., Pinder, S., Bavington, C., Morrison, P. & Nuki, G. (1997) Scand. J. Immunol. 45, 213–220. [DOI] [PubMed] [Google Scholar]
- 38.Bussolino, F., Wang, J. M., Defilippi, P. Turrini, F., Sanavio, F., Edgell, C. J., Aglietta, M. Arese, P., & Mantovani, A. (1989) Nature 337, 471–473. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, S., Kobayashi, M., Chiba, K., Horiuchi, I., Wang, J., Kondoh, T., Hashino, S., Tanaka, J., Hosokawa, M. & Asaka, M. (2002) Blood 99, 1863–1865. [PubMed] [Google Scholar]
- 40.Sugimori, N., Nakao, S., Yachie, A., Niki, T., Takami, A., Yamazaki, H., Miura, Y., Ueda, M., Shiobara, S. & Matsuda, T. (1999) Bone Marrow Transplant. 23, 119–124. [DOI] [PubMed] [Google Scholar]
- 41.Natori, T., Sata, M., Washida, M., Hirata, Y., Nagai, R. & Makuuchi, M. (2002) Biochem. Biophys. Res. Commun. 297, 1058–1061. [DOI] [PubMed] [Google Scholar]
- 42.Morikawa, K., Miyawaki, T., Oseko, F., Morikawa, S. & Imai, K. (1993) Eur. J. Haematol. 51, 144–151. [DOI] [PubMed] [Google Scholar]
- 43.Ellerin, T., Rubin, R. H. & Weinblatt, M. E. (2003) Arthritis Rheum. 48, 3013–3022. [DOI] [PubMed] [Google Scholar]