Abstract
Few studies have focused on falls among home care (HC) clients with neurological conditions. This study identified factors that increase risk of falling among HC clients with no recent history of falls, and explored whether risk profiles varied among those with dementia or parkinsonism compared to those without selected neurological conditions. A retrospective cohort design was used and analysis of data from community-based HC clients across Ontario was conducted on a sample of ambulatory clients with dementia, parkinsonism, or none of the selected neurological conditions. Data were obtained from the Resident Assessment Instrument for HC (RAI-HC) assessment. The outcome used in multivariable analyses was whether clients fell during follow-up. Unsteady gait was a strong predictor of falls across all three groups. Co-morbid parkinsonism most strongly predicted falls in the dementia group. Clients with borderline intact to mild cognitive impairment had higher odds of falling within the parkinsonism and comparison groups.
Keywords: aging, dementia, falls, home care, interRAI, Parkinson’s disease
Among older adults, falls are common and can cause serious injuries, including hip fractures (Luukinen, Koski, Honkanen, & Kivela, 1995; Tinetti & Williams, 1997). Recent estimates in Ontario show that approximately 30 per cent of home care (HC) clients fall annually (Canadian Institute for Health Information, 2014). A history of a recent fall (e.g., within the past six months to one year) has consistently been identified as a strong risk factor for subsequent falls among older adults (Lundebjerg, 2001). However, identifying contributing factors and preventing a fall is important since this initial event can lead to fear of falling, activity restrictions, and reduced physical functioning (Arfken, Lach, Birge, & Miller, 1994 ; Friedman, Munoz, West, Rubin, & Fried, 2002). Parkinsonism, including Parkinson’s disease (PD), and dementia have been cited as independent predictors of falls (Deandrea et al., 2010; Mahoney, Sager, Dunham, & Johnson, 1994; Robbins et al., 1989); however, it is unclear whether these individuals present with unique factors contributing to fall risk, and require targeted falls prevention strategies.
The annual prevalence of falls among those with cognitive impairment and dementia is approximately 60 per cent (Tinetti, Speechley, & Ginter, 1988; van Dijk, Meulenberg, van de Sande, & Habbema, 1993). Evidence suggests that the link between cognitive impairment and falls is impaired executive function (Mirelman et al., 2012 ), which can limit the ability to perform tasks, such as activities of daily living (ADLs), which require input from multiple domains and impose a high cognitive load (Muir et al., 2012; Taylor, Delbaere, Lord, Mikolaizak, & Close, 2013). Falls are also common among people with PD, with up to 68 per cent of people with PD falling annually (Wood, Bilclough, Bowron, & Walker, 2002). Individuals with PD present with motor and gait impairments that increase their risk for falls by threefold, in contrast to individuals without PD (Deandrea et al., 2010 ).
Although there is an abundance of research examining falls risk in population-based studies of healthy, community-dwelling older adults or institutionalized individuals, there is limited research examining risk factors in community-dwelling adults receiving HC who have impairments or conditions that place them at higher risk for falls than healthy, community-dwelling older adults (Arfken et al., 1994; Deandrea et al., 2010; Mahoney et al., 1994; Robbins et al., 1989). The prevalence and importance of risk factors, and associated falls prevention strategies, may vary by setting or clinical profile. Further, falls history may dominate among risk factors when frequent fallers are included, making it difficult to identify contributing factors associated with new or first-time falls. Relatively few studies have examined what factors predispose older adults with dementia or PD to falls (Baltadjieva, Giladi, Gruendlinger, Peretz, & Hausdorff, 2006; Buchner & Larson, 1987; Voss et al., 2012), or whether all fallers have clusters of similar risk factors regardless of dementia or PD.
This study aimed to identify risk factors for falls among HC clients with no recent history of falls, and to explore whether risk factors were different in individuals with dementia or parkinsonism, compared to individuals with neither of these conditions.
Methods
Study Design and Sample
A retrospective cohort design was employed with secondary analysis of Resident Assessment Instrument for Home Care (RAI-HC) data for HC clients receiving services in Ontario for at least 60 consecutive days (i.e., long-stay clients). In Ontario, HC services are accessed through 14 different Community Care Access Centres (CCACs). The CCACs are local agencies that help people in Ontario access home and community care services, as well as long-term care, by coordinating and arranging for these services. Each CCAC has its own distinct geographic boundary within the province to identify the area they service. The level and types of services a client receives are determined by care coordinators, who are trained to complete the RAI-HC within seven days of a client’s admission to HC and semi-annually thereafter for each long-stay client (Carpenter & Hirdes, 2013; Morris et al., 1997). Clients were excluded if they did not have at least two consecutive assessments completed during their most recent admission period, and if the length of time between those two assessments was > 365 days. An admission period, or episode of care, refers to the period of time between when a client is admitted for home care and when a client is subsequently discharged. A single client may have multiple admission periods.
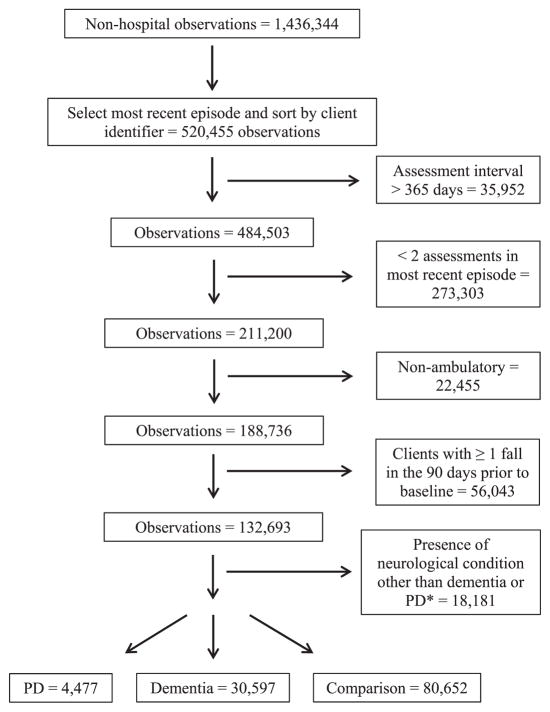
Non-ambulatory clients were excluded because preliminary analyses revealed that wheelchair use significantly reduced the risk for falls. A client was defined as ambulatory if any of the following were checked off for the “primary mode of locomotion indoors” item at baseline: no assistive device used, cane used, or walker/ crutch used. Only clients who had not experienced a fall 90 days prior to their second most recent assessment within the dataset (hereafter referred to as “baseline”) were included in our study ( Figure 1 ).
Figure 1. Flow diagram depicting selection of clients used for analyses.
PD = Parkinson’s disease
*Other neurological conditions: Huntington’s disease, muscular dystrophy, epilepsy, cerebral palsy, traumatic brain injury, spinal cord injury, stroke, multiple sclerosis, and amyotrophic lateral sclerosis.
Diagnoses were determined on the basis of a list of conditions on a client’s most recent RAI-HC in the database that were indicated as present according to physician records, hospitalization due to the condition within the past 90 days, or whether treatment/monitoring was required by an HC professional. Previous research supports the validity for RAI-HC diagnostic data relative to hospital administrative records (Foebel et al., 2013). An HC client was considered to have dementia if the “Alzheimer’s disease” or “dementia other than Alzheimer’s” items from the RAI-HC were selected. Clients were considered to have parkinsonism if the “parkinsonism” item from the Disease Diagnoses section was selected. The comparison group did not have a diagnosis of any of the following neurological conditions: dementia, parkinsonism, Huntington’s disease, muscular dystrophy, epilepsy, cerebral palsy, traumatic brain injury, spinal cord injury, stroke, multiple sclerosis, and amyotrophic lateral sclerosis. These conditions were selected based on priority conditions identified in previous research supported by the Public Health Agency of Canada (Caesar-Chavannes and Macdonald, 2013).
Measures
The RAI-HC is a validated, reliable assessment instrument (Hirdes et al., 2008; Landi et al., 2000; Morris et al., 1997; Poss et al., 2008) used to assess all long-stay HC clients in Ontario. The assessment is completed by case managers who help coordinate home care services based on determining a client’s needs and eligibility for services. The RAI-HC captures various aspects of health status, such as disease diagnoses, physical function measures, cognitive/behavioural status, and service utilization, to aid in care planning and quality monitoring by a client’s case manager. The study dataset included longitudinal RAI-HC assessments for clients who received CCAC services from January 1, 2002, to December 31, 2010. This study received approval from the University of Waterloo’s Office of Research Ethics (Project # 18324).
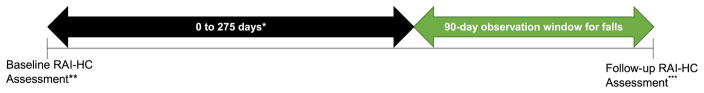
Potential risk factors for falls were selected on the basis of previous research (Deandrea et al., 2010; Fletcher & Hirdes, 2002; Lewis, Moutoux, Slaughter, & Bailey, 2004). With the exception of other neurological conditions, which were ascertained from the client’s most recent assessment in their latest admission period, risk factors were ascertained from each client’s baseline RAI-HC assessment, defined as the second last assessment in their most recent episode of care (see Figure 2 ).
Figure 2. Observation windows and assessments used in multivariable and descriptive analyses.
* Depending on the length of time between assessments, which was limited to a maximum of 365 days.
** The second most recent assessment in the client’s most recent admission period identified between January 1, 2002, and December 31, 2010. This assessment was used to identify risk factors for falls.
*** The most recent assessment in the client’s most recent admission period identified between January 1, 2002, and December 31, 2010. This assessment was used to determine whether falls had occurred in the previous 90 days and to determine the major diagnostic categories of interest (i.e., dementia, parkinsonism, and no neurological conditions).
Sociodemographic and Extrinsic Factors
Sociodemographic risk factors examined were age group, gender, and living arrangement (i.e., whether the client had informal support and time spent alone during the day). Environmental hazards in the home, such as loose carpeting, were categorized according to number of hazards: 0–1 and ≥ 2, based on a previous study (Fletcher & Hirdes, 2002 ).
Health Characteristics and Medication Use
Health conditions examined included arthritis, cardiovascular disease, diabetes, dizziness, other neurological conditions, visual impairment, self-rated health, unsteady gait, and pain. The presence of daily pain (and severity level) was captured by the validated interRAI Pain Scale Score (Fries, Simon, Morris, Flodstrom, & Bookstein, 2001). A single variable was created for cardiovascular conditions that included any of the following conditions: hypertension, congestive heart failure, and peripheral vascular disease from the Disease Diagnoses section of the RAI-HC. The presence of other neurological conditions listed above was included as a binary risk factor for falls (present/absent) in those with dementia and/ or parkinsonism because it was hypothesized that co-morbid diagnosis of another neurological condition(s) could further increase the risk of falls in these groups. Medication exposure in the seven days prior to baseline was captured both by drug number (9+ drugs, including over-the-counter and/or prescribed medications) and by binary variables indicating the presence/ absence of drug classes (including antipsychotics, anxiolytics, antidepressants, hypnotics, and analgesics).
Embedded in the RAI-HC assessment is the Changes in Health, End-stage disease, and Symptoms and Signs (CHESS) scale, a measure of health instability ranging from 0 (none) to 5 (very high) (Hirdes, Frijters, & Teare, 2003). The CHESS score has been shown to have comparable performance to other frailty measures for predicting adverse outcomes in HC clients (Armstrong, Stolee, Hirdes, & Poss, 2010 ). In addition, it has been shown to be predictive of mortality among persons with neurological conditions in facility and community-based care settings (Hirdes, Poss, Mitchell, Korngut, & Heckman, 2014).
Mental Health and Behavioural Factors
Mental health and behavioural risk factors examined included depressive symptoms and wandering. The categories for the wandering item in the RAI-HC were collapsed as: “any wandering in the last 3 days” versus “no wandering in the last 3 days.” Wandering aimlessly without supervision may increase the likelihood that clients with PD or unsteady gait will fall. Robinovitch et al. (2013) reported that in long-term care homes, forward walking was the activity most frequently performed by residents prior to a fall. Depressive symptoms were captured with the RAI Depression Rating Scale (DRS) (Burrows, Morris, Simon, Hirdes, & Phillips, 2000; Szczerbinska, Hirdes, & Zyczkowska, 2012). The DRS ranges from 0–14 with a cutpoint of ≥ 3 used to indicate the presence of clinically meaningful depressive symptoms.
Physical and Cognitive Function Factors
In addition to the CHESS and DRS scales, other validated functional scales embedded within the RAI-HC assessment and examined here included (a) the Activities of Daily Living (ADL) Self-Performance Hierarchy Scale (range 0 = independent to 6 = total dependence) (Landi et al., 2000; Morris, Fries, & Morris, 1999) and (b) the Cognitive Performance Scale (CPS; range 0 = cognitively intact to 6 = very severe cognitive impairment) (Gruber-Baldini, Zimmerman, Mortimore, & Magaziner, 2000; Landi et al., 2000). All interRAI scales we used in this study were collapsed using cutpoints reported in previous work (Hirdes, Mitchell, Maxwell, & White, 2011 ). A client’s difficulty climbing stairs was captured by the “stair climbing” item; the categories were collapsed to two levels: “without help” and “with help/did not occur”. Bladder and bowel incontinence were also examined as risk factors for falls. Worsening bladder continence was included to determine if worsening incontinence predicted falls better than absolute level of incontinence.
The outcome was whether or not the client had fallen 90 days prior to their most recent assessment (hereafter referred to as “follow-up”), which was obtained from the “number of falls in the last 90 days” item on their follow-up assessment. Although the provincial standard for completing assessments is an initial assessment within seven days of being approved for home care services and once every six months thereafter, the time span between assessments varies based on the clinical complexity of the client or any major changes in the client’s health, with more complex clients typically assessed more frequently. The median (25th–75th percentile) for the length of time between the assessments included in these analyses was 194 (156–240) days. Figure 2 shows a summary of the observation windows and assessments used in the analyses.
Statistical Analysis
We used descriptive statistics to present baseline characteristics for each group. Within each diagnostic group, we used odds ratios to identify factors that significantly increased falls at the bivariate level. Significant risk factors at the bivariate level were then entered into a generalized estimating equation (GEE) model, with an exchangeable correlation matrix, to determine factors that predicted falls. We used a GEE rather than logistic regression to account for correlations among data within geographic regions (e.g., due to similar assessment practices within CCACs). An α= 0.01 was set for statistical significance at the bivariate and multivariable levels because of the large sample size. We tested final models for collinearity using the condition index, with a condition index of ≥ 30 to indicate the presence of severe collinearity requiring removal of one of the collinear variables from the GEE model.
We only included age and gender in multivariable models if they improved the model and were significant correlates of being a faller. Since the assessment interval was inconsistent, though normally distributed, it may have affected the falls outcome because a longer time period would provide more opportunity to be classified as a faller. Therefore, we included the assessment interval as a covariate in the GEE models as long as it improved the model and was statistically significant. All statistics were performed using SAS version 9.2.
Results
Table 1 presents the baseline characteristics for all three diagnostic groups and the percentage of clients who fell within each category for each baseline characteristic. The proportion of clients experiencing a fall during follow-up among HC clients in the dementia (n = 30,597), parkinsonism (n = 4,477), and the comparison groups (n = 80, 652) was 21.9 per cent, 32.2 per cent, and 19.1 per cent, respectively.
Table 1.
Baseline characteristics and distribution of falls outcome among ambulatory home care clients in each diagnostic group
| Characteristics | Dementia % (n) | Falls in Dementia % (n) | Parkinsonism% (n) | Falls in Parkinsonism % (n) | Comparison % (n) | Falls in Comparison % (n) |
|---|---|---|---|---|---|---|
| n | 30,597 | 21.9 (6,697) | 4,477 | 32.2 (1,443) | 80,652 | 19.1 (15,400) |
| Age (years) | ||||||
| < 65 | 2.8 (842) | 17.6 (148) | 4.6 (207) | 33.3 (69) | 15.6 (12,539) | 13.6 (1,700) |
| ≥ 65 to < 75 | 10.4 (3,191) | 17.4 (555) | 17.9 (799) | 32.3 (258) | 13.9 (11,240) | 16.3 (1,827) |
| ≥ 75 to < 85 | 46.2 (14,141) | 21.7 (3,064) | 50.3 (2,253) | 31.3 (706) | 33.4 (26,947) | 19.0 (5,129) |
| ≥85 to < 95 | 37.8 (11,559) | 23.6 (2,723) | 25.8 (1,156) | 34.3 (396) | 32.9 (26,558) | 22.2 (5,890) |
| ≥ 95 | 2.8 (858) | 23.5 (202) | 1.4 (61) | 23.0 (14) | 4.2 (3,353) | 25.4 (851) |
| Female | 65.1 (19,919) | 21.0 (4,189) | 51.5 (2,304) | 30.9 (711) | 70.5 (56,901) | 19.2 (10,949) |
| ≥ 2 Environmental Hazards | 2.0 (597) | 26.5 (158) | 3.5 (157) | 30.6 (48) | 2.8 (2,252) | 21.1 (475) |
| Time Client Is Alone Daily | ||||||
| Never / Hardly Ever | 50.8 (15,535) | 21.9 (3,408) | 47.4 (2,123) | 31.4 (667) | 31.7 (25,536) | 17.9 (4,581) |
| About One Hour | 16.2 (4,956) | 22.9 (1,137) | 17.1 (764) | 33.3 (254) | 10.9 (8,795) | 19.6 (1,720) |
| Long Periods of Time | 25.5 (7,808) | 21.8 (1,702) | 24.9 (1,116) | 33.3 (372) | 35.1 (28,330) | 20.1 (5,688) |
| Always | 7.5 (2,298) | 19.6 (450) | 10.6 (474) | 31.7 (150) | 22.3 (17,990) | 19.0 (3,410) |
| Absence of Informal Support Vision | 0.8 (254) | 13.0 (33) | 1.4 (64) | 25.0 (16) | 2.9 (2,366) | 14.2 (335) |
| Adequate | 73.4 (22,449) | 20.9 (4,681) | 67.8 (3,036) | 32.4 (983) | 73.9 (59,589) | 18.2 (10,832) |
| Impaired / Moderately Impaired | 23.0 (7,037) | 24.6 (1,734) | 28.6 (1,279) | 32.1 (410) | 22.0 (17,737) | 21.6 (3,839) |
| Highly / Severely Impaired | 3.6 (1,110) | 25.4 (282) | 3.6 (162) | 30.9 (50) | 4.1 (3,324) | 21.9 (728) |
| Diabetes | 18.9 (5,775) | 22.5 (1,297) | 21.0 (941) | 34.0 (320) | 25.1 (20,202) | 20.4 (4,124) |
| Arthritis | 42.7 (13,072) | 23.8 (3,109) | 49.7 (2,227) | 31.8 (708) | 57.1 (46,058) | 20.5 (9,437) |
| Hip Fracture | 2.7 (813) | 23.2 (189) | 4.1 (182) | 29.1 (53) | 3.8 (3,054) | 20.5 (625) |
| Cardiovascular Condition(s) | 64.4 (19,700) | 22.6 (3,882) | 62.3 (2,790) | 31.8 (758) | 71.1 (57,315) | 20.0 (10,255) |
| Unsteady Gait | 44.8 (13,693) | 26.9 (3,684) | 77.2 (3,454) | 34.0 (1,175) | 51.4 (41,414) | 22.4 (9,275) |
| Pain Scale Scorea | ||||||
| 0 | 58.8 (17,983) | 20.4 (3,672) | 39.5 (1,768) | 33.5 (593) | 30.3 (24,458) | 17.5 (4,280) |
| 1–2 | 38.0 (11,639) | 23.7 (2,760) | 51.2 (2,290) | 31.5 (722) | 56.1 (45,206) | 19.4 (8,784) |
| 3 | 3.2 (972) | 27.2 (264) | 9.4 (419) | 30.6 (128) | 13.6 (10,978) | 21.3 (2,335) |
| CHESS Scoreb | ||||||
| 0 | 35.3 (10,787) | 20.9 (2,252) | 36.8 (1,647) | 31.3 (516) | 39.1 (31,515) | 17.1 (5,384) |
| 1–2 | 56.0 (17,125) | 22.1 (3,787) | 56.0 (2,508) | 32.5 (814) | 52.7 (42,486) | 20.0 (8,487) |
| 3–5 | 8.8 (2,682) | 24.5 (657) | 7.2 (322) | 35.1 (113) | 8.2 (6,648) | 23.0 (1,529) |
| Dizziness or Lightheadedness | 8.9 (2,714) | 27.6 (748) | 17.4 (779) | 34.4 (268) | 14.7 (11,812) | 21.8 (2,574) |
| Parkinsonism | 4.0 (1,214) | 34.5 (419) | 100.0 (4,477) | 32.2 (1,443) | N/A | N/A |
| Dementia | 100.0 (30,597) | 21.9 (6,697) | 27.1 (1,214) | 34.5 (419) | N/A | N/A |
| Other Neurological Condition(s) | 18.1 (5,532) | 24.5 (1,356) | 17.3 (776) | 30.9 (240) | N/A | N/A |
| Poor Self-Rated Health | 6.6 (2,029) | 26.7 (542) | 22.6 (1,010) | 30.1 (304) | 20.5 (16,514) | 20.7 (3,414) |
| Fear of Falling | 30.5 (9,344) | 26.5 (2,474) | 55.0 (2,464) | 33.6 (829) | 37.7 (30,400) | 22.0 (6,678) |
| Bladder Continence | ||||||
| Continent / Continent with Catheter | 51.3 (15,692) | 19.1 (3,004) | 47.0 (2,102) | 31.1 (654) | 67.2 (54,230) | 17.3 (9,354) |
| Usually Continent / Occasionally Incontinent | 25.5 (7,811) | 24.1 (1,879) | 29.7 (1,329) | 32.6 (433) | 21.3 (17,212) | 22.2 (3,821) |
| Frequently Incontinent / Completely | 23.2 (7,094) | 25.6 (1,814) | 23.4 (1,046) | 34.0 (356) | 11.4 (9,211) | 24.2 (2,225) |
| Worsening Bladder Continence | 14.1 (4,307) | 26.9 (1,158) | 13.3 (595) | 37.7 (224) | 6.4 (5,144) | 24.4 (1,255) |
| Bowel Continence | ||||||
| Continent / Continent with Ostomy | 77.8 (23,807) | 21.2 (5,036) | 85.4 (3,822) | 32.5 (1,243) | 92.2 (74,345) | 18.8 (13,939) |
| Usually Continent / Occasionally Incontinent | 14.6 (4,457) | 24.7 (1,101) | 10.1 (454) | 32.2 (146) | 5.7 (4,579) | 23.8 (1,091) |
| Frequently Incontinent / Completely Incontinent / Did Not Occur | 7.6 (2,330) | 24.0 (558) | 4.5 (201) | 26.9 (54) | 2.1 (1,727) | 21.4 (370) |
| ≥ 9 Medications | 37.5 (11,487) | 24.5 (2,810) | 53.5 (2,393) | 33.3 (796) | 48.2 (38,844) | 21.1 (8,212) |
| Medications | ||||||
| Antipsychotic / Neuroleptic | 21.5 (6,582) | 22.1 (1,456) | 15.4 (691) | 32.3 (223) | 5.4 (4,337) | 19.2 (834) |
| Anxiolytic | 12.8 (3,920) | 22.9 (899) | 18.5 (830) | 32.1 (266) | 18.0 (14,506) | 20.8 (3,010) |
| Antidepressant | 27.0 (8,251) | 23.7 (1,957) | 29.6 (1,325) | 34.3 (454) | 19.0 (15,333) | 22.9 (3,512) |
| Hypnotic or Analgesic | 10.1 (3,083) | 23.8 (734) | 13.8 (619) | 31.8 (197) | 13.8 (11,102) | 20.8 (2,313) |
| Wandering | 9.9 (3,030) | 22.5 (683) | 2.7 (119) | 45.4 (54) | 0.3 (27) | 21.5 (58) |
| DRS Scorec | ||||||
| 0 | 59.0 (18,038) | 21.6 (3,894) | 60.4 (2,705) | 32.9 (891) | 67.5 (54,449) | 18.5 (10,047) |
| 1–2 | 25.8 (7,886) | 22.2 (1,751) | 24.3 (1,086) | 30.9 (336) | 20.5 (16,532) | 19.9 (3,291) |
| 3+ | 15.3 (4,668) | 22.5 (1,051) | 15.3 (686) | 31.5 (216) | 12.0 (9,650) | 21.3 (2,056) |
| ADLs Scored | ||||||
| 0 | 47.3 (14,478) | 20.4 (2,957) | 49.0 (2,194) | 30.6 (671) | 80.5 (64,880) | 18.6 (12,067) |
| 1–2 | 39.6 (12,108) | 22.8 (2,763) | 35.9 (1,608) | 34.2 (550) | 15.9 (12,830) | 21.7 (2,778) |
| 3–4 | 12.4 (3,780) | 24.8 (938) | 13.8 (619) | 33.0 (204) | 3.4 (2,750) | 19.3 (531) |
| 5–6 | 0.8 (229) | 17.0 (39) | 1.3 (56) | 32.1 (18) | 0.2 (188) | 11.7 (22) |
| ADLs Decline | 37.0 (11,309) | 23.2 (2,619) | 38.9 (1,740) | 32.9 (572) | 28.0 (22,543) | 20.9 (4,718) |
| CPS Scoree | ||||||
| 0 | 1.5 (467) | 23.3 (109) | 31.0 (1,388) | 28.8 (400) | 63.8 (51,488) | 17.4 (8,962) |
| 1–2 | 58.2 (17,812) | 22.1 (3,931) | 54.4 (2,437) | 34.5 (841) | 33.7 (27,167) | 22.3 (6,057) |
| 3–4 | 26.5 (8,118) | 21.3 (1,729) | 9.8 (438) | 30.1 (132) | 1.8 (1,422) | 20.4 (290) |
| 5–6 | 13.7 (4,199) | 22.1 (928) | 4.8 (214) | 32.7 (70) | 0.7 (574) | 15.9 (91) |
| Worsening Decision Making Stair Climbing | 38.0 (11,619) | 21.4 (2,489) | 15.6 (698) | 33.7 (235) | 5.7 (4,571) | 24.4 (1,116) |
| Without Help | 47.4 (14,485) | 18.8 (2,723) | 29.7 (1,331) | 31.4 (418) | 43.9 (35,385) | 16.7 (5,912) |
| With Help / Did Not Occur | 52.7 (16,109) | 24.7 (3,974) | 70.3 (3,146) | 32.6 (1,025) | 56.1 (45,261) | 21.0 (9,486) |
| Assessment interval (days)f | 181.9 (77.2) | N/A | 188.4 (75.1) | N/A | 197.7 (74.7) | N/A |
Range 0–3;
Changes in Health, End-stage disease, Signs, and Symptoms Scale; range 0–5;
Depression Rating Scale; range 0–14;
Activities of Daily Living Hierarchy Scale; range 0–6;
Cognitive Performance Scale; range 0–6;
Mean (SD)
N/A = not applicable
Unsteady gait was a common and strong predictor of being a faller across all three subgroups (see Tables 2, 3 and 4 ). In addition, older age, male gender, antidepressant use, bladder incontinence, and the use of ≥ 9 medications significantly increased the odds of falling in both the dementia and comparison groups. Further, the presence of borderline intact to mild cognitive impairment (CPS scores 1–2) increased the odds of falling in the parkinsonism (OR: 1.27; 95% CI: 1.09–1.48), and comparison groups (OR: 1.15; 95% CI: 1.11–1.19).
Table 2.
Final adjusted model for ambulatory home care clients with dementiaa
| Risk Factor | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Age (years) | ||
| < 65 | 1.07 (0.85–1.34) | 0.58 |
| ≥ 65 to < 75 (reference) | 1.00 | |
| ≥ 75 to < 85 | 1.26 (1.16–1.37) | < 0.001 |
| ≥ 85 to < 95 | 1.35 (1.24–1.46) | < 0.001 |
| ≥ 95 | 1.31 (1.06–1.63) | 0.01 |
| Male (reference: female) | 1.20 (1.15–1.25) | < 0.001 |
| Absence of Informal Support (reference: presence of support) | 0.59 (0.40–0.87) | 0.007 |
| Parkinsonism (reference: not present) | 1.63 (1.45–1.84) | < 0.001 |
| Unsteady Gait (reference: not present) | 1.43 (1.35–1.50) | < 0.001 |
| Arthritis (reference: not present) | 1.09 (1.05–1.13) | < 0.001 |
| Bladder Continence | ||
| Continent / Continent with Catheter (reference) | 1.00 | |
| Usually Continent / Occasionally Incontinent | 1.17 (1.10–1.25) | < 0.001 |
| Frequently Incontinent / Completely Incontinent / Did Not Occur | 1.19 (1.14–1.26) | < 0.001 |
| Worsening Bladder Continence (reference: not present) | 1.16 (1.10–1.23) | < 0.001 |
| Dizziness or Lightheadedness (reference: not present) | 1.23 (1.09–1.38) | < 0.001 |
| ≥ 9 Medications (reference: 0–8 medications) | 1.11 (1.07–1.14) | < 0.001 |
| Antidepressant (reference: not present) | 1.11 (1.04–1.19) | 0.003 |
| ADL Scoreb | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.01 (0.95–1.08) | 0.75 |
| 3–4 | 0.99 (0.89–1.10) | 0.88 |
| 5–6 | 0.58 (0.41–0.82) | 0.002 |
| Stair Climbing (reference: no difficulty climbing) | 1.14 (1.07–1.22) | < 0.001 |
Adjusted for assessment interval;
Activities of Daily Living Hierarchy Scale; range 0–6
Table 3.
Final model for ambulatory home care clients with parkinsonism
| Risk Factor | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Unsteady Gait (reference: not present) | 1.46 (1.26–1.68) | < 0.001 |
| CPS Scorea | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.27 (1.09–1.48) | 0.002 |
| 3–4 | 0.97 (0.75–1.26) | 0.82 |
| 5–6 | 1.08 (0.80–1.46) | 0.59 |
| Wandering (reference: no wandering) | 1.94 (1.21–3.10) | 0.006 |
Cognitive Performance Scale; range 0–6
Table 4.
Final adjusted model for ambulatory home care clients in the comparison groupa
| Risk Factor | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Age (years) | ||
| < 65 | 0.87 (0.79–0.95) | 0.002 |
| ≥ 65 to < 75 (reference) | 1.00 | |
| ≥ 75 to < 85 | 1.18 (1.13–1.24) | < 0.001 |
| ≥ 85 to < 95 | 1.42 (1.37–1.47) | < 0.001 |
| ≥ 95 | 1.69 (1.56–1.83) | < 0.001 |
| Male (reference: female) | 1.12 (1.07–1.18) | < 0.001 |
| Absence of Informal Support (reference: presence of support) | 0.81 (0.74–0.89) | < 0.001 |
| Time Client Is Alone Daily | ||
| Never or Hardly Ever (reference) | 1.00 | |
| About One Hour | 1.05 (1.00–1.09) | 0.05 |
| Long Periods of Time | 1.13 (1.08–1.18) | < 0.001 |
| Always | 1.07 (1.02–1.12) | 0.002 |
| Unsteady Gait (reference: not present) | 1.31 (1.27–1.35) | < 0.001 |
| CHESS Scoreb | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.07 (1.04–1.11) | < 0.001 |
| 3–5 | 1.17 (1.09–1.25) | < 0.001 |
| Bladder Continence | ||
| Continent / Continent with Catheter (reference) | 1.00 | |
| Usually Continent / Occasionally Incontinent | 1.17 (1.11–1.23) | < 0.001 |
| Frequently Incontinent / Completely Incontinent / Did Not Occur | 1.28 (1.23–1.34) | < 0.001 |
| Pain Scale Scorec | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.05 (1.02–1.09) | 0.006 |
| 3 | 1.16 (1.10–1.23) | <0.001 |
| Vision | ||
| Adequate (reference) | 1.00 | |
| Impaired / Moderately Impaired | 1.06 (1.03–1.10) | < 0.001 |
| Highly / Severely Impaired | 1.09 (1.01–1.18) | 0.03 |
| Dizziness or Lightheadedness (reference: not present) | 1.10 (1.06–1.14) | < 0.001 |
| Diabetes (reference: not present) | 1.12 (1.08–1.16) | < 0.001 |
| ≥ 9 Medications (reference: 0–8 medications) | 1.11 (1.08–1.15) | < 0.001 |
| Antidepressant (reference: not present) | 1.33 (1.27–1.39) | < 0.001 |
| CPS Scored | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.15 (1.11–1.19) | < 0.001 |
| 3–4 | 1.15 (1.05–1.25) | 0.002 |
| 5–6 | 1.05 (0.78–1.41) | 0.73 |
| ADL Scoree | ||
| 0 (reference) | 1.00 | |
| 1–2 | 1.01 (0.98–1.05) | 0.40 |
| 3–4 | 0.86 (0.77–0.97) | 0.02 |
| 5–6 | 0.55 (0.37–0.83) | 0.004 |
Adjusted for assessment interval and gender;
Changes in Health, End-stage disease, Signs, and Symptoms Scale; range 0–5;
Range 0–3;
Cognitive Performance Scale; range 0–6;
Activities of Daily Living Hierarchy Scale; range 0–6
A co-morbid diagnosis of parkinsonism was the strongest predictor of falls among ambulatory clients with dementia (OR: 1.63; 95% CI: 1.45–1.84), followed by unsteady gait (OR: 1.43; 95% CI: 1.35–1.50). Being dependent on others to perform ADLs (ADLs score 5–6) significantly reduced the odds of falling (OR: 0.58; 95% CI: 0.41–0.82), as did the absence of an informal caregiver (OR: 0.59; 95% CI: 0.40–0.87).
Wandering behaviour almost doubled the odds of falling in ambulatory clients with parkinsonism (OR: 1.94; 95% CI: 1.21–3.10). Among those with parkinsonism, compared to cognitively intact, ambulatory clients, those with borderline intact to mild cognitive impairment (CPS scores 1–2) were at significantly increased odds of falling (OR: 1.27; 95% CI: 1.09–1.48), while those with severe to very severe cognitive impairment (CPS scores 5–6) were not at risk (OR: 1.08; 95% CI: 0.80–1.46). In the comparison group, being dependent on others to perform ADLs (ADL score 5–6) reduced the odds of falling (OR: 0.55; 95% CI: 0.37–0.83).
We conducted a sensitivity analysis to determine whether the findings would be different if clients with other neurological conditions were removed from the dementia and parkinsonism groups. In the dementia group, the ORs for the variables absence of informal support, dizziness, and arthritis were lower than in the original analysis and did not cross 1, but were not statistically significant at the p < .01 level. All other variables remained statistically significant (results not shown).
Discussion
This study reveals that among HC clients, individuals with parkinsonism have the highest proportion of fallers among those without a recent history of falls, and risk factors for falls among individuals with parkinsonism may differ compared to individuals with dementia, or without these neurological conditions. Further, a co-morbid diagnosis of parkinsonism was the strongest predictor of falls in the dementia group. Therefore, it may be prudent to consider individuals with parkinsonism at high risk of falls, including those with dementia and co-existing parkinsonism. Importantly, unsteady gait was a common and strong predictor of falls even in the absence of a reported falls history. Mild but not severe cognitive impairment was a common risk factor in the parkinsonism and comparison groups. Other risk factors – including bladder incontinence, antidepressant use, use of ≥ 9 medications, male gender, and age – increased the odds of falling in the dementia and comparison groups.
Unsteady gait is likely to represent an important marker of imminent falls among HC clients who have not fallen recently. The association between unsteady gait and falls was greater among subgroups with dementia and parkinsonism than in clients without these conditions. Individuals with PD often have gait asymmetry, short strides, and increased stride time duration, all of which tend to worsen as their disease progresses (Baltadjieva et al., 2006). The link between dementia and impaired gait is likely mediated, in part, by impaired executive function that may lead to falls in situations where dual tasking is required and attentional demands are higher. Older adults with mild cognitive impairment and Alzheimer’s disease have significantly decreased gait velocity, increased stride time, and increased stride time variability under dual-task conditions in contrast to cognitively intact older adults (Muir et al., 2012). These findings suggest that unsteady gait is an important risk factor to target for falls prevention programs in HC settings.
Ambulatory HC clients with parkinsonism had a unique profile of risk factors compared to the other two groups in that relatively few risk factors were associated with falls. Moreover, wandering – a behaviour likely associated with co-morbid cognitive impairment, or behavioural sleep disorders common among people with PD (Roychowdhury & Forsyth, 2012; Thorpy, 2004) – was a predictor of falls only in those with parkinsonism. Interestingly, mild cognitive impairment but not co-morbid dementia diagnosis was associated with increased odds of falling in those with parkinsonism. A similar phenomenon was reported in a study of nursing home residents, and it was suggested that this is likely because those with severe cognitive impairment are less likely to be moving around and performing ADLs themselves and are therefore less likely to fall than older adults who maintain some level of functioning but are still mildly impaired (Nazir, Mueller, Perkins, & Arling, 2012). Together, the profile of risk factors for the parkinsonism group suggests a link between certain aspects of gait and cognition that may be compounded in people with neurodegenerative disorders such as parkinsonism and some forms of dementia, thus increasing their risk for falls.
Variability in the factors that contribute to falling among HC clients may be explained by the impairments present. For example, the use of ≥ 9 medications and unsteady gait were more prevalent among HC clients in the comparison group than clients in the dementia group and increased the odds of falling. The relationship between polypharmacy and falls is poorly understood. Some hypothesize that taking more medications increases the likelihood of taking psychotropic medications, such as antidepressants, which are known to increase falls risk (Ziere et al., 2006). Others suggest that taking multiple medications is associated with having multiple conditions that are related to falls (Husson et al., 2013). Being male or experiencing bladder incontinence increased the odds of falling in the dementia and comparison groups but not in the parkinsonism group. Our observed association between gender and falls is consistent with a previous study of HC clients that included individuals with and without dementia (Fletcher & Hirdes, 2002).
Some researchers attribute higher odds of falling among older males to their risk-taking behaviours, or to the fact that women may be more likely to limit their activity due to fear of falling (Fletcher & Hirdes, 2002 ). The presence of higher levels of bladder incontinence was also a relatively strong predictor of falls in the comparison and dementia groups only, and this has previously been reported in individuals with and without dementia (Foley et al., 2012; Lee et al., 2011). Incontinence is likely related to falls through older adults’ rushing to the bathroom to avoid episodes of incontinence (Brown et al., 2000). However, there is no evidence yet that incontinence programs may reduce the risk of falls among HC clients.
The strengths of this study lie in its large sample size and use of data from comprehensive, standardized health assessments completed by trained health professionals. This is one of the few studies that focused on HC clients and excluded clients who were non-ambulatory and clients with a recent history of falls, which, in addition to the relatively short 90-day period for observation of falls, may explain the relatively small odds ratios reported here in contrast to other studies that included individuals with a history of falls or a follow-up period of six months to one year (Lundebjerg et al., 2001). Nonetheless, it is important to determine whether risk factors for future falls can be identified among persons who have not fallen previously. The present results suggest that future falls are not entirely stochastic events among HC clients and that assessments like the RAI-HC may be used to identify potentially modifiable risk factors for falls among HC clients that might cluster in subgroups of individuals, such as clients with dementia and parkinsonism. We attempted to examine, in individuals with no recent fall, whether certain factors cluster in individuals with parkinsonism and dementia and differ from the cluster of factors that increase the risk for falls among older adults without certain neurological conditions. The results of this study can help inform whether risk modification strategies can be applied globally to all older adults, or if they should be tailored somewhat based on clinical presentation. The RAI-HC can be used to monitor changes in the modifiable risk factors we identified in this study over time in order to allocate appropriate falls prevention strategies to clients who may need them the most.
A few limitations should be acknowledged. The RAI-HC only captures falls 90 days prior to an assessment, suggesting we did not capture all falls given that the median time between assessments was 194 days; however, this limitation is addressed in newer versions of the instrument not yet used in Ontario. Therefore, it is possible that individuals with only one fall occurring soon after the first assessment would be misclassified as a non-faller, and individuals with recurrent falls would be over-represented in the “fallers” group. This study did not capture the time since diagnosis or the stage of parkinsonism, nor did it distinguish subtypes of dementia. The type or stage of neurologic disease may impact the level of physical impairment that contributes to falls (Aarsland & Kurz, 2010). The RAI-HC captures parkinsonism, which includes PD and secondary parkinsonism, suggesting that these results apply only to ambulatory HC clients with parkinsonism rather than to clients with PD. The external validity of the study may have been compromised by the exclusion of clients who had only a single assessment and by the selection of the most recent admission period, as this represents a different time point for each client within the HC system.
Identifying risk factors for falls is important for creating targeted falls prevention programs; however, there is little evidence to suggest that falls can be prevented in older adults with dementia (Oliver et al., 2007; Winter, Watt, & Peel, 2013). People with dementia may require more encouragement and individual supervision to adhere to an exercise program for fall prevention (Mirolsky-Scala, & Kraemer, 2009; Suttanon, Hill, Said, Byrne, & Dodd, 2012), which ultimately may lead to an increased burden on informal caregivers and increased costs associated with preventing falls in this population. Moreover, very few studies have been conducted on fall prevention interventions for people with PD, and among those that have, none have shown statistically significant reductions in falls following the intervention (Allen et al., 2010; Ashburn et al., 2007; Goodwin et al., 2011; Protas et al., 2005). However, there is moderate evidence that physical activity and exercise can lessen postural instability and improve balance task performance in those with mild to moderate PD (Dibble, Addison, & Papa, 2009), suggesting that if these are risk factors for falls, then falls could theoretically be prevented by improvements in these domains.
In summary, ambulatory clients with parkinsonism and no recent falls history appear to be at a particularly high risk for their first fall, suggesting they should be a priority for falls prevention programs. Notably, older adults with parkinsonism may present with few risk factors compared to those without parkinsonism. Unsteady gait was a strong predictor of falls in all groups even in the absence of a falls history. Mild but not severe cognitive impairment increases the risk for falls. Other factors that may increase the odds of falling in clients with and without dementia include age, antidepressant use, use of ≥ 9 medications, male gender, and bladder incontinence. Additional research is needed to confirm these findings, with the goal of guiding falls prevention practices in HC in the future.
Footnotes
This study is part of the National Population Health Study of Neurological Conditions. Funding for the study was provided by the Public Health Agency of Canada. The opinions expressed in this publication are those of the authors/researchers and do not necessarily reflect the official views of the Public Health Agency of Canada. We acknowledge the Neurological Health Charities Canada for their contribution to the success of this project. John P. Hirdes holds the Ontario Home Care Research and Knowledge Exchange Chair funded by the Ontario Ministry of Health and Long Term Care. We gratefully acknowledge funding from the Schlegel-UW Research Institute for Aging, the Canadian Institutes of Health Research, and the Ontario Ministry of Research and Innovation.
References
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathology. 2010;20(3):633–639. doi: 10.1111/j.1750-3639.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JCT, … Fung VS. The effects of an exercise program on fall risk factors in people with Parkinson’s disease: A randomized controlled trial. Movement Disorders. 2010;25(9):1217–1225. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- Arfken CL, Lach HW, Birge SJ, Miller JP. The prevalence and correlates of fear of falling in elderly persons living in the community. American Journal of Public Health. 1994;84(4):565–570. doi: 10.2105/ajph.84.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JJ, Stolee P, Hirdes JP, Poss JW. Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age and Ageing. 2010;39(6):755–758. doi: 10.1093/ageing/afq121. [DOI] [PubMed] [Google Scholar]
- Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78(7):678–684. doi: 10.1136/jnnp.2006.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. European Journal of Neuroscience. 2006;24(6):1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- Brown JS, Vittinghoff E, Wyman JF, Stone KL, Nevitt MC, Ensrud KE, Grady D. Urinary incontinence: Does it increase risk for falls and fractures? Journal of the American Geriatrics Society. 2000;48(7):721–725. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB. Falls and fractures in patients with Alzheimer-type dementia. Journal of the American Medical Association. 1987;257(11):1492–1495. [PubMed] [Google Scholar]
- Burrows AB, Morris JN, Simon SE, Hirdes JP, Phillips C. Development of a minimum data set-based depression rating scale for use in nursing homes. Age and Ageing. 2000;29(2):165–172. doi: 10.1093/ageing/29.2.165. [DOI] [PubMed] [Google Scholar]
- Caesar-Chavannes CR, MacDonald S. Cross-Canada forum–national population health study of neurological conditions in Canada. Chronic Diseases and Injuries in Canada. 2013;33(3):188–191. [PubMed] [Google Scholar]
- Canadian Institute for Health Information. Preventing falls: From evidence to improvement in Canadian health care. 2014 Retrieved from https://www.accreditation.ca/sites/default/files/falls-joint-report-2014-en.pdf.
- Carpenter I, Hirdes JP. A good life in old age? Monitoring and Improving Quality in Long-Term Care. Paris, France: OECD; 2013. Using interRAI assessment systems to measure and maintain quality of long-term care; p. 93. [Google Scholar]
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: A systematic review and meta-analysis. Epidemiology. 2010;21(5):658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: A systematic review across the disability spectrum. Journal of Neurologic Physical Therapy. 2009;33(1):14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Hirdes JP. Risk factors for falling among community-based seniors using home care services. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57(8):M504–M510. doi: 10.1093/gerona/57.8.m504. [DOI] [PubMed] [Google Scholar]
- Foebel AD, Hirdes JP, Heckman GA, Kergoat MJ, Patten S, Marrie RA. Diagnostic data for neurological conditions in interRAI assessments in home care, nursing home and mental health care settings: A validity study. BMC Health Services Research. 2013;13(1):457. doi: 10.1186/1472-6963-13-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AL, Loharuka S, Barrett JA, Mathews R, Williams K, Mcgrother CW, Roe BH. Association between the geriatric giants of urinary incontinence and falls in older people using data from the Leicestershire MRC incontinence study. Age and Ageing. 2012;41(1):35–40. doi: 10.1093/ageing/afr125. [DOI] [PubMed] [Google Scholar]
- Friedman SM, Munoz B, West SK, Rubin GS, Fried LP. Falls and fear of falling: Which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. Journal of the American Geriatrics Society. 2002;50(8):1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x. [DOI] [PubMed] [Google Scholar]
- Fries BE, Simon SE, Morris JN, Flodstrom C, Bookstein FL. Pain in U.S. nursing homes: Validating a pain scale for the Minimum Data Set. Gerontologist. 2001;41(2):173–179. doi: 10.1093/geront/41.2.173. [DOI] [PubMed] [Google Scholar]
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: A pragmatic randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry. 2011;82(11):1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Zimmerman SI, Mortimore E, Magaziner J. The validity of the Minimum Data Set in measuring the cognitive impairment of persons admitted to nursing homes. Journal of the American Geriatrics Society. 2000;48(12):1601–1606. doi: 10.1111/j.1532-5415.2000.tb03870.x. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: A new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. 2003;51(1):96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Ljunggren G, Morris JN, Frijters DH, Finne Soveri H, Gray L, Gilgen R. Reliability of the interRAI suite of assessment instruments: A 12-country study of an integrated health information system. BMC Health Services Research. 2008;8:277. doi: 10.1186/1472-6963-8-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirdes JP, Mitchell L, Maxwell CJ, White N. Beyond the “iron lungs of gerontology”: Using evidence to shape the future of nursing homes in Canada. Canadian Journal on Aging. 2011;30(3):371–390. doi: 10.1017/S0714980811000304. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Poss JW, Mitchell L, Korngut L, Heckman G. Use of the interRAI CHESS scale to predict mortality among persons with neurological conditions in three care settings. PLoS One. 2014;9(6):e99066. doi: 10.1371/journal.pone.0099066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson N, Watfa G, Laurain M, Perret-Guillaume C, Niemier J, Miget P, Benetos A. Characteristics of polymedicated (≥ 4) elderly: A survey in a community-dwelling population aged 60 years and over. Journal of Nutrition, Health and Aging. 2013;18(1):87–91. doi: 10.1007/s12603-013-0337-8. [DOI] [PubMed] [Google Scholar]
- Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, … Bernabei R. Minimum data set for home care: A valid instrument to assess frail older people living in the community. Medical Care. 2000;38(12):1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Lee CY, Chen LK, Lo YK, Liang CK, Chou MY, Lo CC, … Lin YT. Urinary incontinence: An under-recognized risk factor for falls among elderly dementia patients. Neurological Urodynamics. 2011;30(7):1286–1290. doi: 10.1002/nau.21044. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Moutoux M, Slaughter M, Bailey SP. Characteristics of individuals who fell while receiving home health services. Physical Therapy. 2004;84(1):23–32. [PubMed] [Google Scholar]
- Lundebjerg N. Guideline for the prevention of falls in older persons. Journal of the American Geriatrics Society. 2001;49(5):664–672. [PubMed] [Google Scholar]
- Luukinen H, Koski K, Honkanen R, Kivela S. Incidence of injury-causing falls among older adults by place of residence: A population-based study. Journal of the American Geriatrics Society. 1995;43(8):871–876. doi: 10.1111/j.1532-5415.1995.tb05529.x. [DOI] [PubMed] [Google Scholar]
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. Journal of the American Geriatrics Society. 1994;42(3):269–274. doi: 10.1111/j.1532-5415.1994.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, … Hausdorff JM. Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PLoS ONE. 2012;7(6):1–8. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirolsky-Scala G, Kraemer T. Fall management in Alzheimer-related dementia: A case study. Journal of Geriatric Physical Therapy. 2009;32(4):181–189. doi: 10.1519/00139143-200932040-00007. [DOI] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54(11):M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Steel K, Ikegami N, Bernabei R, Carpenter GI, … Topinková E. Comprehensive clinical assessment in community setting: Applicability of the MDS-HC. Journal of the American Geriatrics Society. 1997;45(8):1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait and Posture. 2012;35(1):96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Nazir A, Mueller C, Perkins A, Arling G. Falls and nursing home residents with cognitive impairment: New insights into quality measures and interventions. Journal of the American Medical Directors Association. 2012;13(9):819.e1–819.e6. doi: 10.1016/j.jamda.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Oliver D, Connelly JB, Victor CR, Shaw FE, Whitehead A, Genc Y, … Gosney MA. Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: Systematic review and meta-analyses. British Medical Journal. 2007;334(7584):82–85. doi: 10.1136/bmj.39049.706493.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss JW, Jutan NM, Hirdes JP, Fries BE, Morris JN, Teare GF, Reidel K. A review of evidence on the reliability and validity of Minimum Data Set data. Healthcare Management Forum. 2008;21(1):33–39. doi: 10.1016/S0840-4704(10)60127-5. [DOI] [PubMed] [Google Scholar]
- Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20(3):183–190. [PubMed] [Google Scholar]
- Robbins AS, Rubenstein LZ, Josephson KR, Schulman BL, Osterweil D, Fine G. Predictors of falls among elderly people. Results of two population-based studies. Archives of Internal Medicine. 1989;149(7):1628–1633. [PubMed] [Google Scholar]
- Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, Loughin M. Video capture of the circumstances of falls in elderly people residing in long-term care: An observational study. The Lancet. 2013;381(9860):47–54. doi: 10.1016/S0140-6736(12)61263-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, Forsyth DR. Sleep disturbance in Parkinson disease. Journal of Clinical Gerontology and Geriatrics. 2012;3(2):53–61. [Google Scholar]
- Suttanon P, Hill KD, Said CM, Byrne KN, Dodd KJ. Factors influencing commencement and adherence to a home-based balance exercise program for reducing risk of falls: Perceptions of people with Alzheimer’s disease and their caregivers. International Psychogeriatrics. 2012;24(7):1172–1182. doi: 10.1017/S1041610211002729. [DOI] [PubMed] [Google Scholar]
- Szczerbinska K, Hirdes JP, Zyczkowska J. Good news and bad news: Depressive symptoms decline and undertreatment increases with age in home care and institutional settings. American Journal of Geriatric Psychiatry. 2012;20(12):1045–1056. doi: 10.1097/JGP.0b013e3182331702. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Delbaere K, Lord SR, Mikolaizak AS, Close JCT. Physical impairments in cognitively impaired older people: Implications for risk of falls. International Psychogeriatrics. 2013;25(1):148–156. doi: 10.1017/S1041610212001184. [DOI] [PubMed] [Google Scholar]
- Thorpy MJ. Sleep disorders in Parkinson’s disease. Clinical Cornerstone. 2004;6(Suppl 1A):S7–S15. doi: 10.1016/s1098-3597(04)90013-0. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New England Journal of Medicine. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. New England Journal of Medicine. 1997;337(18):1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- van Dijk PTM, Meulenberg OGRM, van de Sande HJ, Habbema JDF. Falls in dementia patients. The Gerontologist. 1993;33(2):200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- Voss TS, Elm JJ, Wielinski CL, Aminoff MJ, Bandyopadhyay D, Chou KL, … Tilley BC. Fall frequency and risk assessment in early Parkinson’s disease. Parkinsonism and Related Disorders. 2012;18(7):837–841. doi: 10.1016/j.parkreldis.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Watt K, Peel NM. Falls prevention interventions for community-dwelling older persons with cognitive impairment: A systematic review. International Psychogeriatrics. 2013;25(2):215–227. doi: 10.1017/S1041610212001573. [DOI] [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: A prospective multidisciplinary study. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72(6):721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziere G, Dieleman JP, Hofman A, Pols HAP, Van Der Cammen TJM, Stricker BHC. Poly-pharmacy and falls in the middle age and elderly population. British Journal of Clinical Pharmacology. 2006;61(2):218–223. doi: 10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]