Abstract
Conflicting evidence suggests that bone lead or blood lead may reduce areal bone mineral density (BMD). Little is known about how lead at either compartment affects bone structure. This study examined postmenopausal women (N = 38, mean age 76 ± 8, body mass index (BMI): 26.74 ± 4.26 kg/m2) within the Hamilton cohort of the Canadian Multicentre Osteoporosis Study (CaMos), measuring bone lead at 66% of the non-dominant leg and at the calcaneus using 109Cadmium X-ray fluorescence. Volumetric BMD and structural parameters were obtained from peripheral quantitative computed tomography images (200 μm in-plane resolution, 2.3 ± 0.5 mm slice thickness) of the same 66% site and of the distal 4% site of the tibia length. Blood lead was measured using atomic absorption spectrometry and blood-to-bone lead partition coefficients (PBB, log ratio) were computed. Multivariable linear regression examined each of bone lead at the 66% tibia, calcaneus, blood lead and PBB as related to each of volumetric BMD and structural parameters, adjusting for age and BMI, diabetes or antiresorptive therapy. Regression coefficients were reported along with 95% confidence intervals. Higher amounts of bone lead at the tibia were associated with thinner distal tibia cortices (−0.972 (−1.882, −0.061) per 100 μg Pb/g of bone mineral) and integral volumetric BMD (−3.05 (−6.05, −0.05) per μg Pb/g of bone mineral). A higher PBB was associated with larger trabecular separation (0.115 (0.053, 0.178)), lower trabecular volumetric BMD (−26.83 (−50.37, −3.29)) and trabecular number (−0.08 (−0.14, −0.02)), per 100 μg Pb/g of bone mineral after adjusting for age and BMI, and remained significant while accounting for diabetes or use of antiresorptives. Total lead exposure activities related to bone lead at the calcaneus (8.29 (0.11, 16.48)) and remained significant after age and antiresorptives-adjustment. Lead accumulated in bone can have a mild insult on bone structure; but greater partitioning of lead in blood versus bone revealed more dramatic effects on both microstructure and volumetric BMD.
Keywords: Bone lead content, Blood lead concentration, XRF, Bone structure, pQCT, Bone mineral density
Introduction
The heavy metal lead (Pb) is found residentially in the contaminated dust of older homes built before the 1960s, due primarily to the remnants of lead-based paints [1]. These remains can also leach into soil and subsequently into running water, and when in higher concentrations, present in air, which has been linked to higher blood lead levels [1,2]. Exposure to lead can be elevated for contractors reconstructing home exteriors [3], in addition to those working in the automobile [4], ammunition [5] and battery manufacturing [6] industries. Lead has primarily direct effects on impairing the immune and central nervous systems. An increase in lead concentrations in blood by 10 to 20 μg/dL led to a 2.6-point decrease in IQ score in children as shown in one meta-analysis [7]. Other well-documented effects on the body at higher concentrations include anemia, renal dysfunction, peripheral neuropathy, hypertension, and teratogenicities [8].
At low concentrations, lead is bound to red blood cells and is subsequently delivered in plasma after saturation at equilibrium. Plasma transfer of lead into bone, through partition exchange, results in the replacement of calcium within hydroxyapatite crystals by lead [9]. A three-compartment model of human lead metabolism and exchange developed by Rabinowitz et al. illustrates the mean lifetime exposure of lead within soft tissue, blood, and bone [10]. Over time, bone serves as the largest volume for lead distribution with a total burden as high as 90–95% by adulthood [11]. Despite the more prominent presence of lead within bone compared to any other part of the body, the effects of lead on bone have not been extensively studied. With increased bone turnover in altered endocrine states such as osteoporosis [12], the release of lead from bone into plasma can be accelerated, resulting in potential for higher blood, immune, and central nervous system exposure to lead with aging. In extreme cases such as hyperthyroidism [13] and pregnancy [14] where bone metabolism is accelerated, acute blood lead poisoning can occur.
Bone lead can be measured non-invasively using 109Cadmium (Cd) X-ray fluorescence (XRF) at sites where soft tissue is minimal, such as the anterior aspect of the mid-tibia, calcaneus, and patella [15]. Lead detection by XRF operates on the principle that lead atoms absorb incident photons of a given energy from the radioactive 109Cd source and emit a characteristic lower energy, specific to the lead atom. From spectral data collected by Germanium detectors arranged in backscatter configuration, the lead Kα1 transition energy peak at 75.0 keV can be measured. To obtain an accurate representation of the amount of lead, the area under this curve (AUC) is normalized to the total energy peak AUC related to elastic scattering of photons due to interaction with bone mineral; hence, lead content is expressed as μg lead/g of bone mineral [8].
One previous study quantified a modest relationship between a higher amount of bone lead content at the tibia [16] and a lower areal bone mineral density (aBMD) measured at the lumbar spine by dual-energy X-ray absorptiometry (DXA). However, no study has examined the relationship between bone lead content and structural properties of bone. Although two studies of exposed individuals showed that blood lead content was associated with a lower aBMD at the lumbar spine [17,18], two other studies did not reveal a significant relationship between blood lead and aBMD [16,19]. The conflicting evidence that blood lead may have an effect on aBMD may be explained by the cohort’s level of exposure. No study has shown any association between either blood or bone lead content and bone structure in any population. The present study aimed to determine how bone lead content at the mid-tibia and at the calcaneus is related to bone microstructure and volumetric density at the ultradistal tibia in a random sample of women above 50 years of age. To investigate the partitioning of bone lead into the blood, blood lead content was also examined independently and in conjunction with bone lead as a blood:bone lead partition coefficient to determine its effect on bone structure and volumetric density.
Methods
Study design
This cross-sectional observational cohort study quantified the relationships between bone or blood lead content and each of bone structure and volumetric density measured within the same period. All study procedures were performed in a cohort (Hamilton, ON, Canada) representing an intended population of peri- and post-menopausal Canadian women. All measurements were collected within 1.5 years. Women ≥50 years of age enrolled in the Canadian Multicentre Osteoporosis Study (CaMos) and living within a 50 km radius of the Hamilton CaMos site were considered eligible to participate (N = 340) in this study. Among these, 60 randomly selected women were invited to participate. Of those invited, 38 agreed to participate. Study declines were mostly related to high study procedure burden. No exclusion criteria were imposed. CaMos is an ongoing, prospective population-based cohort study of community-dwelling, randomly selected women and men ≥25 years of age at nine major Canadian cities. The main CaMos objectives, methodology and sampling framework are described in detail elsewhere [20]. Participants volunteered in the completion of a peripheral quantitative computed tomography (pQCT) (XCT2000, Stratec Medizintechnik, Pforzheim, Germany) scan to measure bone apparent microstructure and volumetric bone mineral density (vBMD) and an XRF scan to obtain bone lead content at the mid-tibia and calcaneus. Height and weight were obtained on a standiometer and standard scale, respectively. Body mass index was computed as weight (kg) divided by height (m) squared.
pQCT scans of the distal and mid-tibia
All pQCT scans were performed on the non-dominant leg at 4% and 66% of the tibial length measured from the distal articulating aspect of the medial malleolus towards the medial condyle of the tibial plateau. A single 2.3 ± 0.5 mm transaxial slice was obtained at each region of interest using an X-ray beam energy of 38 kVp, a tube current of 0.3 mA, reconstructed by filtered back-projection on a matrix of 256 × 256. The 4% site, which was used to derive bone apparent microstructure, was scanned at 10 mm/s CT speed to yield an in-plane resolution of 200 μm; the 66% site was scanned at 20 mm/s CT speed to yield an in-plane resolution of 500 μm and was used to derive cortical geometry and volumetric density information. Hydroxyapatite phantoms were assessed on days in which scans were obtained. Only images with no discontinuities in the cortical bone were accepted for image analyses. Integral (vBMDi), cortical (vBMDc), and trabecular (vBMDtr) vBMD measures were computed using Stratec Analysis Software v6.0 (Orthometrix Inc., White Plains, NY, USA) after an edge-detection-based segmentation algorithm separated soft tissue from bone using a threshold of 280 mg/cm3. Primary measures of apparent trabecular microstructure (trabecular separation (Tb.Sp), bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th)) and cortical thickness (Ct.Th) were computed with the custom software package, pQCT OsteoQ (Inglis Software Solutions Inc., Hamilton, ON), which applied a combination of threshold-based and region-growing algorithms. All bone apparent microstructure measurements have previously been validated and all outcomes shown here have demonstrated short-term reproducibility within 5% [21].
XRF scans of tibia and calcaneus
A single operator completed all XRF scans using an 88.04 keV 109Cd source yielding between 0.744 and 0.580 GBq of radioactivity between the first and last scans performed in this study (5 months) respectively. With participants sitting up-right in a padded chair, the 66% site of the tibia and lateral aspect of the calcaneus of the same leg were positioned and braced in an axial plane perpendicular to the 109Cd source. Measurement sites were swabbed with isopropyl alcohol to minimize surface contaminants. Integration time at both the calcaneus and 66% tibia sites was 30 min, as previously suggested [22]. Distance from anatomy to source was calibrated to limit dead time to between 10 and 30%. Quality control was performed on a daily basis using a custom bone mineral phantom with known concentrations of lead content (ppm).
Spectral readings from germanium detectors were analyzed using Genie2000 software (Canberra Industries Inc., USA). A Marquardt nonlinear least-squares regression curve was fit to spectral data to estimate peak amplitudes and AUCs [23]. K-shell lead X-ray peak AUCs were normalized to the total energy peak AUC found in the emission spectrum. The normalized ratio was converted to μg lead/g bone mineral by calibration against the lead-bone mineral phantom. Each bone lead measurement included an uncertainty interval, which reflected the counting statistics of the lead X-ray peak, the elastic peak, and linear calibration. Because actual number of counts may fall below those predicted by the background curve, bone lead measurement values <0 μg lead/ g bone mineral were possible. Bone lead measurements of the calcaneus and tibia have previously been shown to be reproducible with a median uncertainty of 4.26 μg lead/g bone [23] and valid as compared to atomic absorption spectrometry of autopsy samples [24].
Blood lead measurement
Refrigerated whole-blood samples (2–8 °C) were analyzed under graphite-furnace atomic absorption spectrometry (GFAAS) using a single Perkins & Elmer 600 instrument. The minimum detectable limit of this technique was 2.01 μg/dL blood lead. Blood lead units were converted to μg/dL to be consistent with units reported in the literature.
Medical history questionnaires
A questionnaire was designed to collect information on the degree of exposure of individuals to lead during their lifetime. A composite score of residential exposure to lead was formulated for each dwelling occupied (current, childhood and other), computed as a function of the number of years occupying the dwelling, the location (small town, suburb or inner city), type of water source (hard, soft), whether a water treatment or filtration device was used, type of water consumed (tap, bottled, treated), type of plumbing (lead, lead soldering, non-lead), presence of lead based paint in the interior and exterior of the home. Total occupational exposure of lead was estimated by multiplying years in occupation and a self-report of whether or not the occupation exposed them to lead. Total activities exposure to lead was computed by summing the participation (yes (1), no (0)) in each of 13 activities (home renovations, interior decorating, jewelry or badge making, SCUBA diving, fishing, electronics fabrication, metalwork, car maintenance, hunting/ shooting/ammunition handling, cosmetics used from the Middle East, Southeast Asia, India, the Dominican Republic or Mexico, use of leaded crystal ware, gardening, use of a soldering iron or gun). Duration of antiresorptive therapy, calcium and vitamin D3 supplementation information was collected. Use of other medications and medical history including ascertained incident fragility fractures within the last 14 years was obtained from the CaMos database. Fragility fractures were defined as non-traumatic fractures occurring as the result of a fall from standing height or less, excluding any fractures of the skull, fingers and toes. All study procedures were overseen and approved by the St. Joseph’s Healthcare Research Ethics Board in Hamilton (08-3073).
Data analysis
Blood lead and bone lead were expressed as a blood:bone lead partition coefficient (PBB = LOG(blood lead content [μg/dL]/bone lead content [μg/g bone])) after first linear transforming bone lead content values such that all values were >0.0 μg lead/g bone. Larger PBB values were indicative of a greater proportion of lead in blood versus bone. A linear regression analysis determined the relationship between mid-tibial or calcaneal bone lead content derived from XRF and each of bone structural or densitometric parameters derived from pQCT at the 4% and 66% sites of the tibia. The same analyses were performed with blood lead and the PBB in place of bone lead content. Linear regression analyses were also used to examine the relationship between lead exposure variables and each of blood lead and bone lead content at the calcaneus and mid-tibia. Regression coefficients were reported along with 95% confidence intervals and model fit quantified by R2 with and without adjusting for age and BMI. Because diabetes was identified in 3 individuals, this was also explored as a covariate in a third model that included age. In post-hoc analyses, to explore the relationship between bone lead at the calcaneus in explaining a potential inverse relationship at the tibia, a linear regression analysis was performed between these two variables as well.
Results
Participant characteristics
Women in this study were on average within the marginally over-weight range of BMI. Over a quarter of the women were on bisphosphonate (10/38 (26.3%)) or estrogen replacement (2/38 (5.3%)) therapy, while a large proportion was taking calcium and vitamin D3 supplements (Table 1). None of the participants used selective estrogen receptor modulators, bone formation agents, or aromatase inhibitors, but one used glucocorticoids for less than 2 months. Of the 38 women, 19 have had a history of fragility fractures within the last 14 years and only 7 of those were on bisphosphonate or estrogen replacement therapy. None of the participants had kidney disease, metabolic bone disease, rheumatoid arthritis, thyroid diseases, osteoarthritis, eating disorders, had an organ transplant or any neuromuscular disorders. However, 3 women (7.9%) had type 2 diabetes, 1 had type 1 diabetes (2.6%), 2 had cancer (5.3%) and 5 had a cardiovascular event or stroke (13.2%).
Table 1.
Participant anthropometrics and bone characteristics. pQCT images of the 4% distal tibia were used to compute volumetric bone mineral density (vBMD) and bone mineral content (vBMC), as well as a series of apparent bone microstructure (trabecular separation (Tb.Sp), trabecular thickness (Tb.Th), bone volume fraction (BV/TV), trabecular number (Tb.N)), cortical thickness (Ct.Th) and mechanical properties as represented by the polar stress–strain index (SSIp). All normally distributed variables were reported using parametric statistics (means and standard deviations (SD)). Non-normally-distributed variables were reported using non-parametric statistics (median and Q1–Q3) where indicated by *.
| Variable | N | Mean/median | SD/Q1–Q3 | Minimum | Maximum |
|---|---|---|---|---|---|
| Age2011 (years) | 38 | 76 | 8 | 62 | 89 |
| BMI (kg/m2) | 38 | 26.74 | 4.26 | 19.16 | 35.98 |
| Duration of antiresorptive therapy (years) * | 38 | 0.0 | 0.0–3.5 | 0.0 | 22.0 |
| Duration of Vitamin D3 supplementation (years)* | 38 | 3.0 | 0.0–10.0 | 0.0 | 40.0 |
| Duration of calcium supplementation (years)* | 38 | 4.5 | 0.0–10.0 | 0.0 | 40.0 |
| pQCT volumetric bone densities | |||||
| vBMDi(mg/cm3) | 37 | 298.02 | 53.93 | 137.20 | 389.80 |
| vBMCi(mg) | 37 | 229.32 | 38.86 | 121.69 | 305.50 |
| vBMDtr(mg/cm3) | 37 | 180.46 | 26.77 | 99.50 | 220.60 |
| vBMCtr(mg) | 37 | 106.15 | 27.62 | 63.00 | 186.65 |
| vBMDc(mg/cm3) | 37 | 927.62 | 35.55 | 859.90 | 978.60 |
| vBMCc(mg) | 37 | 62.82 | 22.04 | 12.19 | 106.15 |
| pQCT apparent bone microstructure | |||||
| Tb.Sp (mm)* | 37 | 0.457 | 0.405–0.542 | 0.333 | 1.168 |
| Tb.Th (mm) | 37 | 0.373 | 0.0416 | 0.263 | 0.461 |
| Ct.Th (mm) | 37 | 1.141 | 0.172 | 0.757 | 1.482 |
| SSIp (mm3) | 37 | 103,212 | 38,011 | 48,854 | 208,014 |
| BV/TV (fraction) | 37 | 0.440 | 0.080 | 0.185 | 0.570 |
| Tb.N (#/mm2)* | 37 | 1.20 | 1.10–1.28 | 0.70 | 1.38 |
Bone and blood lead distributions were variable across the cohort with calcaneal bone lead being more variable than tibial bone lead (Table 2). Bone lead at the calcaneus was marginally higher than that at the mid-tibia (p = 0.076). Exposure to residential sources of lead was the highest in childhood residences compared to current or other dwellings occupied. Only two participants reported occupational exposure to lead for durations of 15, and 20 years. Of the 38 participants, 8 were noted to have participated in one activity that involved exposure to lead. Only 4 participants had a history of smoking. These activity-associated exposure levels are reported along with exposure to lead in residential dwellings in Table 3.
Table 2.
Summary of bone lead, blood lead and bone:blood lead partition characteristics. Bone lead measurements obtained at the mid-tibia and at the calcaneus were quantified from XRF-derived spectral data, normalized to total energy peaks and calibrated to μg lead/g bone mineral units based on a phantom with known bone lead concentrations. Blood lead values obtained by a standard medical lab were converted to μg/dL units. Blood:bone lead partition coefficients (PBB) were computed as base 10 log of the ratio of blood to bone lead.
| Variable | N | Mean/median | SD/Q1–Q3 | Minimum | Maximum |
|---|---|---|---|---|---|
| Mid-tibia bone Pb (μg Pb/g bone) | 38 | 3.25 | 6.03 | −9.50 | 14.23 |
| Calcaneus bone Pb (μg Pb/g bone) | 36 | 7.10 | 10.50 | −14.04 | 27.27 |
| Blood Pb (μg/dL)a | 37 | 1.69 | 1.36 | 0.00b | 7.25 |
| Blood:tibia lead ratio* | 35 | 0.13 | 0.10–0.19 | 0.07 | 0.43 |
| Blood:calcaneus lead ratio* | 34 | 0.11 | 0.07–0.19 | 0.05 | 2.15 |
| PBB tibia (log units) | 35 | −1.99 | 0.43 | −2.65 | −0.84 |
| PBB calcaneus (log units) | 34 | −2.04 | 0.78 | −3.02 | 0.77 |
Due to left censoring of blood lead data with undetectable values below the minimal detectable limit of 2.01 μg/dL, a weighted means and standard deviation was computed here assuming undetectable values yield a mean of 1.00 μg/dL (range of 0.00 to 2.01 μg/dL) and standard deviation of 1.41 μg/dL.
Actual minimum value is somewhere between 0.00 and 2.01 μg/dL. All normally distributed variables were reported using parametric statistics (means and standard deviations (SD)). Non-normally-distributed variables were reported using non-parametric statistics (median and Q1–Q3) where indicated by *.
Table 3.
Exposure of women to different sources of lead. A composite scoring system was used to compute residential exposure to lead in each type of dwelling (current, childhood and other) occupied = # of years occupying dwelling × [((location (small town 1, suburb 2, inner city 3) + water source (hard 2, soft1))/5) × (water filtration at tap (yes 0, no 1)) × (main water treatment (yes 0, no 1)) × (plumbing type (lead 3, lead soldering 2, non-lead 1)/3) × (water consumed (tap 1, bottled or treated 0)) + interior lead-based paint (yes 1, no 0) + exterior lead-based paint (yes 1, no 0)]. The maximum exposure index was 3 (exposure to lead in water, in indoor and outdoor lead-based paint) multiplied by number of years occupied in the dwelling. Occupational exposure to lead was based on self-report of whether or not they were in an occupation that exposed them to lead (yes 1, no 0) × number of years in that occupation. Total activity lead exposure was based on the sum of whether (1) or not (0) they participated in each of 13 activities including: home renovations; interior decorating; jewelry or badge making; SCUBA diving; fishing; electronics fabrication; metalwork; car maintenance; hunting/shooting/ammunition handling; cosmetics used from Middle East, Southeast Asia, India, the Dominican Republic or Mexico; use of leaded crystal ware; gardening; and use of a soldering iron or gun.
| Exposure methods | N | Mean | SD | Median | Q1 | Q3 | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Current Res.Pb Exp. | 38 | 8.39 | 20.36 | 4.00 | 0.00 | 6.20 | 0.00 | 104.00 |
| Childhood Res.Pb Exp. | 37 | 35.19 | 22.12 | 37.33 | 19.60 | 51.20 | 0.00 | 89.60 |
| Other Res.Pb Exp. | 38 | 7.23 | 18.97 | 1.60 | 0.00 | 2.93 | 0.00 | 84.00 |
| Occupational Pb Exp. | 38 | 0.92 | 4.00 | 0.00 | 0.00 | 0.00 | 0.00 | 20.00 |
| Total activities Pb Exp. | 38 | 0.21 | 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
Bone and blood lead relationships
Contrary to the hypothesized relationship between bone lead and bone quality, a higher amount of bone lead at the calcaneus was associated with higher trabecular vBMD, integral BMC (Table 4), smaller trabecular separation, bone volume fraction, and trabecular number (Table 5) at the 4% distal tibia after accounting for age and BMI. However, accounting for diabetes, only the association between calcaneal bone lead and integral vBMC remained significant. Adjusting for antiresorptive therapy revealed a significant effect of bone lead at the calcaneus and trabecular vBMC. In contrast, a higher amount of mid-tibial bone lead was associated with thinner cortices at the 4% distal tibia (Table 5, Fig. 1) – an observation that coincided with the study hypotheses, and which remained significant after accounting for age and BMI, age and diabetes, or age and antiresorptive therapy. Bone lead at the mid-tibia did not associate with any bone properties at this same 66% region of interest as measured by pQCT, which consists only of cortical bone. However, after accounting for age and antiresorptive therapy, an association between higher mid-tibial bone lead and lower integral vBMC was observed. Despite the fact that blood lead did not display any associations with vBMD or bone apparent structure (data not shown), a larger PBB(calcaneus) was significantly associated with larger trabecular separation, and lower trabecular vBMD, integral vBMC (Table 4), trabecular thickness, bone volume fraction, and trabecular number at the 4% distal tibia (Table 5). These relationships were all preserved after accounting for age and BMI. Including diabetes as a covariate did not render relationships of PBB(calcaneus) and each of trabecular vBMD, Tb.Sp, BV/TV or Tb.N to be non-significant. Including antiresorptives as a covariate maintained statistical significance for the same measures except BV/TV which was only marginally significant. Bone measures at the 66% site did not associate with PBB(calcaneus). However, vBMDC displayed a marginally significant relationship after adjusting for age and antiresorptive therapy (B = −16.32 (−35.84, 3.18)) or age and diabetes (−16.46 (−34.92, 1.99)). Unlike the calcaneus, PBB(tibia) did not associate with any structural or vBMD measures at either 4% or 66% tibia sites.
Table 4.
Lead content as related to volumetric bone density measurements. Bone lead at the mid-tibia, bone lead at the calcaneus and the blood:bone lead partition coefficients (PBB) were each related to volumetric bone mineral density (vBMD) integral (vBMDi), trabecular (vBMDtr) and cortical (vBMDC) values, and volumetric bone mineral content (vBMC) integral (vBMCi), trabecular (vBMCtr) and cortical (vBMCC) values. All base (left) and covariate adjusted models were reported with unstandardized regression coefficients and 95% confidence intervals. All coefficients were expressed per 1 μg lead/g of bone mineral difference.
| Variable | N | R2 | Unstandardized B (95% CI) |
B (95% CI) Age & BMI-adjusted |
B (95% CI) Age & diabetes-adjusted |
B (95% CI) Age & antiresorptives-adjusted |
|---|---|---|---|---|---|---|
| Bone Pb at calcaneus | ||||||
| vBMDi | 34 | 0.020 | 0.74 (−1.13, 2.61) | 1.35 (−0.48, 3.17) | 0.68 (−1.14, 2.50) | 0.27 (−1.65, 2.18) |
| vBMDc | 34 | 0.009 | −0.33 (−1.56, 0.90) | −0.26 (−1.49, 0.97) | −0.27 (−1.45, 0.91) | −0.72 (−1.85, 0.41) |
| vBMDtr | 34 | 0.090 | 0.78 (−0.11, 1.67) | 1.07 (0.20, 1.94) | 0.77 (−0.13, 1.68)b | 0.63 (−0.31, 1.58) |
| vBMCi | 34 | 0.118 | 1.32 (0.02, 2.62) | 1.66 (0.36, 2.95) | 1.36 (0.06, 2.66) | 1.33 (−0.04, 2.70)b |
| vBMCc | 34 | 0.001 | −0.07 (−0.84, 0.69) | 0.12 (−0.61, 0.86) | −0.07 (−0.77, 0.63) | −0.23 (−0.96, 0.51) |
| vBMCtr | 34 | 0.101 | 0.85 (−0.06, 1.76)b | 0.93 (−0.04, 1.90)b | 0.90 (−0.02, 1.83)b | 0.99 (0.01, 1.97) |
| Bone Pb at tibia | ||||||
| vBMDi | 34 | 0.065 | −2.22 (−5.18, 0.73) | −1.58 (−4.75, 1.59) | −2.10 (−5.16, 0.96) | −3.05 (−6.05, −0.05) |
| vBMDc | 34 | 0.018 | −0.77 (−2.77, 1.23) | −0.26 (−1.90, 1.39) | −0.09 (−2.14, 1.96) | −0.75 (−2.68, 1.18) |
| vBMDtr | 34 | 0.015 | −0.54 (−2.06, 0.98) | −0.07 (−2.15, 2.02) | −0.45 (−2.09, 1.19) | −0.86 (−2.47, 0.75) |
| vBMCi | 34 | 0.013 | −0.73 (−2.94, 1.47) | −0.13 (−2.50, 2.25) | −0.31 (−2.67, 2.04) | −0.63 (−3.04, 1.77) |
| vBMCc | 34 | 0.102 | −1.13 (−2.30, 0.04) | 0.75 (−0.92, 2.42) | −0.84 (−2.01, 0.33) | −1.15 (−2.32, 0.01)b |
| vBMCtr | 34 | 0.014 | 0.51 (−1.01, 2.03) | −0.66 (−1.87, 0.55) | 0.77 (−0.86, 2.39) | 0.81 (−0.90, 2.51) |
| Blood:bone (calcaneus) lead partition coefficient (PBB) | ||||||
| vBMDi | 34 | 0.115 | −24.49 (−49.80, 0.81) | −26.83 (−50.37, −3.29) | −21.74 (−46.60, 3.11)b | −18.28 (−44.61, 8.05) |
| vBMDc | 34 | 0.003 | −2.42 (−19.58, 14.74) | −2.66 (−19.25, 13.93) | −1.67 (−18.28, 14.95) | −15.78 (−34.85, 3.28) |
| vBMDtr | 34 | 0.182 | −15.05 (−26.95, −3.14) | −16.14 (−27.32, −4.97) | −14.40 (−26.58, −2.23) | −13.09 (−25.81, −0.36) |
| vBMCi | 34 | 0.104 | −16.31 (−34.11, 1.49) | −17.62 (−34.65, −0.58) | −15.62 (−33.67, 2.43)b | −6.81 (−21.35, 7.72) |
| vBMCc | 34 | 0.017 | −3.71 (−14.27, 6.84) | −4.53 (−14.08, 5.02) | −2.54 (−12.35, 7.28) | 3.52 (−12.70, 19.73) |
| vBMCtr | 34 | 0.024 | −5.53 (−18.82, 7.77) | −5.77 (−19.54, 8.00) | −6.34 (−20.06, 7.38) | −1.39 (−11.90, 9.12) |
Bold
indicates relationships that were significant at the α = 0.05 level.
indicates relationships that were significant at the α = 0.10 level.
Table 5.
Lead content as related to bone apparent structure measurements. Bone lead at the mid-tibia, bone lead at the calcaneus and the blood:bone lead partition coefficients (PBB) were each related to trabecular separation (Tb.Sp), trabecular thickness (Tb.Th), bone volume fraction (BV/TV), trabecular number (Tb.N), cortical thickness (Ct.Th) and mechanical properties as represented by the polar stress–strain index (SSIp). All base (left) and covariate adjusted models were reported with regression coefficients and 95% confidence intervals. Coefficients were expressed per 1 μg lead/g of bone mineral difference unless otherwise indicated.
| Variable | N | R2 | Unstandardized B (95% CI) |
B (95% CI) Age & BMI-adjusted |
B (95% CI) Age & diabetes-adjusted |
B (95% CI) Age & antiresorptives adjusted |
|---|---|---|---|---|---|---|
| Bone Pb at calcaneus | ||||||
| Tb.Sp/100 μg Pb | 34 | 0.100 | −0.508 (−1.057, 0.041) | −0.716 (−1.226, −0.206) | −0.517 (−1.062, 0.028)b | −0.473 (−1.049, 0.104) |
| Tb.Th/100 μg Pb | 34 | 0.050 | 0.090 (−0.052, 0.231) | 0.122 (−0.022, 0.267)b | 0.080 (−0.061, 0.221) | 0.058 (−0.093, 0.209) |
| Ct.Th/100 μg Pb | 34 | 0.000 | 0.010 (−0.696, 0.715) | 0.214 (−0.371, 0.799) | −0.020 (−0.687, 0.646) | −0.028 (−0.667, 0.611) |
| SSIp | 34 | 0.026 | 591 (−726, 1907) | 498 (−910, 1905) | 666 (−670, 2003) | 951 (−434, 2335) |
| BV/TV/100 μg Pb | 34 | 0.072 | 0.211 (−0.061, 0.482) | 0.296 (0.028, 0.563) | 0.204 (−0.069, 0.478) | 0.167 (−0.122, 0.456) |
| Tb.N/100 μg Pb | 34 | 0.066 | 0.37 (−0.13, 0.88) | 0.54 (0.06, 1.02) | 0.39 (−0.11, 0.89) | 0.36 (−0.17, 0.89) |
| Bone Pb at tibia | ||||||
| Tb.Sp/100 μg Pb | 36 | 0.058 | 0.645 (−0.260, 1.550) | 0.391 (−0.572, 1.355) | 0.526 (−0.441, 1.493) | 0.734 (−0.234, 1.702) |
| Tb.Th/100 μg Pb | 36 | 0.003 | −0.035 (−0.268, 0.198) | −0.040 (−0.293, 0.214) | −0.070 (−0.313, 0.173) | −0.121 (−0.371, 0.129) |
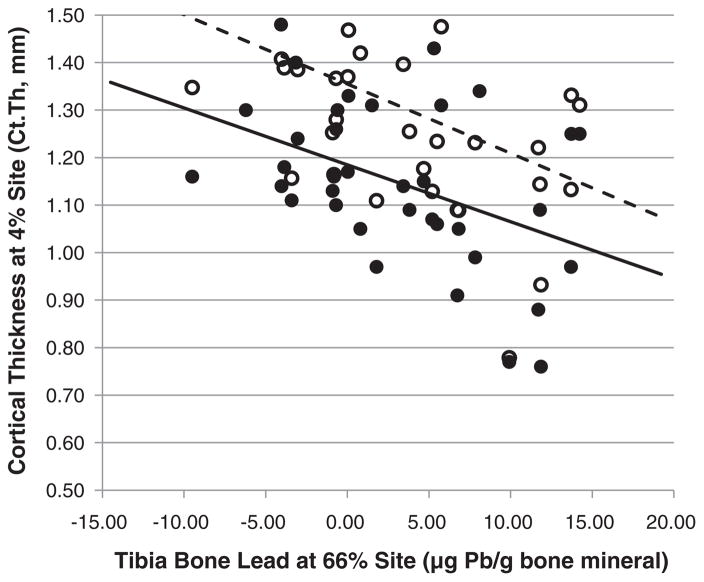
| Ct.Th/100 μg Pb | 36 | 0.186 | −1.192 (−2.060, −0.323) | −0.972 (−1.882, −0.061) | −1.364 (−2.379, −0.348) | −1.316 (−2.262, −0.370) |
| SSIp | 36 | 0.021 | 881 (−1228, 2990) | 987 (−1336, 3311) | 1153 (−1103, 3408) | 1582 (−711, 3875) |
| BV/TV/100 μg Pb | 36 | 0.020 | −0.184 (−0.635, 0.267) | −0.114 (−0.604, 0.376) | −0.176 (−0.658, 0.306) | −0.288 (−0.771, 0.195) |
| Tb.N/100 μg Pb | 36 | 0.040 | −0.49 (−1.32, 0.34) | −0.23 (−1.11, 0.65) | −0.33 (−1.22, 0.56) | −0.49 (−1.38, 0.41) |
| Blood:bone (calcaneus) lead partition coefficient (PBB) | ||||||
| Tb.Sp | 32 | 0.240 | 0.107 (0.036, 0.179) | 0.115 (0.053, 0.178) | 0.104 (0.033, 0.176) | 0.104 (0.028, 0.179) |
| Tb.Th | 32 | 0.126 | −0.019 (−0.039, 0.000) | −0.021 (−0.040, −0.002) | −0.018 (−0.037, 0.002)b | −0.016 (−0.037, 0.005) |
| Ct.Th | 32 | 0.032 | −0.041 (−0.125, 0.042) | −0.050 (−0.125, 0.026) | −0.029 (−0.109, 0.050) | −0.036 (−0.126, 0.054) |
| SSIp | 32 | 0.012 | 5522 (−13,102, 24,147) | 5921 (−13,405, 25,248) | 4255 (−14,966, 23,477) | 1684 (−18,363, 21,731) |
| BV/TV | 32 | 0.166 | −0.042 (−0.079, −0.007) | −0.046 (−0.080, −0.013) | −0.040 (−0.077, −0.004) | −0.038 (−0.076, 0.001)b |
| Tb.N | 32 | 0.139 | −0.07 (−0.14, −0.01) | −0.08 (−0.14, −0.02) | −0.07 (−0.14, 0.00) | −0.07 (−0.14, 0.00y) |
Bold indicates relationships that were significant at the α = 0.05 level.
indicates relationships that were significant at the α = 0.10 level.
Fig. 1.
Illustration of the relationship between bone lead at the mid-tibia and cortical thickness. A linear regression analysis was performed to determine the relationship between bone lead at the mid-tibia and cortical thickness at the distal 4% tibia. Results were similar for Ct.Th obtained at two different slices of the 4% distal tibia, separated by 5 mm. Open circle and dashed line = more proximal slice; closed circle and solid line = more distal slice.
Post-hoc analyses
Upon closer examination of bone lead at the two locations, there was no significant inverse relationship between the amount of lead within the calcaneus and the mid-tibia (p = 0.574). There was also no significant association between blood lead and either bone lead at the mid-tibia (p = 0.472) or at the calcaneus (p = 0.767). There was no difference in the amount of tibial bone lead or calcaneal bone lead between those who have or have not taken antiresorptives, even after age adjustment; though there appeared to be a trend (p = 0.082) towards smaller bone lead at the mid-tibia in those having taken antiresorptives (1.72 ± 6.75 μg/dL) compared to those who have not (3.96 ± 5.67 μg/dL). Similarly, there was a marginally larger PBB in those treated with antiresorptives. Most of the lead exposure variables computed were not associated with bone or blood lead levels, except total activities exposure to lead was related to bone lead content at the calcaneus (8.29 (0.11, 16.48)) but not at the tibia. This relationship was preserved after accounting for age and antiresorptive therapy (12.53 (4.06, 21.01)) but only marginally significant after accounting for age and diabetes (8.55 (−0.07, 17.16)).
Discussion
Tibia bone lead relationship with bone density and structure
Exposure to residential, industrial, and environmental sources of lead remains an overlooked public health concern. While half-life of lead is short within the blood stream (35 days), blood lead levels can remain elevated due to internal tissue mobilization – such as from bone. In the present study, blood lead levels were mostly negligible or low for our cohort of unexposed women. In contrast to blood lead, bone lead half-life is in the decades [25]. Put into context, the larger amount of bone lead at the tibia observed in the present study, being associated with smaller cortical thickness and lower integral vBMD after adjusting for age and antiresorptives, was consistent with the hypothesis that bone lead stored for long periods may have a negative effect on bone integrity. Mechanistic evidence to support this association was presented by Beier et al. who saw that lead caused a decrease in osteoblast cell number, leading to reduced bone formation, corresponding to elevated levels of sclerostin and reduced β-catenin and Runx2 signaling in bone precursors [26]. The loss of bone structural integrity could also be explained by lead’s activation of the peroxisome proliferator-activated receptor (PPAR)-γ pathway, leading to preferential differentiation of mesenchymal stem cells to adipocytes rather than to osteocytes[26]. Cortical thickness was the primary variable that showed any effect due to bone lead, and is also one of the most clinically impacted structural outcomes previously reported to relate directly to fracture risk [27,28]. Trabecular bone exhibits a higher turnover than cortical bone. It is possible that bone lead has a longer half-life in cortical compared to trabecular bone, therefore, exhibiting more of its bone toxicities on the cortical shell. This proposition is supported by evidence from a study by Brito et al. who measured a faster rate of trabecular bone lead transfer to blood than from cortical bone to blood [29].
Calcaneus bone lead relationship with volumetric bone density and structure
The observation that bone lead at the calcaneus was associated with positive effects on bone structure and density at the distal tibia was unexpected. Although the effect sizes for vBMD were small (<1% difference), effects for bone apparent microstructure were between 5% and 15% of mean measurements per 10 μg of lead/g of bone mineral. In either case, accounting for diabetes and antiresorptive therapy attenuated these positive effects of lead on bone density and structure, which suggests that confounding by these variables was possible. It was postulated that a larger sequestration of lead into the calcaneus may avert accumulation in the tibia, thus rescuing any potential lead-mediated toxicities on tibia bone structure. However, we did not observe a significant inverse relationship between bone lead at the calcaneus versus the tibia. While we did not directly measure bone lead at the distal tibia –only lead at the 66% site of the tibia was quantified – it is possible that bone lead at the calcaneus may still relate inversely to the distal tibia rather than at the mid-tibia. At this time, distal tibia bone lead measures are difficult to perform due to the larger amount of soft tissue around the ankle compared to the anterior aspect of the mid-tibia. Without a thorough investigation of how site-specific sources of lead affect the skeleton systemically versus site-specifically, it remains difficult to conclude how lead may mediate its toxic effects on bone structure and density.
Blood:bone lead partitioning and its relationship with trabecular bone density and structure
The significant relationships between higher blood:calcaneal bone lead partition coefficients and poorer trabecular structural integrity may support the above proposition at the end of the Tibia bone lead relationship with bone density and structure section that lead may be in smaller amounts in trabeculae. A larger distribution of lead within blood versus bone may be indicative of a higher discharge of lead from bone, possibly due to greater trabecular bone turnover. The larger trabecular separation, smaller number of trabeculae, and lower bone volume fraction provide evidence that greater bone loss was present. Although no bone turnover markers were measured in the present study, the cohort consisted of women half of whom had fractures within the last 14 years, and many of whom were not on antiresorptive therapy. One study by Gulson showed data that suggested that bisphosphonate therapy can prevent the release of lead from bone into the bloodstream [30]. However, individuals on antiresorptive therapy in the present cohort showed marginally larger bone lead content than those not on antiresorptives. That said, it is possible that those not on antiresorptives are healthier – a proper control group will be required to better support this proposition. Incidentally, adjusting for antiresorptive therapy did not abolish some of the relationships between blood:calcaneal bone lead and bone apparent microstructure. On the contrary, it is possible that a higher partitioning of lead from bone into blood may result in larger negative effects on bone, mediated by blood through more systemic pathways, rather than on local mechanisms related to the presence of lead in bone. One study by Potula et al. showed a large effect of blood lead levels in former smelter workers on reducing spinal aBMD measured by DXA [17].
The direction of causation between the presence of lead in blood and bone structure is difficult to establish in the present study without performing a longitudinal study. This challenge was similarly noted in a previous study by Adachi et al. measuring lead in patients with Paget’s disease [31]. Effect sizes for the relationship between bone apparent microstructure and PBB were variable – with 1 unit in PBB (i.e. 10 μg/dL lead in blood per 1 μg lead/g of bone mineral) being associated with as much as 5% (BV/TV) to 25% (Tb.Sp) difference in terms of the means of bone structure values. Meanwhile for bone density, effect sizes were closer to 10% decreases from the mean per unit increase in PBB. The fact that the same blood:bone partition coefficient calculated using tibial bone lead did not associate with any bone outcome is consistent with the lack of associations observed with tibia versus calcaneal bone lead alone. It may also be possible that the smaller variability in lead content at the mid-tibia compared to the calcaneus limited the ability to observe significant effects.
Exposure to lead and bone lead measurements
The lack of an association between lead exposure level variables and all bone or blood lead measurements may be due to the homogeneity in lead exposure among the women studied. Investigations that specifically examined exposed populations such as those in polluted areas [18] or those working in the mining industry [17] saw much larger effect sizes than those observed here. In fact, Brito et al. modeled exposure data and showed that half-lives for bone lead in the cortex and trabeculae are longer for individuals who are exposed to lead over a longer period (half-life: 47.5 ± 2.3 years) compared to those with acute exposure (half-life: 6.5 ± 0.7 years) [29]. Total lead exposure activities did associate with bone lead at the calcaneus after accounting for age and use of antiresorptive therapy. This self-reported activities index could be useful for future examinations on lead exposure. No standardized lead exposure questionnaire had previously been created, making comparisons with previous studies’ lead exposure levels difficult. The lead exposure variables computed in this study were formulated on logic, weighted by severity of exposure and adjusted for years of exposure. Specifically calculated weights or propensity scores were unavailable, which could have improved the accuracy of representing lead exposure in this cohort.
Study limitations and future directions
The biggest limitation of this study is the fact that bone lead and bone structure and density information were not measured at the same region of interest. Due to the requirement of bone lead measurements to be performed at sites where there is minimal soft tissue, this procedure limited the region of interest at which bone structure and density can be quantified. This study was performed in a small sample of postmenopausal women, limiting the statistical power to perform adjustment of a larger number of covariates. Since measurements were performed on only one occasion, this cross-sectional analysis did not permit true measurement of lead partitioning and transfer kinetics, as had been assessed in studies by Brito et al. [29,32]. In particular, the partition coefficient, PBB, assumed that the transfer of lead from bone to blood was computed at steady-state. Longitudinal assessments would also be able to draw more conclusive evidence on the direction of causality between blood:bone lead partitioning and bone deterioration. Information on DXA areal BMD and bone turnover markers could better gauge the role of bone turnover status on the retention of lead in bone. The most recent BMD available from these participants were from four years ago.
Given that this cohort consisted of marginally overweight women, it is possible that the larger BMI would contribute towards greater uncertainties in the measured bone lead values from XRF, which was demonstrated by Ahmed et al. [22]. Future studies examining bone microstructure and volumetric densities in exposed versus unexposed cohorts will provide greater evidence towards bone lead’s direct effects on bone quality versus potential indirect effects mediated through lead released into the bloodstream. It will be important for future analyses to consider effects for cortical versus trabecular bone separately, and to explore the possibility of adjusting for lead-mediated overestimation of bone density values.
Conclusions
The present study shows evidence that bone lead accumulated from exposure over time can have detrimental effects on bone by reducing cortical thickness and integral volumetric bone density. Loss of cortical thickness and density has been associated with an increased fracture risk. Consequently, those exposed to higher concentrations of lead in the environment may translate to having a higher risk of fracturing their bones. A large enough study to support this extension of knowledge is difficult to complete with limited accessibility to XRF technology. This study also saw greater partitioning of lead into blood versus bone in those with weaker bone structure and lower volumetric bone densities, suggesting that lead release from bone in postmenopausal women may be of a potential concern. Although blood lead levels measured in this cohort were not high, lead released into the blood may exert systemic effects on bone mediated through other pathways that must be explored.
Acknowledgments
This research project is dedicated to the late Dr. Colin E. Webber, who had contributed to the development of XRF technology and to bone lead investigations at McMaster University. CaMos participants are thanked for their volunteerism. Lesley Egden is kindly thanked for her contributions to training on XRF operation and data analysis. Andy Kin On Wong held a Vanier CGS Award at the time that this project was completed (CGV-104858).
Abbreviations
- XRF
109Cd X-ray fluorescence
- pQCT
Peripheral quantitative computed tomography
- aBMD/vBMD
Areal/volumetric bone mineral density
- CaMos
Canadian Multicentre Osteoporosis Study
- AUC
Area under the curve
- BMI
Body mass index
- Ct.Th
Cortical thickness
- BV/TV
Bone volume fraction
- Tb.N
Trabecular number
- Tb.Sp
Trabecular separation
- Tb.Th
Trabecular thickness
- PBB
Blood to bone lead partition coefficient
- DXA
Dual energy X-ray absorptiometry
Footnotes
Conflicts of interest
Andy Kin On Wong, Karen A. Beattie, Aakash Bhargava, Marco Cheung, Colin E. Webber, David Chettle, Alexandra Papaioannou, and Jonathan D. Adachi declare that they have no conflicts of interest.
References
- 1.Lucas JP, Bellanger L, Le Strat Y, Le Tertre A, Glorennec P, Le Bot B, et al. Source contributions of lead in residential floor dust and within-home variability of dust lead loading. Sci Total Environ. 2014;470–471:768–79. doi: 10.1016/j.scitotenv.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Brink LL, Talbott EO, Sharma RK, Marsh GM, Wu WC, Rager JR, et al. Do US ambient air lead levels have a significant impact on childhood blood lead levels: results of a national study. J Environ Public Health. 2013;2013:278042. doi: 10.1155/2013/278042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention. Children with elevated blood lead levels related to home renovation, repair, and painting activities—New York State, 2006–2007. MMWR Morb Mortal Wkly Rep. 2009;58:55–8. [PubMed] [Google Scholar]
- 4.Suplido ML, Ong CN. Lead exposure among small-scale battery recyclers, automobile radiator mechanics, and their children in Manila, the Philippines. Environ Res. 2000;82:231–8. doi: 10.1006/enrs.1999.4024. [DOI] [PubMed] [Google Scholar]
- 5.Fillion M, Blais JM, Yumvihoze E, Nakajima M, Workman P, Osborne G, et al. Identification of environmental sources of lead exposure in Nunavut (Canada) using stable isotope analyses. Environ Int. 2014;71:63–73. doi: 10.1016/j.envint.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Barman T, Kalahasthi R, Rajmohan HR. Effects of lead exposure on the status of platelet indices in workers involved in a lead-acid battery manufacturing plant. J Expo Sci Environ Epidemiol. 2014;24:629–33. doi: 10.1038/jes.2014.4. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- 8.Ambrose TM, Al-Lozi M, Scott MG. Bone lead concentrations assessed by in vivo X-ray fluorescence. Clin Chem. 2000;46:1171–8. [PubMed] [Google Scholar]
- 9.Wittmers LE, Jr, Aufderheide AC, Wallgren J, Rapp G, Jr, Alich A. Lead in bone. IV. Distribution of lead in the human skeleton. Arch Environ Health. 1988;43:381–91. doi: 10.1080/00039896.1988.9935855. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–70. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip AT, Gerson B. Lead poisoning–Part II Effects and assay. Clin Lab Med. 1994;14:651–70. [PubMed] [Google Scholar]
- 12.Tsaih SW, Korrick S, Schwartz J, Lee ML, Amarasiriwardena C, Aro A, et al. Influence of bone resorption on the mobilization of lead from bone among middle-aged and elderly men: the Normative Aging Study. Environ Health Perspect. 2001;109:995–9. doi: 10.1289/ehp.01109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman RH, White R, Kales SN, Hu H. Lead poisoning from mobilization of bone stores during thyrotoxicosis. Am J Ind Med. 1994;25:417–24. doi: 10.1002/ajim.4700250309. [DOI] [PubMed] [Google Scholar]
- 14.Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Korsch MJ, Vimpani G. Pregnancy increases mobilization of lead from maternal skeleton. J Lab Clin Med. 1997;130:51–62. doi: 10.1016/s0022-2143(97)90058-5. [DOI] [PubMed] [Google Scholar]
- 15.Fleming DE, Mills CE. A 4 × 500 mm2 cloverleaf detector system for in vivo bone lead measurement. Med Phys. 2007;34:945–51. doi: 10.1118/1.2436973. [DOI] [PubMed] [Google Scholar]
- 16.Theppeang K, Glass TA, Bandeen-Roche K, Todd AC, Rohde CA, Links JM, et al. Associations of bone mineral density and lead levels in blood, tibia, and patella in urban-dwelling women. Environ Health Perspect. 2008;116:784–90. doi: 10.1289/ehp.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potula V, Kleinbaum D, Kaye W. Lead exposure and spine bone mineral density. J Occup Environ Med. 2006;48:556–64. doi: 10.1097/01.jom.0000222556.89044.90. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Wang K, Wang Z, Gan C, He P, Liang Y, et al. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone. 2014;63:76–80. doi: 10.1016/j.bone.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Kang MH, Park SM, Oh DN, Kim MH, Choi MK. Dietary nutrient and food intake and their relations with serum heavy metals in osteopenic and osteoporotic patients. Clin Nutr Res. 2013;2:26–33. doi: 10.7762/cnr.2013.2.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreiger N, Tenenhouse A, Joseph L, MacKenzie T, Poliquin S, Brown JP, et al. Research notes: The Canadian Multicentre Osteoporosis Study (CaMos) — background, rationale, methods. Can J Aging. 1999;18:12. [Google Scholar]
- 21.Wong AK, Beattie KA, Min KK, Webber CE, Gordon CL, Papaioannou A, et al. A trimodality comparison of volumetric bone imaging technologies. Part I: short-term precision and validity. J Clin Densitom. 2014;18:124–35. doi: 10.1016/j.jocd.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed N, Osika NA, Wilson AM, Fleming DE. In vivo K-shell X-ray fluorescence bone lead measurements in young adults. J Environ Monit. 2005;7:457–62. doi: 10.1039/b418385a. [DOI] [PubMed] [Google Scholar]
- 23.McNeill FE, Stokes L, Chettle DR, Kaye WE. Factors affecting in vivo measurement precision and accuracy of 109Cd K x-ray fluorescence measurements. Phys Med Biol. 1999;44:2263–73. doi: 10.1088/0031-9155/44/9/313. [DOI] [PubMed] [Google Scholar]
- 24.Somervaille LJ, Chettle DR, Scott MC. In vivo measurement of lead in bone using x-ray fluorescence. Phys Med Biol. 1985;30:929–43. doi: 10.1088/0031-9155/30/9/005. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JR, Reigart JR, Ebeling M, Hulsey TC. Time required for blood lead levels to decline in nonchelated children. J Toxicol Clin Toxicol. 2001;39:153–60. doi: 10.1081/clt-100103831. [DOI] [PubMed] [Google Scholar]
- 26.Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, et al. Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect. 2013;121:97–104. doi: 10.1289/ehp.1205374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–8. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 28.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 29.Brito JA, McNeill FE, Webber CE, Chettle DR. Grid search: an innovative method for the estimation of the rates of lead exchange between body compartments. J Environ Monit. 2005;7:241–7. doi: 10.1039/b416054a. [DOI] [PubMed] [Google Scholar]
- 30.Gulson B, Mizon K, Smith H, Eisman J, Palmer J, Korsch M, et al. Skeletal lead release during bone resorption: effect of bisphosphonate treatment in a pilot study. Environ Health Perspect. 2002;110:1017–23. doi: 10.1289/ehp.021101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi JD, Arlen D, Webber CE, Chettle DR, Beaumont LF, Gordon CL. Is there any association between the presence of bone disease and cumulative exposure to lead? Calcif Tissue Int. 1998;63:429–32. doi: 10.1007/s002239900552. [DOI] [PubMed] [Google Scholar]
- 32.Brito JA, McNeill FE, Chettle DR, Webber CE, Vaillancourt C. Study of the relationships between bone lead levels and its variation with time and the cumulative blood lead index, in a repeated bone lead survey. J Environ Monit. 2000;2:271–6. doi: 10.1039/b002855j. [DOI] [PubMed] [Google Scholar]