Abstract
Stimulation of the CD28 costimulatory receptor can lead to an increased surface lipid raft expression in T lymphocytes. Here, we demonstrate that CD28 itself is recruited to lipid rafts in both Jurkat and peripheral blood T lymphocytes. This recruitment of CD28 is triggered by engagement with either anti-CD28 mAbs or a natural ligand of CD28, B7.2 (CD86). All detectable tyrosine-phosphorylated CD28 is in the lipid raft fractions, as is all of the CD28 associated with phosphatidylinositol 3-kinase, which is recruited to CD28 by tyrosine phosphorylation. Targeting the CD28 cytoplasmic domain to lipid rafts results in its tyrosine phosphorylation, indicating that tyrosine phosphorylation of CD28 may occur after translocation to lipid rafts. Studies with Jurkat cells deficient in Lck and CD45 demonstrate that movement of CD28 into lipid rafts does not require Lck and CD45 and can occur despite reduction of CD28 tyrosine phosphorylation to below the levels of detection. Analysis of murine CD28 mutants reveals a correlation between translocation to lipid rafts and costimulation of IL-2 production. Taken together with the known importance of lipid rafts in T cell activation, these observations suggest that translocation to lipid rafts may play an important role in CD28 signaling.
Naïve, resting T lymphocytes require stimulation of both the T cell receptor (TCR) and CD28 for optimal proliferation and differentiation. Through interactions with its ligands B7.1 (CD80) and B7.2 (CD86) on the surface of antigen-presenting cells, CD28 delivers a costimulus that increases production of cytokines such as IL-2, enhances expression of the IL-2 receptor, and induces anti-apoptotic mediators (1, 2). TCR and CD28 signals must occur concurrently and in close proximity for maximal T cell activation (3).
After interaction with ligands, CD28 recruits a number of signaling intermediates including phosphatidylinositol 3-kinase (PI3K), adapter Grb-2, and the serine/threonine phosphatase PP2A (4–8). CD28 also may interact with protein tyrosine kinases Lck and Itk tyrosine kinases and the phosphatase MKP6 (9–11). The exact role of these intermediates, however, is uncertain.
One recent area of signaling research has focused on the role of lipid rafts (also known as glycosphingolipid/cholesterol-enriched microdomains, detergent-insoluble glycolipid-enriched domains, and detergent-resistant membranes) in signal transduction by membrane receptors (12, 13). Lipid rafts are heterogeneous patches of cell membranes rich in protein, cholesterol, and sphingolipids. The rafts are characterized as gel-like, liquid-ordered regions surrounded by the more fluid areas of the plasma membrane, and they are closely packed with cholesterol and sphingolipids and are resistant to extraction with nonionic detergents. Proteins with saturated acyl chains (like those with glycosylphosphatidylinositol anchorage or with palmitoylation/myristoylation modifications) favor partitioning to the tightly packed lipid environment of the rafts (14–17). Lipid rafts seem to play a critical role in T cell activation (18–26). Key signaling membrane receptors such as TCR and CD2 move to rafts after activation, and drugs that interfere with raft formation block signaling from these receptors (17, 24–26).
CD28 has been reported to induce expression of rafts and direct the colocalization of TCR complexes with the rafts in the immunological synapse (27). In this report, we demonstrate ligand-induced movement of CD28 to lipid rafts in both peripheral blood lymphocytes and Jurkat T cells and explore the structure–function requirements of CD28 for this behavior in Jurkat cells.
Materials and Methods
Antibodies and Reagents. mAbs to murine CD28 (37.51) and human CD28 (9.3) were gifts from J. Allison (University of California, Berkeley) and the Fred Hutchinson Cancer Center (Seattle), respectively. The horseradish peroxidase (HRP)-conjugated antiphosphotyrosine mAb 4G10-HRP was obtained from Upstate Biotechnology (Lake Placid, NY). Rabbit antisera against p85α subunit of PI3K (p85α Z-8), goat antisera to hCD28 (CD28 N-20) and mCD28 (CD28 M-20), anti-actin mAb, HRP-conjugated anti-goat Ig, and anti-mouse Ig were purchased from Santa Cruz Biotechnology. UCHT1 (anti-human CD3ε) was purchased from BD Biosciences (Franklin Lakes, NJ). Ionomycin was from Calbiochem. Phorbol 12-myristate 13-acetate, HRP-conjugated cholera toxin B subunit, and anti-FLAG M2 were from Sigma.
Jurkat E6-1 T cells and Rat2 embryonic fibroblast cells and their derivative cell lines have been described (28). Mutants of Jurkat cells, JCaM1 and J45.01, were purchased (American Type Culture Collection). JCaM1-Lck cells were a kind gift from A. Weiss (University of California, San Francisco). Human peripheral blood lymphocytes were prepared from a single-step Ficoll density centrifugation, followed by separation of monocytes by culture-plastic adherence for 2 h.
Lck-CD28 chimera was constructed by incorporating the Lck myristoylation signal sequence at the N-terminal end of the mCD28 intracellular portion and a FLAG sequence at its C terminus. The addition of first 12 aa of Lck was performed by PCR amplification by using a forward sense primer: 5′-ATATATAAGCTTATGGGCTGTGTCTGCAGCTCAAACCCTGAAGATGACGGATCCAATAGTAGAAGGAACAGACTCCTTC-3′ (containing a HindIII site, first 12 aa of Lck, and the sequence NSRRNRLL of mCD28). The antisense primer used for the PCR reaction was 5′-AAAATCTAGATCACTTGTCATCGTCGTCCTTGTAGTCGGTACCGGGGCGGTACGCTGCAAAG-3′ (includes an XbaI site, the FLAG tag, and C-terminal 6 aa of mCD28 with the original stop codon replaced by the FLAG tag). The PCR amplification was performed on pBluescript II KS+ plasmid (Stratagene) containing the cDNA for WT mCD28 (29). The PCR product was subcloned into the HindIII and XbaI of pBluescript II KS+. The insert was subcloned into the XhoI–XbaI site of pEFIRES-P expression vector (30) (a gift of S. Hobbs, Institute of Cancer Research, London). The construct was introduced into Jurkat E6-1 cells by electroporation and selected in 0.4 μg/ml puromycin.
Membrane Fractionation and Western Blotting. The insoluble (“raft”) fraction was prepared as described (17). Cells were lysed for 30 min on ice in 50 mM Tris·Cl (pH 8.0), 1% Nonidet P-40, 20 mM EDTA supplemented with one tablet for 50 ml of Protease Inhibitor Mixture (Roche Biochemicals) and 1 mM Na3VO4. The lysates were then centrifuged at 13,000 × g for 10 min at 4°C. The pellet was saved and designated as the “insoluble” fraction. The proteins from this fraction were released by sonication on ice by using a microprobe in the following buffer: 20 mM Tris·Cl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% deoxycholate, 1 mM EDTA plus protease inhibitors, and 1 mM Na3VO4. After sonication, the mix was left on ice for 30 min at 4°C and centrifuged for 10 min at 4°C. The supernatant was used for subsequent immunoprecipitations.
For the total fraction of proteins, the cells were lysed in 20 mM Tris·Cl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% deoxycholate, 1 mM EDTA plus protease inhibitors, and 1 mM Na3VO4 for 30 min on ice. After centrifugation at 13,000 × g for 10 minat4°C, the supernatants were saved and designated as the total fraction.
Sucrose-density gradient fractionation of membrane lipids was performed by using a published protocol with modification (17). After antibody stimulation of the cells, they were rinsed in complete RPMI medium 1640 and resuspended in 1 ml of ice-cold sucrose gradient (SG) buffer (20 mM Tris·Cl, pH7.5/150 mM NaCl/1mM Na3VO4/5 mM EDTA and protease inhibitors) supplemented with 2% Nonidet P-40. After a 30-min incubation on ice, the lysates were homogenized with 20 strokes of a loose-fitting Dounce homogenizer. They were then mixed with an equal volume of 85% sucrose (wt/vol) made in SG buffer. The mix was then placed in the bottom of an SW40Ti centrifuge tube and overlaid with 6.5 ml of 30% sucrose (wt/vol), followed by 3.5 ml of 5% sucrose (wt/vol) solution made in SG buffer. The gradient was then spun at 100,000 × g for 20 h at 4°C in an ultracentrifuge. Fractions of 1 ml each were sequentially collected from the top. N-octyl-β-d-glucoside (Sigma) was added to 1% (wt/vol) to each fraction and left for 30 min on ice before immunoprecipitation.
Immunoprecipitations were performed as described (28). The % translocation to the detergent-insoluble fraction was quantitated by densitometric scanning of blots from the x-ray films exposed to the chemiluminescent signal of CD28 immunoprecipitated from the total fraction and the insoluble fraction. The films were exposed multiple times to obtain a linear signal for the exposures.
Cell Activation and Treatment with Enzyme Inhibitors/Activators. Pervanadate was freshly prepared by addition of 25 μl of 500 mM Na3VO4 (Sigma) (final, 20 mM) to 574 μl of PBS and 1 μlof30% (wt/wt) H2O2 solution (Sigma) (final, 16.3 mM), incubating for 15 min at room temperature. Free H2O2 was neutralized by the addition of 200 μg/ml catalase (Sigma). Before treatment, cells were washed twice with ice-cold complete RPMI medium 1640 and resuspended at a density of 1 × 108 cells per ml. Pervanadate was then added at 1:100 dilution to the cell suspension and incubated for 10 min at room temperature. The reaction was halted by placing the cells on ice for 5 min with subsequent washing with ice-cold complete RPMI medium 1640 before cell lysis.
IL-2 Measurements. Production of IL-2 by Jurkat cells was induced as before (28) with the additional inclusion of crosslinking by a secondary anti-mouse antibody depending on the experiment. The crosslinking was performed by the addition of 40 μg/ml of the secondary antibody to the cells 10 min after the addition of 5 μg/ml of anti-hCD28 or anti-mCD28 antibodies at 37°C in the presence of 1 ng/ml phorbol 12-myristate 13-acetate plus 0.5 μM ionomycin. IL-2 levels were measured by an ELISA kit (BioSource International, Camarillo, CA).
Results
Stimulation of CD28 by mAb Induces Its Translocation to Lipid Rafts. When Jurkat cells or peripheral T cells are lysed in buffer containing 1% Nonidet P-40, <20% of CD28 is found in the detergent-insoluble fraction that contains lipid raft components (Fig. 1). Stimulation of CD28, however, has a dramatic effect on its localization in this assay. When Jurkat cells or peripheral T cells are stimulated with an anti-CD28 mAb before lysis, a substantial proportion (60–80%) of CD28 is found in the detergent-insoluble fraction (Fig. 1). Another group has previously shown no translocation of CD28 on cell-surface activation in Jurkat cells (31). The basis for this discrepancy is uncertain.
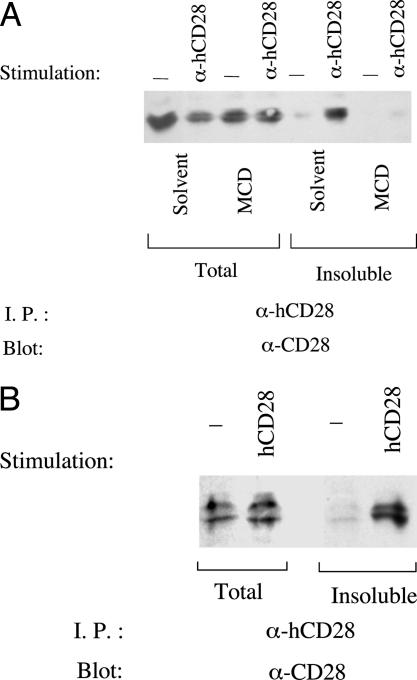
Fig. 1.
Stimulation of CD28 induces its translocation to lipid rafts. (A) After mAb-mediated stimulation, CD28 translocates to a detergent-insoluble fraction, and translocation is inhibited by dispersing lipid rafts with methyl-β-cyclodextrin (MCD). Resting or anti-CD28 mAb-stimulated Jurkat T cells (4 × 107 cells per sample) were treated with 10 mM methyl-β-cyclodextrin or DMSO as the carrier solvent (used at 0.1% final concentration) for 15 min. After lysis, CD28 was immunoprecipitated from total or detergent-insoluble fractions and then revealed by immunoblotting (see Materials and Methods). (B) hCD28 in resting peripheral blood T lymphocytes also translocates to the detergent-insoluble fraction after stimulation with anti-CD28 mAb.
Methyl-β-cyclodextrin perturbs the integrity of lipid rafts by extracting cholesterol from biological membranes (17). Pretreatment of Jurkat cells with methyl-β-cyclodextrin abrogates the translocation of CD28 to the detergent-insoluble fraction, consistent with the possibility that mAb-mediated stimulation of CD28 triggers its movement into lipid rafts (Fig. 1A). To explore this further, we used sucrose-density gradient fractionation of membranes to analyze the subcellular localization of CD28. The great majority of CD28 from unstimulated Jurkat cells colocalizes with actin in the soluble fractions, and <20% of CD28 colocalizes with components of lipid rafts such as Lck and GM1 gangliosides (Fig. 2 Top). When membranes are obtained from Jurkat cells that have been stimulated with an anti-CD28 mAb, however, a substantial proportion (60–80%) of CD28 colocalizes with the lipid raft fractions (Fig. 2 Middle). Taken together, these data indicate that mAb-mediated stimulation of CD28 triggers its translocation to lipid rafts.
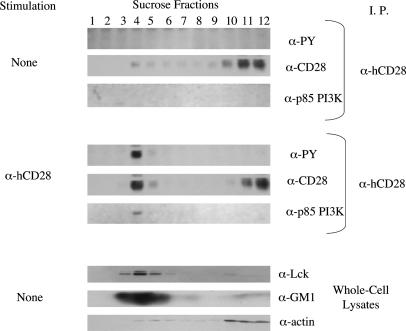
Fig. 2.
mAb-mediated stimulation of hCD28 in Jurkat T cells leads to its translocation to lipid rafts. Jurkat cells (108 cells per ml) were incubated with medium alone or with anti-CD28 mAb for 5 min at 37°C. The lysates of the cells were then prepared for sucrose centrifugation according to Materials and Methods. CD28 immunoprecipitates of the 12 fractions were analyzed by immunoblotting with antiphosphotyrosine (PY), anti-CD28, and anti-PI3K p85. Each fraction was also subjected to blotting with anti-Lck, anti-actin, and HRP-conjugated cholera toxin B, which marks the presence of GM1.
Tyrosine-Phosphorylated CD28 Is Found in Lipid Rafts. After mAb-mediated stimulation of CD28, all detectable tyrosine-phosphorylated CD28 is in the lipid raft fractions, as is all of the CD28 associated with PI3K, which is recruited to CD28 by tyrosine phosphorylation (Fig. 2 Middle).
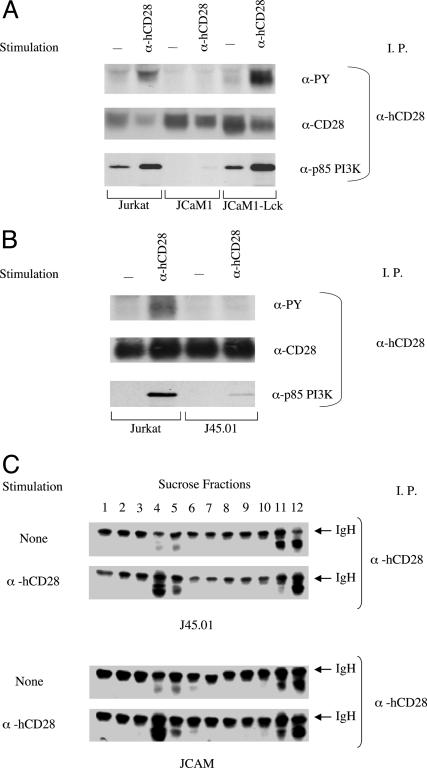
Translocation of CD28 to Lipid Rafts Does Not Require Lck or CD45. Stimulation by an anti-CD28 mAb does not result in detectable tyrosine phosphorylation of CD28 in JCaM1 cells, a mutant of Jurkat that lacks Lck (32), but does when Lck expression is reconstituted in JCaM1 (Fig. 3A). Despite the absence of detectable tyrosine phosphorylation, mAb-mediated stimulation triggers the translocation of CD28 to lipid rafts in JCaM1 (Fig. 3C). Similarly, CD28 is not tyrosine-phosphorylated at detectable levels after stimulation of J45.01, a Jurkat mutant that lacks CD45, a transmembrane tyrosine phosphatase required for activity of Lck and other Src-like kinases (Fig. 3B). Nonetheless, we find that stimulation of CD28 on J45.01 triggers its translocation into lipid rafts (Fig. 3C). Translocation of CD28 to lipid rafts, therefore, does not require Lck or CD45. We do observe a low level of mAb-induced recruitment of PI3K to CD28 in JCaM1 and J45.01, suggesting either that tyrosine phosphorylation occurs below the level of detection in these cells or that a low level of PI3K can associate with CD28 independently of tyrosine phosphorylation.
Fig. 3.
Lck and CD45 are required for the tyrosine phosphorylation of CD28 but not for its translocation into lipid rafts. The Jurkat mutants, JCaM1 (Lck-negative) and JCaM1-Lck (JCaM1 with Lck expression restored) (A), and J45.01, lacking in CD45 expression (B), were stimulated with anti-CD28 mAb or left unstimulated for 5 min in a 37°C bath. For each sample, 108 cells per ml were used. After the stimulation, CD28 was immunoprecipitated and analyzed by immunoblotting as indicated. (C) J45.01 and JCaM1 were stimulated with anti-CD28 mAb or left unstimulated as in Fig. 3. Each sample used 108 cells per ml. After the 5-min stimulation, the cells were washed once and lysed; the lysates were fractionated on a sucrose gradient according to the Materials and Methods. CD28 was immunoprecipitated from each fraction and revealed by immunoblotting.
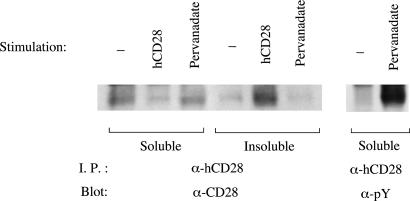
To examine the effects of CD28 tyrosine phosphorylation in the absence of CD28 ligation, we used pervanadate, a tyrosine phosphatase inhibitor that markedly increases the tyrosine phosphorylation of CD28 when added to Jurkat T cells (Fig. 4). Despite this increased tyrosine phosphorylation, CD28 does not localize to lipid rafts in pervanadate-treated cells. These results suggest that tyrosine phosphorylation of CD28 is not sufficient to trigger movement to lipid rafts. We cannot, however, exclude the possibility that pervanadate simultaneously affects other molecules that influence pathways for translocation.
Fig. 4.
Pervanadate-induced tyrosine phosphorylation state of CD28 does not lead to movement to the detergent-insoluble fraction. Jurkat cells at 108 cells per ml in complete RPMI medium 1640 were treated with pervanadate or with hCD28 Ab or left unstimulated. After lysis in 1% Nonidet P-40, CD28 was immunoprecipitated from the detergent-soluble and -insoluble fractions and then analyzed by immunoblotting as indicated.
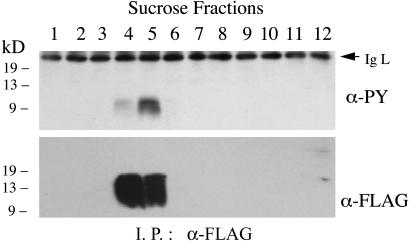
A CD28 Chimera Targeted to Lipid Rafts Is Tyrosine-Phosphorylated. The studies with JCaM1 and J45.01 demonstrate that mAb-induced translocation of CD28 to lipid rafts can occur when its tyrosine phosphorylation is dramatically reduced or absent, suggesting that phosphorylation is a consequence of translocation to rafts. To determine whether localization of CD28 within lipid rafts is sufficient to induce its tyrosine phosphorylation, we used the N-terminal myristoylation sequence of Lck to target the CD28 cytoplasmic domain to lipid rafts. When expressed in Jurkat cells, a chimera composed of the Lck myristoylation sequence, the cytoplasmic domain of CD28, and a FLAG epitope localizes to the lipid raft fraction. This chimera is tyrosine-phosphorylated, demonstrating that lipid rafts contain protein tyrosine kinases that can phosphorylate CD28 (Fig. 5).
Fig. 5.
A chimera containing the CD28 cytoplasmic domain localizes to lipid rafts and is tyrosine-phosphorylated. Lysates of unstimulated Jurkat T cells that stably express a Lck-CD28-FLAG chimera (described in Materials and Methods) were fractionated on a sucrose gradient. Anti-FLAG antibody was used to immunoprecipitate the chimera, which was then analyzed by immunoblotting with antibodies to phosphotyrosine and the FLAG epitope.
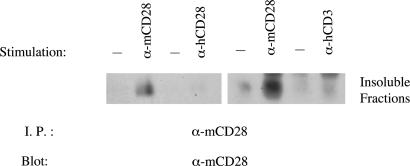
Ligated CD28 Molecules Move into Lipid Rafts. Ligand-induced translocation of CD28 into lipid rafts could require ligation of the individual CD28 molecules that move into rafts or could generate an intracellular signal that triggers the general movement of CD28, ligated or not, into rafts. To help distinguish between these possibilities, we used Jurkat cells that have been transfected with a gene encoding WT murine CD28 (mCD28) and, therefore, express mCD28 and the endogenous human CD28 (hCD28). Like hCD28, mCD28 translocates to the detergent-insoluble fraction after stimulation with a mAb specific for mCD28 and lysis of cells in 1% Nonidet P-40 (Fig. 6). When these hCD28+mCD28+ cells are stimulated with a mAb to hCD28, however, mCD28 does not move into detergent-insoluble fraction, a finding consistent with the model that ligation of individual CD28 molecules is required for translocation to rafts (Fig. 6). We find that stimulation of CD3ε also fails to induce translocation to the detergent-insoluble fraction of mCD28 (Fig. 6) or hCD28 (data not shown), indicating that TCR signals alone do not cause the movement of CD28 into lipid rafts.
Fig. 6.
Stimulation of either hCD28 or hCD3 on hCD28+mCD28+ Jurkat cells fails to induce the translocation of mCD28 to the detergent-insoluble fraction. Jurkat cells expressing WT mouse CD28 (mCD28) and endogenous human CD28 (hCD28) were stimulated with an anti-mCD28 mAb, an anti-hCD28 mAb, or an anti-hCD3 or left unstimulated. The cells were lysed, and the detergent-insoluble fractions were analyzed for the presence of mCD28 by immunoprecipitation followed by immunoblotting.
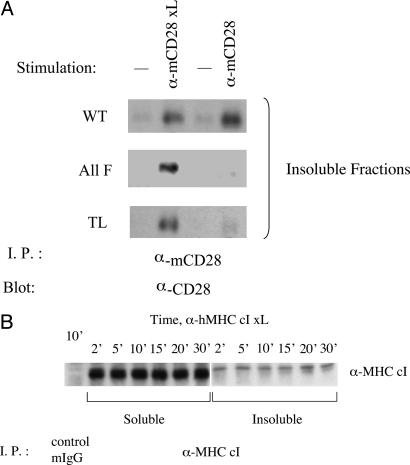
Translocation to Lipid Rafts Induced by Anti-CD28 mAb or B7.2 Requires Tyr-188 of the CD28 Cytoplasmic Domain. To examine CD28 structural requirements for translocation to lipid rafts, we studied Jurkat cells that express mutants of mCD28 and stimulated mCD28 by the addition of an anti-mCD28 mAb alone or an anti-mCD28 mAb crosslinked by a second-step antiserum. Mutation of all four cytoplasmic tyrosines to phenylalanine (ALL F mutant) abrogates the ability of the anti-mCD28 mAb alone to trigger the movement of mCD28 into the detergent-insoluble fraction (Fig. 7A). Indeed, the behavior of the ALL F mutant is indistinguishable from the tailless (TL) mutant, which deletes all but four residues of the cytoplasmic domain (Fig. 7A). Crosslinking the anti-mCD28 mAb, however, overcomes this requirement for CD28 cytoplasmic domain residues. When the anti-mCD28 mAb is crosslinked by a second-step antiserum to mouse Ig, the ALL F and TL mutants translocate to the detergent-insoluble fraction (Fig. 7A). This translocation of CD28 in response to crosslinked mAb seems to be specific. In contrast to results with CD28, secondary crosslinking of anti-MHC class I mAb does not trigger the movement of MHC class I molecules to the detergent-insoluble fraction (Fig. 7B).
Fig. 7.
(A) Translocation of mCD28 to the detergent-insoluble fraction in response to anti-mCD28 mAb requires cytoplasmic domain tyrosine residues, but increasing the strength of mAb crosslinking overcomes this requirement. Jurkat cells expressing WT, all-phenylalanine mutant (All F), and TL versions of mCD28 were stimulated by anti-mCD28 mAb alone (α-mCD28) or anti-mCD28 mAb crosslinked by goat anti-mouse antibody (α-mCD28 xL). The remaining samples were left untreated. The presence of mCD28 in the detergent-insoluble fractions was determined by immunoprecipitation and immunoblotting. (B) MHC class I molecules do not translocate to lipid rafts after extensive crosslinking. Jurkat cells were stimulated with an anti-MHC class I mAb plus secondary crosslinking at 37°C for the indicated times and then lysed. The presence of MHC class I molecules in the detergent-soluble and -insoluble fractions was determined by immunoprecipitation and immunoblotting.
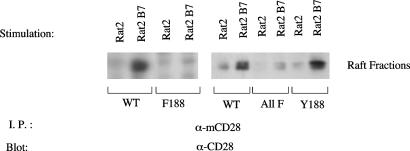
To determine whether the translocation of CD28 triggered by mAb-mediated stimulation also occurs after engagement by natural ligands, we incubated mCD28-expressing Jurkat cells with Rat2 cells (which do not express B7 molecules) or with Rat2 cells that express murine B7.2 after gene transfer (28). Translocation to lipid rafts was assessed by sucrose-density gradient fractionation of membranes. Interaction with the B7.2-expressing Rat2 cells, but not control Rat2 cells, induces the translocation of WT mCD28 to lipid rafts (Fig. 8). Mutation of Tyr-188 to phenylalanine (Phe-188 mutant) or of all four tyrosines (ALL F mutant) severely impairs but does not completely abrogate B7.2-induced movement of mCD28 into lipid rafts. In contrast, engagement by B7.2 triggers the movement of the Tyr-188 mutant into rafts, and the Tyr-188 mutant moves into rafts and WT mCD28 (Fig. 8). Therefore, optimal B7.2-induced translocation to lipid rafts depends on Tyr-188, but there seems to be a mechanism by which B7.2 can induce movement into lipid rafts independently of CD28 cytoplasmic tyrosines.
Fig. 8.
Interaction with natural ligand of CD28, hB7.2, induces translocation of CD28 to lipid rafts, and mutation of Tyr-188 (Y188) impairs this translocation. Jurkat T cells expressing WT, Phe-188, the all-phenylalanine mutant (ALL F), or Tyr-188 versions of mCD28 were cospun for 5 min with Rat2 cells expressing hB7.2 or parental Rat2 cells at 37°C. The Jurkat cells and the Rat2 cells were present at a 1:1 ratio, with 4 × 107 cells for each. The cell pellets were then lysed, and membranes were subjected to sucrose-density gradient fractionation. CD28 was immunoprecipitated from the pooled fractions that contain lipid rafts and revealed by immunoblotting.
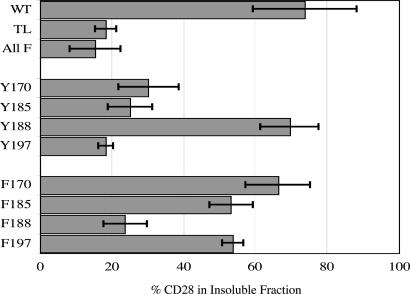
To determine the importance of individual tyrosine residues for translocation in response to anti-mCD28 mAb alone, we analyzed two types of mutants: the Y mutants, in which tyrosine is retained at the designated site with the remaining three tyrosines mutated to phenylalanine, and the F mutants, which have a single phenylalanine substitution at the designated residue. The Tyr-188 mutant translocates to the detergent-insoluble fraction as effectively as WT mCD28, and mutation of Tyr-188 to phenylalanine (Phe-188 mutant) severely impairs mAb-induced translocation (Fig. 8). A tyrosine at position 188, therefore, is necessary for translocation, and Tyr-188 allows translocation even when all other tyrosine residues have been mutated to phenylalanine. These results are specific for Tyr-188. In contrast to the results obtained with Tyr-188, none of the other Tyr mutants translocates to the detergent-insoluble fraction (Fig. 9). Moreover, single phenylalanine substitutions at positions 170, 185, or 197 have little or no effect on mAb-induced translocation of mCD28 to the detergent-insoluble fraction (Fig. 9).
Fig. 9.
Translocation of mCD29 to the detergent-insoluble fraction in response to anti-mCD28 mAb requires Tyr-188 but not the other CD28 cytoplasmic tyrosines. Jurkat cells expressing either WT mCD28 or 1 of 10 mCD28 mutants were stimulated with anti-mCD28 mAb without secondary crosslinking. After lysis, CD28 was immunoprecipitated from total or detergent-insoluble fractions and then revealed by immunoblotting; the percentage of translocation was calculated as described in Materials and Methods. Shown are the mean and standard deviations of at least three independent experiments except for Phe-185 and Phe-197 (two independent experiments each).
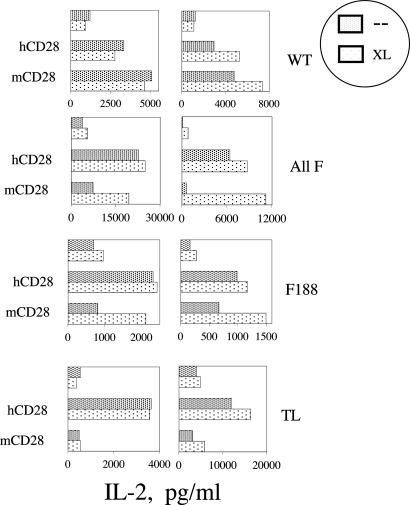
Crosslinking Anti-CD28 mAb with a Second-Step Antibody Enhances the Costimulatory Ability of the ALL F and Tyr-188 Mutants. When stimulated by mAb, WT mCD28 expressed by Jurkat delivers a costimulus for IL-2 production comparable to that of the endogenous hCD28. We have reported that mutation of Tyr-188 (but no other individual cytoplasmic tyrosine) impairs the ability of anti-mCD28 mAb to deliver this costimulatory signal (28). Conversely, we have found that the Tyr-188 mutant can deliver a full costimulus for IL-2 production, but no other Y mutant can (28, 29). In response to stimulation by mAb alone, therefore, both translocation to lipid rafts and delivery of a costimulus require Tyr-188.
To explore further the relationship between translocation and costimulation, we took advantage of the ability of secondary crosslinking to induce movement of mCD28 mutants into lipid rafts (Fig. 7A). Secondary crosslinking does not affect the ability of hCD28 or WT mCD28 to costimulate IL-2 production (Fig. 10). The effects of crosslinking on the ALL F and Phe-188 mutants, however, are striking; when stimulated by a mAb and a second-step antiserum, each mutant delivers a costimulus comparable to that of the endogenous hCD28 (Fig. 10). The costimulus delivered by secondary crosslinking requires sequences within the cytoplasmic domain of mCD28 and is abrogated by the TL mutation.
Fig. 10.
Secondary crosslinking of anti-mCD28 mAb overcomes the requirement for Tyr-188 in the costimulation of IL-2 by mCD28. Jurkat cells expressing WT, the all-phenylalanine mutant (All F), the Tyr-188 to phenylalanine mutant (Phe-188), or TL versions of mCD28 were stimulated with the combination of 1 μg/ml phorbol 12-myristate 13-acetate and 0.5 μM ionomycin. mAbs to either the endogenous hCD28 or to mCD28 were added as indicated either alone (–) or with a crosslinking goat anti-mouse antibody (XL). The levels of IL-2 in the supernatants were quantitated by ELISA. Shown are the results of experiments with two independently derived clones for each mCD28 mutant.
Discussion
Here we demonstrate that CD28 itself translocates to the lipid raft fraction upon stimulation with anti-CD28 mAb or with B7.2 (CD86)-bearing cells. Movement of CD28 into lipid rafts correlates with its costimulation of IL-2 production by Jurkat cells in that CD28 mutations that abolish the translocation also impair this costimulatory function.
In studies of Jurkat cells, mutation of the CD28 cytoplasmic residue Tyr-188 to phenylalanine abrogates translocation to lipid rafts in response to anti-CD28 mAb and impairs translocation induced by B7.2. In an earlier study, we demonstrated that Tyr-188 is the major phosphorylation site upon activation of CD28 (28). These observations suggest a model in which ligand-induced phosphorylation of Tyr-188 triggers the movement of CD28 to lipid rafts. Several findings, however, argue against this model. First, Lck and CD45 are required for the tyrosine phosphorylation of CD28 but are not required for translocation to lipid rafts. These findings are in sharp contrast to studies demonstrating that TCR movement to lipid rafts requires Src kinases and is sensitive to the Src kinase inhibitor PP1 (24). Second, inducing ligand-independent tyrosine phosphorylation of CD28 by addition of pervanadate does not trigger the translocation of CD28. Finally, targeting the CD28 cytoplasmic domain to lipid rafts leads to its tyrosine phosphorylation, suggesting that tyrosine phosphorylation of CD28 is a consequence of its movement into lipid rafts. Taken together, these observations raise the possibility that Tyr-188 participates in the translocation process through interactions that do not require its phosphorylation. Clathrin-dependent endocytosis of CTLA-4 provides an example of a tyrosine-dependent but phosphorylation-independent event that affects the intracellular localization of a member of the CD28 family (33–36).
The addition of a secondary crosslinking antibody overcomes the requirement for Tyr-188 in mAb-induced translocation of CD28 to rafts. This effect of secondary crosslinking seems to have specificity in that secondary crosslinking of a mAb to MHC class I does not induce the translocation of MHC class I molecules to rafts. Moreover, although mutation of Tyr-188 clearly impairs translocation of CD28 induced by B7.2, it does not completely abrogate it. Thus, the effect of secondary crosslinking may have physiological relevance. One possibility is that the extent of crosslinking of CD28 by its natural ligands B7.1 and B7.2, in turn determined by their surface density on the antigen-presenting cells, influences the mechanism responsible for translocation of CD28 to lipid rafts. Under conditions of low ligand density, the mechanism that requires Tyr-188 dominates; conversely, when ligand density is high, translocation becomes independent of Tyr-188 (and, possibly, the CD28 cytoplasmic domain). The secondary crosslinking antibody not only overcomes the requirement for Tyr-188 in the translocation of CD28 to lipid rafts but also allows CD28 to costimulate IL-2 in the absence of cytoplasmic Tyr residues. Once located in rafts, therefore, ligated CD28 may be able to engage tyrosine-independent pathways, perhaps by means of interactions between its diproline motifs and SH3 domain-bearing signaling molecules, that are sufficient to costimulate IL-2 in Jurkat cells (9, 10).
An important caveat of these investigations is their reliance on Jurkat cells for studies of the structure–function relationships of translocation and of costimulation. Although our data demonstrate that CD28 translocates to the lipid rafts of peripheral blood T cells and Jurkat cells (Fig. 1), the structural requirements for translocation and signaling may differ between primary T cells and transformed T cell lines such as Jurkat. Clearly, the role of the lipid rafts and their dynamic interactions with CD28 merits a closer examination.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 AI26644 and by the Rosalind Russell Arthritis Foundation.
Abbreviations: TCR, T cell receptor; PI3K, phosphatidylinositol 3-kinase; HRP, horseradish peroxidase; TL, tailless.
References
- 1.June, C. H., Bluestone, J. A., Nadler, L. M. & Thompson, C. B. (1994) Immunol. Today 15, 321–331. [DOI] [PubMed] [Google Scholar]
- 2.Chambers, C. A. & Allison, J. P. (1999) Curr. Opin. Cell Biol. 11, 203–210. [DOI] [PubMed] [Google Scholar]
- 3.Michel, F. & Acuto, O. (2002) Sci. STKE 144, PE35. [DOI] [PubMed] [Google Scholar]
- 4.Chuang, E., Fisher, T. S., Morgan, R. W., Robbins, M. D., Duerr, J. M., Vander Heiden, M. G., Gardner, J. P., Hambor, J. E., Neveu, M. J. & Thompson, C. B. (2000) Immunity 13, 313–322. [DOI] [PubMed] [Google Scholar]
- 5.Schneider, H., Cai, Y. C., Prasad, K. V., Shoelson, S. E. & Rudd, C. E. (1995) Eur. J. Immunol. 25, 1044–1050. [DOI] [PubMed] [Google Scholar]
- 6.Lee, K. M., Chuang, E., Griffin, M., Khattri, R., Hong, D. K., Zhang, W., Straus, D., Samelson, L. E., Thompson, C. B. & Bluestone, J. A. (1998) Science 282, 2263–2266. [DOI] [PubMed] [Google Scholar]
- 7.Schneider, H. & Rudd, C. E. (2000) Biochem. Biophys. Res. Commun. 269, 279–283. [DOI] [PubMed] [Google Scholar]
- 8.Truitt, K. E., Hicks, C. M. & Imboden, J. B. (1994) J. Exp. Med. 179, 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holdorf, A. D., Green, J. M., Levin, S. D., Denny, M. F., Straus, D. B., Link, V., Changelian, P. S., Allen, P. M. & Shaw, A. S. (1999) J. Exp. Med. 190, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marengere, L. E., Okkenhaug, K., Clavreul, A., Couez, D., Gibson, S., Mills, G. B., Mak, T. W. & Rottapel, R. (1997) J. Immunol. 159, 3220–3229. [PubMed] [Google Scholar]
- 11.Marti, F., Krause, A., Post, N. H., Lyddane, C., Dupont, B., Sadelain, M. & King, P. D. (2001) J. Immunol. 166, 197–206. [DOI] [PubMed] [Google Scholar]
- 12.Simons, K. & Ikonen, E. (1997) Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. A. & London, E. (1998) Annu. Rev. Cell Dev. Biol. 14, 111–136. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers, W. & Rose, J. K. (1996) J. Cell Biol. 135, 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran, M. & Miceli, M. C. (1998) Immunity 9, 787–796. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, W., Trible, R. P. & Samelson, L. E. (1998) Immunity 9, 239–246. [DOI] [PubMed] [Google Scholar]
- 17.Xavier, R., Brennan, T., Li, Q., McCormack, C. & Seed, B. (1998) Immunity 8, 723–732. [DOI] [PubMed] [Google Scholar]
- 18.Horejsi, V., Drbal, K., Cebecauer, M., Cerny, J., Brdicka, T., Angelisova, P. & Stockinger, H. (1999) Immunol. Today 20, 356–361. [DOI] [PubMed] [Google Scholar]
- 19.Fragoso, R., Ren, D., Zhang, X., Su, M. W., Burakoff, S. J. & Jin, Y. J. (2003) J. Immunol. 170, 913–921. [DOI] [PubMed] [Google Scholar]
- 20.Arcaro, A., Gregoire, C., Boucheron, N., Stotz, S., Palmer, E., Malissen, B. & Luescher, I. F. (2000) J. Immunol. 165, 2068–2076. [DOI] [PubMed] [Google Scholar]
- 21.Cinek, T. & Horejsi, V. (1992) J. Immunol. 149, 2262–2270. [PubMed] [Google Scholar]
- 22.van't Hof, W. & Resh, M. D. (1999) J. Cell Biol. 145, 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabouridis, P. S., Magee, A. I. & Ley, S. C. (1997) EMBO J. 16, 4983–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montixi, C., Langlet, C., Bernard, A. M., Thimonier, J., Dubois, C., Wurbel, M. A., Chauvin, J. P., Pierres, M. & He, H. T. (1998) EMBO J. 17, 5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janes, P. W., Ley, S. C. & Magee, A. I. (1999) J. Cell Biol. 147, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, H. & Reinherz, E. L. (2001) J. Biol. Chem. 276, 18775–18785. [DOI] [PubMed] [Google Scholar]
- 27.Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. (1999) Science 283, 680–682. [DOI] [PubMed] [Google Scholar]
- 28.Sadra, A., Cinek, T., Arellano, J. L., Shi, J., Truitt, K. E. & Imboden, J. B. (1999) J. Immunol. 162, 1966–1973. [PubMed] [Google Scholar]
- 29.Truitt, K. E., Nagel, T., Suen, L. F. & Imboden, J. B. (1996) J. Immunol. 156, 4539–4541. [PubMed] [Google Scholar]
- 30.Hobbs, S., Jitrapakdee, S. & Wallace, J. C. (1998) Biochem. Biophys. Res. Commun. 252, 368–372. [DOI] [PubMed] [Google Scholar]
- 31.Yashiro-Ohtani, Y., Zhou, X. Y., Toyo-Oka, K., Tai, X. G., Park, C. S., Hamaoka, T., Abe, R., Miyake, K. & Fujiwara, H. (2000) J. Immunol. 164, 1251–1259. [DOI] [PubMed] [Google Scholar]
- 32.Straus, D. B. & Weiss, A. (1992) Cell 70, 585–593. [DOI] [PubMed] [Google Scholar]
- 33.Bradshaw, J. D., Lu, P., Leytze, G., Rodgers, J., Schieven, G. L., Bennett, K. L., Linsley, P. S. & Kurtz, S. E. (1997) Biochemistry 36, 15975–15982. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Y. & Allison, J. P. (1997) Proc. Natl. Acad. Sci. USA 94, 9273–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang, E., Alegre, M. L., Duckett, C. S., Noel, P. J., Vander Heiden, M. G. & Thompson, C. B. (1997) J. Immunol. 159, 144–151. [PubMed] [Google Scholar]
- 36.Shiratori, T., Miyatake, S., Ohno, H., Nakaseko, C., Isono, K., Bonifacino, J. S. & Saito, T. (1997) Immunity 6, 583–589. [DOI] [PubMed] [Google Scholar]