Abstract
Semaphorin 3B (SEMA3B) is a secreted member of the semaphorin family, important in axonal guidance. We and others have shown that SEMA3B can act as a tumor suppressor by inducing apoptosis either by reexpression in tumor cells or applied as a soluble ligand. The common method of inactivation of SEMA3B is by allele loss and tumor-acquired promoter methylation. We studied the mechanism of SEMA3B-induced tumor cell apoptosis and found that vascular endothelial growth factor (VEGF)165 significantly decreased the proapoptotic and antimitotic effect of transfected or secreted SEMA3B on lung and breast cancer cells. VEGF165 binds to neuropilin, receptors for SEMA3B, and we found that SEMA3B competed for binding of 125I-VEGF165 to lung and breast cancer cells. We also found that small interfering RNA knockdown of tumor-produced VEGF-A or the use of an anti-VEGF neutralizing antibody (Ab) significantly inhibited tumor cell growth in vitro. By contrast, VEGF121, a VEGF variant that lacks binding to neuropilin (NP)-1 or NP-2 receptors, was not expressed in tumor cells and had no effect on SEMA3B growth-suppressing activities. In conclusion, we hypothesize that VEGF165, produced by tumor cells, acts as an autocrine survival factor and that SEMA3B mediates its tumor-suppressing effects, at least in part, by blocking this VEGF autocrine activity.
Semaphorin 3B (SEMA3B) is located at 3p21.3, a site of very frequent allele loss and/or promoter methylation in the early pathogenesis of lung and breast cancer (1-3). SEMA3B encodes a protein with tumor suppressor activity for lung cancer (4). Treatment with exogenously added SEMA3B or introduction of a plasmid encoding SEMA3B into H1299 non-small cell lung cancer (NSCLC) cells led to induction of apoptosis and a dramatic decrease in colony formation (2, 4). By contrast, tumor-acquired SEMA3B missense mutations have lost this activity. Independent studies in ovarian cancer by Tse et al. (5) demonstrated that SEMA3B also inhibited ovarian tumor formation in a xenograft model. Additionally, expression of SEMA3B in a p53-negative glioblastoma cell line was increased after reexpression of p53, suggesting that SEMA3B might also act as a mediator of p53 tumor-suppressor activity (6). However, because H1299 cells are p53 null, SEMA3B can induce marked tumor suppression even in the absence of p53.
SEMA3B is a secreted protein that belongs to the class 3 semaphorins (7, 8). Although the major role of SEMA3 proteins is to guide axons, they are also involved in diverse processes such as immune modulation (9, 10), organogenesis (11), neuronal apoptosis (12), and drug resistance (2, 13). SEMA3 members form complexes with two types of cell surface receptors: neuropilins (NP-1 and NP-2) and plexins (14-16). Neuropilins provide binding sites for SEMA3 whereas plexins are necessary for signal transduction by SEMA3B (17-19). In addition to semaphorins, NP-1 and NP-2 also bind several members of the vascular endothelial growth factor (VEGF) family, including VEGF-A (20, 21), VEGF-B, and placental growth factor (PIGF) (22, 23), all of which are angiogenic factors. VEGF-A is an endothelial cell (EC) agonist and is essential for vasculogenesis, angiogenesis, and wound healing and plays a role in tumor angiogenesis. VEGFR-1 (Flt-1) and VEGFR-2 (KDR) are the classic receptors for VEGF-A. VEGF-A isoforms (e.g., VEGF165 and VEGF121) are created by alternative mRNA splicing and possess identical affinity for all VEGF receptors (24). By contrast, NPs bind VEGF165 but not VEGF121, the shortest isoform that lacks a heparin-binding domain (20). In vitro studies show that VEGF165 interaction with NPs enhances VEGF-A effects, transduced through VEGFR-2, such as chemotaxis, EC survival, and angiogenesis (21, 25). In vivo studies show that NP-1 overexpression or silencing leads to abnormalities in blood vessel formation and the cardiovascular system during embryogenesis, indicating the importance of NP-1 interaction with the members of the VEGF family during development (26).
SEMA3A has been the most studied protein of the SEMA3 subclass due to its demonstrated role in axon guidance. It has been suggested that VEGF165 is able to antagonize the proapoptotic and inhibitory effects of SEMA3A in the nervous system because both proteins share the same binding domain on cell-surface receptors (27). Porcine and rat aortic endothelial cell expressing NP-1 and VEGFR-2 respond to exogenously added SEMA3A by decreasing cell migration, as well as microvessel and lamellipodia formation (28). These effects were abolished by VEGF165 (28). A similar pattern is observed in medulloblastoma cells expressing NP-1 and VEGFR-1; SEMA3A induces apoptosis in these cells whereas VEGF165 antagonizes this effect by promoting cell proliferation and survival (29). VEGFs are indirect promoters of tumor growth in vivo by inducing angiogenesis that is crucial for supporting expansion of tumor mass and metastases. In addition, members of the VEGF family support tumor growth directly by acting as autocrine survival factors for those malignant cells that express VEGF or NP receptors (30-32). VEGF and SEMA3A are antagonistic autocrine NP-1 ligands that regulate breast carcinoma cell migration (33). The existence of common receptor(s) for both VEGF and semaphorin(s) implies that these factors might compete for the same binding sites on the cell surface.
Tumor cells produce VEGF, which could act as an autocrine survival factor as well as stimulate tumor angiogenesis. Because SEMA3B exerts an anti-tumor, antiproliferative and proapoptotic effect on lung and ovarian cancer cells in vitro, we hypothesized that SEMA3B effects on tumor cell growth and viability might be due to its ability to block autocrine VEGF survival pathway by competing for the same receptor(s). The present study was undertaken to characterize the interacting effects of SEMA3B activity and VEGF165 in NSCLC and breast cancer. Our findings indicate that SEMA3B causes apoptosis in these two common human cancers and that this effect can be overridden by VEGF165. These data imply an interacting role of these two proteins during tumorigenesis, providing a mechanism for the tumor-suppressing activity of SEMA3B.
Materials and Methods
Materials. We obtained human VEGF (VEGF165) from Pepro-Tech (Rocky Hill, NJ), tissue culture and transfection materials from Invitrogen, 125I-labeled VEGF165 from PerkinElmer Life Science, chemiluminescence film (Hyperfilm ECL) from Amersham Pharmacia, and other chemicals from Sigma, Invitrogen, and Bio-Rad.
Antibodies (Ab). Rabbit polyclonal and mouse monoclonal Ab were generated by standard methods using a mixture of three peptides (rabbit) derived from the sequence of human SEMA3B [CGHRAEEPVLRL; CGRIEDGKGKSPYDPRHRAA (peptide for mouse monoclonal); CALQSLPLESRRKGRNRRTHAPEP] as described in Tomizawa et al. (4). The SEMA3B rabbit polyclonal serum containing the Ab was used as a neutralizing agent, and the SEMA3B mouse monoclonal Ab was used for Western blots. Preimmunized rabbit serum was used as a control. SEMA3B rabbit polyclonal Ab specificity was determined by using transfected cells with SEMA3B, and neutralization ability was studied by cells treated with or without SEMA3B-CM. Mouse anti-human VEGF-A monoclonal Ab were purchased from Oncogene (Ab-5 for neutralization and Ab-3 for Western blot). Anti-VEGF 2C3 Ab is a mouse monoclonal Ab that inhibits the binding of human VEGF-A to receptors (VEGFR-2 and neuropilins). Pro-caspase 3 and cleaved caspase-3 Ab were purchased from Cell Signaling Technology (Beverly, MA).
Cell Lines. Cell lines included lung cancers NCI-H1299, NCIH157, and HCC44 and breast cancer cell lines H1806, HCC1569, HCC1437, and HCC2185 from the Hamon Center Repository; Cos7 monkey kidney cells and breast cancer cells MDA-MB-231 from the American Type Culture Collection (ATCC); and human umbilical vascular endothelial cells (HUVEC) from Clonetics (San Diego). Lung cancer cells and Cos-7 were grown in RPMI medium 1640 supplemented with 5% FBS (R5 medium). HUVEC were grown in Endothelial Cell Basal Media from Cambrex (East Rutherford, NJ), and MDA-MB-231 cells were grown in MEM supplemented with l-glutamine and 10% FBS.
Expression Plasmids. Genes encoding human wild-type (WT) SEMA3B, mutant SEMA3B containing single missense mutations D397H (SEMAMUT1) and T415I (SEMAMUT2), and WT p53, VEGF121, and VEGF165 were inserted into pcDNA3 expression vector (Promega).
RT-PCR. Total RNA was extracted by using RNeasy Mini kit (Qiagen, Valencia, CA). RT-PCR was performed by using the SuperScript One Step RT-PCR Systems (Invitrogen), and amplification products were resolved on 1% agarose gels. A schedule for typical RT-PCR consisted of 1 h of reverse transcription at 42°C, 1 min of denaturation at 95°C, 1 min of annealing, and 1 min of extension at 72°C. All samples analyzed by RT-PCR were also tested for GAPDH expression to confirm the integrity of the RNA.
Small Interfering RNA (siRNA). The siRNA (sense and antisense strands) was obtained from the Center for Biomedical Inventions (University of Texas Southwestern Medical Center). The sense strands sequences were the following: VEGF, 5′-AUGUGAAUGCAGACCAAAGAATT and control, 5′-GAUAGACAAAUGACGAAUGCGUATT. In vitro transfection was performed by using the Oligofectamine reagent from Invitrogen. Thirty percent confluent cells in six-well plates were treated with 100 nM siRNA, the cells were washed after 6 h, and the experiment ended after 72 h.
Western Blot Analysis. Cell extracts were made in Nonidet P-40 extraction buffer (40 mM Hepes-NaOH, pH 7.4/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS/150 mM NaCl/protein inhibitors), and whole cell extracts (50-75 μg of protein) were separated on 10% SDS/PAGE gels and transferred to Hybond-P membrane (Schleicher & Schuell). Membranes were blocked for 30 min with 5% dry milk in 0.1% Tween 20 in Tris-buffered saline, incubated at room temperature for 2 h with monoclonal anti-SEMA3B Ab, then 40 min with horseradish peroxidase-labeled anti-mouse IgG (Amersham Pharmacia), and developed by Super Signal Chemiluminescence substrate (Pierce).
Cos7 Conditioned Medium (CM) Preparation. Cos7 cells were transfected with the vector pcDNA3 (negative control), or plasmids encoding SEMA3B, SEMA3Bmut, VEGF121, and VEGF165. Medium was collected 48 h posttransfection. SEMA3B present in the medium was determined by Western blot and by neutralizing its effect with the anti-SEMA3B rabbit polyclonal Ab. Semiquantitative assay showed an average of 15-40 ng/ml SEMA3B in the CM after transfection. Levels of VEGF isoforms and SEMA3B in the medium were determined by Human VEGF Accucyte EIA kit (Oncogene, San Diego, CA) and Western Blot, respectively.
Binding of 125I-VEGF165 to MDA-MB-231, H157, and H1299 Cells. Cells were seeded in 12- or 24-well plates and allowed to grow to confluence (2-5 × 105 cells per well). Cells were washed with cold PBS followed by washing with binding buffer (DMEM/0.1% gelatin/20 mM Hepes, pH 7.2/1 μg/ml heparin). To determine nonspecific binding, cells were preincubated with 100-fold molar excess of cold VEGF165 or SEMA3B-CM for 30 min at 4°C. 125I-VEGF165 (80-100 pM) was added and incubated for 2 h at 4°C, cells were washed three times with cold PBS containing 0.1% BSA (BSA-PBS), the plates were shaken with lysis buffer (1% Triton X-100 in 0.1% BSA-PBS) for 30 min at room temperature, and 350 μl from each well was counted in a Cobra II Auto Gamma counter (Packard).
Transfections. Cells were transfected by using Lipofectamine (Invitrogen) according to the manufacturer's instructions and analyzed 48 h after transfection.
Cell Growth Assay Using the Cos7 CM. H1299 and MDA-MB-231 cells were seeded in six-well plates at a density of 10,000 cells per well in the presence of CM from Cos7 cells, transfected either with vector, SEMA3B, SEMA3Bmut, VEGF121, or VEGF165, diluted 1:2 with medium, and cells were counted 5 days later. Assays done in triplicate were repeated at least two times.
Cell-Cycle Analysis. Cells were harvested 72 h after transfection, fixed with 70% ethanol, treated with 5 mg/ml RNase A (Sigma), stained with 50 μg/ml propidium iodide, and analyzed by flow cytometry for DNA synthesis and cell-cycle status (FACSCalibur instrument and cellquest software, Becton Dickinson).
Results
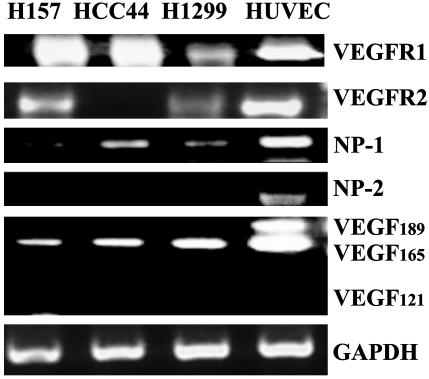
Expression of VEGFR-1, VEGFR-2, NP-1, NP-2, and VEGF Isoforms in NSCLC. Because SEMA3B was likely to compete with endogenously expressed VEGF-A for VEGF receptors R1 and R2 as well as NP-1 and NP-2, we determined, by using RT-PCR, which isoforms of VEGF-A and which receptors are expressed in the human tumor cells selected for functional studies. HUVEC expressed all of the genes and was used as a positive control (Fig. 1) (34, 35). VEGFR-1 and NP-1 were detected in all three NSCLC lines whereas none of the lines expressed NP-2; VEGFR-2 was detected in NSCLC H157 and H1299 cells but not in NSCLC HCC44 cells; and all NSCLC lines expressed VEGF165 but not VEGF121 or VEGF189 isoforms (Fig. 1). Determined by immunoassay, H1299 and Cos7 cells both endogenously produced 10-12 ± 1ng/ml VEGF165 into the culture media under control conditions. By contrast, VEGF165 plasmid transfected H1299 cells secreted ≈200 ± 15 ng/ml VEGF165 into culture medium (a 20-fold increase in production compared with endogenous levels).
Fig. 1.
Expression of VEGFR-1, VEGFR-2, NP-1, NP-2, and VEGF-A isoforms in lung cancer cells and HUVEC. RNA was collected from dividing cells, and the presence or absence of VEGF-A isoforms and receptors was determined by RT-PCR. Primers to GAPDH were used to confirm integrity of RNA. HUVEC were used as a positive control for gene expression.
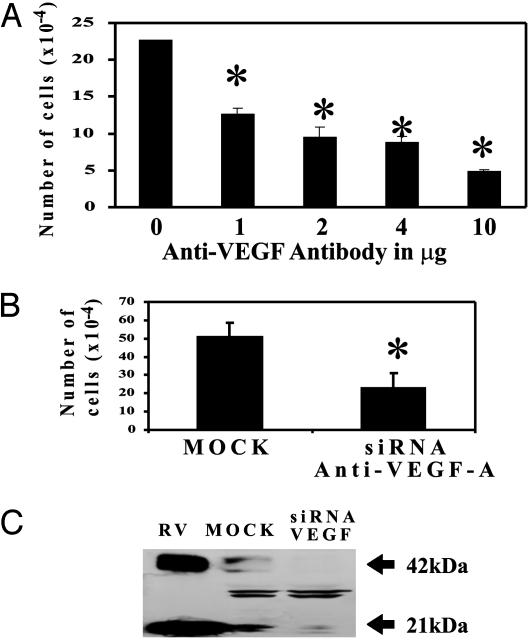
VEGF-A Plays a Role in the Survival of H1299 Lung Cancer Cells. H1299 cells express detectable amounts of VEGF-A under normal culture conditions. We wished to determine the effect on H1299 cell proliferation after VEGF-A removal. We have used two approaches to this, siRNA knockdown of VEGF-A and an anti-VEGF-A neutralizing Ab (Ab-5). By using techniques that remove or neutralize the biological activity of VEGF-A, we observed a >50% decrease in cancer cell proliferation after 3 days of treatment or transfection (Fig. 2A and B). A decrease in endogenous VEGF protein levels was seen after transfection of VEGF siRNA but not after control siRNA treatment (Fig. 2C). These data suggest that tumor cell produced VEGF has an important role in the survival or growth of NSCLC H1299.
Fig. 2.
VEGF-A plays a role in the survival of H1299 lung cancer cells. H1299 cells were seeded and treated with anti-VEGF Ab or transfected with siRNA for VEGF-A (see Materials and Methods). (A) Quantification of cell proliferation after H1299 cells were treated with anti-VEGF Ab for 4 days. The Ab inhibition of cell proliferation was dose-dependent. (B) Quantification of cell proliferation assay after H1299 cells were transfected with siRNA control (MOCK) and VEGF-A. (C) Western blot analysis for VEGF after 72 h of MOCK or VEGF siRNA transfection (180 μg of protein) and recombinant VEGF (RV) as a positive control. *, P < 0.05, t test.
Presence of SEMA3B Protein in the CM from Cos7 Cells. CM derived from Cos7 cells transfected with the SEMA3B expression vector and purified on an anti-SEMA3B Ab column showed a band that reacted with anti-SEMA3B Ab (Fig. 3). We collected the Cos7 cell-derived CM containing SEMA3B (SEMA3B-CM) at 48 h posttransfection to use as a source of exogenously added SEMA3B protein in all assays described below. The medium from Cos7 cells transfected with empty vector (Control-CM) was used as a negative control. We used this affinity-purified protein to estimate that 15-40 ng/ml SEMA3B was in the CM.
Fig. 3.
Expression of SEMA3B protein after transfection and protein purification of Cos7 CM. Cos7 cells were transfected with SEMA3B expression plasmid. Shown is Western blot analysis for Cos7 CM after transfection with SEMA3B expression plasmid and anti-SEMA3B column protein purification.
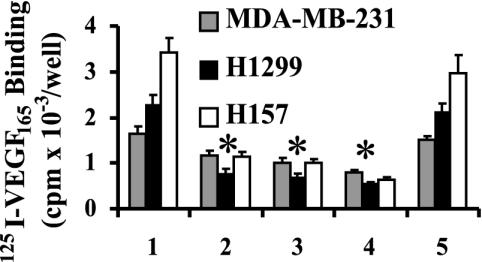
SEMA3B Inhibits 125I-VEGF165 Binding to Lung and Breast Cancer Cells. Cell-surface binding of 125I-VEGF165 was tested on the NSCLCs H1299 and H157 and breast cancer MDA-MB-231 cells (Fig. 4) with and without the addition of SEMA3B. The MDA-MB-231 line was chosen because these cells express NP-1 and NP-2 but not VEGFR-1 or VEGFR-2, thus enabling us to distinguish between signaling pathways induced by different VEGF receptors. Specific 125I-VEGF165 binding was observed in all cell lines after 2 h of incubation with 100 pM of labeled VEGF165 (Fig. 4, lane 1). SEMA3B-CM decreased the specific binding of 125IVEGF165 to all cell lines (Fig. 4, lanes 2-4) with maximal inhibition of 50% for MDA-MB-231 and 65-75% for H1299 and H157, respectively. By contrast, Cos7 control-CM had no significant effect on 125I-VEGF165 binding (Fig. 4, lane 5). These data suggest that VEGF165 and SEMA3B share binding sites on these human tumor cells as was previously observed for SEMA3A and VEGF165 on NP receptors (36).
Fig. 4.
Competition of SEMA3B and 125I-VEGF165 for binding to the cell surface receptors on MDA-MB-231, H1299, and H157 cells. MDA-MB-231 (gray), H1299 (black), and H157 (white) cells were incubated for 2 h with 125I-VEGF165 and SEMA3B-CM in the binding buffer, and the bound 125IVEGF165 was detected by using a γ counter. 125I-VEGF165 binding with SEMA3B-CM added at 5%, 15%, and 30% of total volume per well (200 μl) (lanes 2-4), which CM from the Cos7 cells transfected with empty vector served as a control (lane 5). Nonspecific binding was determined in the presence of 100-fold molar excess of unlabeled VEGF and was subtracted from the experimental values. We observed a nonspecific binding ranging from 12% to 40% when compared with specific binding from 125I-VEGF165 alone.
VEGF165 Antagonizes SEMA3B-Mediated Effects on Tumor Cell Proliferation. SEMA3B gene and protein expression was not detected in the majority of NSCLC, including H1299 and H157 (2, 4). SEMA3B-CM inhibited proliferation of H1299 cells by 50% compared with vector control whereas rabbit anti-SEMA3B Ab neutralized this effect (Table 1). Control IgG from preimmune rabbits had no effect on the inhibitory effect of the SEMA3B-CM or vector control (data not shown). These results demonstrate that SEMA3B protein present in the CM of SEMA3B-transfected Cos7 cells is responsible for the antiproliferative effect. Treatment with mutant SEMA3Bmut1 vector showed no effect in cell number when compared with CM. The antiproliferative effect of SEMA3B-CM was overridden by cotreatment with VEGF165-CM. By contrast, cotreatment with VEGF121, which does not bind to NP-1 and NP-2, had no significant effect on cell proliferation as compared with SEMA3B-CM alone. Transfection with VEGF121 or VEGF165 alone did not affect cell proliferation in the absence of SEMA3BCM. The specificity of the VEGF165 reversal of SEMA3B growth inhibition was confirmed by treating with monoclonal anti-VEGF Ab 2C3 (37), which gave similar cell numbers as treatment with SEMA3B alone. In contrast to the anti-VEGF neutralizing Ab Ab-5, the anti-VEGF Ab 2C3 does not inhibit cell proliferation. The lack of effect of VEGF121 indicates that only a NP-binding VEGF isoform (VEGF165) is able to counteract the effects of SEMA3B.
Table 1. Effect of SEMA3B-CM and VEGF isoforms 165 and 121 on proliferation in H1299 lung and MDA-MB-231 breast cancer cells.
| Treatment* | H1299 Cell no. (× 10−4) (P value)† | MDA-MB-231 Cell no. (× 10−4) (P value)† |
|---|---|---|
| Control-CM‡ | 11 ± 1.3 (−) | 16 ± 1.0 (−) |
| SEMA3B-CM‡ | 6 ± 0.6 (0.002)§ | 5 ± 2.0 (0.002)§ |
| SEMA3Bmut1-CM¶ | 11 ± 1.0 (0.0005)∥ | 15 ± 0.5 (0.0005)∥ |
| SEMA3B-CM plus anti-SEMA3B** | 11 ± 1.5 (0.004)∥ | 13 ± 1.0 (0.004)∥ |
| VEGF121 | 11 ± 0.8 (NS)†† | 16 ± 1.0 (NS)†† |
| VEGF165 | 11 ± 0.8 (NS)†† | 16 ± 2.0 (NS)†† |
| VEGF121 plus SEMA3B-CM | 6 ± 1.2 (0.004)‡‡ | 8 ± 0.3 (0.003)‡‡ |
| VEGF165 plus SEMA3B-CM | 12 ± 1.5 (0.0002)∥ | 15 ± 0.6 (0.003)∥ |
| VEGF165 plus anti-VEGF-A Ab | 12 ± 0.9 (NS)§§ | 14 ± 0.8 (NS)§§ |
| VEGF165 plus SEMA3B-CM plus anti-VEGF Ab** | 7 ± 0.6 (0.001)¶¶ | 4 ± 0.9 (0.001)¶¶ |
CM from cells transfected with various plasmids as specified above was added to MDA-MB-231 and H1299 cells.
P value was calculated by using a two-tailed Student's t test.
Cos7 cells were transfected with vector control or with SEMA3B expression vector, and CM was collected 48 h thereafter.
Significant decrease as compared with Control-CM.
SEMA3B-mut-CM (D397H) is inactive as described in Materials and Methods and Results.
Significant increase as compared with SEMA3B-CM.
Anti-SEMA3B or anti-VEGF (30 μg/ml) antibody 2C3 was used as neutralizing agent for SEMA3B-CM and VEGF165, respectively.
Nonsignificant as compared with control.
Significant decrease when compared with cells treated with VEGF121.
Nonsignificant as compared with VEGF165.
Significant decrease as compared with VEGF165 plus SEMA3B-CM.
Similarly, transfection with SEMA3B led to an 65-80% decrease in colony number for H1299 and H157 cells compared with untreated cells or cells transfected with empty vector (H1299 vector control 200 ± 18, SEMA3B transfected 42 ± 5.1, H157 vector control 230 ± 2.3, and SEMA3B transfected 78 ± 7.2 colonies, respectively). When cells were cotransfected with both VEGF165 and SEMA3B plasmids, the number of colonies was not significantly different from that in the control cells whereas cotransfection of SEMA3B and VEGF121 showed reduced colony number, indicating that VEGF121 isoform does not modulate the inhibitory effect of SEMA3B (H1299 SEMA3BVEGF121 44 ± 7 colonies and for H157 85 ± 11 colonies). Taken together, these data suggest that SEMA3B is a potent growth inhibitory factor for human NSCLC cells and that VEGF165, but not the VEGF121 isoform, overcomes the SEMA3B growth inhibitory effect.
The Effect of SEMA3B and VEGF165 on MDA-MB-231 Breast Cancer Cells Lacking VEGFR-1 and VEGFR-2. Growth inhibition of 50-60% by SEMA3B-CM was also observed in five breast cancer lines (see Materials and Methods for list of cell lines). In contrast to NSCLC cells described above that express NP-1 as well as one or both classic VEGF receptors, MDA-MB-231 breast cancer cells express only NP-1 and NP-2 proteins (20, 21). We confirmed by RT-PCR that MDA-MB-231 does not express VEGFR-1 or VEGFR-2. We used this line to demonstrate that SEMA3B induced growth inhibition and that VEGF165 reversal of this effect can be mediated through neuropilin receptors alone and does not have to involve VEGFR-1 or VEGFR-2 (Table 1). Thus, treatment with SEMA3B-CM caused a 50% reduction in MDA-MB-231 cell number (while SEMA3Bmut-CM was inactive) and the rabbit anti-SEMA3B Ab (10 μg/ml) blocked the SEMA3B-CM growth inhibition. VEGF165 (but not VEGF121) reversed the SEMA3B growth inhibition, which in turn was neutralized by the anti-VEGF Ab 2C3. When the anti-VEGF Ab 2C3 Ab was added to MDA-MB-231 cells in the absence of SEMA3B but in the presence of VEGF165, it had no effect on cell growth, and VEGF165 or VEGF121 treatment alone did not alter breast cancer cell growth (Table 1). Taken together, these data show that SEMA3B-CM has an antiproliferative effect on MDA-MB-231 breast cancer cells expressing NP-1 and NP-2 but not VEGFR-1 and VEGFR-2, and that VEGF165 but not VEGF121 is able to antagonize this effect.
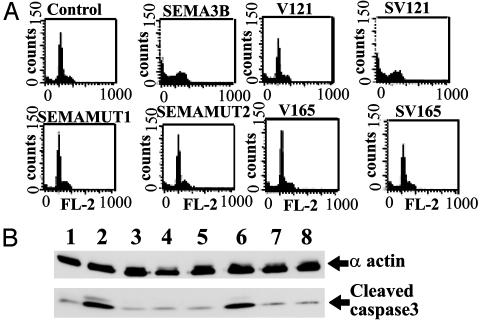
SEMA3B Induces Apoptosis of H1299 Lung Carcinoma Cells and VEGF165 Abolishes This Effect. We examined whether VEGF165 could decrease apoptosis induced in lung cancer cells by SEMA3B by using fluorescence-activated cell sorter (FACS) analysis and caspase 3 activation assays (4) (Fig. 5). H1299 cells were transfected with vector control and SEMA3B for 72 h in the presence or absence of cotransfected VEGF121 or VEGF165. More than 94% of the vector control cells were alive by the end of the 72-h period, with only 6% of cells displaying signs of apoptotic death (Fig. 5A). As expected, SEMA3B treatment increased the number of tumor cells in apoptosis (sub G0 peak, Fig. 5A) by 40%. VEGF121 and VEGF165 given alone did not alter apoptosis or the cell cycle. Cotransfection of VEGF165 with SEMA3B reversed the effect of SEMA3B on apoptosis induction whereas cotransfection with VEGF121 did not (Fig. 5A). Increase in the caspase-3 activity that arises after the cleavage of pro-caspase-3 is a hallmark of induced apoptosis and is considered to be a point of no return. Transfection of H1299 cells with SEMA3B increased caspase-3 activity relative to basal levels in the control cells whereas SEMA3B mutants had no effect (Fig. 5B). However, cotransfection of SEMA3B with VEGF165 antagonized the SEMA3B effect on caspase-3 cleavage (Fig. 5B). These data demonstrate that SEMA3B induced caspase-3-dependent apoptotic pathway in H1299 cells and that this effect was reversed by VEGF165.
Fig. 5.
SEMA3B induces caspase-3-dependent apoptosis and VEGF165 antagonizes this effect. (A) Flow cytometry analysis of apoptosis induction in H1299 cells 72 h posttransfection. NSCLC H1299 cells were transfected with pcDNA3 (vector control) SEMA3B, VEGF121 (V121), SEMA3B plus V121 (SV121), SEMAMUT1, SEMAMUT2, VEGF165 (V165), and SEMA3B plus V165 (SV165), and FACS assay was performed to determine induction of apoptosis. The number of tumor cells undergoing apoptosis are indicated by sub G0 peak. (B) Caspase-3 cleavage increase detected by Western blotting as a hallmark of apoptotic activation. Vectors used in transfection of H1299 cells are as follows: lane 1 (vector control), lane 2 (SEMA3B), lane 3 (SEMAMUT1), lane 4 (SEMAMUT2), lane 5 (V121), lane 6 (SEMA3B plus V121), lane 7 (V165), and lane 8 (SEMA3B + V165).
Discussion
SEMA3B, encoded at 3p21.3, is a candidate tumor suppressor gene (4, 5). Evidence in support of this finding includes the following: frequent allele loss at the SEMA3B 3p21.3 locus; frequent loss of SEMA3B expression in lung cancer secondary to tumor-acquired promoter methylation; occasional somatically acquired tumor SEMA3B mutations; and inhibition of tumor cell growth in vitro and in vivo coupled with dramatic ability to induce tumor cell apoptosis after transfection or exposure to SEMA3B protein (3-5). In the present work, we have shown that reexpression of WT but not mutant SEMA3B induces apoptosis in lung and breast cancer cells and that this effect is reversed by coexpression of VEGF165 but not VEGF121. We also have shown that siRNA-mediated VEGF knockdown or neutralizing anti-VEGF Ab inhibit lung cancer cell proliferation in vitro. SEMA3B inhibits VEGF165 binding to receptors on these tumor cells, and the effect can be mediated by NP receptors alone in the absence of VEGF receptor expression. These results suggest that tumor cell-produced VEGF-A is a tumor cell survival or growth factor and that SEMA3B acts through a VEGF-regulated system to mediate its tumor suppressor effects. These findings accord with prior observations by Bachelder et al. that NP-1 supports a VEGF signaling pathway that is critical for breast carcinoma cell survival (32) and that VEGF and SEMA3A are antagonistic NP-1 ligands that regulate breast carcinoma cell migration (33). Also, SEMA3A induces apoptosis in neurons (38, 39), competes with VEGF-A binding on porcine aortic endothelial, medulloblastoma, and human embryonic kidney 293 cells, and affects cell survival and migration (27-29). Recent research also shows SEMA3F (whose gene is located ≈70 kb telomeric of SEMA3B in the same 3p21.3 region) and VEGF as having opposing effects on cell attachment and motility in breast cancer lines MCF-7 and C100 (40). In these studies, SEMA3F inhibited lamellipodia formation, membrane ruffling, and cell-cell contacts through interaction with NP-1 (40).
Based on these observations and prior findings, it seems that SEMA3B functions as a suppressor of tumor growth by inducing apoptosis potentially in premalignant as well as malignant cells. Additional support for our hypothesis came when a p53-negative glioblastoma cell line after reintroduction of p53 showed increased expression of SEMA3B. Furthermore, UV radiation and doxorubicin treatment also induced SEMA3B in MCF-7 breast cancer cells (6). These findings suggested that SEMA3B could be mediating a p53 DNA damage response. However, SEMA3B induces apoptosis in the NSCLC H1299 cell line, which is null for p53, demonstrating that reexpression of SEMA3B alone is sufficient for induction of apoptosis. Because SEMA3B and VEGF165 share similar binding sites on NP-1 and NP-2 proteins, we also propose that VEGF, which is frequently expressed in human cancers including lung and breast cancer (41-45), would compete with SEMA3B for binding on the cell surface, and would antagonize the negative regulatory effects of SEMA3B on cell growth.
Based on the present findings and published observations, we propose the following working model. During the course of carcinogenesis, DNA damage and mutations occur, activating the p53 pathway, among other things, leading to increased expression of SEMA3B. SEMA3B induction would in turn lead to induction of apoptosis, removing damaged cells. However, premalignant cells that had undergone 3p21.3 allele loss, mutation, and/or SEMA3B promoter methylation become haploin-sufficient for SEMA3B, or lose this control mechanism entirely. Likewise, expression of VEGF165 by premalignant cells or by neighboring cells would bypass this effect. Thus, either or both loss of SEMA3B expression and/or VEGF165 overexpression would lead to an outgrowth of cells with genetic abnormalities. However, in many lung and breast cancers, it seems the combination of loss of SEMA3B expression, VEGF overproduction, and p53 mutation occur together. This result would indicate that, even though these mechanisms are interrelated, they also have other separate growth control properties that must be eliminated for a clinically evident malignancy to develop. Finally, the ability of exogenously added SEMA3B to induce apoptosis in full-fledged tumor cells with numerous genetic and epigenetic changes and expressing endogenous VEGF165 suggests the potential use of SEMA3B as a systemic therapeutic agent.
Acknowledgments
We thank W. C. Lai, B. Gao, and. K. Tomenga for SEMA3B Ab development and R. Brekken for discussions. This work was supported by National Cancer Institute Grant CA71618, Lung Cancer Specialized Programs of Research Excellence Grant P50 CA70907, the Susan G. Komen Foundation, and Department of Defense Grant BC020841.
Abbreviations: SEMA3B, semaphorin 3B; NSCLC, non-small cell lung cancer; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; NP, neuropilin; CM, conditioned medium; hUVEC, human umbilical vein endothelial cells; siRNA, small interfering RNA.
References
- 1.Lerman, M. I. & Minna, J. D. (2000) Cancer Res. 60, 6116-6133. [PubMed] [Google Scholar]
- 2.Sekido, Y., Bader, S., Latif, F., Chen, J. Y., Duh, F. M., Wei, M. H., Albanesi, J. P., Lee, C. C., Lerman, M. I. & Minna, J. D. (1996) Proc. Natl. Acad. Sci. USA 93, 4120-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroki, T., Trapasso, F., Yendamuri, S., Matsuyama, A., Alder, H., Williams, N. N., Kaiser, L. R. & Croce, C. M. (2003) Cancer Res. 63, 3352-3355. [PubMed] [Google Scholar]
- 4.Tomizawa, Y., Sekido, Y., Kondo, M., Gao, B., Yokota, J., Roche, J., Drabkin, H., Lerman, M. I., Gazdar, A. F. & Minna, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 13954-13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse, C., Xiang, R. H., Bracht, T. & Naylor, S. L. (2002) Cancer Res. 62, 542-546. [PubMed] [Google Scholar]
- 6.Ochi, K., Mori, T., Toyama, Y., Nakamura, Y. & Arakawa, H. (2002) Neoplasia 4, 82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolodkin, A. L., Matthes, D. J. & Goodman, C. S. (1993) Cell 75, 1389-1399. [DOI] [PubMed] [Google Scholar]
- 8.Puschel, A. W. (1996) Eur. J. Neurosci. 8, 1317-1321. [DOI] [PubMed] [Google Scholar]
- 9.Hall, K. T., Boumsell, L., Schultze, J. L., Boussiotis, V. A., Dorfman, D. M., Cardoso, A. A., Bensussan, A., Nadler, L. M. & Freeman, G. J. (1996) Proc. Natl. Acad. Sci. USA 93, 11780-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumanogoh, A. & Kikutani, H. (2001) Trends Immunol. 22, 670-676. [DOI] [PubMed] [Google Scholar]
- 11.Behar, O., Golden, J. A., Mashimo, H., Schoen, F. J. & Fishman, M. C. (1996) Nature 383, 525-528. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardini, V. & Fankhauser, C. (1999) Mol. Cell. Neurosci. 14, 301-316. [DOI] [PubMed] [Google Scholar]
- 13.Yamada, T., Endo, R., Gotoh, M. & Hirohashi, S. (1997) Proc. Natl. Acad. Sci. USA 94, 14713-14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamagnone, L., Artigiani, S., Chen, H., He, Z., Ming, G. I., Song, H., Chedotal, A., Winberg, M. L., Goodman, C. S., Poo, M., et al. (1999) Cell 99, 71-80. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi, T., Fournier, A., Nakamura, F., Wang, L. H., Murakami, Y., Kalb, R. G., Fujisawa, H. & Strittmatter, S. M. (1999) Cell 99, 59-69. [DOI] [PubMed] [Google Scholar]
- 16.Rohm, B., Ottemeyer, A., Lohrum, M. & Puschel, A. W. (2000) Mech. Dev. 93, 95-104. [DOI] [PubMed] [Google Scholar]
- 17.Ohta, K., Mizutani, A., Kawakami, A., Murakami, Y., Kasuya, Y., Takagi, S., Tanaka, H. & Fujisawa, H. (1995) Neuron 14, 1189-1199. [DOI] [PubMed] [Google Scholar]
- 18.Winberg, M. L., Noordermeer, J. N., Tamagnone, L., Comoglio, P. M., Spriggs, M. K., Tessier-Lavigne, M. & Goodman, C. S. (1998) Cell 95, 903-916. [DOI] [PubMed] [Google Scholar]
- 19.Maestrini, E., Tamagnone, L., Longati, P., Cremona, O., Gulisano, M., Bione, S., Tamanini, F., Neel, B. G., Toniolo, D. & Comoglio, P. M. (1996) Proc. Natl. Acad. Sci. USA 93, 674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soker, S., Fidder, H., Neufeld, G. & Klagsbrun, M. (1996) J. Biol. Chem. 271, 5761-5767. [DOI] [PubMed] [Google Scholar]
- 21.Soker, S., Takashima, S., Miao, H. Q., Neufeld, G. & Klagsbrun, M. (1998) Cell 92, 735-745. [DOI] [PubMed] [Google Scholar]
- 22.Migdal, M., Huppertz, B., Tessler, S., Comforti, A., Shibuya, M., Reich, R., Baumann, H. & Neufeld, G. (1998) J. Biol. Chem. 273, 22272-22278. [DOI] [PubMed] [Google Scholar]
- 23.Makinen, T., Olofsson, B., Karpanen, T., Hellman, U., Soker, S., Klagsbrun, M., Eriksson, U. & Alitalo, K. (1999) J. Biol. Chem. 274, 21217-21222. [DOI] [PubMed] [Google Scholar]
- 24.Houck, K. A., Ferrara, N., Winer, J., Cachianes, G., Li, B. & Leung, D. W. (1991) Mol. Endocrinol. 5, 1806-1814. [DOI] [PubMed] [Google Scholar]
- 25.Yamada, Y., Takakura, N., Yasue, H., Ogawa, H., Fujisawa, H. & Suda, T. (2001) Blood 97, 1671-1678. [DOI] [PubMed] [Google Scholar]
- 26.Kitsukawa, T., Shimono, A., Kawakami, A., Kondoh, H. & Fujisawa, H. (1995) Development (Cambridge, U.K.) 121, 4309-4318. [DOI] [PubMed] [Google Scholar]
- 27.Gu, C., Limberg, B. J., Whitaker, G. B., Perman, B., Leahy, D. J., Rosenbaum, J. S., Ginty, D. D. & Kolodkin, A. L. (2002) J. Biol. Chem. 277, 18069-18076. [DOI] [PubMed] [Google Scholar]
- 28.Miao, H. Q., Soker, S., Feiner, L., Alonso, J. L., Raper, J. A. & Klagsbrun, M. (1999) J. Cell Biol. 146, 233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagnard, D., Vaillant, C., Khuth, S. T., Dufay, N., Lohrum, M., Puschel, A. W., Belin, M. F., Bolz, J. & Thomasset, N. (2001) J. Neurosci. 21, 3332-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masood, R., Kundra, A., Zhu, S., Xia, G., Scalia, P., Smith, D. L. & Gill, P. S. (2003) Int. J. Cancer 104, 603-610. [DOI] [PubMed] [Google Scholar]
- 31.Zhu, Z., Hattori, K., Zhang, H., Jimenez, X., Ludwig, D. L., Dias, S., Kussie, P., Koo, H., Kim, H. J., Lu, D., et al. (2003) Leukemia 17, 604-611. [DOI] [PubMed] [Google Scholar]
- 32.Bachelder, R. E., Crago, A., Chung, J., Wendt, M. A., Shaw, L. M., Robinson, G. & Mercurio, A. M. (2001) Cancer Res. 61, 5736-5740. [PubMed] [Google Scholar]
- 33.Bachelder, R. E., Lipscomb, E. A., Lin, X., Wendt, M. A., Chadborn, N. H., Eickholt, B. J. & Mercurio, A. M. (2003) Cancer Res. 63, 5230-5233. [PubMed] [Google Scholar]
- 34.Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shibuya, M. & Heldin, C. H. (1994) J. Biol. Chem. 269, 26988-26995. [PubMed] [Google Scholar]
- 35.Deroanne, C. F., Bonjean, K., Servotte, S., Devy, L., Colige, A., Clausse, N., Blacher, S., Verdin, E., Foidart, J. M., Nusgens, B. V. & Castronovo, V. (2002) Oncogene 21, 427-436. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura, F., Tanaka, M., Takahashi, T., Kalb, R. G. & Strittmatter, S. M. (1998) Neuron 21, 1093-1100. [DOI] [PubMed] [Google Scholar]
- 37.Brekken, R. A., Overholser, J. P., Stastny, V. A., Waltenberger, J., Minna, J. D. & Thorpe, P. E. (2000) Cancer Res. 60, 5117-5124. [PubMed] [Google Scholar]
- 38.Shirvan, A., Shina, R., Ziv, I., Melamed, E. & Barzilai, A. (2000) Brain Res. Mol. Brain Res. 83, 81-93. [DOI] [PubMed] [Google Scholar]
- 39.Shirvan, A., Ziv, I., Fleminger, G., Shina, R., He, Z., Brudo, I., Melamed, E. & Barzilai, A. (1999) J. Neurochem. 73, 961-971. [DOI] [PubMed] [Google Scholar]
- 40.Nasarre, P., Constantin, B., Rouhaud, L., Harnois, T., Raymond, G., Drabkin, H. A., Bourmeyster, N. & Roche, J. (2003) Neoplasia 5, 83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassano, A., Bagala, C., Battelli, C., Schinzari, G., Quirino, M., Ratto, C., Landriscina, M. & Barone, C. (2002) Anticancer Res. 22, 2179-2184. [PubMed] [Google Scholar]
- 42.Mamluk, R., Gechtman, Z., Kutcher, M. E., Gasiunas, N., Gallagher, J. & Klagsbrun, M. (2002) J. Biol. Chem. 277, 24818-24825. [DOI] [PubMed] [Google Scholar]
- 43.Igarashi, H., Esumi, M., Ishida, H. & Okada, K. (2002) Cancer 95, 47-53. [DOI] [PubMed] [Google Scholar]
- 44.Padro, T., Bieker, R., Ruiz, S., Steins, M., Retzlaff, S., Burger, H., Buchner, T., Kessler, T., Herrera, F., Kienast, J., et al. (2002) Leukemia 16, 1302-1310. [DOI] [PubMed] [Google Scholar]
- 45.Mineta, H., Miura, K., Ogino, T., Takebayashi, S., Misawa, K. & Ueda, Y. (2002) Anticancer Res. 22, 1039-1044. [PubMed] [Google Scholar]