Abstract
Dendritic cells (DCs) are important for initiating immune responses, in part through their ability to acquire and shuttle antigen to the draining lymph node (DLN). The mobilization of DCs to the DLN is complex and remains to be fully elucidated during infection. Herein described is the use of an innovative, simple assay that relies on the fluorochrome 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) to track the migration of DCs during footpad infection with Mycobacterium bovis Bacille Calmette-Guérin (BCG) in C57BL/6 mice. This assay enables the characterization of skin DC sub-populations that actively relocate to the draining, popliteal LN in response to BCG. This protocol originates from a BCG model where migratory skin DCs were identified by flow cytometry. The assay is amiable to the study and identification of DCs or other cells that home to the popliteal LN after inoculation of microbes, their metabolites or other inflammatory stimuli in the footpad, and consequently to study factors that regulate the migration of these cells.
Keywords: Infection, Issue 116, Dendritic cells, CFSE labeling, migration, lymph node, BCG, mouse, skin
Introduction
DCs localized in body surfaces sense microbes or their products, and in doing so mobilize via lymphatic vessels to the draining LN (DLN) 1,2. This relocation is needed for transport of microbial antigen to the DLN and the consequent priming of immune responses to the invading microbe. Indeed, blockade or failure of DCs to migrate from the infected tissue to the DLN mutes the T-cell response 3-5. Accordingly, assays designed to track migration at the single-cell level are useful to help ascribe migratory function to a given DC subset.
There is a large body of data on the mobilization of skin DCs to the DLN. The latter accounts for much of the knowledge on DC migration from inflamed or infected sites 2. This is not surprising since the skin houses a large population of DCs and is a highly accessible surface for experimentation in the laboratory mouse. Several techniques have thus been developed to assess the migration of murine DCs from the skin to the DLN 2. FITC skin painting is by far the most commonly used approach. In this assay the fluorochrome fluorescein-5-isothiocyanate (FITC) is prepared in a mixture with acetone and a contact irritant. This cocktail is applied to depilated or shaved skin and the accumulation of FITC-labeled cells is in turn analyzed in the DLN. Migration can also be studied by injecting the skin with fluorochrome-tagged nano- or microparticles, which enables the tracking of phagocytic cells that have internalized the fluorescent particles in the skin. Lymphatic vessels can also be cannulated to directly extract migrating DCs from lymph. This is however technically challenging, especially in mice. The number of DCs obtained for subsequent analysis is also a limiting factor.
In spite of many previous studies on skin DC migration, there remains a paucity of information regarding skin DC mobilization to DLN following vaccination with Bacille Calmette-Guérin (BCG), the live, attenuated strain of Mycobacterium bovis used to vaccinate against M. tuberculosis. BCG is inoculated in the skin, causing a limited infection that triggers a T helper-cell type 1 response. Both the DC sub-populations that mobilize to the DLN from the BCG-infected skin and the factors that regulate their migration during this process are not fully understood. In light of the above, a simple but novel method was developed where the fluorochrome 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) is injected in situ to track the migration of DCs into the DLN 3. C57BL/6 mice are injected in the hind footpad with an inoculum of BCG and the day before sacrifice, animals are injected in the same footpad with a high concentration of CFSE. Twenty-four hours later animals are sacrificed and the draining popliteal LN (pLN) removed and prepared for flow cytometry. This assay enables the identification of cells that migrate overnight from the footpad skin to the DLN (Figure 1).
Furthermore, gene-targeted mice can be used to help identify factors required for cells to mobilize to the DLN. Using this assay, the authors of this report have previously found that EpCAMlow CD11bhigh migratory skin DCs transport BCG to the DLN in a process regulated by Interleukin-1 receptor and the intracellular adaptor molecule MyD88 3. The protocol for this CFSE-based migration assay which originates from the above report by Bollampalli et al.3 where flow cytometry was used to detect and analyze migratory skin DCs, is herein described. The advantages and disadvantages of this assay are discussed.
Protocol
NOTE: C67BL/6 mice were used for the experiments shown in this manuscript. These animals were house under specific pathogen-free conditions at the MTC animal facility, Karolinska Institutet, Stockholm, Sweden. Animal experiments were performed according to the directives and guidelines of the Swedish Board of Agriculture, the Swedish Animal Protection Agency, and Karolinska Institutet. Experiments were approved by the Stockholm North Animal Ethics Council.
1. Preparation of CFSE Stocks and Storage
Prepare a 5 mM stock of 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) in dimethyl sulfoxide. Vortex. Dispense 100 µl aliquots into sterile, 0.5 ml screw cap microtubes. Store aliquots at -80 °C.
2. Mice
Use inbred male or female mice between 8 and 12 weeks of age. Sex- and age-matched animals are preferred. Make sure the necessary animal ethical permits are in place before the initiation of experiments.
3. Immobilization of Animals with Isoflurane Gas Anesthesia
Load the anesthesia unit with isoflurane. Fill the 10 ml gas-tight glass syringe with isoflurane. Avoid introducing air bubbles. Connect the syringe and liquid inlet with the supplied tubing and move the pusher forward until the liquid in the connecting tube is visibly just about to enter the vaporizer. NOTE: Do not store isoflurane in the syringe when not in use.
Anesthetize animals with isoflurane in the induction chamber, with an airflow maintained at approximately 400 - 500 ml/min and the concentration of isoflurane at 3.5%. NOTE: The unit will not operate without airflow.
Once the animals are asleep, turn the stopcock on the anesthesia unit to route the isoflurane gas to the mask piece. Check that the mask piece is intact to avoid leakage of gas. Airflow can be maintained at 400 ml/min, alternatively decreased to 200 ml/min. Reduce isoflurane concentration to 2.6%. NOTE: Anesthetic effect can be increased and/or decreased by adjusting the flow rate.
4. BCG Injections and Recovery
Visually confirm and perform tests such as the toe pinch reflex test to ascertain that mice are immobilized/asleep on the isoflurane mask piece before performing injections.
Inoculate in the hind footpad, 1 x 106 Colony-forming units of Mycobacterium bovis Bacille Calmette-Guérin (BCG) in 30 µl of PBS using a syringe fitted with a 29 G x ½ inch needle. NOTE: The generation of mycobacterial stocks is briefly described in the original description of this procedure 3, and in substantial detail elsewhere 6. BCG is also readily available from commercial sources. The authors recommend that BCG be pulse-sonicated or passed through a syringe to disrupt clumps prior to its inoculation into mice. BCG is reiterated here as a microbial stimuli given its employment in the original description of this protocol 3 but the authors certainly encourage the testing of other microbial stimuli.
Perform the same procedure described above for the injection of PBS into control mice.
Monitor animals after injections to confirm that recovery from anesthesia is successful and that animals can support themselves on the injected, hind footpad.
5. CFSE Injections
- Preparation of CFSE for Injections:
- Thaw a vial of 5 mM CFSE stock solution from -80 °C. Dilute the CFSE 10-fold in PBS. Resuspend the solution thoroughly before filling the syringe in preparation for the injection.
- CFSE Injection and Recovery:
- Repeat the procedure for anesthetizing animals in section 3. Inject 20 µl of 0.5 mM CFSE in the hind footpad which previously received BCG or PBS. NOTE: The injection of CFSE should be performed the day before (24 hr) the planned date for sacrifice.
6. Surgical Removal of Popliteal Lymph Node (pLN)
NOTE: Use of sterilized surgical instruments and their repeated decontamination in 70% ethanol are encouraged throughout this step.
Euthanize the mice. Spray the animal with 70% ethanol. Pin the animal in the dorsal position, on a dissection board. NOTE: The authors can recommend euthanasia through cervical dislocation.
Carefully make a vertical incision in the hind thigh. Clear muscle and fat using scissors and tweezers to expose and consequently excise the pLN located deep in the fat pouch, in the hollow of the knee.
Transfer the pLN to 0.5 ml sterile PBS, on ice.
7. Generation of Single-cell Suspension from pLN
Homogenize the pLN through a 70-micron cell strainer placed in a 6 well-plate containing 5 ml PBS or fluorescence-activated cell sorting (FACS) buffer (PBS containing 2% fetal calf serum (FCS), 5 mM ethylenediaminetetraacetic acid (EDTA), and 1 mM sodium azide). Homogenize through the strainer using the back end of a 3 ml syringe. NOTE: Sodium azide is toxic and should be handled with caution.
Wash the strainer with another 5 ml of PBS or FACS buffer, collect the material in a 15 ml tube and pellet the cells at 277 x g (1,200 rpm, R = 172 mm) for 5 min, 4 °C. Alternatively, homogenize pLNs directly in microcentrifuge tubes using polypropylene homogenizers.
Based on the pellet size, resuspend the pLN suspension in an appropriate volume of PBS or FACS buffer, e.g., 200 - 1,000 µl. Use a counting chamber to quantify the total cell number obtained from each LN suspension. Use trypan blue to exclude dead/dying cells.
8. Cell Staining and Flow Cytometry
Transfer 1 - 10 x 106 cells to 5 ml round-bottom polystyrene tubes. Wash with FACS buffer, and pellet by centrifugation at 277 x g (1,200 rpm, R= 172 mm) for 5 min, 4 °C.
Discard the supernatant and resuspend the pellet in 50 - 100 µl of antibody cocktail (see Dendritic cell [DC] markers used for surface staining, Materials Table) containing 0.5 - 1 µg of anti-mouse CD16/CD32 (to block unspecific Fc-mediated interactions) in FACS buffer for 30 - 45 min, on ice. Protect samples from light.
After incubation, wash cells with FACS buffer and pellet by centrifugation at 277 x g (1,200 rpm, R = 172 mm) for 5 min, 4 °C.
Resuspend cells in 50 - 100 µl of FACS buffer. Acquire data on a flow cytometer 7-10. NOTE: For excellent reviews on flow cytometry we refer the reader to the following publications that relay both theoretical and practical information on this method.
Representative Results
The CFSE-based migration assay (Figure 1) was used together with flow cytometry to detect and characterize the CFSE-labeled cells that appear in the pLN after BCG infection in the footpad. A large portion of CFSE labeling in the pLN is found on MHC-IIhigh and CD11c+/low cells (Figure 2A), consistent with skin DCs that have migrated to skin DLN 11. Both the frequency and total number of CFSE-labeled, MHC-IIhigh CD11c+/low cells are increased in pLNs from BCG-infected mice compared to PBS-injected controls (Figure 2B-C), suggesting that BCG enhances the relocation of these cells to the DLN. Since this assay measures migration during a 24-hr period, it was possible to establish that skin DCs are still entering the DLN 5 days after infection (Figure 2C). This extends DC migration in the current model well beyond the timeline for initial activation of antigen-specific CD4+ T cells in the pLN 3. Further, migratory skin DCs express elevated levels of co-stimulatory molecules CD80 and CD86 (Figure 2D). This is consistent with previous observations from skin explant cultures and FITC skin painting 11, supporting the concept that migratory DCs are activated cells. Importantly, both LN-resident DCs (MHC-II+ CD11chigh cells) and plasmacytoid DCs (MHC-IIlow CD11clow PDCA-1+ cells), which enter inflamed LNs through high endothelial venules and not afferent lymphatics 12, where negative for CFSE (Figure 2EF), suggesting indeed that CFSE labeling occurred mainly in the footpad.
Given that MHC-IIhigh CD11c+/low cells represent not one but multiple DC sub-populations 13, the additional surface markers CD103, EpCAM (CD326) and CD11b were included in the antibody staining panel used for flow cytometric detection (Materials Table) to further characterize the population of migratory DCs (Figure 3). In doing so, a sub-population of EpCAMlow CD11bhigh cells was identified as the main migratory skin DC subset in response to BCG infection (Figure 3).
The flow cytometric data shown here was acquired on a flow cytometer equipped with 4 lasers (488, 532, 640 and 405 nm). The data was analyzed with cell analysis software. Other multi-color flow cytometers than the one specified in the Materials Table can be employed for the successful detection of the markers mentioned in the above studies, or the detection of other markers, on other cell types for that matter. For the protocol described herein, a cytometer capable of detecting 6 different fluorochromes is necessary. Importantly, the clones for each antibody used are specified (Materials Table). The choice of fluorochrome conjugate needs to be considered as this may depend on the lasers and detection channels on the flow cytometer available. Similarly, antibodies need to be titrated to determine the most appropriate dilutions for staining.
Population frequencies in BCG- and PBS-injected samples were graphed and used together with total cell counts to calculate absolute numbers of defined, gated subsets of DCs. The significance of differences in data group means was analyzed by Student' s t test or ANOVA where appropriate, with a cut-off of p < 0.05. Outliers were excluded from analysis following Grubbs' s test for outliers.
 Figure 1. Outline: BCG Footpad Infection Model and CFSE-Based Migration Assay. BCG is inoculated in the hind footpad of C57BL/6 mice. At different time points after infection, and 24 hr before sacrifice, the fluorochrome CFSE is injected in the same footpad that previously received BCG. The draining, popliteal lymph node is isolated and CFSE-labeled cells characterized by flow cytometry. Please click here to view a larger version of this figure.
Figure 1. Outline: BCG Footpad Infection Model and CFSE-Based Migration Assay. BCG is inoculated in the hind footpad of C57BL/6 mice. At different time points after infection, and 24 hr before sacrifice, the fluorochrome CFSE is injected in the same footpad that previously received BCG. The draining, popliteal lymph node is isolated and CFSE-labeled cells characterized by flow cytometry. Please click here to view a larger version of this figure.
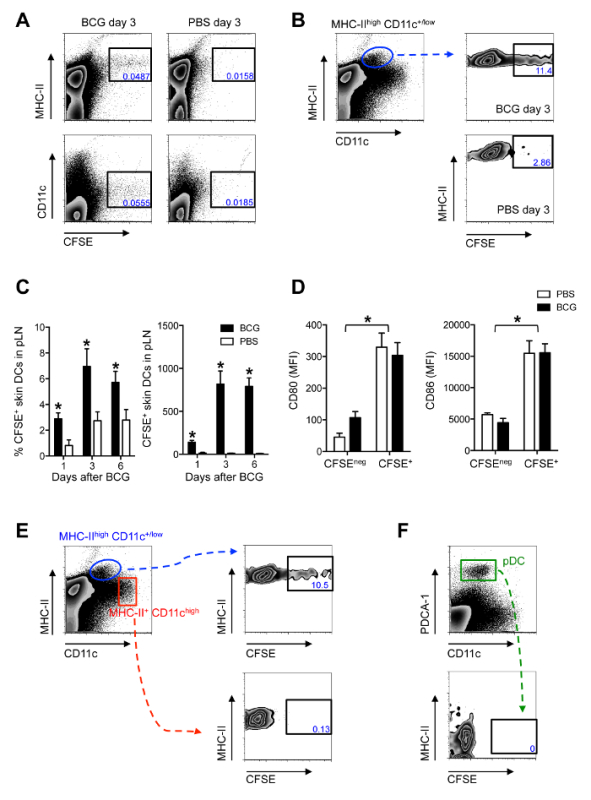 Figure 2. Migratory Skin DCs are a Major Population Relocating to the DLN After BCG Footpad Infection. C57BL/6 mice were infected with 1 x 106 CFUs of BCG in the footpad. Twenty-four hours before sacrifice, animals were injected with 0.5 mM CFSE in the same footpad. Popliteal LNs were harvested, homogenized into single-cell suspensions and subjected to flow cytometry. For measurements made 1 day after BCG, CFSE was injected 2hr after BCG. (A and B) Zebra plots showing predominant expression of CFSE on MHCIIhigh and CD11c+/low cells in BCG-draining pLN 3 days after infection. (C) Frequency and total number of CFSE-labeled MHCIIhigh CD11c+/low cells in pLN from BCG- and PBS-injected mice were graphed at different time points. (D) Mean fluorescence intensity (MFI) for CD80 and CD86 was determined on CFSE+ and CFSEneg MHC-IIhigh CD11c+/low skin DCs in pLN 3 days after BCG infection. (E) CFSE expression within skin DCs (MHC-IIhigh CD11c+/low), LN-resident DCs (MHC-II+ CD11chigh) and (F) plasmacytoid DCs (MHC-IIlow CD11clow PDCA-1+) was determined by flow cytometry 3 days after BCG. For each experiment, at least 5 mice were used for BCG-infected groups and 3 for PBS-injected controls. Bars indicate standard error of the mean. Total number of CFSE-labeled cells within each subset are given in Bollampalli et al., PLoS Pathog 2015, 11:e1005206 3. * Denotes statistically significant differences between BCG-infected and PBS controls. One of two independent experiments shown. Figure adapted from Bollampalli et al., PLoS Pathog. 2015, 11:e1005206 3, with permission from the publisher. Please click here to view a larger version of this figure.
Figure 2. Migratory Skin DCs are a Major Population Relocating to the DLN After BCG Footpad Infection. C57BL/6 mice were infected with 1 x 106 CFUs of BCG in the footpad. Twenty-four hours before sacrifice, animals were injected with 0.5 mM CFSE in the same footpad. Popliteal LNs were harvested, homogenized into single-cell suspensions and subjected to flow cytometry. For measurements made 1 day after BCG, CFSE was injected 2hr after BCG. (A and B) Zebra plots showing predominant expression of CFSE on MHCIIhigh and CD11c+/low cells in BCG-draining pLN 3 days after infection. (C) Frequency and total number of CFSE-labeled MHCIIhigh CD11c+/low cells in pLN from BCG- and PBS-injected mice were graphed at different time points. (D) Mean fluorescence intensity (MFI) for CD80 and CD86 was determined on CFSE+ and CFSEneg MHC-IIhigh CD11c+/low skin DCs in pLN 3 days after BCG infection. (E) CFSE expression within skin DCs (MHC-IIhigh CD11c+/low), LN-resident DCs (MHC-II+ CD11chigh) and (F) plasmacytoid DCs (MHC-IIlow CD11clow PDCA-1+) was determined by flow cytometry 3 days after BCG. For each experiment, at least 5 mice were used for BCG-infected groups and 3 for PBS-injected controls. Bars indicate standard error of the mean. Total number of CFSE-labeled cells within each subset are given in Bollampalli et al., PLoS Pathog 2015, 11:e1005206 3. * Denotes statistically significant differences between BCG-infected and PBS controls. One of two independent experiments shown. Figure adapted from Bollampalli et al., PLoS Pathog. 2015, 11:e1005206 3, with permission from the publisher. Please click here to view a larger version of this figure.
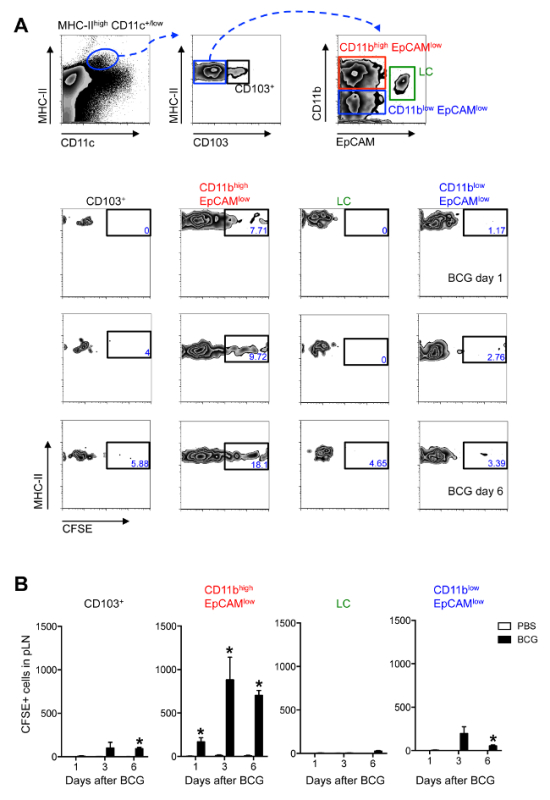 Figure 3. EpCAMlow CD11bhigh DCs are the Main Skin DC Subset Trafficking to the DLN After BCG Footpad Infection. C57BL/6 mice were injected with BCG and CFSE as in Figure 2. (A) Zebra plots showing gating strategy employed (top panel) and frequency of CFSE+ cells within each, defined subset at different time points after BCG infection (bottom panels). Frequencies within each subset may vary depending on the time point after infection. After 3 days of BCG one can expect to find approximately 0.2% MHC-IIhigh CD11c+/low cells among all LN cells. Of these, approximately 6% are CD103+ cells. For the subsets found within the MHC-IIhigh CD103negative gate, one can find approximately 42% CD11bhigh EpCAMlow cells, 27% CD11blow EpCAMlow cells and 7% LCs. (B) Total numbers of CFSE+ cells within each, defined subset at different time points after BCG infection is shown. For each experiment, at least 5 mice were used for BCG-infected groups and 3 for PBS controls. Bars indicate standard error of the mean. * Denotes statistically significant differences relative to PBS-injected controls. One of three independent experiments shown. Figure re-printed from Bollampalli et al., PLoS Pathog 2015, 11:e1005206 3, with permission from the publisher. Please click here to view a larger version of this figure.
Figure 3. EpCAMlow CD11bhigh DCs are the Main Skin DC Subset Trafficking to the DLN After BCG Footpad Infection. C57BL/6 mice were injected with BCG and CFSE as in Figure 2. (A) Zebra plots showing gating strategy employed (top panel) and frequency of CFSE+ cells within each, defined subset at different time points after BCG infection (bottom panels). Frequencies within each subset may vary depending on the time point after infection. After 3 days of BCG one can expect to find approximately 0.2% MHC-IIhigh CD11c+/low cells among all LN cells. Of these, approximately 6% are CD103+ cells. For the subsets found within the MHC-IIhigh CD103negative gate, one can find approximately 42% CD11bhigh EpCAMlow cells, 27% CD11blow EpCAMlow cells and 7% LCs. (B) Total numbers of CFSE+ cells within each, defined subset at different time points after BCG infection is shown. For each experiment, at least 5 mice were used for BCG-infected groups and 3 for PBS controls. Bars indicate standard error of the mean. * Denotes statistically significant differences relative to PBS-injected controls. One of three independent experiments shown. Figure re-printed from Bollampalli et al., PLoS Pathog 2015, 11:e1005206 3, with permission from the publisher. Please click here to view a larger version of this figure.
Discussion
noDCs are specialized antigen-presenting cells that can respond to microbial challenge at body surfaces by acquiring microbial antigen and migrating to DLN via lymphatics. There, DCs present this antigen to T cells in a manner that drives primary T-cell responses. The process of DC migration from tissue to DLN is therefore highly important for understanding how a productive T-cell response is initiated. Methods to measure DC migration are consequently very useful in unfolding the above.
A mouse model was employed to study skin DC migration to DLN following infection with BCG, a live vaccine that is injected clinically in the skin. In line, BCG is injected in the hind footpad to mimic this cutaneous route of vaccination. A simple assay was developed and consequently incorporated to this model to investigate the migration of skin DC sub-populations from the BCG inoculation site in the footpad skin to the draining pLN. This protocol relies on injecting the fluorochrome CFSE into the same footpad that previously received BCG and then isolating the draining pLN 24 hr later to analyze the presence of CFSE-labeled cells by flow cytometry. Although not described in the protocol, LNs from this setup can also be prepared for analysis by confocal microscopy, to study migration processes such as the positioning of migratory cells in the LN 3. Furthermore, similar results on BCG-triggered skin DC migration have been obtained using both BCG Pasteur 1173P2 3 and BCG SSI 1331 14.
CFSE is commonly used to label cells in vitro and to track such labeled cells in vivo after cell transfers 15. In the current protocol though, it was used to directly label skin cells in situ. The molecular basis of CFSE labeling is similar to FITC, which is also an amine-reactive fluorescent dye. CFSE is however preferred over FITC for conjugation as it creates very stable carboxamide bonds 16. Moreover, CFSE is highly membrane permeable and passively diffuses into cells. Once inside, it forms intracellular amine (fluorescent) conjugates that are retained in the labeled cell 15.
The CFSE-based migration assay developed by the authors enables the identification of cells relocating from skin to DLN within a 24-hr period in response to BCG. It thus measures acute migration during a defined timeframe and allows one to investigate the ability of different, injected stimuli to trigger migration. This assay is different from the classical technique of FITC skin painting, which measures a cumulative migration event triggered by cutaneous, topical application of a contact-sensitizing agent. Thus, the CFSE-based migration assay can be employed to identify migratory skin DCs or other cells that home to the pLN after the injection of microbes, microbial products or other inflammatory stimuli in the footpad. Indeed, both neutrophils and γ:δ T cells have been shown to relocate from skin to DLN in response to BCG 17,18. In fact, the assay was recently used in a co-infection setting to show that a gut-localized nematode infection could mute BCG-triggered skin DC migration to DLN 14.
DC migration is often assessed between 24 to 72 hr in FITC skin painting since FITC-labeled DCs are difficult to detect 4 to 5 days after painting 19. Loss of the CFSE signal on labeled DCs is not an issue in the assay presented here since analysis of migration was restricted to 24 hr; the reason behind this being that the authors wanted to specifically study "overnight" migration events. Others interested in this assay are however encouraged to investigate earlier or even later time points for CFSE-based tracking. Moreover, since CFSE is introduced by injection it can be given at any defined time. This flexibility allowed the authors to assess DC migration between day 5 and 6 after BCG infection (Figure 2C) 3. Injecting CFSE could potentially limit the cell types accessible in situ to the labeling reaction. For instance Langerhans cells (LCs), which are DCs that reside in the top layer of the skin, the epidermis, migrate poorly in response to BCG in the CFSE-based migration assay (Figure 3) 3. However, during FITC skin painting, DCs positioned in the dermis, the layer below the epidermis, are readily labeled and migrate to the DLN faster than LCs 20,21. This suggests that cells can become fluorochrome-labeled without necessarily needing to localize to the layer of the skin where the labeling solution is applied.
As a model for BCG infection, the mouse ear provides a bona fide intradermal route of inoculation that is more in line with the clinical administration of BCG as a tuberculosis vaccine. Footpad injection on the other hand, is likely a combination of the intradermal and subcutaneous routes. That said, it requires skill and training to correctly and reproducibly deliver BCG to the dermis 22, so in clinical practice it may not always be given truly intradermal but rather subcutaneous or a combination of both. As an inoculation site the footpad does have two very clear advantages over the ear that merit mentioning. First, the immune response is concentrated to the draining pLN following a footpad injection and this facilitates the examination of responses. The authors of this report found the pLN to be the first and main LN draining the footpad 3. Others have suggested split drainage between the popliteal and subiliac LNs after injection of Evan's blue 23. Nevertheless, in regards to the ear, there is more than one ear DLN in mice and these may not be regularly present or easy to locate 24. The latter clearly introduces a liability in studies seeking to investigate responses in DLN. Second, larger volumes can be inoculated into the footpad (10 - 50 µl) compared to the ear. This in turn both minimizes the liability of introducing major alterations in lymphatic flow and of having to work with very small injection volumes, which is in itself an issue when it comes to inoculating large quantities of mycobacteria. In the ear where injection volumes have to be small (1-10 µl), even 5 µl may alter lymphatic flow and potentially force a larger amount of the injected material directly into the DLN in an uncontrolled way. It is therefore important to account for injection volumes. This is especially important in experiments addressing the contribution of cells in ferrying bacteria to the LN. In the BCG footpad model, some BCG may have reached the DLN in the absence of cellular transport. This is based on the observation that one can still detect BCG in the DLN of mice where cell migration from the footpad was entirely ablated by local injection of pertussis toxin 3. Whether this is an indication that BCG can directly access lymphatics and reach the DLN in a truly cell-free manner remains to be determined since it cannot be excluded that mycobacteria in this case where given access to lymphatics by force of the injected volume.
A practical consideration for immunizations in the ear or footpad is that animals have to be adequately immobilized for injections to be carried out correctly and in a reproducible manner. Isoflurane gas is described in the current protocol as a method to restrain animals in preparation for footpad injections. The relatively rapid induction and recovery times for isoflurane-mediated anesthesia make it a popular method, but similar to other anesthetic agents, it affects physiology, which may interfere with experimental results 25. Indeed, both volatile and injectable anesthetics decrease lymph flow by abolishing voluntary muscle movements, reducing muscle tone, and decreasing lymphangion contraction 26. Furthermore, a 5-fold reduction in migration to DLN of DCs adoptively transferred in the footpad has been reported in anesthetized animals 27. Given the above, and since the handling procedures prior to anesthesia are likely to cause more stress to the animals than the injection itself, which is both rapid to administer and does not require a recovery step, the possibility to adequately restrain the animal without anesthetics should be considered. A mouse restrainer customized for footpad injections, where the animal can be rapidly brought to an immobilized position and inoculated in the footpad within 30 to 40 sec would be ideal to inject the mouse in the hind footpad without altering other aspects of its physiology in the process, and would be extremely useful in studies addressing cell migration through lymphatics.
In conclusion, this report highlights a novel protocol for tracking skin DCs during their mobilization to the DLN using an assay that monitors migration during a defined 24-hr window. This CFSE-based migration assay allows detection and analysis of migratory cells by flow cytometry, as detailed here, as well as by confocal microscopy 3. It provides a flexible platform for studying a variety of injected stimuli and their ability to trigger DC migration, and importantly, can be employed to track not only DCs but also other populations moving from skin to DLN during different experimental settings.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by Karolinska Institutet, a project grant from the Swedish Research Council VR (Dnr 2014-2794) and a KID Doctoral Grant from Karolinska Institutet (All to A.G.R). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Bollampalli VP, et al. BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAMlow CD11bhigh Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming. PLoS Pathog. 2015;11:e1005206. doi: 10.1371/journal.ppat.1005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Larsen MH, Biermann K, Jacobs WR., Jr Laboratory maintenance of Mycobacterium tuberculosis. Curr Protoc Microbiol. 2007;Chapter 10:Unit 10A 11. doi: 10.1002/9780471729259.mc10a01s6. [DOI] [PubMed] [Google Scholar]
- Tung JW, et al. Modern flow cytometry: a practical approach. Clin Lab Med. 2007;27:453–468. doi: 10.1016/j.cll.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- Sharrow SO. Overview of flow cytometry. Curr Protoc Immunol. 2002;Chapter 5:Unit 5.1. doi: 10.1002/0471142735.im0501s50. [DOI] [PubMed] [Google Scholar]
- Holmes K, Lantz LM, Fowlkes BJ, Schmid I, Giorgi JV. Preparation of cells and reagents for flow cytometry. Curr Protoc Immunol. 2001;Chapter 5:Unit 5.3. doi: 10.1002/0471142735.im0503s44. [DOI] [PubMed] [Google Scholar]
- Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Blasius AL, Mak TW, Cella M, Colonna M. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J Exp Med. 2005;202:687–696. doi: 10.1084/jem.20051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri S, et al. Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol. 2010;88:366–375. doi: 10.1038/icb.2010.34. [DOI] [PubMed] [Google Scholar]
- Obieglo K, et al. Chronic Gastrointestinal Nematode Infection Mutes Immune Responses to Mycobacterial Infection Distal to the Gut. J Immunol. 2016;196(5) doi: 10.4049/jimmunol.1500970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR, Glidden MH, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009;Chapter 4:Unit4.9. doi: 10.1002/0471142735.im0409s84. [DOI] [PubMed] [Google Scholar]
- Banks PR, Paquette DM. Comparison of three common amine reactive fluorescent probes used for conjugation to biomolecules by capillary zone electrophoresis. Bioconjug Chem. 1995;6:447–458. doi: 10.1021/bc00034a015. [DOI] [PubMed] [Google Scholar]
- Nakamizo S, et al. Dermal Vgamma4(+) gammadelta T cells possess a migratory potency to the draining lymph nodes and modulate CD8(+) T-cell activity through TNF-alpha production. J Invest Dermatol. 2015;135:1007–1015. doi: 10.1038/jid.2014.516. [DOI] [PubMed] [Google Scholar]
- Abadie V, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Edwards AJ, Watkins MC, Asherson GL. Distribution of immunogenic cells after painting with the contact sensitizers fluorescein isothiocyanate and oxazolone. Different sensitizers form immunogenic complexes with different cell populations. Immunology. 1980;39:21–27. [PMC free article] [PubMed] [Google Scholar]
- Shklovskaya E, Roediger B, Fazekasde St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MI, Iritani BM, Ruddell A. Lymph node mapping in the mouse. J Immunol Methods. 2008;332:170–174. doi: 10.1016/j.jim.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Gargiulo S, et al. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 2012;53:E55–E69. doi: 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Tal O, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]


