Abstract
Here, we demonstrate a detailed protocol for the radiosynthesis of a 125I-labeled azide prosthetic group and its application to the efficient radiolabeling of DBCO-group-functionalized gold nanoparticles using a copper-free click reaction. Radioiodination of the stannylated precursor (2) was carried out by using [125I]NaI and chloramine T as an oxidant at room temperature for 15 min. After HPLC purification of the crude product, the purified 125I-labeled azide (1) was obtained with high radiochemical yield (75 ± 10%, n = 8) and excellent radiochemical purity (>99%). For the synthesis of radiolabeled 13-nm-sized gold nanoparticles, the DBCO-functionalized gold nanoparticles (3) were prepared by using a thiolated polyethylene glycol polymer. A copper-free click reaction between 1 and 3 gave the 125I-labeled gold nanoparticles (4) with more than 95% of radiochemical yield as determined by radio-thin-layer chromatography (radio-TLC). These results clearly indicate that the present radiolabeling method using a strain-promoted copper-free click reaction will be useful for the efficient and convenient radiolabeling of DBCO-group-containing nanomaterials.
Keywords: Chemistry, Issue 116, Radiolabeling, Radioisotope, Radiotracer, Radioactive iodine, Bioorthogonal reaction, Copper-free click reaction, Prosthetic group, Azide, Gold nanoparticles
Introduction
The strain-promoted copper-free click reaction between azides and cyclooctynes has been extensively applied to the efficient bioorthogonal labeling of a wide range of biomolecules, nanomaterials, and living subjects1-7. Due to the excellent site-specificity and rapid reaction rate of this conjugation reaction, it has also been used to synthesize radiolabeled tracers. A few 18F-labeled azide or DBCO prosthetic groups have been prepared for in vitro labeling of various cancers targeting peptides and antibodies, as well as for in vivo pre-targeted imaging of tumors8-13. In addition to these examples, the same conjugation reaction was applied to the metal-radioisotope-labeling of nanomaterials for positron emission tomography (PET) imaging studies14-16.
For several decades, radioactive iodines have been used for biomedical research and clinical trials through PET imaging (124I), single-photon emission computed tomography (SPECT) imaging (123I, 125I), and thyroid cancer treatment (131I)17-21. Therefore, an efficient method for radioactive iodine labeling is fundamentally important for various investigations, including molecular imaging studies, analysis of organ distribution of biomolecules, biomarker identification, and drug development. A copper-free click reaction strategy could be used in radioactive iodine labeling. However, this application has not been investigated as extensively as 18F-labeled biomolecules22-23. Here, we will provide a step-by-step protocol for the synthesis of an 125I-labeled azide for radiolabeling of DBCO-group-derived molecules. The procedures in the present report will include radioiodination of the stannylated precursor, purification steps with HPLC, and solid phase extraction. We also demonstrate efficient radiolabeling of DBCO-group-modified 13-nm-sized gold nanoparticles using the 125I-labeled azide. The detailed protocol in this report will help synthetic chemists understand a new radiolabeling methodology for the synthesis of radiolabeled products.
Protocol
Caution: The oxidized form of radioactive iodine is quite volatile and must be handled with adequate lead shields and lead vials. All radiochemical steps should be carried out in a well-ventilated charcoal-filtered hood, and the experimental procedures need to be monitored by radioactivity detection devices.
1. Preparation of Chemicals and the Reverse Phase Cartridge for the Synthesis of the 125I-labeled Azide
- Preparation of reagents in solution
- Dissolve 1 mg of the azide precursor (2) in 150 µl absolute ethanol (Figure 1). NOTE: A detailed synthetic procedure for the azide precursor (2) was reported in the previous paper22.
- Dissolve 1 mg chloramine T in 20 µl of 1x phosphate buffer saline (pH = 7.4).
- Dissolve 2 mg sodium metabisulfite in 20 µl H2O.
- Preparation of the cartridge
- Wash the tC18 cartridge with 10 ml absolute ethanol followed by 10 ml H2O. Do not dry the matrix of the cartridge with air.
2. Radiosynthesis of the 125I-labeled Azide Prosthetic Group
- Radioiodination reaction of the precursor
- Add the azide precursor solution (1 mg in 150 µl of absolute ethanol) and acetic acid (10 µl) to a 1.5 ml microcentrifuge tube.
- Add 150 MBq of [125I]NaI in 0.1 M NaOH (50 µl) to the reaction mixture.
- Add a chloramine T solution (1 mg in 20 µl of 1x phosphate buffer saline) and close the microcentrifuge tube containing reaction mixture.
- Incubate the reaction mixture at room temperature for 15 min until the radioiodination reaction is completed.
- Add a sodium metabisulfite solution (2 mg in 20 µl H2O) to the reaction mixture to quench the radioiodination reaction.
- Withdraw 0.2 µl of the crude product and then dilute it with 100 µl of solution (H2O/CH3CN, 1:1) for HPLC analysis. NOTE: For all HPLC experiments, use 0.1% formic acid containing H2O (solvent A) and 0.1% formic acid containing acetonitrile (solvent B) as eluents.
- Analyze the diluted crude product by using a reverse-phase analytical radio-HPLC (C18 reverse-phase column; flow rate: 1 ml/min; eluent gradient: 20% solvent B for 0-2 min, 20-80% solvent B for 2-22 min, 80-100% solvent B for 22-23 min, and 100% solvent B for 23-28 min; retention time: 16.4 min) (Figure 2).
- Purification of the crude product with a preparative HPLC NOTE: Provide enough lead shielding around HPLC parts such as the injector, column, detector, collection vials, and the container in which the effluent is collected.
- Withdraw the entire reaction mixture into an HPLC vial. Rinse the reaction tube with acetonitrile (0.5 ml) and add the rinse into the same injection vial. Dilute the collected solution with H2O (1 ml).
- Inject the crude product onto a preparative radio-HPLC (C18 reverse-phase column; flow rate: 10 ml/min; eluent gradient: 20% solvent B for 0-2 min, 20-80% solvent B for 2-22 min, 80-100% solvent B for 22-23 min, and 100% solvent B for 23-28 min).
- Collect the radioactive peak representing the 125I-labeled azide (1) (tR under these HPLC conditions is 17.8-18.8 min) in a glass test tube (Figure 2).
- Measure the radiochemical yield of the fraction using a radioactivity dose calibrator according to the manufacturer's protocol.
- Inject the purified product onto an analytical radio-HPLC using the same HPLC conditions for determining the radiochemical purity of the product.
- Solid phase extraction of the product
- Dilute the fraction containing the desired product (1) with 40 ml pure H2O.
- Add the diluted solution into a preconditioned tC18 cartridge.
- Wash the cartridge with an additional 15 ml H2O.
- Elute the product (1) trapped in the cartridge with 2 ml acetone into a 10-ml glass vial that is protected by a lead shield. Measure the radioactivity of the eluted product using a radioactivity dose calibrator according to the manufacturer's protocol. NOTE: Dimethyl sulfoxide (DMSO) or absolute ethanol can also be used for elution of the product from the cartridge. Approximately 5-10% of the radioactivity normally sticks to the cartridge, and the remaining radiolabeled product cannot be fully eluted by using excess amounts of organic solvent.
- Evaporate the acetone with a stream of nitrogen or argon gas.
- Dissolve the residue with DMSO (100-200 µl) for the next radiolabeling step.
3. Synthesis of DBCO-group-conjugated Gold Nanoparticles
- Surface modification of 13-nm-sized gold nanoparticles with DBCO-group-containing polyethylene glycol
- Prepare sodium-citrate-stabilized gold nanoparticles (3) (average size = 13 nm) according to a previous report24.
- Add an aqueous solution of Tween 20 (1 mM, 1.5 ml) to the citrate-stabilized gold nanoparticles (10 nM, 15 ml). Shake the solution for 20 min on an orbital shaker.
- Add an aqueous solution of DBCO-group-containing polyethylene glycol thiol (average molecular weight = 5,000, 100 μM, 1.5 ml). Shake the solution for 2 hr on an orbital shaker.
- Purification of the DBCO-group-modified gold nanoparticles
- Purify the DBCO-group-modified gold nanoparticles (4) by successive centrifugation (11,400 x g, 15 min x 3).
- Decant the supernatant and add pure water for resuspension of the gold nanoparticle pellets.
4. Radiolabeling of DBCO-group-modified Gold Nanoparticles via the Copper-free Click Reaction
- Synthesis of 125I-labeled gold nanoparticles using the 125I-labeled azide (1)
- Prepare a concentrated solution of DBCO-group-modified gold nanoparticles by using centrifugation (11,400 x g, 15 min), and adjust the concentration of the gold nanoparticles to 2 µM.
- Add 4.1 MBq of the 125I-labeled azide (1) in DMSO (5 µl) to a suspension of gold nanoparticles (4) (2 μM, 50 µl).
- Incubate the resulting reaction mixture at 40 °C for 60 min.
- Withdraw an aliquot (0.2 µl) from the crude product and apply it onto a silica-coated thin-layer chromatography (TLC) plate.
- Develop the TLC plate using ethyl acetate as a mobile phase.
- Place the TLC plate on a radio-TLC scanner and run the scanner to monitor the radiolabeling reaction (Figure 3) according to the manufacturer's protocol.
- Purification of the crude product
- Purify the reaction mixture containing the 125I-labeled gold nanoparticles (4) by centrifugation (11,400 x g, 15 min).
- Decant the supernatant and add pure water for resuspension of the gold nanoparticle pellets.
- Withdraw an aliquot (0.2 µl) from the purified product and apply it onto a silica-coated TLC plate.
- Develop the TLC plate using ethyl acetate as the mobile phase.
- Place the TLC plate on a radio-TLC scanner and run the scanner to determine the radiochemical yield and radiochemical purity of the 125I-labeled gold nanoparticles (4) (Figure 3) according to the manufacturer's protocol.
Representative Results
The radioiodination reaction of the stannylated precursor (2) was carried out using 150 MBq of [125I]NaI, acetic acid, and chloramine T at room temperature for 15 min to provide the radiolabeled product (1). After preparative HPLC purification of the crude mixture, the desired product was obtained with 75 ± 10% (n = 8) of radiochemical yield. Analytical HPLC revealed that the radiochemical purity of the 125I-labeled product was more than 99% (Figure 2), and the observed specific radioactivity of product 1 is 40.7 MBq/µmol. Solid phase extraction of the fraction containing the purified product by using the cartridge provided an acetone solution of 1. Using a stream of nitrogen or argon gas, the organic solvent can be evaporated, and then the residue can be dissolved again in DMSO or absolute ethanol for the next step.
For 125I-labeling of polyethylene-glycol-modified gold nanoparticles, the DBCO-group-modified gold nanoparticles were prepared by the procedure shown in Figure 1. An excess amount of polyethylene glycol thiol (MW 5,000) with DBCO groups was reacted with the citrated, stabilized 13-nm gold nanoparticles. After the modification step, the product was purified by successive centrifugation to give the DBCO-functionalized gold nanoparticles (3). In the radiolabeling step, 3.7 MBq of 1 was added to 2 µM of 3 (~400 µM of the DBCO groups), and the labeling reaction was carried out at 40 °C for 1 hr. Radio-TLC analysis showed that more than 95% of 1 was reacted with the DBCO-group-functionalized gold nanoparticles (3) within 60 min. The reaction was carried out for 60 min, and then the crude product was purified by centrifugation. 125I-labeled gold nanoparticles (4) were obtained with >99% (n = 4) radiochemical yield as determined by radio TLC (Figure 3).
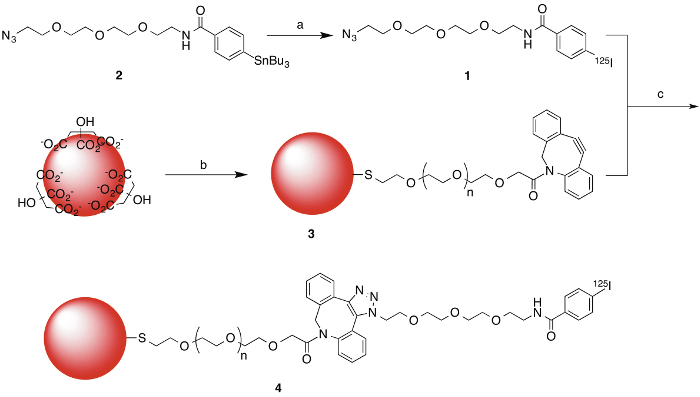 Figure 1. Radiosynthesis of the 125I-labeled azide (1) and 125I-labeled gold nanoparticles (4). Reagents and conditions: (a) [125I]NaI, acetic acid, chloramine T, RT, 15 min, 75 ± 10% (n = 8) radiochemical yield; (b) DBCO-PEG-SH (MW 5,000), H2O, RT, 2 hr; ~40 °C, 60 min, >99% radiochemical yield. Please click here to view a larger version of this figure.
Figure 1. Radiosynthesis of the 125I-labeled azide (1) and 125I-labeled gold nanoparticles (4). Reagents and conditions: (a) [125I]NaI, acetic acid, chloramine T, RT, 15 min, 75 ± 10% (n = 8) radiochemical yield; (b) DBCO-PEG-SH (MW 5,000), H2O, RT, 2 hr; ~40 °C, 60 min, >99% radiochemical yield. Please click here to view a larger version of this figure.
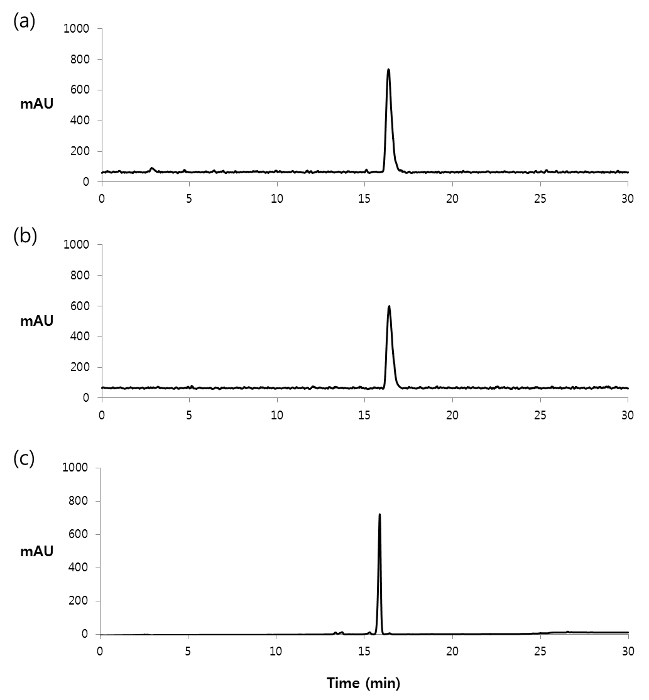 Figure 2. Analytical HPLC chromatogram of the 125I-labeled azide (1). (a) Radiochromatogram of the crude product. (b) Radiochromatogram of the purified product. (c) UV chromatogram (254 nm) of the purified product. Please click here to view a larger version of this figure.
Figure 2. Analytical HPLC chromatogram of the 125I-labeled azide (1). (a) Radiochromatogram of the crude product. (b) Radiochromatogram of the purified product. (c) UV chromatogram (254 nm) of the purified product. Please click here to view a larger version of this figure.
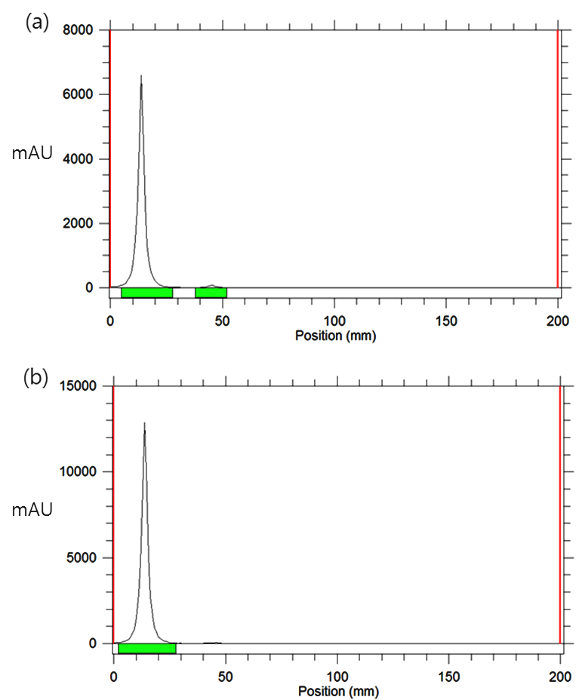 Figure 3. Radio-TLC results of the 125I-labeled gold nanoparticles (4) (Rf of 4 = 0.05, Rf of 1 = 0.45, eluent: ethyl acetate) (a) after a 60 min reaction and (b) after purification. Please click here to view a larger version of this figure.
Figure 3. Radio-TLC results of the 125I-labeled gold nanoparticles (4) (Rf of 4 = 0.05, Rf of 1 = 0.45, eluent: ethyl acetate) (a) after a 60 min reaction and (b) after purification. Please click here to view a larger version of this figure.
Discussion
In general, the observed radiochemical yield of the purified 125I-labeled azide (1) was 75 ± 10% (n = 8). The radiolabeling was accomplished with 50-150 MBq of radioactivity, and the radiochemical results are quite consistent. If [125I]NaI (t1/2 = 59.4 d) that underwent radioactive decay for more than a month was used in the radioiodination reaction, the radiochemical yield of 1 was observed to be slightly decreased (53-65%). Therefore, it is recommended that [125I]NaI be utilized as soon as it is produced or is delivered to the lab in order to obtain optimized radiochemical yield. In addition, a freshly prepared chloramine T solution should also be used in the reaction to obtain the desired radiochemical yield.
Because the precursor (2) was quite hydrophobic, 150 µl absolute ethanol should be added to dissolve 1 mg of 2 in the radioiodination reaction. Otherwise, the whole reaction mixture could become turbid after adding an aqueous solution of the oxidant and [125I]NaI. Decreased solubility of the precursor often results in low radiochemical yield of 1. DMSO can also be used for dissolving 2 in the radiolabeling step. In addition, acetic acid should be added to the precursor solution to obtain high radiochemical yield in the radioiodination step.
Before using preparative HPLC for purification of the crude product containing 1, the reverse phase HPLC column needs to be washed with solvents A and B (flow rate: 10 ml/min; eluent gradient: 100% solvent B for 0-10 min, 100-0% solvent B for 10-25 min, and 0% solvent B for 25-30 min) to remove trace amount of impurities from the system. Next, the reverse phase HPLC column is equilibrated with 20% solvent B in 80% solvent A for at least 20 min to obtain the consistent retention time of 1.
The fraction containing purified 1 should be diluted with more than 4 times the volume of H2O in the solid phase extraction procedure. Otherwise, some of the purified product cannot be trapped in the tC18 cartridge. When acetone is used to elute purified 1 from the cartridge, the final volume can be reduced by evaporation of acetone with a stream of nitrogen or argon gas at ambient temperature.
Among several radioactive iodines, 125I was selected and used in the current research. Different kinds of iodine radioisotopes need to be tested using the present method in other biological and medical studies (e.g.,124I for PET imaging, 131I for therapeutic purposes).
As far as we understand, the present radiolabeling protocol is the first report describing detailed synthetic steps for a radioiodine-labeled azide group. Recently, we published another azide prosthetic group, which has a different structure23. However, the radiolabeled azide (1) in the current method provided slightly better radiochemical results than the other in terms of radiolabeling efficiency with DBCO-group-containing molecules. Existing prosthetic groups (i.e., N-hydroxysuccinimide and maleimide) for the labeling of radioactive iodine could not provide site-specificity. However, the present method demonstrates straightforward radiolabeling efficiency along with excellent bioorthogonality. Since the azide functional group is known to be highly stable in physiological conditions and in vivo environments, the radiolabeled product (1) can be utilized in pre-targeted in vivo imaging studies. We anticipate that this method will be efficiently applied to both in vitro and in vivo iodine radioisotope labeling of biomolecules and nanomaterials that contain a strained cyclooctyne structure.
Based on the specific radioactivity of 1, the calculated molar ratio of 125I and gold nanoparticles is ~1:1. 125I-labeled gold nanoparticles (4) can be used in molecular imaging and biodistribution studies of nanomaterials. The current method can also be applied to radioactive iodine labeling of different sizes and shapes of gold nanomaterials.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the National Research Foundation of Korea, funded by the government of the Republic of Korea, (Grant nos. 2012M2B2B1055245 and 2012M2A2A6011335) and by the RI-Biomics Center of Korea Atomic Energy Research Institute.
References
- Jewett JC, Bertozzi CR. Cu-free Click Cycloaddition Reactions in Chemical Biology. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets MF, et al. Bioconjugation with Strained Alkenes and Alkyne. Acc. Chem. Res. 2011;44:805–815. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]
- Sletten EM, Bertozzi CR. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011;44:666–676. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, et al. Bioorthogonal Cu-Free Click Chemistry in vivo for Tumor-Targeted Delivery of Nanoparticles. Angew. Chem. Int. Ed. 2012;51:11836–11840. doi: 10.1002/anie.201206703. [DOI] [PubMed] [Google Scholar]
- Chang PV, et al. Copper-Free Click Chemistry in Living Animals. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostic HE, Smith MD, Poloukhtine AA, Popik VV, Best MD. Membrane Labeling and Immobilization via copper-free Click Chemistry. Chem. Commun. 2012;48:1431–1433. doi: 10.1039/c1cc14415d. [DOI] [PubMed] [Google Scholar]
- Someya T, Ando A, Kimoto M, Hirao I. Site-Specific Labeling of RNA by Combining Genetic Alphabet Expansion Transcription and Copper-Free Click Chemistry. Nucl. Acids Res. 2015;43:6665–6676. doi: 10.1093/nar/gkv638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, et al. Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew. Chem. Int. Ed. 2013;52:10549–10552. doi: 10.1002/anie.201304026. [DOI] [PubMed] [Google Scholar]
- Sachin K, et al. F-18 Labeling Protocol of Peptides Based on Chemically Orthogonal Strain-Promoted Cycloaddition under Physiologically Friendly Reaction Conditions. Bioconjugate Chem. 2012;23:1680–1686. doi: 10.1021/bc3002425. [DOI] [PubMed] [Google Scholar]
- Evans HL, et al. Copper-Free Click - A Promising Tool for Pre-targeted PET Imaging. Chem. Commun. 2012;48:991–993. doi: 10.1039/c1cc16220a. [DOI] [PubMed] [Google Scholar]
- Campbell-Verduyn LS, et al. Strain-Promoted Copper-Free "Click" Chemistry for 18F Radiolabeling of Bombesin. Angew. Chem. Int. Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- Arumugam S, Chin J, Schirrmacher R, Popik VV, Kostikov AP. 18F]Azadibenzocyclooctyne ([18F]ADIBO): A Biocompatible Radioactive Labeling Synthon for Peptides using Catalyst Free [3+2] Cycloaddition. Bioorg. Med. Chem. Lett. 2011;21:6987–6991. doi: 10.1016/j.bmcl.2011.09.126. [DOI] [PubMed] [Google Scholar]
- Bouvet V, Wuest M, Wuest F. Copper-Free Click Chemistry with the Short-Lived Positron Emitter Fluorine-18. Org. Biomol. Chem. 2011;9:7393–7399. doi: 10.1039/c1ob06034a. [DOI] [PubMed] [Google Scholar]
- Satpati D, Bauer N, Hausner SH, Sutcliffe JL. Synthesis of [64Cu]DOTA-ADIBON3-Ala-PEG28-A20FMDV2 via Copper-Free Click Chemistry for PET Imaging of Integrin αvβ6. J. Radioanal. Nucl. Chem. 2014;302:765–771. [Google Scholar]
- Lee DE, et al. Facile Method To Radiolabel Glycol Chitosan Nanoparticles with 64Cu via Copper-Free Click Chemistry for MicroPET Imaging. Mol. Pharmaceutics. 2013;10:2190–2198. doi: 10.1021/mp300601r. [DOI] [PubMed] [Google Scholar]
- Zeng D. 64Cu Core-Labeled Nanoparticles with High Specific Activity via Metal-Free Click Chemistry. ACS Nano. 2012;6:5209–5219. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, et al. Radiosynthesis and in vivo Evaluation of [125I]2-4(iodophenethyl)-2-Methylmalonic Acid as a Potential Radiotracer for Detection of Apoptosis. J. Radioanal. Nucl. Chem. 2016;308:23–29. [Google Scholar]
- Adam MJ, Wilbur DS. Radiohalogens for Imaging and Therapy. Chem. Soc. Rev. 2005;34:153–163. doi: 10.1039/b313872k. [DOI] [PubMed] [Google Scholar]
- Jeon J, et al. Radiosynthesis of 123I-Labeld Hesperetin for Biodistribution Study of Orally Administered Hesperetin. J. Radioanal. Nucl. Chem. 2015;306:437–443. [Google Scholar]
- Kil KE, et al. Development of [123I]IPEB and [123I]IMPEB as SPECT Radioligands for Metabotropic Glutamate Receptor Subtype. ACS Med. Chem. Lett. 2014;5:652–656. doi: 10.1021/ml500007z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, et al. The Utility of I-123 Pretherapy Scan in I-131 Radioiodine Therapy for Thyroid Cancer. Thyroid. 2012;22:304–309. doi: 10.1089/thy.2011.0203. [DOI] [PubMed] [Google Scholar]
- Jeon J, et al. Efficient Method for Iodine Radioisotope Labeling of Cyclooctyne-Containing Molecules using Strain-Promoted Copper-Free Click Reaction. Bioorg. Med. Chem. 2015;23:3303–3308. doi: 10.1016/j.bmc.2015.04.045. [DOI] [PubMed] [Google Scholar]
- Choi MH, et al. Synthesis and Evaluation of an 125I-Labeled Azide Prosthetic Group for Efficient and Bioorthogonal Radiolabeling of Cyclooctyne-Group Containing Molecules using Copper-Free Click Reaction. Bioorg. Med. Chem. Lett. 2016;26:875–878. doi: 10.1016/j.bmcl.2015.12.073. [DOI] [PubMed] [Google Scholar]
- Kim YH, et al. Tumor Targeting and Imaging Using Cyclic RGD-PEGylated Gold Nanoparticle Probes with Directly Conjugated Iodine-125. Small. 2011;7:2052–2060. doi: 10.1002/smll.201100927. [DOI] [PubMed] [Google Scholar]


