Abstract
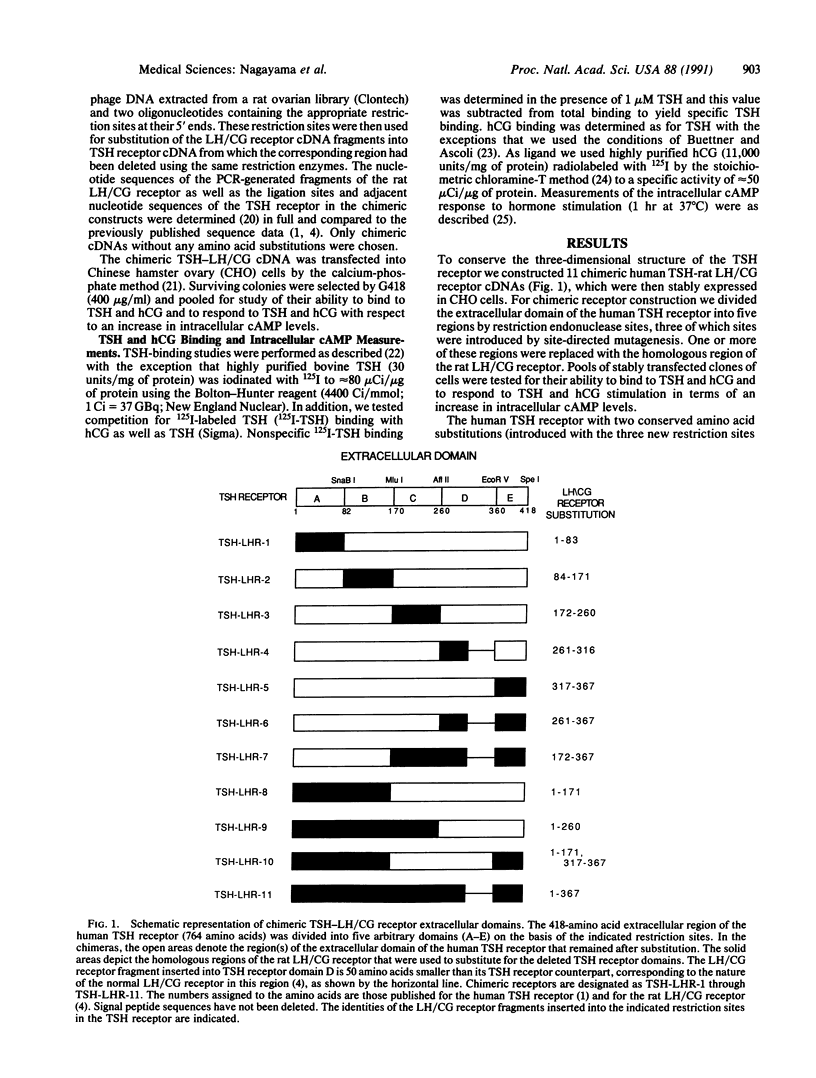
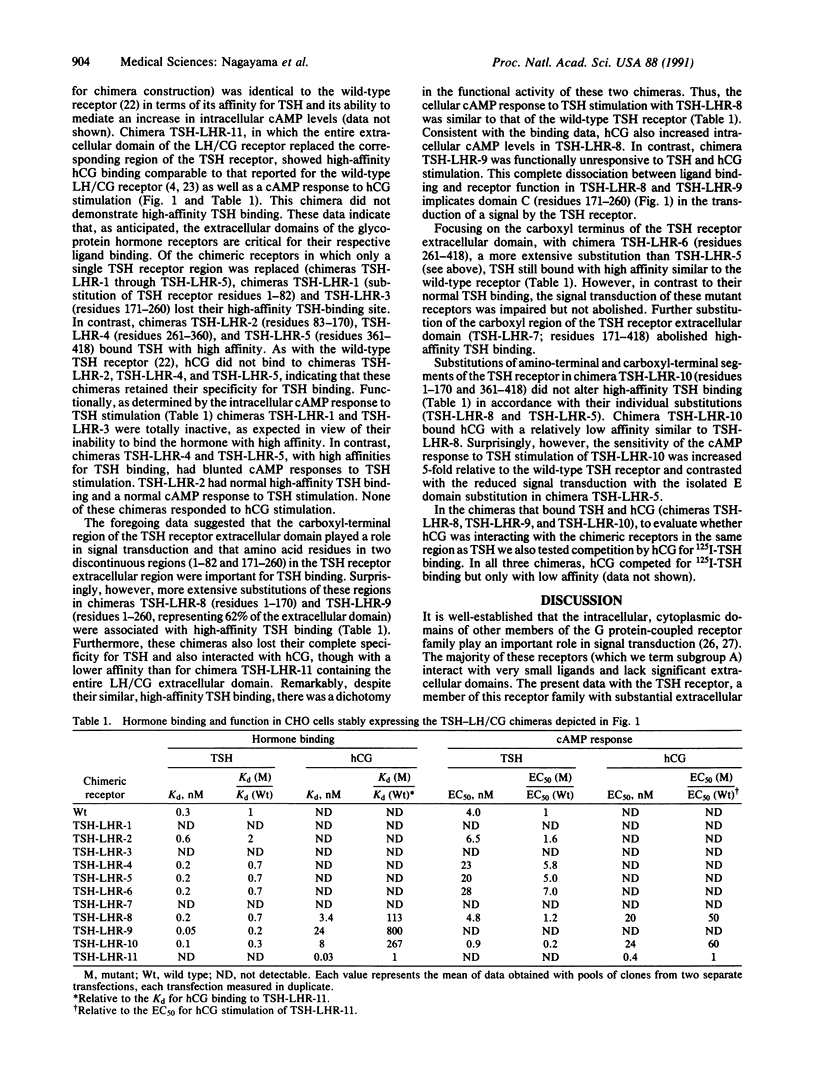
To define the sites in the extracellular domain of the human thyrotropin (TSH) receptor that are involved in TSH binding and signal transduction we constructed chimeric thyrotropin-luteinizing hormone/chorionic gonadotropin (TSH-LH/CG) receptors. The extracellular domain of the human TSH receptor was divided into five regions that were replaced, either singly or in various combinations, with homologous regions of the rat LH/CG receptor. The chimeric receptors were stably expressed in Chinese hamster ovary cells. The data obtained suggest that the carboxyl region of the extracellular domain (amino acid residues 261-418) and particularly the middle region (residues 171-260) play a role in signal transduction. The possibility is also raised of an interaction between the amino and carboxyl regions of the extracellular domain in the process of signal transduction. With respect to hormone binding, substitution of the entire extracellular domain of the LH/CG receptor for the corresponding region of the TSH receptor resulted in high-affinity human CG binding with complete loss of TSH binding. Surprisingly, however, there was at least one chimera with a substitution at each of the five domains that still retained high-affinity TSH binding. Substitution of residues 1-170 of the TSH receptor with the corresponding region of the LH/CG receptor was associated with the retention of high-affinity TSH binding but ligand specificity was lost in that TSH and human CG could interact functionally with the receptor. In summary, these studies suggest that the middle region and carboxyl half of the extracellular domain of the TSH receptor are involved in signal transduction and that the TSH-binding region is likely to span the entire extracellular domain, with multiple discontinuous contact sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buettner K., Ascoli M. Na+ modulates the affinity of the lutropin/choriogonadotropin receptor. J Biol Chem. 1984 Dec 25;259(24):15078–15084. [PubMed] [Google Scholar]
- Chazenbalk G. D., Nagayama Y., Kaufman K. D., Rapoport B. The functional expression of recombinant human thyrotropin receptors in nonthyroidal eukaryotic cells provides evidence that homologous desensitization to thyrotropin stimulation requires a cell-specific factor. Endocrinology. 1990 Sep;127(3):1240–1244. doi: 10.1210/endo-127-3-1240. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. C., Henner D. J., Wells J. A. Engineering human prolactin to bind to the human growth hormone receptor. Science. 1990 Mar 23;247(4949 Pt 1):1461–1465. doi: 10.1126/science.247.4949.1461. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Jhurani P., Ng P., Wells J. A. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989 Mar 10;243(4896):1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Ferrero E., Hsieh C. L., Francke U., Goyert S. M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990 Jul 1;145(1):331–336. [PubMed] [Google Scholar]
- Finke R., Seto P., Rapoport B. Evidence for the highly conformational nature of the epitope(s) on human thyroid peroxidase that are recognized by sera from patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1990 Jul;71(1):53–59. doi: 10.1210/jcem-71-1-53. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Amir S. M., Petersen A. W., Ingbar S. H. Preparation of biologically active 125I-TSH. Endocrinology. 1974 Nov;95(5):1228–1233. doi: 10.1210/endo-95-5-1228. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hirayu H., Magnusson R. P., Rapoport B. Studies on the mechanism of desensitization of the cyclic AMP response to TSH stimulation in a cloned rat thyroid cell line. Mol Cell Endocrinol. 1985 Aug;42(1):21–27. doi: 10.1016/0303-7207(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Libert F., Lefort A., Gerard C., Parmentier M., Perret J., Ludgate M., Dumont J. E., Vassart G. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1250–1255. doi: 10.1016/0006-291x(89)92736-8. [DOI] [PubMed] [Google Scholar]
- Loosfelt H., Misrahi M., Atger M., Salesse R., Vu Hai-Luu Thi M. T., Jolivet A., Guiochon-Mantel A., Sar S., Jallal B., Garnier J. Cloning and sequencing of porcine LH-hCG receptor cDNA: variants lacking transmembrane domain. Science. 1989 Aug 4;245(4917):525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K. C., Sprengel R., Phillips H. S., Köhler M., Rosemblit N., Nikolics K., Segaloff D. L., Seeburg P. H. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989 Aug 4;245(4917):494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- Misrahi M., Loosfelt H., Atger M., Sar S., Guiochon-Mantel A., Milgrom E. Cloning, sequencing and expression of human TSH receptor. Biochem Biophys Res Commun. 1990 Jan 15;166(1):394–403. doi: 10.1016/0006-291x(90)91958-u. [DOI] [PubMed] [Google Scholar]
- Nagayama Y., Kaufman K. D., Seto P., Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1184–1190. doi: 10.1016/0006-291x(89)92727-7. [DOI] [PubMed] [Google Scholar]
- Parmentier M., Libert F., Maenhaut C., Lefort A., Gérard C., Perret J., Van Sande J., Dumont J. E., Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989 Dec 22;246(4937):1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Braun T., Nikolics K., Segaloff D. L., Seeburg P. H. The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol. 1990 Apr;4(4):525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth H. L., Chazenbalk G. D., Nagayama Y., Russo D., Rapoport B. An insertion in the human thyrotropin receptor critical for high affinity hormone binding. Science. 1990 Sep 21;249(4975):1423–1425. doi: 10.1126/science.2169649. [DOI] [PubMed] [Google Scholar]



