Abstract
Stroke is a leading cause of death worldwide and continues to be one of the major causes of long-term adult disabilities. About 87% of strokes are ischemic in origin and occur in the territory of the middle cerebral artery (MCA). Currently the only Food and Drug Administration (FDA) approved drug for the treatment of this devastating disease is tissue plasminogen activator (tPA). However, tPA has a small therapeutic window for administration (3 - 6 hr), and is only effective in 4% of the patients who actually receive it. Current research focuses on understanding the pathophysiology of stroke in order to find potential therapeutic targets. Thus, reliable models are crucial, and the MCA occlusion (MCAo) model (also termed the intraluminal filament or suture model) is deemed to be the most clinically relevant surgical model of ischemic stroke, and is fairly non-invasive and easily reproducible. Typically the MCAo model is used with rodents, especially with mice due to all the genetic variations available for this species. Here we describe (and present in the video) how to successfully perform the MCAo model (with reperfusion) in mice to generate reliable and reproducible data.
Keywords: Medicine, Issue 116, stroke, mouse, transient ischemia reperfusion, middle cerebral artery occlusion, intraluminal filament, intraluminal suture
Introduction
Stroke is the fifth leading cause of death worldwide, with one person dying from the disease every 4 minutes. Over 800,000 Americans suffer a stroke every year, which is not only devastating for the patient, but also for their families. Stroke is the main cause of adult disability and the annual expenditure is estimated to be in the order of $ 36.5 billion1 despite very few treatment options being available.
Tissue plasminogen activator (tPA) is the only Food and Drug Administration (FDA) licensed drug for ischemic stroke. However, it is only effective if administered to patients within 3-6 hours from the onset of the stroke, and in these cases it benefits only 4% of patients2. Therefore, it is imperative that reproducible, clinically relevant animal models of stroke are used to aid in the development of potential therapeutic strategies and treatments for this disease. It is important to note that in vitro models, whilst useful in modeling certain aspects of cerebral dysfunction, are not capable of recapitulating the complex physiological interactions that occur in brain and periphery following a stroke. Consequently, in vivo models are essential.
The most common type of stroke is ischemic in origin, accounting for 87% of total strokes. Other strokes are intracerebral hemorrhage (9%) and subarachnoid hemorrhage (4%), and are caused most often by an emboli to the middle cerebral artery (MCA). This is attributable to the prominent curve at the root of MCA, which causes laminar blood flow entering the brain to become disrupted. The MCA arises from the internal carotid artery (ICA) and routes along the lateral sulcus, where it branches and projects to the basal ganglia and the lateral surfaces of the frontal, parietal and temporal lobes, including the primary motor and sensory cortex. The Circle of Willis is created by posterior cerebral arteries being connected to the cerebral arteries and the posterior communicating arteries.
The intraluminal filament or suture model of MCAo is one of the most widely used in stroke research. However, there are a couple of different variations to this model, and these are based on whether the microfilament is inserted into the external carotid artery (ECA, termed the Longa method)3, or whether it is inserted into the ICA (termed the Koizumi method)4. In Koizumi's method, the common carotid artery (CCA) on the side of the surgery must be permanently tied if the filament is removed to prevent bleeding from the incision in the CCA, while in Longa's method it is the ECA that must be permanently tied5. Here the Longa method will be used as we feel this is a far superior and a more clinically relevant surgical model of ischemic stroke. Furthermore, the use of a silicon-tipped monofilament, especially with the Longa method, produces very reproducible MCAo as opposed to the flame-blunted monofilaments, which often produce incomplete occlusion and/or subarachnoid hemorrhage6.
The intraluminal filament method can be used as a model of permanent or transient occlusion4,6. To perform the transient model, the filament is removed after a period of ischemia (e.g., 30 min, 60 min, or 2 hr), and reperfusion is allowed to happen. This model, to some extent, simulates the restoration of blood flow after spontaneous or therapeutic intervention (e.g., tPA administration) to lyse a thromboembolic clot in humans. For the permanent model, the filament is simply left in place for a period of time (e.g., 24 hr), so no reperfusion occurs. Another advantage of the intraluminal filament method is the fact that a craniotomy does not need to be performed, allowing the skull to be left intact and avoiding any changes in intracranial pressure and temperature.
In this video we demonstrate how to perform the Longa intraluminal filament method to induce MCAo and reperfusion. We also show how to perform the 18-point neurological score and determine the infarct volume using 2,3,5-triphenyltetrazalium chloride (TTC) staining.
Protocol
This protocol and the experiments reported in the video were approved by the LSUHSC-S Institutional Animal Care and Use Committee and are in compliance with the guidelines of NIH.
NOTE: Male C57BL/6 mice weighing 25 - 29 g were used in this study. The mice were maintained on a standard chow pellet diet with free access to water, under a 12 hr light/dark cycle in individually ventilated cages. The procedure will be performed under sterile conditions using sterile techniques (e.g., sterile gloves, sterile instruments).
1. Pre-surgical Preparations
Induce anesthesia using a combination of ketamine (150 mg/kg) and xylazine (10 mg/kg) injected intraperitoneally (i.p.). Monitor depth of anesthesia by foot pinch initially every 3 - 5 min and every 10 min once anesthesia is achieved. While the animal is under anesthesia, administer a sterile ocular ointment to prevent dryness. The initial dose of ketamine/xylazine usually lasts about 30-40 minutes. An additional dose can be administered if needed, which is verified by the animal’s response to a foot pinch.
Place mice in a supine position on a temperature regulated heat mat and maintain body temperature at 36.5 ± 0.1 °C, which is verified using a rectal probe.
Shave the neck and disinfect the skin with 70% ethyl alcohol.
2. Occlusion of the MCA (Figure 1)
Make a midline neck incision using Iris straight scissors and retract (using retractors) the soft tissues to expose the vessels.
Dissect the CCA and the ECA from surrounding tissue using Dumont forceps without damaging the vagus nerve.
Make a temporary suture by tying a loose knot around the CCA using 6-0 silk.
Make a permanent suture around the ECA and the smaller vessels extending from it by tightly ligating the vessels (distal to the bifurcation of the CCA).
Make a suture around the ECA proximal to the CCA bifurcation.
Place a microvessel clip around the internal carotid artery (ICA) and the pterygopalatine artery (PPA).
Make a small incision in the ECA using micro dissecting spring scissors and insert a 180 μm silicon-tipped monofilament. Make the cut as close to the permanent suture as possible for easier manipulation of the filament.
Tighten the temporary suture around the ECA with the filament inserted and remove the microvessel clip.
Cut the ECA between the permanent, distal suture and the entry point of the filament using micro dissecting spring scissors.
Guide the filament through the ICA until resistance is felt (approx. 9 - 10 μm beyond the bifurcation of the CCA) in the MCA. NOTE: In the event too much resistance is felt while the majority of the filament is still visible, the filament may have entered the PPA. If this happens, pull the filament back to the bifurcation, gently push forward into the ICA using Dumont forceps, and advance the filament until it can be visualized in the ICA.
3. Reperfusion
After a 30-min occlusion period, remove the filament by gently pulling it back using Dumont forceps and secure the suture around the open end of the ECA.
Remove the temporary suture around the CCA by carefully loosening the ligature using Dumont forceps and blood flow is resumed through the CCA.
Close the incision with a continuous surgical suture. Closure of the skin can be accomplished by either continuous or interrupted sutures. Skin staples are also an acceptable method.
Inject mice with 1 ml of saline subcutaneously as volume replenishment and with the analgesic carprofen (5 mg/Kg, s.c.) for relief of pain and discomfort from the surgical procedure, Signs that indicate additional pain relief is needed include hunched back, ungroomed coat, decreased activity, abnormal posturing, and decreased appetite.
Observe mice throughout the recovery from the anesthesia in a heated 30 °C cage using a heat lamp regulated using a temperature controller and place mashed chow in a petri dish on the floor of the cage to encourage eating. The mice will be housed one per cage during the reperfusion period.
4. Sham Surgery
Subject mice to the same procedure without the monofilament insertion.
5. Post-operative Neurological Scores (Table 1; Figure 2)
Neurologically evaluate mice after the appropriate reperfusion period using an 18-point scoring system assessing the General; Motor; Sensory; Proprioception. A higher neurological score corresponds to decreased neurological function. NOTE: Mice that are judged unresponsive and unable to walk will be euthanized. Other criteria for humane euthanasia will include a weight loss of greater than 20%, respiratory distress, and infection around the surgical area. A CO2 chamber will be used for euthanasia and the physical method to confirm death will be cervical dislocation or thoracotomy.
6. Measurement of Brain Infarct Volume (Figure 3)
Induce anesthesia using a combination of ketamine (150 mg/kg) and xylazine (10 mg/kg) injected i.p. Monitor depth of anesthesia by foot pinch initially every 3 - 5 min and every 10 min once anesthesia is achieved.
Place mice in a supine position and cut the skin from the abdomen to the neck followed by the peritoneum using iris straight scissors.
Lift up the sternum using Dumont forceps and cut the ribs to expose the heart.
Open the left and the right side of the chest using two pairs of hemostats, exposing the heart.
Insert a 26 G needle attached to a 5 ml syringe into the left ventricle and cut the right atrium. Perfuse with room temperature normal saline or phosphate buffered saline (PBS) until the fluid becomes clear (usually 3 - 5 min).
Remove the skull carefully away from the brain using iris straight scissors and Dumont forceps.
Cut off the olfactory bulbs and the cerebellum using a razor blade so that the brain will fit in the matrix. Use this matrix to slice the brain into even segments. Chill the matrix before use to keep the tissue cool. Place the brain into the matrix and set it on ice.
Slice the brain into 2 mm coronal segments using two razor blades. Make the first cut using a razor blade beginning 2 mm from the top and leave this razor blade in place. Make another 2 mm cut behind the first. Remove the first razor blade with the tissue attached. Repeat this process until all tissue has been sliced. NOTE: There should be 4 - 5 segments when finished.
Place the segments into 24-well plate containing 2% 2,3,5-triphenyltetrazalium chloride (TTC), which is then placed in a shallow water bath at 37 °C for 20 min. Placing each segment into an individual well helps to keep them in the order in which they were cut. Ensure that the TTC solution completely covers the tissue segments. After 10 min turn all the slices over.
Place a small amount of 10% formalin into the wells of a new 24-well plate and transfer the segments to this plate in the order in which they were cut.
- Scan the segments into the computer and analyze the infarct size as a percentage of the whole brain slice6 using ImageJ analysis software (NIH 1.57 Image Software)6.
- Outline the infarcted area to generate an area measurement. Next measure the entire contralateral hemisphere to determine the area. Divide the infarct area by the area of the contralateral hemisphere and multiply by 100 to determine the infarct volume.
Representative Results
Mice underwent 30-min MCAo-induced brain ischemia (Figure 1) followed by a period of reperfusion (24 hr and 1 wk are presented here, but the length of reperfusion can be varied). The mortality during MCAo was minimal (approximately 2%). Post ischemia, the mortality rate (within the first 24 hr) was around 26%.
Laser Doppler flowmetry was used to confirm blood flow perfusion in the MCA territory before and after MCAo/reperfusion. Figure 4 demonstrates clearly that when the temporary CCA tie is released after 30 min of ischemia for filament removal and reperfusion to occur, there is a surge in perfusion, which reached < 100% of baseline perfusion (i.e., prior to surgery) 5 min into reperfusion.
24 hr after stroke onset (prior to measurement of infarct volume) neurological scores pertaining to general, sensory, motor and proprioceptive deficits were assessed (Table 1). This scoring system is intended to provide objective ('yes or no') criteria for assessment. Figure 2 shows that the score for mice (n= 27) at 24 hr was 12.56 ± 0.7, and remained high 1 week later (11.50 ± 1.5). A higher neurological score corresponds to decreased neurological function.
Infarct volumes mirrored similar patterns to the neurological score at 24 hr, i.e., greater than sham animals. 24 hr after MCAo, mice had large infarct volumes (Figure 3: 15.9 ± 2.6%), which were heightened 1 week post stroke (28.5 ± 1.9%).
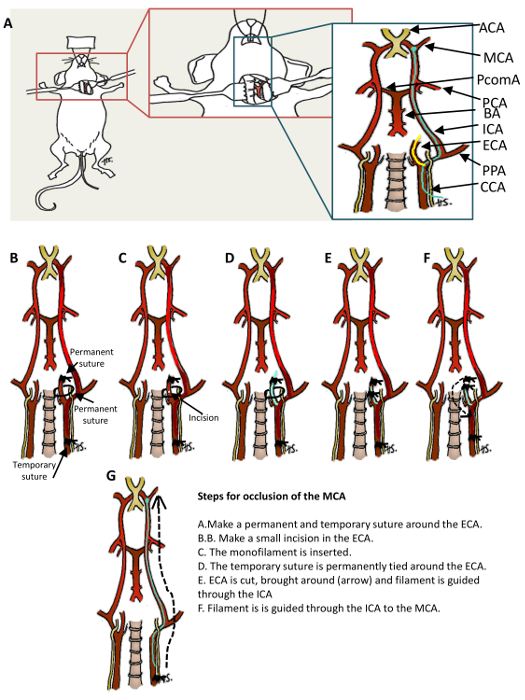 Figure 1: Schematic Showing the Location of Middle Cerebral Artery Occlusion (MCAo) Surgery and Large-vessel Vasculature of the Cerebral Circulation.A. Surgical MCAo is achieved via insertion of a filament into the external carotid artery and then into the proximal middle carotid artery. B-G. Steps for inducing MCAo. Anterior communication artery (ACA); middle cerebral artery (MCA); posterior communication artery (PcomA); posterior cerebral artery (PCA); basilar artery (BA); internal carotid artery (ICA); external carotid artery (ECA); pterygopalatine artery (PPA); internal carotid artery (ICA). (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
Figure 1: Schematic Showing the Location of Middle Cerebral Artery Occlusion (MCAo) Surgery and Large-vessel Vasculature of the Cerebral Circulation.A. Surgical MCAo is achieved via insertion of a filament into the external carotid artery and then into the proximal middle carotid artery. B-G. Steps for inducing MCAo. Anterior communication artery (ACA); middle cerebral artery (MCA); posterior communication artery (PcomA); posterior cerebral artery (PCA); basilar artery (BA); internal carotid artery (ICA); external carotid artery (ECA); pterygopalatine artery (PPA); internal carotid artery (ICA). (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
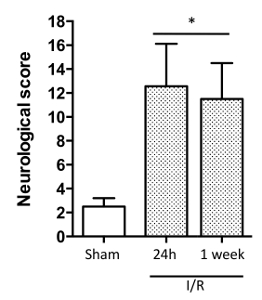 Figure 2: The 18-point Stroke Score. This comprehensive stroke score assesses functional improvements in general, and sensory, motor and proprioceptive aspects of mouse behavior following a stroke. Data are mean ± SEM. *P < 0.05. n = 3 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
Figure 2: The 18-point Stroke Score. This comprehensive stroke score assesses functional improvements in general, and sensory, motor and proprioceptive aspects of mouse behavior following a stroke. Data are mean ± SEM. *P < 0.05. n = 3 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
 Figure 3: Infarct Volumes of C57BL/6 mice 24 hr after MCAo. Mice were subjected to 30-min MCAo and 24 hr or 1 wk of reperfusion. A) Brains were removed, sectioned and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC). In living tissue, dehydrogenases enzymatically reduce TTC to 1,3,5-triphenylformazaon (TPF), which is a red color, but in ischemic tissue the enzyme is not functional, so the tissue remains white. B) The graph shows an increase in infarct volume in mice having had a stroke. Data are mean ± SEM. **P < 0.002, ****P < 0.0001 versus sham; n = 4 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. Scale bar = 1 cm (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
Figure 3: Infarct Volumes of C57BL/6 mice 24 hr after MCAo. Mice were subjected to 30-min MCAo and 24 hr or 1 wk of reperfusion. A) Brains were removed, sectioned and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC). In living tissue, dehydrogenases enzymatically reduce TTC to 1,3,5-triphenylformazaon (TPF), which is a red color, but in ischemic tissue the enzyme is not functional, so the tissue remains white. B) The graph shows an increase in infarct volume in mice having had a stroke. Data are mean ± SEM. **P < 0.002, ****P < 0.0001 versus sham; n = 4 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. Scale bar = 1 cm (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
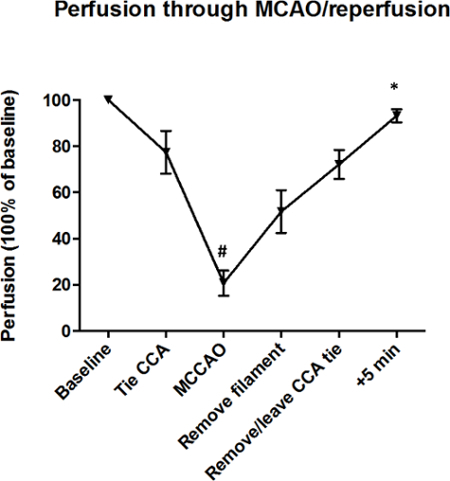 Figure 4: Perfusion of MCA Territory Throughout MCAo and Reperfusion. Mice were subjected to 30-min MCAo, and < 5 min reperfusion. Baselines were normalized to 100%. As expected, perfusion 5 min into the reperfusion period was higher than MCAo, i.e., when no perfusion of the tissue occurred. Data are mean ± SEM. *P < 0.05. n = 3 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
Figure 4: Perfusion of MCA Territory Throughout MCAo and Reperfusion. Mice were subjected to 30-min MCAo, and < 5 min reperfusion. Baselines were normalized to 100%. As expected, perfusion 5 min into the reperfusion period was higher than MCAo, i.e., when no perfusion of the tissue occurred. Data are mean ± SEM. *P < 0.05. n = 3 mice/group. The data was analyzed using ANOVA plus a Bonferroni post hoc test. (Modified with permission from Smith et al., reference #5.) Please click here to view a larger version of this figure.
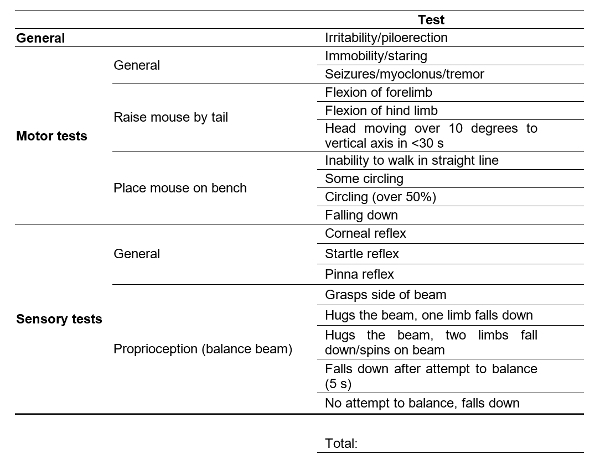 Table 1: Neurological Scoring System. One point given for a yes answer to each of the tests. Marks out of 18 7,8.
Table 1: Neurological Scoring System. One point given for a yes answer to each of the tests. Marks out of 18 7,8.
| Test | Brief Description | What does it test for? | References |
| Morris water maze | An open-field water-maze procedure where rodents learn to escape from water onto a hidden platform | Spatial memory, movement control, and cognitive mapping | 19 |
| Rotarod | A horizontal rotating rod where rodents must walk forward so as not to fall off | Motor coordination, balance, and grip strength | 20 |
| Pole | Rodents placed on a pole and observed for sliding or falling as they make their way down into a cage | Movement disorders caused by cortical damage | 21, 22 |
| Gait analysis | As rodents cross a glass plate their paw prints are captured to examine their movements | Walking patterns (paw pressure, stride length, width and frequency, toe spread, gait angle, and body rotation) and motor coordination | 23, 24 |
| Sticky label test | Strips of tape applied to the hairless part of forepaw of the rodents to record time to contact each paw, order of contact, time of removal, and order of removal | Forepaw sensitivity and sensorimotor deficits | 25, 26 |
| Corner | Rodents placed between two boards forming a 30° angle; when entering deep into the corner both sides of the vibrissae are stimulated and the rodent rears and turns back to face the open end | Neurodevelopmental disorders and repetitive behaviors (monitoring turns in one direction versus the opposite direction) | 27, 28 |
| Cylinder | Rodents are placed in a glass cylinder with the forelimb activity while rearing against of the wall is recorded | Locomotor asymmetry and evaluate poststroke limb use | 28 |
| Staircase | Rodents placed on a platform in a box with a baited double staircase; rodents are food restricted to promote movement up the stairs to collect the bait | Reaching abilities for each forelimb independently requiring sensory capacities, dexterity, and motor coordination | 25, 29 |
| Ladder | Rodents walk across a horizontal ladder to reach their cage; foot slips while walking across the ladder are recorded | Foot faults during locomotion; forelimb and hindlimb function and coordination | 30 |
Table 2: Examples of Murine Behavioral Tests.
| Suggestion for GLP is as follows: |
| - Specific details of which model of stroke has been chosen (e.g., Longa vs. Koizumi), and the strain, age, sex, weight reported clearly. |
| - Studies performed in a double-blinded fashion. |
| - Power analysis reported. |
| - Criteria for inclusion and exclusion in the study decided prior to the study, and reported. |
| - Animals monitored daily (basic appearance, body weight and behavior) for any signs of distress, pain or sickness. |
| - Reporting of animals excluded from the study. |
| - Randomization of animals into groups. |
| - Reporting of negative and positive results. |
| - Reporting of length of surgery, anesthetic, body temperature and blood gases. |
| - Reporting of environmental enrichment used, if any. |
Table 3: Suggestions for Good Laboratory practice.
Discussion
Since its conception 20 years ago, the MCAo model for human stroke involving insertion of a filament has been used in a huge number of studies. This is mainly due to the fact that it mimics what happens clinically in the most common form of stroke (i.e., ischemic stroke). The striatum is more sensitive to ischemia than the cerebral cortex, and as such, the length of ischemic time will translate into whether both the striatum and the dorsolateral cortex will be affected, or just the striatum. Both infarct and reperfusion times can be varied accordingly, and this offers the researcher the ability to be able to study pathological effects associated with transient ischemic attacks (TIAs) to much larger infarcts.
Over the years modifications and troubleshooting of the filament method of MCAo has shown the importance of the procedure being performed in sterile conditions to avoid the risk of infection9. Results may vary between rodent species, strain (anatomical variations have been shown)10,11, weight, and anesthetics used, but the trends in the results presented here are likely to be reflected in other laboratories where MCAo is performed. Other critical steps to take into account are body temperature, blood pressure and blood gases. It is well established that body temperature affects neurological damage, with smaller lesions associated with hypothermia12 and more severe deficits being presented in hyperthermia13. As such, temperature should be measured and controlled, for example, via the use of animal temperature controllers with a heat pad, so as to maintain body temperature at 36.5 °C. Volume replenishment and the encouragement of eating by placing soften chow at the bottom of the cage are also additional factors to observe. Blood pressure and blood gases6,14 can both easily be measured and monitored using readily available equipment from commercial suppliers.
Limitations of the MCAo technique include properly dissecting the vagus nerve from the arteries without damage and avoiding advancing the filament into the PPA, which can affect not only the ability to induce stroke effectively, but also the survival rate of the mouse. The most important significance with respect to alternative in vivo MCAo methods is the survival rate when using the Longa method described here. We have also found that this corresponds to increased leukocyte-endothelial cell interactions and increased infarct volumes, which make this model, once mastered, an excellent tool for studying potential therapeutic targets for the treatment of stroke.
Although we have not reported any here, a number of behavioral tests can also be performed such as Morris water maze, Rotarod, pole test, gait analysis, sticky label test, corner test, cylinder test, staircase test, and ladder test (see Table 2 for details and references). Sham animals have no problems with completing these behavioral tests; however, stroke animals perform these tests much less successfully. Following on from this, one area of debate is whether environmental enrichment affects not only the reproducibility of the MCAo model, but also whether it will affect the outcome of behavioral tests15. This is unclear, but it is worth standardizing this within the laboratory, and throughout the study.
There are a plethora of different tests available for measuring stroke outcome. We have presented the employment of laser doppler for blood flow measurements, an in-depth 18-point neurological assessment score, and also infarct volume measurements. However, additional options are also useful, such as imaging. We routinely employ the use of intravital fluorescence microscopy to study cellular interactions (characteristic of an inflammatory response)6 within the cerebral microcirculation in real-time, in anesthetized animals5,6,16. MCAo and reperfusion produces a heightened cerebral inflammatory response versus sham animals5,6,16. Other imaging modalities such as magnetic resonance imaging17 and positron emission tomography18 can also be used, as they provide the opportunity for longitudinal studies that are clinically relevant.
Histological analysis of brain tissue, plasma and serum samples should be routinely performed as they allow further characterization of the pathophysiological responses to stroke and the mechanisms involved, but also, and perhaps more importantly, they allow researchers to study the effects of compounds on the outcome of stroke, thus providing important data for potential therapeutic targets for this debilitating and devastating disease. Finally, we believe it is highly advisable for researchers to consider not only the most appropriate stroke model based on the requirements for their study, but also that GLP ("Good Laboratory practice") is made compulsory. See Table 3 for GLP suggestions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by the National Institute of Health, the National Heart Lung and Blood Institute (NIH and NHLBI; HL125572-01A1) and the LSUHSC-S start up fund to F.N.E. Gavins.
References
- Go AS, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MP, et al. Patients with acute stroke trated with intravenous tPS 3-6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249(2):614–623. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema, I: a new experimnetal model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986. pp. 1–8.
- Smith HK, Russell JM, Granger DN, Gavins FNE. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J Neurosci Meth. 2015;249:99–105. doi: 10.1016/j.jneumeth.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21(8):1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Atorvastain induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20(9):1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Liesz A, et al. The spectrum of systemic immune alterations after murine focal ischemia; the immunodepression versus immunomodulation. Stroke. 2009;40(8):2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- Beckmann N. High resolution magnetic resonance angiography non-invasively reveals mouse strain differences in the cerebrovascular anatomy in vivo. Magn Reson Med. 2000;44(2):252–258. doi: 10.1002/1522-2594(200008)44:2<252::aid-mrm12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13(4):683–692. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- Burk J, Burggraf D, Vosko M, Dichgans M, Hamann GF. Protection of cerebral microvasculature after moderate hypothermia following experimental focal cerebral ischemia in mice. Brain Res. 2008. pp. 248–255. [DOI] [PubMed]
- Noor R, Wang CX, Shuaib A. Effects of hyperthemia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett. 2003;349(2):130–132. doi: 10.1016/s0304-3940(03)00802-4. [DOI] [PubMed] [Google Scholar]
- Shin HK, et al. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39(5):1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Würbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods. 2009;6(4):257–261. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- Holloway PM, et al. Both MC1 and MC3 receptors provide protection from cerebral ischemia-reperfusion-induced neutrophil recruitment. Arterioscler Thromb Vasc Biol. 2015;35 doi: 10.1161/ATVBAHA.115.305348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte C, et al. Characterization of the inflammatory response in a photothrombotic stroke model by MRI: implications for stem cell transplantation. Mol Imaging Biol. 2010;13(4):663–671. doi: 10.1007/s11307-010-0395-9. [DOI] [PubMed] [Google Scholar]
- Iwae Y, et al. Glial cell-mediated deterioration and repair of the nervous system after traumatic brain injury in a rat model as assessed by positron emission tomography. J Neurotrauma. 2010;27(8):1463–1475. doi: 10.1089/neu.2009.1196. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mouzon B, et al. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma. 2012;29(18):2761–2773. doi: 10.1089/neu.2012.2498. [DOI] [PubMed] [Google Scholar]
- Fleming S, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human α-synuclein. J Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RKW, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res. 2001;125(1-2):109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Toon L, Silva M, D'Hooge R, Aerts JM, Berckmans D. Automated gait analysis in the open-field test for laboratory mice. Behav Res Methods. 2009;41(1):148–153. doi: 10.3758/BRM.41.1.148. [DOI] [PubMed] [Google Scholar]
- Lubjuhn J, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods. 2009;184(1):95–103. doi: 10.1016/j.jneumeth.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Bouët V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203(2):555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Freret T, et al. Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav Neurosci. 2009;123(1):224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neurosci. 2013;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Balkaya M, Kröber JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow. 2012;33:330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C, et al. Anti-nogo-a antibody infusion 24 hours after experimental stroke imporved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2(13) doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


