Abstract
Apoptosis contributes to myocardial ischemia/reperfusion (MI/R) injury, and both thioredoxin (Trx) and nitric oxide have been shown to exert antiapoptotic effects in vitro. Recent evidence suggests that this particular action of Trx requires S-nitrosation at Cys-69. The present study sought to investigate whether or not exogenously applied Trx reduces MI/R injury in vivo and to which extent this effect depends on S-nitrosation. Adult mice were subjected to 30 min of MI and treated with either vehicle or human Trx (hTrx, 2 mg/kg, i.p.) 10 min before reperfusion. Native hTrx was incorporated into myocardial tissue as shown by immunostaining, and reduced MI/R injury as evidenced by decreased terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining, DNA fragmentation, caspase-3 activity, and infarct size. When hTrx was partially S-nitrosated by preincubation with S-nitrosoglutathione, its cardioprotective effect was markedly enhanced. Treatment with hTrx significantly reduced p38 mitogen-activated protein kinase (MAPK) activity, and this effect was also potentiated by S-nitrosation. To further address the role of S-nitrosation for the overall antiapoptotic effect to Trx, the action of Escherichia coli Trx (eTrx) was investigated in the same model. Whereas eTrx inhibited MI/R-induced apoptosis to a degree similar to hTrx, S-nitrosation of this protein, which lacks Cys-69, failed to further enhance its antiapoptotic action. Collectively, our results demonstrate that systemically applied Trx is taken up by the myocardium to exert potent cardioprotective effects in vivo, offering interesting therapeutic avenues. In the case of hTrx, these effects are further potentiated by S-nitrosation, but this posttranslational modification is not essential for protection.
Keywords: reperfusion injury, apoptosis, antioxidant, posttranslational regulation
Growing evidence from animal experimental and clinical observations indicates that myocardial infarction after ischemia/reperfusion, the single-most important cause of death in the United States, is a consequence not only of necrosis but also apoptosis (1, 2). We and others have recently demonstrated that blocking the signal transduction leading to apoptosis significantly reduces myocardial infarct size and ameliorates cardiac function (3-5), confirming the notion that apoptosis contributes to myocardial ischemia/reperfusion (MI/R) and that antiapoptotic treatment options may improve clinical outcomes in patients with ischemic heart disease.
Thioredoxin (Trx) is a 12-kDa protein ubiquitously expressed in all living cells that fulfills a variety of biological functions related to cell proliferation and apoptosis (6, 7). It is characterized by the highly conserved reduction/oxidation (redox)-active site sequence Trp-Cys-Gly-Pro-Cys-Lys. The two cysteine residues within the redox-active center of Trx (Cys-32 and Cys-35) provide the sulfhydryl equivalents critical to its reducing activity and are oxidized to form a disulfide bridge. The oxidized form (Trx-S2) is reduced back to the dithiol in a NADPH-dependent manner by Trx reductase. Trx acts as a powerful antioxidant and plays an important role in maintaining critical protein thiols in the reduced state. Moreover, it has been shown to scavenge reactive oxygen species (ROS) and to protect against hydrogen peroxide (H2O2), tumor necrosis factor (TNF)-α, and cis-diamminedichloroplatinum (II) (CDDP)-induced cytotoxicity in cultured cells (8-10). In addition to its activity as an oxidoreductase, recent in vitro studies demonstrate that Trx interacts directly with, and inhibits, activity of apoptosis-regulating kinase-1 (ASK-1), a mitogen-activated protein (MAP) kinase kinase kinase that activates two proapoptotic kinases, p38 MAP kinase (MAPK) and c-Jun N-terminal kinase (JNK) (11, 12). Although there is mounting evidence suggesting that ROS triggers post-ischemic myocardial apoptosis and that p38 MAPK and JNK are critical proapoptotic molecules in ischemic/reperfused hearts, the intriguing possibility that Trx may exert antiapoptotic effects and thus reduce MI/R injury in vivo has not been investigated.
Numerous experimental results have demonstrated that low concentrations of NO produced from endothelial NO synthase (eNOS) or pharmacological concentrations of exogenous NO produced by NO donors reduce apoptosis (13, 14). Recent in vitro studies in cultured cells suggested that S-nitrosation, a posttranslational protein modification that involves the covalent attachment of an NO group to a cysteine thiol, may be involved in the antiapoptotic effect of NO. However, biochemical experiments have demonstrated that the function of different proteins can be differentially modulated by S-nitrosation. For example, S-nitrosation of caspases by NO results in their inactivation and thus inhibits apoptosis (15-17). In contrast, S-nitrosation of Ras increases its activity and results in activation of extracellular signal-regulated kinase (ERK) 1/2, an antiapoptotic MAPK (15, 18). Although it is known that human Trx contains multiple cysteines, some or all of which can be S-nitrosated (19), the functional significance of this posttranslational modification has not been evaluated in a pathologically relevant model in vivo. Therefore, the aims of the present study were to (i) determine whether systemic administration of Trx reduces myocardial reperfusion injury by reducing the degree of postischemic myocardial apoptosis; (ii) elucidate whether the antiapoptotic effects of Trx can be modified by S-nitrosation; and (iii) investigate the mechanism(s) by which S-nitrosation may modulate the function of Trx.
Methods
Experimental Protocols. The experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care. Male adult mice were anesthetized with 2% isoflurane. MI was produced by temporarily exteriorizing the heart by means of a left thoracic incision and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery (LAD). After 30 min of MI, the slipknot was released, and the myocardium was reperfused (R) for 3 h or 24 h. Ten minutes before R, mice were randomized to receive either vehicle (PBS, pH 7.5), human Trx (hTrx, 2 mg/kg), or Escherichia coli Trx (eTrx, 2 mg/kg) (20) by i.p. injection. To investigate the effect of S-nitrosation on the antiapoptotic properties of Trx, hTRX or eTRX was incubated with 10 μM S-nitrosoglutathione (GSNO) for 30 min at 37°C in the dark before in vivo administration. In orientating experiments, this particular concentration of GSNO was found to result in an S-nitroso (S-NO) content of hTrx comparable to that estimated to be present in cultured endothelial cells (19). Sham operated control mice (sham MI/R) underwent the same surgical procedures except that the suture placed under the left coronary artery was not tied. At the end of the R period, the ligature around the coronary artery was retied, and 2% Evans blue dye was injected into the left ventricular cavity. The heart was quickly excised, and the I/R cardiac tissue was isolated and processed according to the procedures described below.
Determination of Myocardial Apoptosis. Myocardial apoptosis was qualitatively analyzed by detection of DNA fragmentation (DNA ladders, n = 5-6 per group) and quantitatively analyzed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining (n = 8-10 hearts per group) and caspase-3 activity assay (n = 10-12 per group) as described (21).
Measurement of Infarct Size. Twenty-four hours after reperfusion, mice were anesthetized, and the hearts were excised. Myocardial infarct size was determined by using 2,3,5-triphenyltetrazolium chloride (TTC) staining as described (n = 12-14 per group) (22).
Quantification of S-NO Content and Detection of S-Nitrosated Trx. The S-NO content of GSNO-treated (1-1,000 μM, 37°C, 30 min) Trx was quantified by using gas phase chemiluminescence with reductive sample processing (23). Unreacted GSNO was removed according to the procedure described by Jaffrey et al. (24) or by ultrafiltration over membranes with a 5-kDa cut-off, producing virtually identical results. Protein S-nitrosation was detected by using a Nitro-Glo kit (PerkinElmer), a procedure based on the “biotin switch method” (n = 5-7 hearts per group) (24).
Immunohistochemistry. Paraformadehyde-fixed tissues were cut into semi-thin sections of 4-5 μm thickness and stained with an antibody against hTrx (Research Diagnostic, Flanders, NJ). Immunostaining was developed with a Vectastain ABC kit (Vector Laboratories).
p38 MAPK Activity Assay. p38 MAP kinase activity measurements were performed by using a p38 MAPK assay kit (Cell Signaling Technology, Beverly, MA) as described (n = 5-7 hearts per group) (22).
Statistical Analysis. All values in the text and figures are presented as means ± SEM of n independent experiments. All data (except Western blot density) were subjected to ANOVA followed by Bonferroni correction for post hoc t test. Western blot densities were analyzed with the Kruskal-Wallis test followed by Dunn's post hoc test.
Results
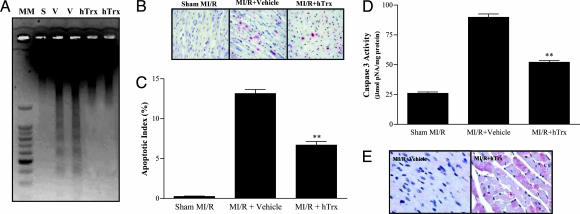
Administration of hTRX Before Reperfusion Reduces MI/R Myocardial Apoptosis. Thirty minutes of MI followed by 3 h of R resulted in significant myocardial apoptosis as evidenced by strong DNA ladder formation (Fig. 1A), a markedly increased number of TUNEL-positive cells (13.2 ± 1.25 vs. 0.24 ± 0.11 in sham MI/R, P < 0.01) (Fig. 1 B and C), and significant caspase-3 activation (3.5-fold increase over sham MI/R, P < 0.01) (Fig. 1D). Administration of hTrx 10 min before R blocked DNA fragmentation (Fig. 1 A), reduced the number of TUNEL-positive cells (6.1 ± 1.7%. P < 0.01 vs. MI/R plus Vehicle) (Figs. 1 B and C), and decreased caspase-3 activity in ischemic/reperfused cardiac tissue (Fig. 1D), providing direct evidence that hTRX has significant antiapoptotic effects after MI/R in vivo.
Fig. 1.
Effect of hTrx on postischemic myocardial apoptosis. (A) Representative photograph of electrophoretic analysis of internucleosomal DNA extracted from sham-operated control hearts (S) or mouse hearts exposed to I/R receiving either vehicle (V) or hTrx. (B) Representative photographs of TUNEL staining. Nuclei of normal cells are stained blue, and apoptotic nuclei are stained red. (C) Summary of TUNEL-positive myocytes from 8-10 mice per group. Assays were performed in a blinded fashion. (D) Summary of caspase-3 activity determined with a colorimetric assay kit. n = 10-12 per group. **, P < 0.01 vs. vehicle group. (E) Representative photographs of hTrx immunostaining confirming uptake of hTrx by cardiomyocytes.
To determine whether the observed cardioprotective effect of systemically applied Trx was due to a local action (for which it has to enter the ischemic/reperfused myocardium), cardiac tissue was examined for the presence of hTrx by using immunohistochemistry. As illustrated in Fig. 1E, no immunostaining was detected in myocardial tissue from mice treated with vehicle, indicating that the anti-human Trx antibody used in the present study does not crossreact with endogenous murine Trx. In contrast, in tissue samples from hTrx-treated animals, strong immunostaining was detected throughout the myocardium, providing direct and compelling evidence for the uptake of Trx by ischemia/reperfused cardiomyocytes. Immunostaining was apparent in both cytosolic and mitochondrial compartments and showed marked variation in intensity between individual cells. These results confirm and extend data from a recent report by using Western blot analysis demonstrating that systemically administered hTrx can enter ischemic/reperfused brain tissue (20).
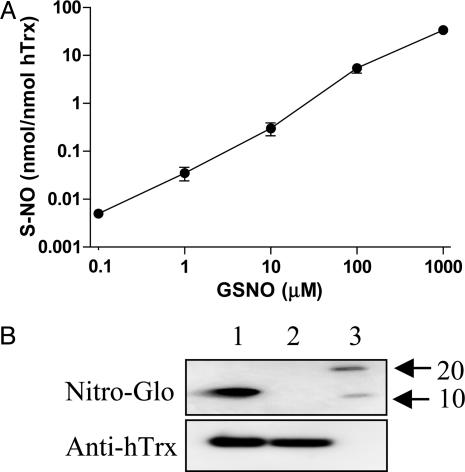
S-Nitrosation of hTrx Potentiates Its Cardioprotective Effects. Only trace amounts of bound NO (<0.1% on a molar basis) were detected in native hTrx by using gas phase chemiluminescence. However, when hTrx was incubated with GSNO its S-NO content increased in a concentration-dependent fashion (Fig. 2A) to a maximum of 2.0 ± 0.1 nmol SNO/nmol hTrx at 1 mM GSNO. With GSNO concentrations up to 100 μM, nitrosation of hTrx seemed to occur exclusively at sulfhydryl moieties as the chemiluminescence signal was completely abolished by preincubation with mercuric chloride (23) (<1% N-nitroso species were formed at 1 mM GSNO; data not shown). Incubation of hTrx with 10 μM GSNO increased its S-NO content to a level (0.30 ± 0.09 nmol/nmol Trx) comparable to the endogenous S-NO content in endothelial cells (19). This preparation of hTrx, which contains ≈6% of its available thiols (hTrx has a total of five reactive cysteines) in the nitrosated state, was thus used in all further in vivo experiments. The results from our biochemical analysis were corroborated by using a modified Western blot technique for detection of S-nitroso proteins. As illustrated in Fig. 2B, no S-nitroso epitopes were detected in native hTrx (lane 2) whereas in vitro exposure of hTrx to 10 μM GSNO resulted in significant Trx S-nitrosation (Fig. 2B). After completion of the Nitro-Glo procedure, the membrane was washed with stripping buffer and reblotted with an antibody against hTrx to ensure that comparable amounts of protein were loaded in each lane (see Fig. 2B Lower). Taken together, these results demonstrate that preincubation of hTrx with 10 μM GSNO at 37°C increased its S-NO content to a level comparable to that seen with endogenous Trx in cultured endothelial cells (19). This concentration is ≈7 times lower than that recently reported when hTrx was treated with 1 mM of GSNO (25).
Fig. 2.
S-Nitrosation of hTrx as measured by chemiluminescence and Western blotting. (A) Extent of S-nitrosation after preincubation of hTrx with increasing concentrations of GSNO as quantified by gas phase chemiluminescence (means ± SEM, n = 3, hTrx, 16.7 μM). (B) Representative Western blots using a Nitro-Glo kit (to detect S-nitrosated protein, Upper) or using an anti-hTrx antibody as primary antibody (to detect hTrx, Lower). Lanes: 1, GSNO-treated hTrx; 2, vehicle-treated hTrx; 3, molecular marker.
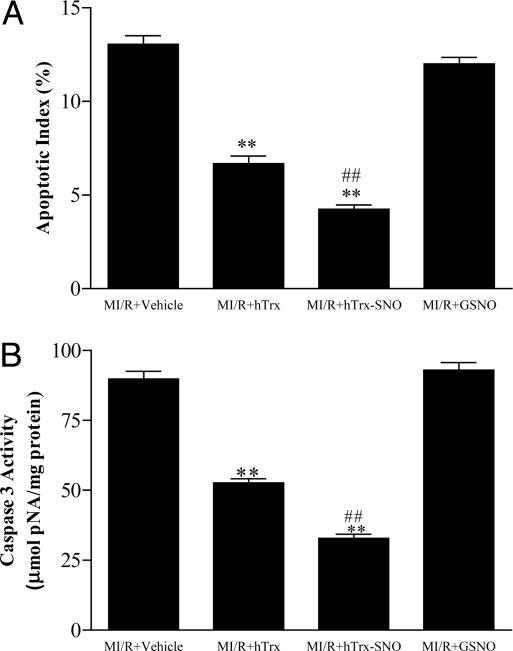
By using cultured endothelial cells, Dimmeler and colleagues (19) have demonstrated that hTrx is selectively S-nitrosated at Cys-69, but not Cys-32 or -35 when exposed to physiological concentrations of NO, and this posttranslational modification was considered essential for maintaining the redox-regulatory and antiapoptotic functions of hTrx (19). In stark contrast, another study (25) reported that treatment of Trx with 1 mM GSNO (a concentration 100 times higher then that used in the present study) markedly increased S-NO content (to ≈2 nmol/nmol protein) and resulted in activation of ASK-1 in vitro (25), suggesting that S-nitrosation of Trx results in activation rather than inhibition of apoptosis. To determine the functional consequence of hTrx S-nitrosation in vivo, we treated animals with partially S-nitrosated hTrx (hTrx-SNO) and compared its cardioprotective effects with that of native hTrx. As summarized in Fig. 3, S-nitrosation markedly enhanced the antiapoptotic effect of hTrx as measured either by TUNEL staining (Fig. 3A), or more specifically, by caspase-3 activation (Fig. 3B). To exclude the possibility that the additional antiapoptotic effect of GSNO-treated hTrx might have been due to unreacted GSNO in solution (although this was determined to be consistently <20 nM by using chemiluminescence), an additional group of animals was treated with the same amount of GSNO without Trx. For this control, a 10-μM solution of GSNO was placed in a 37°C incubator for 30 min before application, and the same volume was injected as for the GSNO-treated Trx experiments. As illustrated in Fig. 4 A and B, the treatment of animals with this low dose of GSNO failed to exert any antiapoptotic effect. This result demonstrated that it is the functional modification of hTrx by S-nitrosation, not GSNO itself, that is responsible for the enhanced antiapoptotic effect observed after exposing hTrx to GSNO.
Fig. 3.
Antiapoptotic effects of Trx and enhancement by S-nitrosation. (A) Effect of hTrx and its S-nitrosated form (hTrx-SNO) on postischemic myocardial apoptosis as assessed by TUNEL-positive myocytes. (B) Effect of hTrx and S-nitrosated hTrx on postischemic myocardial apoptosis as assessed by caspase-3 activity. **, P < 0.01 vs. vehicle group; ##, P < 0.01 vs. MI/R treated with native hTrx.
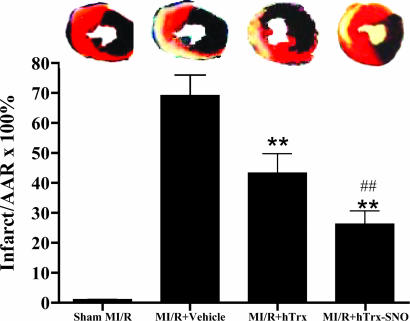
Fig. 4.
Myocardial infarct size in the different treatment groups. (Upper) Representative photomicrographs of heart sections obtained from mice subjected to sham myocardial ischemia/reperfusion (MI/R), MI/R treated with vehicle, MI/R treated with native hTrx, or S-nitrosated hTrx. Blue portion, nonischemic, normal region; red portion, ischemic/reperfused, but not infarcted region; negatively stained portion, ischemic/reperfused, infarcted region. (Lower) Bar chart of myocardial infarct size expressed as percent of total ischemic/reperfused area (area-at-risk; means ± SEM of 10-12 mice per group). **, P < 0.01 vs. vehicle-treated ischemic/reperfused hearts; ##, P < 0.01 vs. hTrx.
To determine whether the antiapoptotic effect of hTrx and its enhancement by S-nitrosation may indeed reduce ultimate myocardial infarction after a prolonged reperfusion period, animals were subjected to 30 min of ischemia, followed by 24 h of reperfusion, and treated with either vehicle, hTrx, or S-nitrosated hTrx. Compared with animals treated with vehicle, the application of hTrx significantly reduced myocardial infarct size after reperfusion. Importantly, in those animals treated with S-nitrosated hTrx, myocardial infarct size was further reduced to a level that was not only significantly less than that of vehicle-treated animals, but also less than the level seen in animals treated with native hTrx (Fig. 4). These results provide direct evidence that in vivo treatment with hTrx before reperfusion significantly reduces myocardial ischemia/reperfusion injury and that these cardioprotective effects can be further potentiated by S-nitrosation of hTrx.
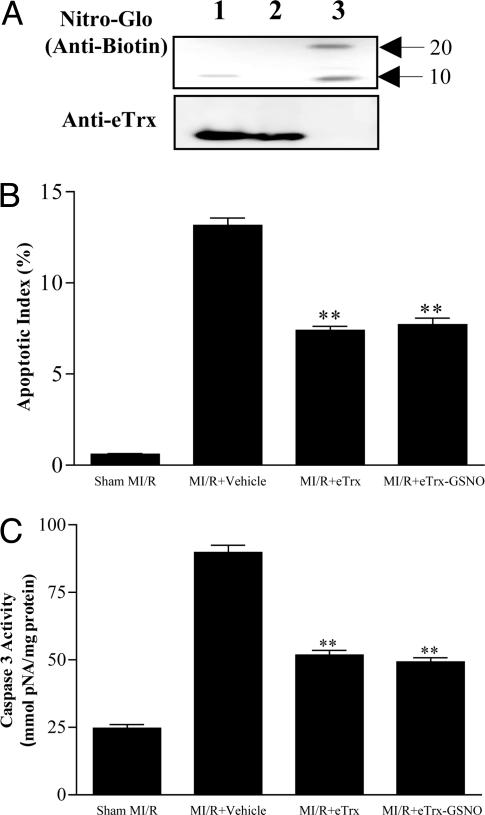
eTrx Has Antiapoptotic Effects Comparable with hTrx but Lacks Potentiation by S-Nitrosation. To further address the possible significance of S-nitrosation of Cys-69 for the overall cardioprotective effect of hTrx, we compared the action of the latter with that of a bacterial isoform isolated from E. coli (eTrx), which lacks this particular cysteine (7). In contrast to the results obtained with hTrx, only a faint SNO-related signal was detected with the Nitro-Glo assay after incubation of eTrx with 10 μM GSNO (Fig. 5A). This result indicate that the two highly conserved catalytically active cysteines, Cys-32 and -35, are either not subject to substantial S-nitrosation or that thiol modifications at these sites are readily reversible, confirming and extending earlier conclusions by Dimmeler and colleagues (19).
Fig. 5.
Incubation of eTrx with GSNO neither causes appreciable protein S-nitrosation (A) nor potentiates its antiapoptotic effect as determined by TUNEL staining (B) and caspase-3 activity (C). **, P < 0.01 vs. vehicle group. Means ± SEM of 10-12 mice per group.
In vivo administration of eTrx before reperfusion resulted in eTrx uptake by cardiomyocytes as evidenced by immunohistochemistry using a monoclonal antibody against eTrx (data not shown), which was associated with a concomitant reduction in apoptosis comparable to that seen in animals treated with hTrx (compare Fig. 3 with Fig. 5 B and C). However, preincubation of eTrx with GSNO failed to enhance its antiapoptotic effect as determined by TUNEL staining and caspase-3 activity (Fig. 5 B and C). Taken together, these results provide strong evidence that the selective S-nitrosation of Cys-69 is responsible for the observed potentiation of cardioprotective action of hTrx. However, they also demonstrate that S-nitrosation at cysteine 69 is not a prerequisite for protection by Trx.
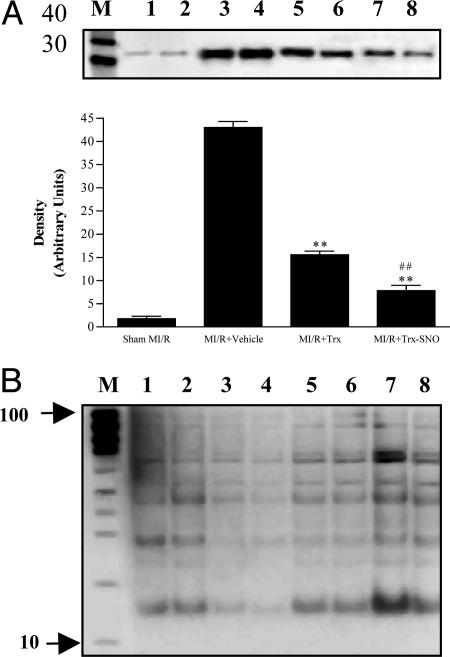
Mechanisms by Which S-Nitrosation Enhances the Antiapoptotic Effect of hTrx. Trx has been shown to exert antiapoptotic effects by means of multiple mechanisms. Among the proposed pathways identified from in vitro experiments, direct antioxidant and ASK-1 inhibitory effects are the two most likely mechanisms by which Trx may exert its antiapoptotic action in ischemia/reperfused hearts. Because p38-MAPK is activated by its upstream kinase, ASK-1, as well as by ROS, we reasoned that measurement of p38-MAPK activity would reflect both antioxidant and ASK-1 inhibitory effects of Trx and thus would be suitable to assess the modulatory effect of S-nitrosation. Consistent with previous results, ischemia/reperfusion resulted in a marked increase in p38-MAPK activity that was significantly reduced by hTrx treatment (Fig. 6A). Most notably, when animals were treated with S-nitrosated hTrx, cardiac p38-MAPK activity was further reduced to a level that was not only lower than the vehicle group, but also significantly lower than animals treated with native hTrx. These data are consistent with the notion that S-nitrosation of hTrx enhances the antioxidant/ASK-1 inhibitory effects and is responsible, at least in part, for enhancement of the cardioprotective effects of hTrx by NO/nitrosation.
Fig. 6.
Potential mechanisms responsible for the cardioprotective effects on hTrx and its modification by S-nitrosation. (A) Effect of hTrx on ischemia/reperfusion-induced p38 MAPK activation and its modification by S-nitrosation. (Upper) Representative Western blot of phosphorylated ATF-2. (Lower) Quantitative densitometry of ≥5 independent experiments. **, P < 0.01 vs. MI/R plus vehicle; ##, P < 0.01 vs. MI/R plus hTrx. (B) Representative Western blots of S-nitrosated proteins from sham MI/R (lanes 1 and 2), MI/R plus vehicle (lanes 3 and 4), MI/R plus hTrx (lanes 5 and 6), and MI/R plus hTrx-SNO (lanes 7 and 8) as detected with a Nitro-Glo kit. An identical amount of protein was loaded on each lane (20 μg per lane).
Previous studies have shown that multiple apoptotic signaling proteins (e.g., caspases and cytochrome c) can be S-nitrosated and their function modified. To determine whether S-nitrosated hTrx may give rise to S-nitrosation of other apoptosis-regulating proteins through transnitrosation reactions, we investigated cardiac tissue protein nitrosation by using the Nitro-Glo assay (identical amount of protein, i.e., 20 μg of protein was loaded on each lane). As illustrated in Fig. 6B, the level of protein S-nitrosation seemed to be significantly reduced after MI/R when compared with sham controls. Treatment with S-nitrosated hTrx markedly enhanced intensity of Nitro-Glo bands, suggesting that S-nitrosated hTrx can result in S-nitrosation of other proteins and restore depleted tissue levels after entering the cardiomyocyte. However, the identities of those endogenous proteins that are S-nitrosated by exogenously administered S-nitrosated hTrx remain to be determined.
Discussion
We have demonstrated that systemic administration of Trx in vivo leads to uptake of this protein by cardiomyocytes where it exerts significant cardioprotective effects when given before R, as evidenced by reduced myocardial apoptosis and decreased myocardial infarct size. Moreover, we have provided direct in vivo evidence that S-nitrosation, likely occurring at Cys-69, significantly enhances the cardioprotective efficacy of human Trx. We have furthermore shown that Trx exerts its protective effect, at least in part, by inhibiting p38-MAPK activation, an effect that is enhanced after S-nitrosation of human Trx. Finally, we have shown that exogenously administered S-nitrosated hTrx can function as a nitrosothiol source to replenish depleted endogenous protein S-nitrosation in the heart. Taken together, our results suggest that systemic administration of Trx during early stages of ischemic events, regardless of whether it is S-nitrosated or not, can protect myocardial tissue from reperfusion-related damage, offering a potentially attractive intervention to improve outcome and prognosis after myocardial ischemia.
The thioredoxin system, including Trx, Trx reductase, and Trx peroxidase, has been shown to play critical roles in maintaining physiologic cardiovascular function as well as protecting cells from oxidative injury under a variety of pathologic conditions. Recent studies have demonstrated that genetic inhibition of endogenous Trx in the heart increases oxidative stress and cardiac hypertrophy (26), and that acute inhibition of Trx abolishes preconditioning-induced cardiac protection (27). Increasing evidence suggests that Trx is a potent endogenous antiapoptotic molecule that exerts its antiapoptotic effects by means of multiple mechanisms. In cultured SH-SY5Y cells, Trx has been shown to block oxidative stress-induced apoptosis by means of up-regulation of Mn-SOD and Bcl-2 expression (28). However, the transcriptional up-regulation of antiapoptotic molecules is not likely to play an important role in the acute treatment model used in the present study. Substantial evidence exists that ROS play a crucial role in the pathogenesis of postischemic myocardial apoptosis, and that activation of p38-MAPK is a critical step in apoptotic signaling in the ischemic/reperfused heart. Therefore, Trx most likely exerts the antiapoptotic effect in the ischemic/reperfused heart by its antioxidant and ASK-1 inhibitory effects. To this end, we here provide direct evidence that treatment with Trx before reperfusion markedly inhibits p38-MAPK activity.
Increasing evidence suggests that S-nitrosation of cysteine thiols may constitute a major route of NO trafficking through which NO-related bioactivity is mediated. This ubiquitous posttranslational regulatory mechanism resembles in several aspects O-phosphorylation and has been shown to affect multiple signal transduction pathways, including those leading to apoptosis (29, 30). Recent biological and in vitro cell culture studies have demonstrated that most caspases can be inhibited by S-nitrosation (14). When a cell receives an apoptotic signal such as Fas-Fas ligand binding, these caspases are rapidly denitrosated and thus activated (15, 17, 31). In addition, NO has been shown to differentially regulate pro- and antiapoptotic members of the MAPK family (15). Specifically, JNK, a proapoptotic MAPK, can be directly nitrosated (at Cys-116) and its activity inhibited (32, 33). In contrast, nitrosation of Ras (at Cys-118) increases its activity and results in activation of extracellular signal-regulated kinase (ERK) 1/2, an antiapoptotic MAPK (15, 18). Most recently, Park et al. (34) reported that, in murine fibrosarcoma L929 cells, NO inhibits hydrogen peroxide-induced apoptosis through S-nitrosation of ASK-1 at Cys-869. Taken together, S-nitrosation has been generally viewed as an antiapoptotic modification of apoptotic signaling molecules, although exceptions exist (35).
Human and other mammalian Trx contain, in addition to the two catalytic site cysteines, three other thiol residues, Cys-62, -69, and -73, that are not found in bacterial Trx. Recent studies indicate that posttranslational modifications of these cysteines significantly affect Trx function. Casagrande et al. (36) reported that, under conditions of oxidative stress, Trx can react with oxidized glutathione at Cys-73 to form a mixed disulfide, resulting in glutathionylation and inhibition of enzymatic activity and function. In human umbilical vein endothelial cells (HUVECs), Trx has been shown to be selectively S-nitrosated at Cys-69 (19), and this S-nitrosation has been considered essential for the antioxidant and antiapoptotic activity of Trx. In contrast, exposing Trx to high levels of GSNO (e.g., 1 mM) may result in excessive S-nitrosation and interferes with its association with ASK-1 (25). These results suggest that different cysteines in Trx exhibit varying sensitivity for S-nitrosation and that their nitrosation status affects Trx function differently. In the present study, Trx was pretreated with GSNO at a concentration that caused <10% of its reactive thiols to become S-nitrosated. Our results clearly demonstrate that S-nitrosation of hTrx markedly increases its cardiovascular protective effects as evidenced by reduced TUNEL-positive staining, lower caspase-3 activity, and smaller infarct size when compared with native hTrx. S-nitrosation and subsequent functional modification do not seem to occur at the catalytic site cysteines (Cys-32 and -35) because pretreatment of eTrx with GSNO failed to modify its cardioprotective effect. Although the present study did not specifically address the question of which of the other cysteines (i.e., Cys-63, -69, or -73) is responsible for this potentiation, Cys-69 is a likely candidate as supported by (i) the lack of potentiation by S-nitrosation of eTrx and (ii) the fact that previous studies have demonstrated that this cysteine is located in a predicted consensus motif for S-nitrosation (19).
The mechanism(s) responsible for the enhanced antiapoptotic and cardioprotective effects of hTrx after S-nitrosation are likely complex. Nevertheless, our results suggest the involvement of at least two mechanisms: S-nitrosation at Cys-69 may promote a conformational change that, akin to phosphorylative modifications (37), allows better access of the substrate to the catalytic site. This assumption is consistent with our finding that S-nitrosation enhances the inhibitory effect of Trx on p38-MAPK activation. An even more intriguing possibility is that S-nitrosated Trx acts as a tissue-specific S-NO donor that inhibits apoptosis by delivering NO+ equivalents to other proapoptotic proteins, such as caspases. This mechanism is supported by our finding that treatment with Trx-SNO seemed to increase the steady-state concentration of S-nitrosated proteins in the ischemic/reperfused heart. Further proteomic analyses are required to determine the identities of the proteins (e.g., caspase 3) that become S-nitrosated by administration of NO-enhanced hTrx. Moreover, other possible mechanisms, including mixed disulfide formation of Trx with glutathione (GSH), formation of nitroxyl [a molecule that has recently been demonstrated to inhibit poly(ADP-ribose) polymerase (PARP)], and apoptosis (38) or inhibition of NADPH oxidase assembly/activity may additionally contribute to the enhanced cardioprotection of S-nitrosated hTrx. Whatever the mechanism(s), one of the most surprising findings of the present study is that systemic application of a protein of the size of Trx survives distribution and cellular uptake to exert protective effects in the cardiomyocyte, and that a relatively small amount of protein-bound NO can further potentiate this effect in vivo. Whether the latter is specific for Trx is unclear at present. Our finding, however, that an equivalent dose of GSNO (which likely results in the formation of SNO-albumin when administered in vivo) failed to afford cardioprotection points to a highly specific effect of protein S-nitrosation, limited to those proteins that can reach targets within the cardiomyocyte. Moreover, the enhanced cardioprotective activity of Trx seems to be unique to S-nitrosation because previous studies by other investigators have clearly demonstrated that oxidation of SH-groups by diamide or thiol alkylation by N-ethylmaleimide (NEM) inhibits, rather than enhances protein activity (39). Clearly, much more needs to be learned about Trx transport across cell membranes and its functional modification by endogenous and exogenous NO. Nevertheless, the results of the present study offer a potentially attractive approach to increasing endogenous protective mechanisms in the heart (without requiring the use of molecular biological techniques) that may ultimately translate into novel therapeutic interventions.
Acknowledgments
This study was supported by National Institutes of Health Grants HL-63828 (to X.L.M.) and HL-69029 (to M.F.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MI/R, myocardial ischemia/reperfusion; Trx, thioredoxin; hTrx, human Trx; eTrx, Escherichia coli Trx; ROS, reactive oxygen species; ASK-1, apoptosis-regulating kinase-1; GSNO, S-nitrosoglutathione; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; S-NO, S-nitroso; MAPK, mitogen-activated protein kinase.
References
- 1.Anversa, P., Cheng, W., Liu, Y., Leri, A., Redaelli, G. & Kajstura, J. (1998) Basic Res. Cardiol. 93, 8-12. [DOI] [PubMed] [Google Scholar]
- 2.Eefting, F., Rensing, B., Wigman, J., Pannekoek, W. J., Liu, W. M., Cramer, M. J., Lips, D. J. & Doevendans, P. A. (2004) Cardiovasc. Res. 61, 414-426. [DOI] [PubMed] [Google Scholar]
- 3.Misra, A., Haudek, S. B., Knuefermann, P., Vallejo, J. G., Chen, Z. J., Michael, L. H., Sivasubramanian, N., Olson, E. N., Entman, M. L. & Mann, D. L. (2003) Circulation 108, 3075-3078. [DOI] [PubMed] [Google Scholar]
- 4.Lee, P., Sata, M., Lefer, D. J., Factor, S. M., Walsh, K. & Kitsis, R. N. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, H456-H463. [DOI] [PubMed] [Google Scholar]
- 5.Gao, F., Gao, E., Yue, T. L., Ohlstein, E. H., Lopez, B. L., Christopher, T. A. & Ma, X. L. (2002) Circulation 105, 1497-1502. [DOI] [PubMed] [Google Scholar]
- 6.Yamawaki, H., Haendeler, J. & Berk, B. C. (2003) Circ. Res. 93, 1029-1033. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren, A. (1985) Annu. Rev. Biochem. 54, 237-271. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda, M., Masutani, H., Nakamura, H., Miyajima, S., Yamauchi, A., Yonehara, S., Uchida, A., Irimajiri, K., Horiuchi, A. & Yodoi, J. (1991) J. Immunol. 147, 3837-3841. [PubMed] [Google Scholar]
- 9.Takeuchi, J., Hirota, K., Itoh, T., Shinkura, R., Kitada, K., Yodoi, J., Namba, T. & Fukuda, K. (2000) Antioxid. Redox Signal. 2, 83-92. [DOI] [PubMed] [Google Scholar]
- 10.Murata, H., Ihara, Y., Nakamura, H., Yodoi, J., Sumikawa, K. & Kondo, T. (2003) J. Biol. Chem. 278, 50226-50233. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono, K. & Ichijo, H. (1998) EMBO J. 17, 2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, Y. & Min, W. (2002) Circ. Res. 90, 1259-1266. [DOI] [PubMed] [Google Scholar]
- 13.Rossig, L., Haendeler, J., Hermann, C., Malchow, P., Urbich, C., Zeiher, A. M. & Dimmeler, S. (2000) J. Biol. Chem. 275, 25502-25507. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler, S., Haendeler, J., Nehls, M. & Zeiher, A. M. (1997) J. Exp. Med. 185, 601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd, C. S. & Cadenas, E. (2002) Biol. Chem. 383, 411-423. [DOI] [PubMed] [Google Scholar]
- 16.Kim, P. K. M., Kwon, Y. G., Chung, H. T. & Kim, Y. M. (2002) Ann. N.Y. Acad. Sci. 962, 42-52. [DOI] [PubMed] [Google Scholar]
- 17.Mannick, J. B., Schonhoff, C., Papeta, N., Ghafourifar, P., Szibor, M., Fang, K. & Gaston, B. (2001) J. Cell Biol. 154, 1111-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun, H. Y., Gonzalez-Zulueta, M., Dawson, V. L. & Dawson, T. M. (1998) Proc. Natl. Acad. Sci. USA 95, 5773-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haendeler, J., Hoffmann, J., Tischler, V., Berk, B. C., Zeiher, A. M. & Dimmeler, S. (2002) Nat. Cell Biol. 4, 743-749. [DOI] [PubMed] [Google Scholar]
- 20.Hattori, I., Takagi, Y., Nakamura, H., Nozaki, K., Bai, J., Kondo, N., Sugino, T., Nishimura, M., Hashimoto, N. & Yodoi, J. (2004) Antioxid. Redox Signal. 6, 81-87. [DOI] [PubMed] [Google Scholar]
- 21.Gao, F., Gong, B., Christopher, T. A., Lopez, B. L., Karasawa, A. & Ma, X. L. (2001) Br. J. Pharmacol. 132, 869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, F., Yue, T. L., Shi, D. W., Christopher, T. A., Lopez, B. L., Ohlstein, E. H., Barone, F. C. & Ma, X. L. (2002) Cardiovasc. Res. 53, 414-422. [DOI] [PubMed] [Google Scholar]
- 23.Feelisch, M., Rassaf, T., Mnaimneh, S., Singh, N., Bryan, N. S., Jourd'heuil, D. & Kelm, M. (2002) FASEB J. 16, 1775-1785. [DOI] [PubMed] [Google Scholar]
- 24.Jaffrey, S. R. & Snyder, S. H. (June 12, 2001) Sci. STKE, 10.1126/stke.2001.86.pl1. [DOI] [PubMed]
- 25.Sumbayev, V. V. (2003) Arch. Biochem. Biophys. 415, 133-136. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto, M., Yang, G., Hong, C., Liu, J., Holle, E., Yu, X., Wagner, T., Vatner, S. F. & Sadoshima, J. (2003) J. Clin. Invest. 112, 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turoczi, T., Chang, V. W., Engelman, R. M., Maulik, N., Ho, Y. S. & Das, D. K. (2003) J. Mol. Cell. Cardiol. 35, 695-704. [DOI] [PubMed] [Google Scholar]
- 28.Andoh, T., Chock, P. B. & Chiueh, C. C. (2002) J. Biol. Chem. 277, 9655-9660. [DOI] [PubMed] [Google Scholar]
- 29.Hess, D. T., Matsumoto, A., Nudelman, R. & Stamler, J. S. (2001) Nat. Cell Biol. 3, E46-E49. [DOI] [PubMed] [Google Scholar]
- 30.Stamler, J. S., Lamas, S. & Fang, F. C. (2001) Cell 106, 675-683. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y. M., Kim, T. H., Chung, H. T., Talanian, R. V., Yin, X. M. & Billiar, T. R. (2000) Hepatology 32, 770-778. [DOI] [PubMed] [Google Scholar]
- 32.Park, H. S., Huh, S. H., Kim, M. S., Lee, S. H. & Choi, E. J. (2000) Proc. Natl. Acad. Sci. USA 97, 14382-14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall, J. P., Merithew, E. & Davis, R. J. (2000) Proc. Natl. Acad. Sci. USA 97, 14022-14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park, H. S., Yu, J. W., Cho, J. H., Kim, M. S., Huh, S. H., Ryoo, K. & Choi, E. J. (2004) J. Biol. Chem. 279, 7584-7590. [DOI] [PubMed] [Google Scholar]
- 35.Schonhoff, C. M., Gaston, B. & Mannick, J. B. (2003) J. Biol. Chem. 278, 18265-18270. [DOI] [PubMed] [Google Scholar]
- 36.Casagrande, S., Bonetto, V., Fratelli, M., Gianazza, E., Eberini, I., Massignan, T., Salmona, M., Chang, G., Holmgren, A. & Ghezzi, P. (2002) Proc. Natl. Acad. Sci. USA 99, 9745-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson, L. N., Noble, M. E. & Owen, D. J. (1996) Cell 85, 149-158. [DOI] [PubMed] [Google Scholar]
- 38.Sidorkina, O., Espey, M. G., Miranda, K. M., Wink, D. A. & Laval, J. (2003) Free Radical Biol. Med. 35, 1431-1438. [DOI] [PubMed] [Google Scholar]
- 39.Wiesel, P., Foster, L. C., Pellacani, A., Layne, M. D., Hsieh, C. M., Huggins, G. S., Strauss, P., Yet, S. F. & Perrella, M. A. (2000) J. Biol. Chem. 275, 24840-24846. [DOI] [PubMed] [Google Scholar]