Abstract
Coimmunoprecipitation of members of the phytochrome red/farred photoreceptor family from plant extracts has been used to analyze their heteromeric binding interactions. Phytochrome (phy)B or phyD apoproteins with six myc epitopes fused to their N termini are biologically active when expressed in Arabidopsis. Immunoprecipitation of either of these tagged proteins from seedling extracts coprecipitates additional type II phytochromes: six myc (myc6)-phyB coprecipitates phyC-phyE; and myc6-phyD coprecipitates phyB and phyE. No interaction of the epitope-tagged proteins with type I phyA was detected. Gel filtration chromatography shows that all five of the Arabidopsis phytochromes are present in seedlings as dimers, and that the heteromeric type II phytochrome complexes migrate at molecular masses characteristic of heterodimers. Similar levels of heterodimer formation are observed in extracts of dark-grown seedlings, where the phytochromes are cytosolic, and light-grown seedlings, where they are predominantly nuclear. These findings indicate that Arabidopsis, which until now has been thought to contain five homodimeric forms of phytochrome, in fact contains multiple species of both homodimeric and heterodimeric phytochromes. The conservation of the phytochrome family throughout angiosperms suggests that heterodimeric red/far-red receptors may be present in many flowering plants.
Phytochromes are soluble chromoprotein photoreceptors that mediate plant responses to red (R) and far-red (FR) light. The light-sensing and biological regulatory activities of phytochromes derive from their photochemical interconversion between a R-absorbing conformation (Pr) and a FR-absorbing conformation (Pfr). Absorption of photons of R converts Pr to Pfr, and absorption of photons of FR converts Pfr back to Pr. For most R/FR plant responses, the Pr form is inactive and the Pfr form is active, so that either the concentration of Pfr or the ratio of Pfr to total phytochrome correlates with the strength of the response. Phytochrome in the Pr conformation is localized to the cytosol, and photoconversion to Pfr results in translocation of the photoreceptor to the nucleus (1, 2). Several candidate phytochrome-interacting proteins have been identified, including the nuclear DNA-binding protein PIF3, the CRY2 blue-light photoreceptor, and the F-box-containing ZTL protein (3, 4). These findings suggest that phytochromes may be directly positioned at light-response gene promoters, and that they may also be directly involved in integrating signaling input from other receptor systems and the circadian clock.
Five genes encoding 120- to 130-kDa phytochrome apoproteins are present in the Arabidopsis genome, PHYA-PHYE (5, 6). Mutations in all five of these genes have been identified, and effects of loss of function for each phytochrome form on plant development and light response have been described (7-11). In addition, the levels of each of the phytochrome A-E holoproteins (phyA-phyE) and their light stabilities have been determined (12). Four of the five, phyB-phyE, are light-stable in the plant and function primarily in regulation of responses to low-fluence R and to the R/FR ratio. In contrast, phyA is rapidly degraded as Pfr and controls plant responses to very low fluence R and high-irradiance FR. PHY gene families similar to the one found in Arabidopsis have been described in many angiosperm plants (13). Comparison of the PHY genes identified in dicots with those found in monocots indicates that three phytochrome types, phyA, phyB/D/E, and phyC, are common to most flowering plants.
Early analysis of the quaternary structure of the abundant form of phytochrome purified from dark-grown oat tissue, now called phyA, showed that it is a dimer (14, 15). Since that time, the assumption has frequently been made that all phytochromes are homodimers, and that the number of molecular species of phytochrome in a plant is a direct reflection of the number of PHY genes. We present evidence here that, in Arabidopsis, the light-stable phyB-phyE phytochromes engage in a variety of heterodimeric interactions, and that the complexity of the phytochrome array is likely higher than has previously been recognized.
Materials and Methods
Plant Materials and Growth Conditions. Arabidopsis thaliana ecotype Nossen (No-0) was used throughout. Extracts for immunoprecipitation (IP) were prepared from seedlings grown on agar medium for 7 days under continuous white light or for 5 days in the dark, as described (12). For hypocotyl length measurements, seedlings were grown on agar medium containing 0.3% sucrose for 5 days under R light as described (12). Hypocotyl lengths of 20-30 seedlings per line were measured under a dissecting microscope. Transformations were performed either by root transformation (16) or by the floral dip method (17).
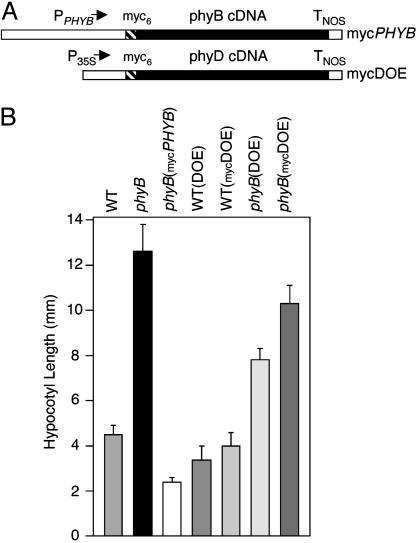
Construction of Epitope-Tagged Transgenes. The coding sequences for six myc (myc6) epitopes (EQKLISEEDL) were multimerized and fused in-frame to the 5′ end of the PHYB cDNA sequence (18) (see Fig. 7, which is published as supporting information on the PNAS web site). The myc6-PHYB coding sequence was cloned in front of the PHYB promoter in the pBI-PB (NdeI) vector (19) to generate the mycPHYB gene (Fig. 1A), and this was transformed into the No-0 phyB-1 host. The WT phyD overexpressor line, designated WT(DOE), is the 35S-phyD no. 2 line described previously (20). The phyB-1 mutant phyD overexpressor line, designated phyB(DOE), was constructed by transforming the 35S-phyD expression vector (20) into the No-0 phyB-1 host. The myc6-PHYD coding sequence was constructed by fusing the sequence coding for the myc6 tag from plasmid pCS2+MT (21) to the 5′ end of the PHYD cDNA sequence (see Fig. 8, which is published as supporting information on the PNAS web site). This sequence was placed under the control of the 35S promoter in the vector pBI123 (20) to produce the mycDOE gene (Fig. 1A). WT(mycDOE) and phyB(mycDOE) lines were produced by transforming this gene into No-0 WT and No-0 phyB-1 hosts.
Fig. 1.
Activities of epitope-tagged phyB and phyD proteins. (A) Structures of the mycPHYB and mycDOE transgenes. myc6 epitopes were translationally fused to the PHYB and PHYD cDNA sequences. PPHYB and P35S represent the PHYB and cauliflower mosaic virus 35S promoter regions, and TNOS is the nopaline synthase terminator sequence. (B) Hypocotyl lengths of seedlings grown for 5 days under 20 μmol·s-1·m-2 R light.
Protein Extraction, IP, and Immunoblot Analysis. Fresh dark- or light-grown seedlings were ground in an ice-cold mortar and pestle in 50 mM Tris (pH 8)/150 mM NaCl/0.1% octylphenoxypolyethoxyethanol CA-630 with complete EDTA-free protease inhibitor mixture (Roche Diagnostics) at tissue weight/buffer volume ratios of 1:1 for dark- and 1:2 for light-grown seedlings. Extracts were centrifuged for 5 min at 12,000 × g in a micro-centrifuge at 4°C, and the supernatants were used directly in IP reactions. One-milliliter aliquots of extracts were precleared by the addition of 20 μl of protein A-agarose beads (Santa Cruz Biotechnology), incubation for 30 min on ice, and centrifugation at 5,000 × g for 5 min. Fifty microliters of tissue culture supernatant of the anti-myc monoclonal line 9E10 (gift of Seth Pincus, Louisiana State University, Baton Rouge) or 1 μg of normal mouse IgG (Santa Cruz Biotechnology) was added to the precleared extract, and the mixture was incubated for 30 min on ice. Twenty microliters of protein A-agarose beads was added, the mixture was incubated on ice with occasional mixing for 1 h, and the beads were pelleted by centrifugation at 5,000 × g for 30 sec at 4°C. The beads were washed four times in 500 μl of extraction buffer. Proteins bound to the beads were eluted by heating at 95°C for 5 min in 2× SDS sample buffer and pelleting the beads. The eluted proteins were analyzed by fractionation on 6% SDS/PAGE, blotting to nitrocellulose and probing with mAbs (12). mAbs were anti-myc 9E10, anti-phyA 073d, anti-phyB B6B3, anti-phyC C11 and C13, anti-phyD 2C1, anti-phyE 7B3, and the universal anti-phy mAb 3B5 (22).
Size-Exclusion Chromatography (SEC). Protein extracts of dark-grown WT or phyB(mycPHYB) seedlings were prepared as above, and 0.5-ml samples containing ≈500 μg of total soluble protein were applied to a Superose 6 (Pharmacia) gel filtration column (25-ml bed volume). The column was eluted with the extraction buffer at 4°C at a rate of 0.5 ml/min, and 1-ml fractions were collected. IP and immunoblot analysis of column fractions were performed as described above. The column was calibrated with protein molecular weight standards (Pharmacia), and immunoblots were scanned and analyzed on an Alphaimager 2200 scanner by using alphaeasefc software (Alpha Innotech, San Leandro, CA).
Results
myc6 Epitope-Tagged phyB and phyD Are Biologically Active. As shown in Fig. 1A, transgenes were constructed consisting of either an N-terminal fusion of six myc epitopes (myc6) to the phyB coding sequence driven by the PHYB promoter region (mycPHYB) or an N-terminal (myc6)-tagged phyD coding sequence driven by the 35S promoter (myc-PHYD overexpressor, or mycDOE). The mycPHYB gene was transformed into a phyB-1-null mutant background, and the hypocotyl growth response of the phyB(mycPHYB) line under R light was compared to that of WT and the untransformed phyB mutant. Fig. 1B shows that the mycPHYB gene fully complements the mutant R hypocotyl elongation phenotype, indicating that it has similar activity to nontagged PHYB. The mycPHYB transgene also complements the elongated rosette leaf morphology and early flowering phyB mutant phenotypes (data not shown). The mycDOE gene was transformed into both the WT and phyB mutant backgrounds. It has been shown that a nontagged DOE transgene in a WT background causes low-level overexpression of phyD and a mild hypersensitivity of hypocotyl growth inhibition under R (20). Fig. 1B illustrates this and also shows that the DOE transgene in a phyB mutant background results in partial complementation of the phyB long-hypocotyl phenotype under R. In both the WT and phyB backgrounds, expression of the mycDOE transgene also causes a reduction in hypocotyl length under R relative to untransformed controls but results in a weaker R hypersensitivity than the DOE gene (Fig. 1B). Hence, although myc6-phyB is equivalent to native phyB in its biological activity, the addition of the epitope tags to phyD appears to have impaired its activity to some extent.
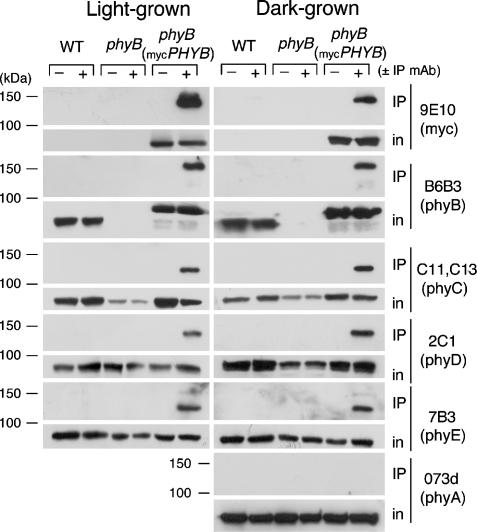
Type II Phytochromes Show Heteromeric Binding Interactions in Vivo. mAbs specific for each of the five Arabidopsis phytochrome apoproteins (phyA-phyE) have been described (22). The WT, phyB, and phyB(mycPHYB) lines were grown under continuous white light or in darkness for 7 days, protein extracts were prepared from the seedlings, and IP was performed with the anti-myc 9E10 mAb. The upper part of Fig. 2 shows that, as expected, the 9E10 mAb precipitates the 138-kDa myc6-phyB protein from a phyB(myc-PHYB) extract but not from extracts of the untransformed lines. The myc6-phyB antigen is also recognized by anti-phyB mAb B6B3. The lower part of Fig. 2 shows that the 120- to 125-kDa phyC-phyE are coprecipitated with myc6-phyB in these samples. No evidence of nonspecific precipitation of these endogenous phytochromes from the WT or phyB extracts by the 9E10 mAb or from the phyB(mycPHYB) extract by nonimmune mouse IgG is observed (Fig. 2). In contrast to phyC-phyE, phyA, which is present at 10- to 60-fold higher concentration than any of the other phytochromes in dark-grown seedlings (12), does not coprecipitate with myc6-phyB (Fig. 2). No difference in the coimmunoprecipitation pattern of the phyC-phyE phytochromes with myc6-phyB is seen between extracts of light- and dark-grown seedlings (Fig. 2). These data suggest that stable binding interactions occur between phyB and other type II phytochromes but not between phyB and type I phyA, and that these interactions occur irrespective of whether the molecules are cytosolic, in dark-grown cells, or nuclear, in light-grown cells (1, 2).
Fig. 2.
Coimmunoprecipitation of phyC-phyE with the myc6-phyB protein from seedling extracts. Samples of extracts of light- and dark-grown seedlings of the WT, phyB mutant, and phyB(mycPHYB) lines were immunoprecipitated with the anti-myc 9E10 mAb or with 1 μg of nonimmune mouse IgG. Aliquots of the input extracted proteins and the immunoprecipitates were separated on 6% SDS gels, blotted, and probed with the indicated antibodies (in, input to IP reaction).
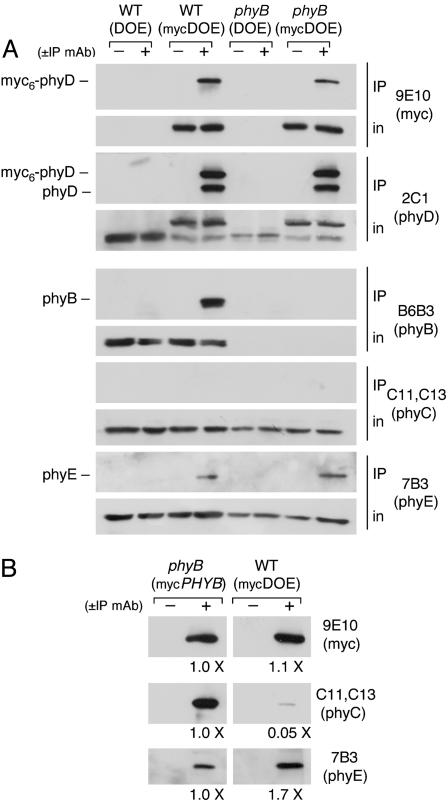
To determine whether these heteromeric phytochrome interactions can be detected when a different type II apoprotein is pulled down, extracts of light-grown seedlings of the WT(myc-DOE) and phyB(mycDOE) lines and the corresponding non-tagged control overexpressor lines were immunoprecipitated with the anti-myc 9E10 mAb. Fig. 3A shows that, when the myc6-phyD protein is pulled down from the mycDOE extracts, endogenous native phyD is coprecipitated, as expected if phyD forms homodimers. In addition, endogenous phyB and phyE, but not phyC, are present in the IP pellets from the mycDOE extracts (Fig. 3A). The presence or absence of phyB, in the WT(myc-DOE) and phyB(mycDOE) lines, respectively, does not significantly alter the coprecipitation characteristics of phyC or phyE with myc6-phyD (Fig. 3A). These data confirm the occurrence of binding interactions among phyB, phyD, and phyE and suggest that these three proteins interact in all possible heteromeric combinations among themselves. Similar results to those shown in Fig. 3A were obtained when dark-grown WT(mycDOE) seedlings were used in IP experiments, and no evidence for any coprecipitation of phyA with myc6-phyD was obtained (data not shown).
Fig. 3.
Binding interactions of type II phytochromes with the myc6-phyD protein. (A) Coimmunoprecipitation of phyB and phyE with the myc6-phyD protein from seedling extracts. Samples of extracts of light-grown seedlings of the WT and phyB mutant lines containing either the DOE or mycDOE transgenes were immunoprecipitated with the anti-myc 9E10 mAb or with nonimmune mouse IgG. Aliquots of the input extracted proteins and the immunoprecipitates were separated on 6% SDS gels, blotted, and probed with the indicated antibodies (in, input to IP reaction). (B) phyC coprecipitates more efficiently with phyB than with phyD. Extracts of dark-grown seedlings expressing similar levels of myc6-phyB or myc6-phyD were immunoprecipitated with the anti-myc 9E10 mAb or with nonimmune mouse IgG. Precipitated proteins were separated on 6% SDS gels, blotted, and probed with the indicated antibodies. Chemiluminescent immunoblot signals of the WT-(mycDOE) IPs relative to the phyB(mycPHYB) IPs were determined by densitometry.
Whereas phyC binds to myc6-phyB (Fig. 2), it does not have a strong affinity for myc6-phyD (Fig. 3A). Very long exposures of phyC blots of the proteins immunoprecipitated by mAb 9E10 from WT(mycDOE) or phyB(mycDOE) extracts indicate that a small amount of phyC is present in those pellets. Therefore, phyC may bind to phyD with low affinity. To compare the relative binding of phyC to myc6-phyB and myc6-phyD, an IP experiment was performed with a phyB(mycPHYB) line and a WT(mycDOE) line that express similar levels of their epitope-tagged transgene products. Fig. 3B shows that, in dark-grown seedlings, 20-fold more phyC is coimmunoprecipitated with myc6-phyB than with an equivalent amount of myc6-phyD. This suggests that there may be a high degree of selectivity to the type II phytochrome interactions. However, because the myc6-phyD protein shows a somewhat reduced biological activity compared to nontagged phyD (Fig. 1B), it is possible its affinity for phyC has been affected by the N-terminal myc6 tag and that native phyD in fact interacts with phyC more strongly than this.
To demonstrate that the phytochrome-binding interactions detected in Figs. 2 and 3 occur in planta, before grinding of the tissue and preparation of the extracts, a tissue-mixing experiment was performed. Seedlings of WT(mycDOE) and phyB- (DOE) and of WT(DOE) and phyB(mycDOE) were mixed before grinding. The mixed-seedling extracts were precipitated with the 9E10 mAb and tested for coprecipitation of phyB, provided by the WT background only, with the myc6-phyD protein, provided by the line containing the mycDOE transgene. Fig. 4 shows that phyB is coprecipitated with myc6-phyD only if they are present together in the seedling before extraction.
Fig. 4.
Tissue-mixing experiment demonstrating in planta phytochrome interactions. Protein extracts were prepared from mixed light-grown seedlings in which phyB and myc6-phyD were expressed either in two different seedling populations (on the left) or in the same seedlings (on the right). Extracts were immunoprecipitated with the anti-myc 9E10 mAb or with nonimmune mouse IgG. Aliquots of the input extracted proteins and the immunoprecipitates were separated on 6% SDS gels, blotted, and probed with the indicated antibodies (in, input to IP reaction).
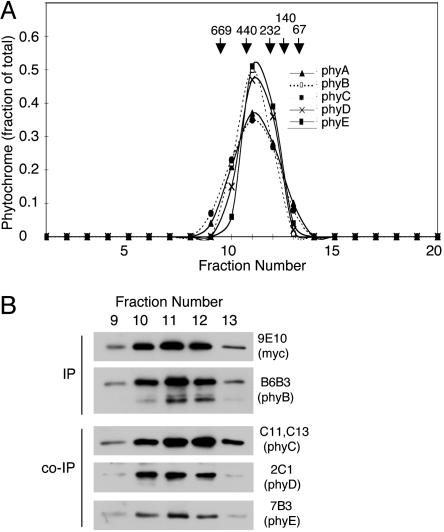
Type II Phytochrome Interactions Represent Heterodimer Formation. Purified native oat phyA has been shown to be a homodimer (14, 15). Overexpressed Arabidopsis phyB also forms homodimeric products in vivo (23). Whether phyC-phyE are also dimers and whether homomeric combinations of these phytochromes occur have not been addressed. The interactions among the type II phytochromes detected in Figs. 2, 3, 4 could take place as formation of heterodimers (such as phyB/C, phyB/D, phyB/E, etc.) or as higher-order dimer-dimer complexes (such as phyB/B binding to phyD/D). Alternatively, completely novel multimeric combinations of the phyB-phyE apoproteins might occur. To distinguish among these possibilities, extracts of dark-grown WT and phyB- (mycPHYB) seedlings were fractionated by SEC, and the migration of the five phytochromes relative to molecular weight standards was monitored by performing immunoblots on column fractions. Fig. 5A shows profiles from scanned immunoblots of column fractions probed with the five phytochrome-specific mAbs. All five native Arabidopsis phytochromes migrate at masses in the range of 300-380 kDa. This corresponds well with the migration of purified oat phyA on SEC, in which an extended structure for the dimer of calculated mass of 250 kDa was found to give an apparent mass of 350-360 kDa on SEC (14, 15). Hence, all five of the Arabidopsis phytochromes are present in dark-grown tissue extracts predominantly as dimers. No evidence for the presence of higher molecular weight complexes containing phytochrome was observed on these blots. To test for the presence of heterodimers in these fractions, an extract of seedlings of the phyB(mycPHYB) line was fractionated by SEC. Column profiles nearly identical to those of the WT were obtained (data not shown). Fractions from throughout the dimer peak were immunoprecipitated with mAb 9E10, and the precipitated proteins were characterized on immunoblots (Fig. 5B). A small amount of degradation of the myc6-phyB protein apparently occurred during fractionation, probably due to removal of the myc tags from the N terminus, resulting in a phyB-reactive band at approximately the position of native phyB. This was seen repeatedly in independent experiments. Nevertheless, in these column fractions, phyC-phyE coimmunoprecipitate with myc6-phyB (Fig. 5B). Once again, no evidence of binding of phyA to myc6-phyB was observed (data not shown). These results demonstrate that the heteromeric phyB/C, phyB/D, and phyB/E complexes migrate in SEC at molecular masses characteristic of dimers. Therefore, the binding interactions between phyB and the other type II apoproteins very likely result in the formation of phytochrome heterodimers.
Fig. 5.
SEC of phytochrome dimers. Extracts of WT Arabidopsis and phyB- (mycPHYB) seedlings were prepared and separated on Superose 6. (A) Profiles of scanned immunoblots for each of the five Arabidopsis phytochromes from SEC of the WT extract. Column fractions were separated on 6% SDS gels, blotted, and probed with the phy-specific mAb. (B) Coimmunoprecipitation of phyC-phyE with myc6-phyB from individual fractions from SEC of an extract of the phyB(mycPHYB) line.
Discussion
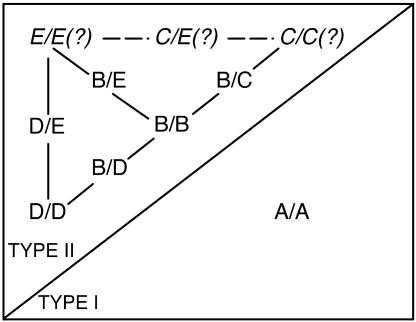
Coimmunoprecipitation experiments with epitope-tagged phyB and phyD apoproteins have shown that the type II light-stable phytochromes in Arabidopsis seedlings form several specific combinations of heterodimers in planta. Fig. 6 illustrates the dimerization interactions that have currently been identified. Overexpressed phyB has previously been shown to form phyB/B homodimers in transgenic plants (23). However, when expressed at a normal WT level, myc6-phyB also forms phyB/C, phyB/D, and phyB/E heterodimers (Figs. 2 and 5). IP of myc6-phyD coprecipitates phyB, phyD, and phyE but only very weakly pulls down phyC (Figs. 3 and 4), demonstrating that phyD/D and phyD/E dimers, along with the phyB/D dimers already mentioned, are present in plant extracts. It is not currently known whether phyE or phyC homodimers or phyC/E heterodimers form. In contrast to these relatively promiscuous binding interactions, no evidence that phyA engages in heteromeric interactions was obtained (Fig. 6). These findings have implications for understanding the diversity and complexity of plant R/FR sensing systems and the molecular mechanisms through which these systems regulate growth and development.
Fig. 6.
Dimerization interactions among the Arabidopsis phytochromes. Homo- and heterodimeric phytochromes that have been shown to be present in Arabidopsis seedlings are illustrated. Type II phytochrome dimer combinations that have not yet been investigated are shown in italics with question marks.
The phyB-phyE phytochromes all have characteristics of “type II” or “green-tissue” forms, in that they are present at moderate to low levels, making them difficult to detect spectroscopically in tissue samples, and are essentially light-stable, falling in abundance only a few-fold when comparing dark- and light-grown plants (12). phyA, on the other hand, is a “type I” or “etiolated-tissue” form, which is present at high levels and is readily detectable spectroscopically in dark-grown plant tissues but is rapidly degraded to very low levels after transfer to light and photoconversion to Pfr. The type I and II phytochromes also appear to differ fundamentally in their photosensory mechanisms. Type II forms are essentially R light sensors, with varying degrees of FR reversibility for different responses, whereas type I phyA can be activated by very low fluences of light from a broad spectral range, including FR, and at least some FR-induced responses are reversible with R (24). In light of our current findings, it appears that phyA may have evolved in plants as a unique single molecular species of light-labile type I R/FR sensor, whereas duplication and divergence of genes encoding the light-stable type II phytochromes has given rise to a complex combinatorial array of R/FR photoreceptor forms. Until the complete range of potential heteromeric combinations of the phytochrome apoproteins has been investigated, however, it remains possible that type I phyA can heterodimerize with phyC or phyE in seedlings or that, under environmental conditions or at plant developmental stages that have not yet been examined, heterodimers of type I and II phytochromes form.
Identification of heterodimeric combinations of phyB-phyE will require a reinterpretation of the single- and multiple-gene phyB-phyE mutant phenotypes in Arabidopsis and other plant species. For example, an Arabidopsis phyB-null mutation, which results in a strong constitutive shade avoidance phenotype (8, 25), would cause loss not only of phyB/B homodimers but also of phyB/C, phyB/D, and phyB/E heterodimers, and it is not clear which aspects of the mutant phenotype correspond with which molecular species of phytochrome. Each of the single-gene Arabidopsis phyC-phyE mutants shows a less dramatic alteration in light-regulated development than the phyB mutant (7, 9-11). This suggests that none of the individual heteromeric forms is likely to have a predominant activity. Nevertheless, it is possible that each heterodimeric form has unique photochemical properties, such as absorption spectra or dark-reversion kinetics, or unique affinities for downstream signaling proteins, and that it is the combined activities of these diverse forms that mediates what has previously been conceived to be phyB function. Similarly, the effects of phyC-phyE mutations and of combinations of phy mutations on photomorphogenic responses will need to be reevaluated. What has previously been interpreted as coaction, cross-talk, or redundancy of individual phytochrome functions may, at least in part, reflect the activities of heterodimeric phytochromes.
To understand the role of apoprotein heterodimerization in phytochrome sensing/signaling, it will be necessary to determine the fractions of the type II phytochromes that are present in plant cells as homodimers relative to the fractions present as each of the heterodimeric combinations and to assess the properties and functions of each of these receptor populations. Both phyB and phyD can homodimerize (ref. 23 and Fig. 3), and preliminary experiments indicate that major fractions of these two forms are present in that state in Arabidopsis seedlings (unpublished work). Indeed, the overlapping but distinctive tissue-specific expression patterns of PHY genes, such as those of PHYB, PHYD, and PHYE (26, 27), likely result in varying fractions of the different homodimeric and heterodimeric phytochromes in different plant tissues and organs. We have shown here that the relative proportions of heterodimers are not dramatically different when comparing dark- and light-grown seedlings. This indicates that heterodimers can form in the cytosol as Pr in the absence of light, that they are likely translocated to the nucleus upon conversion to Pfr, and that the phyB-phyE dimerization affinities are not strongly light-regulated. Nevertheless, with respect to their light-induced cellular relocations, it is possible that the kinetics of photoconversion of the different heterodimers, and therefore the proportions of Pr/Pr, Pr/Pfr, and Pfr/Pfr dimers formed under a given light condition, differ, or that their mechanisms or efficiencies of nuclear transport vary.
The formation of heterodimers of type II phytochromes may also explain several previous observations regarding the stabilities of these proteins and their capacities to be overexpressed in transgenic plants. Hirschfeld et al. (22) described a reduction in the level of phyC and, to a small extent, phyD in phyB-null mutants of Arabidopsis and postulated that this coordination could result from physical interaction of these proteins. The phyC immunoblots in Fig. 2 illustrate this phenomenon. It is now clear that this could result from a reduction in the stability of phyC when phyB is not present with which to heterodimerize. Moreover, it has been noted that, although phyB and phyA are readily overexpressed in transgenic plants, phyC-phyE are not overexpressed to high levels, even when their mRNAs are present at very high levels (20, 28). It is possible that an imbalance in the amounts of these particular phytochromes relative to their dimerization partners leads to instability, and therefore that heterodimerization plays a role in determining the steady-state levels of type II receptors and the ultimate makeup of the phytochrome array.
A variety of experimental approaches, including proteolysis of purified native phyA and expression and analysis of truncated apoproteins in plants, have shown that homodimerization of phyA and phyB is mediated by amino acid sequences in their C-terminal ends (29). Recently, it has been shown that the N terminus of phyB has a high level of light-regulatory activity in the absence of the C-terminal end, but only if it is dimerized and localized to the nucleus with heterologous protein domains (30). Hence, the process of dimerization appears to be an integral aspect of the assembly of a signaling-competent phytochrome. This is consistent with an active role for heterodimerization as a mechanism for generating multiple differentially active phytochrome forms. The phylogeny of PHY genes in angiosperms indicates that PHYB- and PHYC-related coding sequences are found as individual genes or small gene families in most flowering plants (13), suggesting a broadly distributed potential for formation of heterodimers. It is likely that this combinatorial association of type II phytochromes results in greater complexity and versatility of plant light sensing and signaling mechanisms than has previously been recognized.
Supplementary Material
Acknowledgments
We thank Eric Gillitzer and Debbie Willits for suggestions and help with SEC experiments. This work was supported by National Science Foundation Grants IBN-9808801 and IBN-0348913 (to R.A.S.).
Abbreviations: R, red; FR, far-red; phyA-phyE, phytochrome A-E holoproteins; IP, immunoprecipitation; SEC, size-exclusion chromatography; myc6, six myc.
References
- 1.Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S. A. & Nagatani, A. (1999) J. Cell Biol. 145, 437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kircher, S., Gil, P., Kozma-Bognar, L., Fejes, E., Speth, V., Husselstein-Muller, T., Bauer, D., Adam, E., Schäfer, E. & Nagy, F. (2002) Plant Cell 14, 1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quail, P. H. (2002) Nat. Rev. Mol. Cell Biol. 3, 85-93. [DOI] [PubMed] [Google Scholar]
- 4.Gyula, P., Schäfer, E. & Nagy, F. (2003) Curr. Opin. Plant Biol. 6, 446-452. [DOI] [PubMed] [Google Scholar]
- 5.Sharrock, R. A. & Quail, P. H. (1989) Genes Dev. 3, 1745-1757. [DOI] [PubMed] [Google Scholar]
- 6.Clack, T., Mathews, S. & Sharrock, R. A. (1994) Plant Mol. Biol. 25, 413-427. [DOI] [PubMed] [Google Scholar]
- 7.Aukerman, M. J., Hirschfeld, M., Wester, L., Weaver, M., Clack, T., Amasino, R. M. & Sharrock, R. A. (1997) Plant Cell 9, 1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitelam, G. C. & Devlin, P. F. (1997) Plant Cell Environ. 20, 752-758. [Google Scholar]
- 9.Devlin, P. F., Patel, S. R. & Whitelam, G. C. (1998) Plant Cell 10, 1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin, K. A., Davis, S. J., Stoddart, W. M., Vierstra, R. D. & Whitelam, G. C. (2003) Plant Cell 15, 1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monte, E., Alonso, J. M., Ecker, J. R., Zhang, Y., Li, X., Young, J., Austin-Phillips, S. & Quail, P. H. (2003) Plant Cell 15, 1962-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharrock, R. A. & Clack, T. (2002) Plant Physiol. 130, 442-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews, S., Lavin, M. & Sharrock, R. A. (1995) Ann. Mo. Bot. Gard. 82, 296-321. [Google Scholar]
- 14.Lagarias, J. C. & Mercurio, F. M. (1985) J. Biol. Chem. 260, 2415-2423. [PubMed] [Google Scholar]
- 15.Jones, A. M. & Quail, P. H. (1986) Biochemistry 25, 2987-2995. [Google Scholar]
- 16.Wester, L., Somers, D. E., Clack, T. & Sharrock, R. A. (1994) Plant J. 5, 261-272. [DOI] [PubMed] [Google Scholar]
- 17.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, K. & Yaoita, Y. (1997) Nucleic Acids Res. 25, 2231-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharrock, R. A., Clack, T. & Goosey, L. (2003) Plant Mol. Biol. 52, 135-142. [DOI] [PubMed] [Google Scholar]
- 20.Sharrock, R. A., Clack, T. & Goosey, L. (2003) Plant J. 34, 317-326. [DOI] [PubMed] [Google Scholar]
- 21.Turner, D. L. & Weintraub, H. (1994) Genes Dev. 8, 1434-1447. [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld, M., Tepperman, J. M., Clack, T., Quail, P. H. & Sharrock, R. A. (1998) Genetics 149, 523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner, D. & Quail, P. H. (1995) Proc. Natl. Acad. Sci. USA 92, 8596-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinomura, T., Uchida, K. & Furuya, M. (2000) Plant Physiol. 122, 147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed, J. W., Nagpal, P., Poole, D. S., Furuya, M. & Chory, J. (1993) Plant Cell 5, 147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somers, D. E. & Quail, P. H. (1995) Plant J. 7, 413-427. [DOI] [PubMed] [Google Scholar]
- 27.Goosey, L., Palecanda, L. & Sharrock, R. A. (1997) Plant Physiol. 115, 959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin, M., Kuhn, R., Moran, S. & Quail, P. H. (1997) Plant J. 12, 1163-1172. [DOI] [PubMed] [Google Scholar]
- 29.Quail, P. H. (1997) Plant Cell Environ. 20, 657-665. [Google Scholar]
- 30.Matsushita, T., Mochizuki, N. & Nagatani, A. (2003) Nature 424, 571-574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.