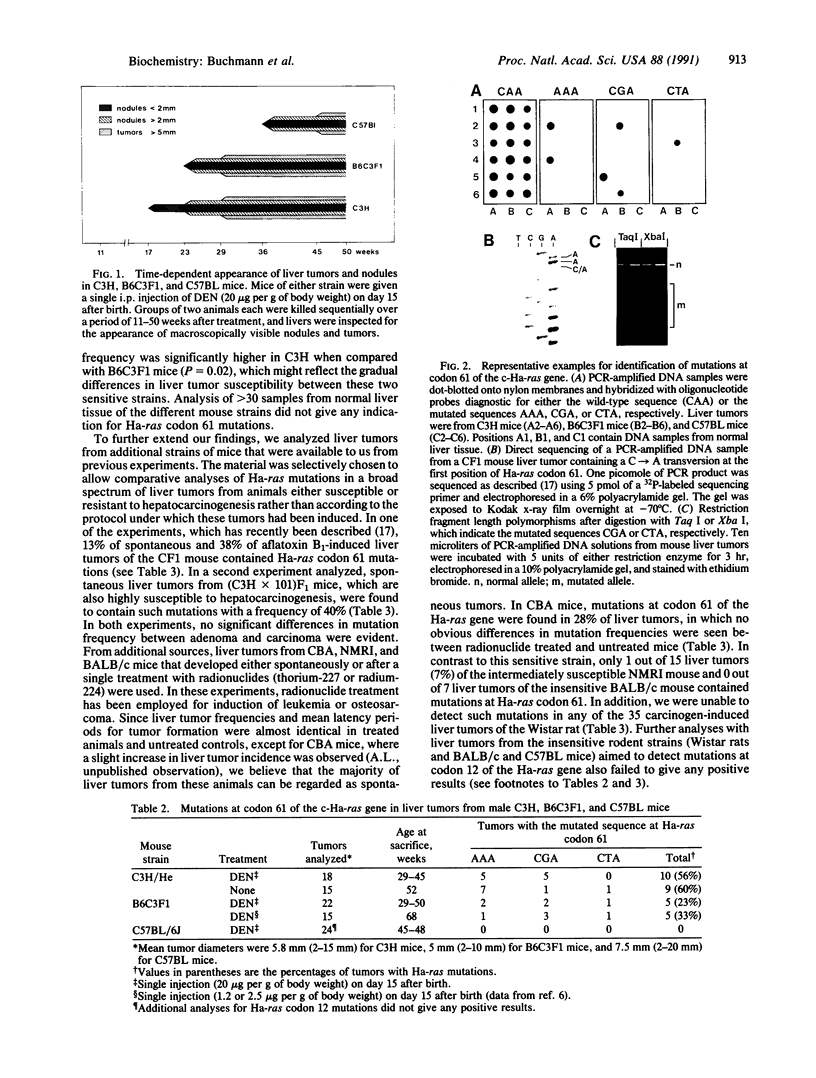
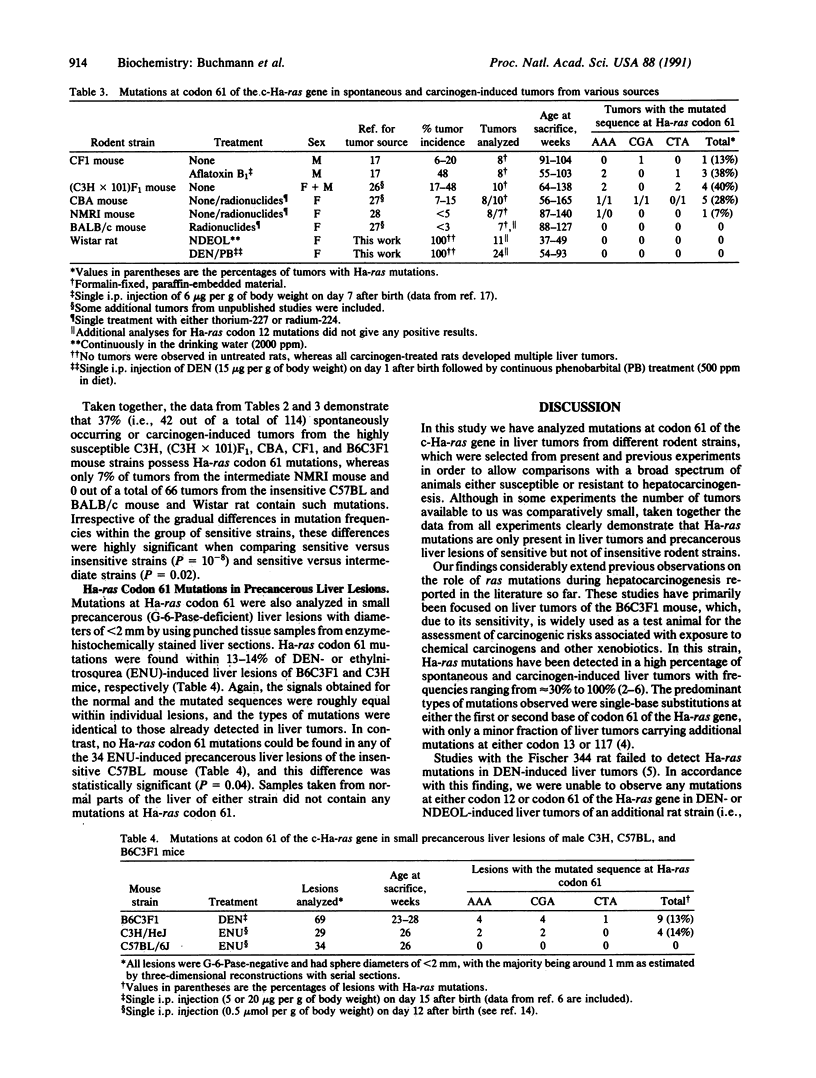
Abstract
The frequency and pattern of mutations at codon 61 of the c-Ha-ras gene have been analyzed in 195 liver tumors and 132 precancerous liver lesions from various rodent strains with differing susceptibility to hepatocarcinogenesis. By using the polymerase chain reaction and allele-specific oligonucleotide hybridization, C----A transversions at the first base and A----T transversions or A----G transitions at the second base of c-Ha-ras codon 61 were detected in 20-60% of spontaneous or carcinogen-induced liver tumors of the C3H/He, CBA, CF1, and B6C3F1 mouse strains, which are highly susceptible to hepatocarcinogenesis. No such mutations, however, could be found in any of the 31 liver tumors of the insensitive C57BL/6J and BALB/c mouse strains or in any of the 35 liver tumors of the comparatively resistant Wistar rat. Further analyses of c-Ha-ras codon 12 mutations in liver tumors from the three insensitive rodent strains also failed to give any positive results. In early precancerous liver lesions, c-Ha-ras codon 61 mutations were found in 13-14% of lesions of the sensitive C3H/He and B6C3F1 mouse strains but not in any of the 34 lesions of the insensitive C57BL/6J mouse. Taken together, our results indicate a close correlation between the mutational activation of the c-Ha-ras gene in liver tumors of the different rodent strains and their susceptibility to hepatocarcinogenesis, whereby the mutations appear to provide a selective growth advantage, leading to a clonal expansion of the mutated liver cell population, only in livers of sensitive but not of insensitive strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Bauer-Hofmann R., Buchmann A., Wright A. S., Schwarz M. Mutations in the Ha-ras proto-oncogene in spontaneous and chemically induced liver tumours of the CF1 mouse. Carcinogenesis. 1990 Oct;11(10):1875–1877. doi: 10.1093/carcin/11.10.1875. [DOI] [PubMed] [Google Scholar]
- Bomhard E., Mohr U. Spontaneous tumors in NMRI mice from carcinogenicity studies. Exp Pathol. 1989;36(3):129–145. doi: 10.1016/s0232-1513(89)80090-8. [DOI] [PubMed] [Google Scholar]
- Brown K., Bailleul B., Ramsden M., Fee F., Krumlauf R., Balmain A. Isolation and characterization of the 5' flanking region of the mouse c-Harvey-ras gene. Mol Carcinog. 1988;1(3):161–170. doi: 10.1002/mc.2940010304. [DOI] [PubMed] [Google Scholar]
- Brown K., Buchmann A., Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A., Mahr J., Bauer-Hofmann R., Schwarz M. Mutations at codon 61 of the Ha-ras proto-oncogene in precancerous liver lesions of the B6C3F1 mouse. Mol Carcinog. 1989;2(3):121–125. doi: 10.1002/mc.2940020303. [DOI] [PubMed] [Google Scholar]
- Deerberg F., Rapp K. G., Pittermann W., Rehm S. Zum Tumorspektrum der Han: WIST-Ratte. Z Versuchstierkd. 1980;22(5-6):267–280. [PubMed] [Google Scholar]
- Dragani T. A., Manenti G., Della Porta G. Genetic susceptibility to murine hepatocarcinogenesis is associated with high growth rate of NDEA-initiated hepatocytes. J Cancer Res Clin Oncol. 1987;113(3):223–229. doi: 10.1007/BF00396377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater N. R., Ginsler J. J. Genetic control of hepatocarcinogenesis in C57BL/6J and C3H/HeJ inbred mice. Carcinogenesis. 1986 Oct;7(10):1701–1707. doi: 10.1093/carcin/7.10.1701. [DOI] [PubMed] [Google Scholar]
- Erfle V., Hehlmann R., Schetters H., Meier A., Luz A. Time course of C-type retrovirus expression in mice submitted to osteosarcomagenic doses of 224radium. Int J Cancer. 1980 Jul 15;26(1):107–113. doi: 10.1002/ijc.2910260117. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Kemp C. J., Ginsler J. J., Drinkwater N. R. Rapid growth of preneoplastic lesions in hepatocarcinogen-sensitive C3H/HeJ male mice relative to C57BL/6J male mice. Carcinogenesis. 1988 Jun;9(6):885–890. doi: 10.1093/carcin/9.6.885. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Winkler M. L., Drinkwater N. R. Partial hepatectomy is a promoter of hepatocarcinogenesis in C57BL/6J male mice but not in C3H/HeJ male mice. Carcinogenesis. 1990 Apr;11(4):589–594. doi: 10.1093/carcin/11.4.589. [DOI] [PubMed] [Google Scholar]
- Kaspareit J., Deerberg F. Tumour incidence and tumour spectrum of male Han:NMRI mice--short communication. Z Versuchstierkd. 1987;30(3-4):105–109. [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Sauer G. The simultaneous extraction of high-molecular-weight DNA and of RNA from solid tumors. Anal Biochem. 1983 Oct 15;134(2):288–294. doi: 10.1016/0003-2697(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Lindamood C., 3rd, Bedell M. A., Billings K. C., Dyroff M. C., Swenberg J. A. Dose response for DNA alkylation, [3H]thymidine uptake into DNA, and O6-methylguanine-DNA methyltransferase activity in hepatocytes of rats and mice continuously exposed to dimethylnitrosamine. Cancer Res. 1984 Jan;44(1):196–200. [PubMed] [Google Scholar]
- Luz A., Murray A. B. Sudden outbreak of a leukemia-like lesion in female CBA mice after repeated injections of 5-azacytidine. J Cancer Res Clin Oncol. 1988;114(5):525–527. doi: 10.1007/BF00391506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Davis E. F., Huber L. J., Kim Y., Wogan G. N. Characterization of c-Ki-ras and N-ras oncogenes in aflatoxin B1-induced rat liver tumors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1104–1108. doi: 10.1073/pnas.87.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. A., Murray A. B., Linzner U., Luz A. Osteosarcoma risk after simultaneous incorporation of the long-lived radionuclide 227Ac and the short-lived radionuclide 227Th. Radiat Res. 1990 Jan;121(1):14–20. [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Maronpot R. R., Anderson M. W., Aaronson S. A. Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):33–37. doi: 10.1073/pnas.83.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Patterson R. M., Maronpot R. R., Aaronson S. A., Anderson M. W. Activated oncogenes in B6C3F1 mouse liver tumors: implications for risk assessment. Science. 1987 Sep 11;237(4820):1309–1316. doi: 10.1126/science.3629242. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sinha S., Webber C., Marshall C. J., Knowles M. A., Proctor A., Barrass N. C., Neal G. E. Activation of ras oncogene in aflatoxin-induced rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3673–3677. doi: 10.1073/pnas.85.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers S. J., Wiseman R. W., Ward J. M., Miller E. C., Miller J. A., Anderson M. W., Eva A. Detection of activated proto-oncogenes in N-nitrosodiethylamine-induced liver tumors: a comparison between B6C3F1 mice and Fischer 344 rats. Carcinogenesis. 1988 Feb;9(2):271–276. doi: 10.1093/carcin/9.2.271. [DOI] [PubMed] [Google Scholar]
- Tarone R. E., Chu K. C., Ward J. M. Variability in the rates of some common naturally occurring tumors in Fischer 344 rats and (C57BL/6N x C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1981 Jun;66(6):1175–1181. doi: 10.1093/jnci/66.6.1175. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Thorpe E., Stevenson D. E. The toxicology of dieldrin (HEOD). I. Long-term oral toxicity studies in mice. Food Cosmet Toxicol. 1973 Jun;11(3):415–432. doi: 10.1016/0015-6264(73)90007-2. [DOI] [PubMed] [Google Scholar]
- Wiseman R. W., Stowers S. J., Miller E. C., Anderson M. W., Miller J. A. Activating mutations of the c-Ha-ras protooncogene in chemically induced hepatomas of the male B6C3 F1 mouse. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5825–5829. doi: 10.1073/pnas.83.16.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]






