Abstract
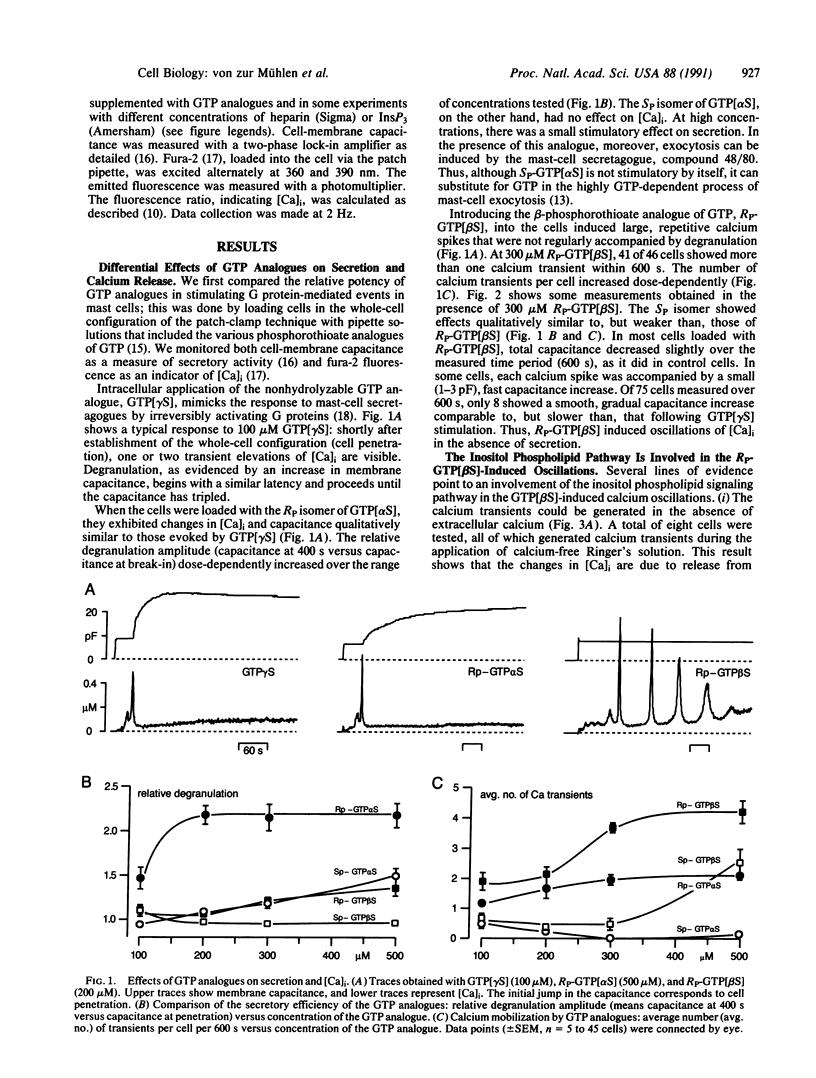
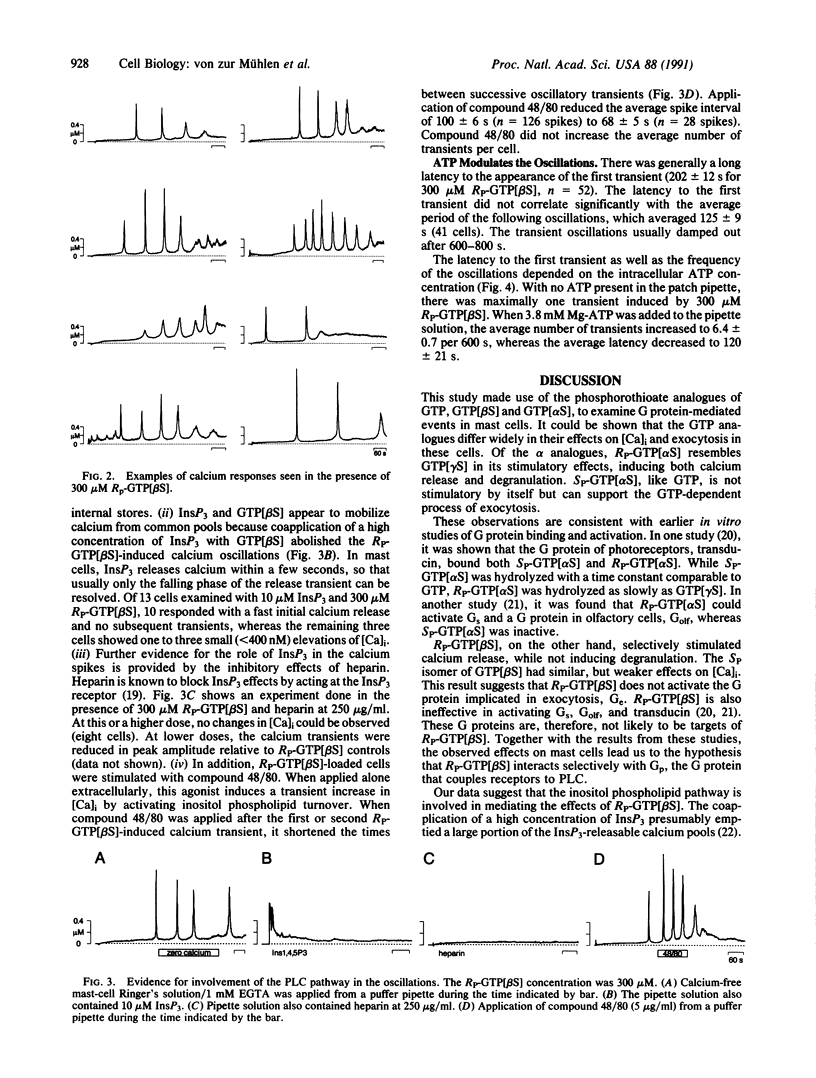
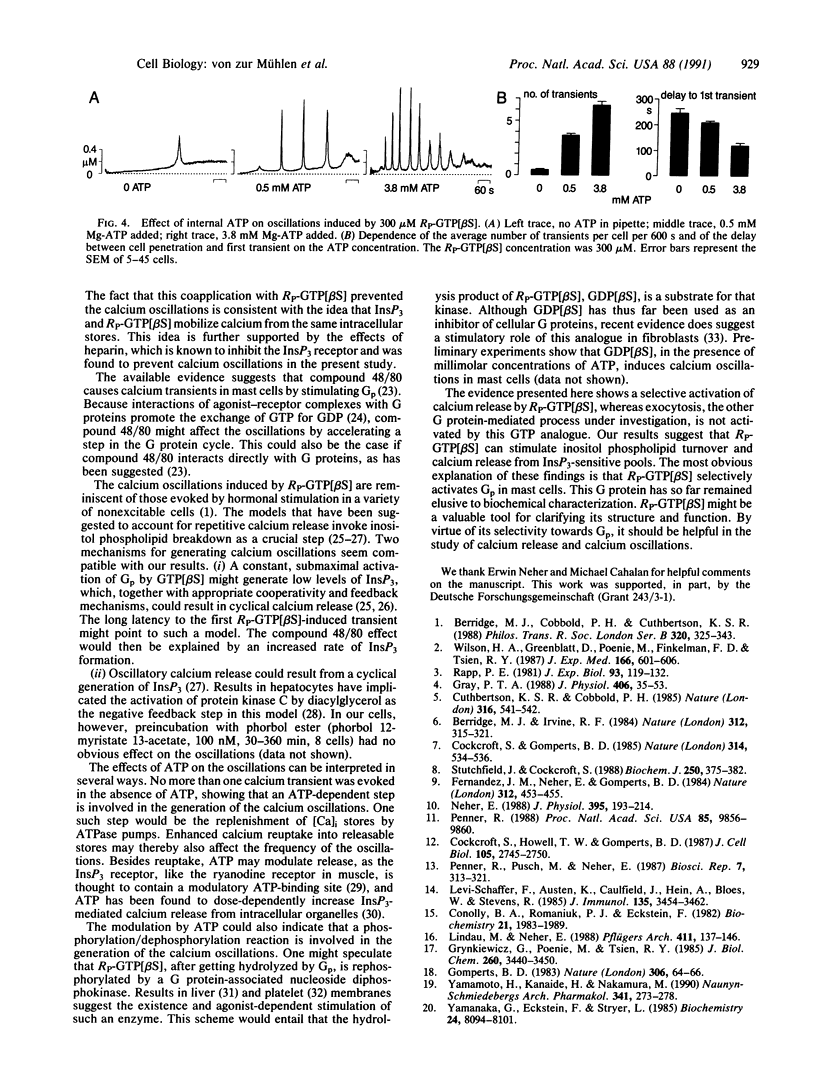
In rat peritoneal mast cells, the activation of GTP-binding proteins (G proteins) by guanosine 5'-[gamma-thio]triphosphate GTP[gamma S] has been found to induce a transient rise in intracellular calcium as well as degranulation. A G protein that couples to phospholipase C (Gp) is thought to mediate the calcium response, whereas degranulation is mediated by a different G protein, termed Ge. In an attempt to activate mast-cell G proteins more selectively, the GTP analogues guanosine 5'-[alpha-thio]triphosphate (GTP[alpha S]) and guanosine 5'-[beta-thio]triphosphate (GTP[beta S]) (RP and SP diastereomers) were introduced into mast cells by means of patch pipettes. Degranulation and free intracellular calcium were monitored by cell capacitance and fura-2 measurements, respectively. It was found that RP-GTP[alpha S], like GTP[gamma S], induced both calcium release and exocytosis. In contrast, RP-GTP[beta S] induced repetitive calcium spikes that were not regularly accompanied by exocytosis. These results suggest that RP-GTP[beta S] selectively activates calcium signaling in mast cells. The RP-GTP[beta S]-induced oscillations were independent of extracellular calcium. They were absent in the presence of heparin or high concentrations of inositol 1,4,5-trisphosphate and modulated by compound 48/80, suggesting the involvement of the inositol phospholipid signaling pathway. Latency of appearance and spiking frequency were markedly modulated by varying the intracellular ATP concentration. The differential activation of intracellular calcium signaling and exocytosis by GTP[beta S] confirms the presence of independent signal-transduction pathways for the two cell responses. RP-GTP[beta S] may prove helpful in the biochemical and molecular characterization of Gp, the as-yet-unidentified G protein that couples receptors to intracellular calcium release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Romaniuk P. J., Eckstein F. Synthesis and characterization of diastereomers of guanosine 5'-O-(1-thiotriphosphate) and guanosine 5'-O-(2-thiotriphosphate). Biochemistry. 1982 Apr 27;21(9):1983–1989. doi: 10.1021/bi00538a002. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jones D. T., Masters S. B., Bourne H. R., Reed R. R. Biochemical characterization of three stimulatory GTP-binding proteins. The large and small forms of Gs and the olfactory-specific G-protein, Golf. J Biol Chem. 1990 Feb 15;265(5):2671–2676. [PubMed] [Google Scholar]
- Kimura N., Shimada N. Evidence for complex formation between GTP binding protein(Gs) and membrane-associated nucleoside diphosphate kinase. Biochem Biophys Res Commun. 1990 Apr 16;168(1):99–106. doi: 10.1016/0006-291x(90)91680-q. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Caulfield J. P., Hein A., Bloes W. F., Stevens R. L. Fibroblasts maintain the phenotype and viability of the rat heparin-containing mast cell in vitro. J Immunol. 1985 Nov;135(5):3454–3462. [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bronner C., Landry Y., Bockaert J., Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990 Jan 1;259(2):260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Guanosine 5'-O-(3-thiotriphosphate) and guanosine 5'-O-(2-thiodiphosphate) activate G proteins and potentiate fibroblast growth factor-induced DNA synthesis in hamster fibroblasts. J Biol Chem. 1990 Jul 15;265(20):11567–11575. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Pusch M., Neher E. Washout phenomena in dialyzed mast cells allow discrimination of different steps in stimulus-secretion coupling. Biosci Rep. 1987 Apr;7(4):313–321. doi: 10.1007/BF01121453. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Higgins B. L. Temperature and nucleotide dependence of calcium release by myo-inositol 1,4,5-trisphosphate in cultured vascular smooth muscle cells. J Biol Chem. 1985 Nov 25;260(27):14413–14416. [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Jakobs K. H. Receptor-regulated formation of GTP[gamma S] with subsequent persistent Gs-protein activation in membranes of human platelets. FEBS Lett. 1989 Mar 13;245(1-2):189–193. doi: 10.1016/0014-5793(89)80219-4. [DOI] [PubMed] [Google Scholar]
- Wilson H. A., Greenblatt D., Poenie M., Finkelman F. D., Tsien R. Y. Crosslinkage of B lymphocyte surface immunoglobulin by anti-Ig or antigen induces prolonged oscillation of intracellular ionized calcium. J Exp Med. 1987 Aug 1;166(2):601–606. doi: 10.1084/jem.166.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Kanaide H., Nakamura M. Heparin specifically inhibits the inositol 1,4,5-trisphosphate-induced Ca2+ release from skinned rat aortic smooth muscle cells in primary culture. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):273–278. doi: 10.1007/BF00180651. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Stereochemistry of the guanyl nucleotide binding site of transducin probed by phosphorothioate analogues of GTP and GDP. Biochemistry. 1985 Dec 31;24(27):8094–8101. doi: 10.1021/bi00348a039. [DOI] [PubMed] [Google Scholar]



