Abstract
Objective
Spinal cord injury causes a drastic loss of motor, sensory and autonomic function. The goal of this project was to investigate the use of intraspinal microstimulation (ISMS) for producing long distances of walking over ground. ISMS is an electrical stimulation method developed for restoring motor function by activating spinal networks below the level of an injury. It produces movements of the legs by stimulating the ventral horn of the lumbar enlargement using fine penetrating electrodes (≤ 50µm diameter).
Approach
In each of five adult cats (4.2–5.5kg), ISMS was applied through 16 electrodes implanted with tips targeting lamina IX in the ventral horn bilaterally. A desktop system implemented a physiologically-based control strategy that delivered different stimulation patterns through groups of electrodes to evoke walking movements with appropriate limb kinematics and forces corresponding to swing and stance. Each cat walked over an instrumented 2.9m walkway and limb kinematics and forces were recorded.
Main Results
Both propulsive and supportive forces were required for over-ground walking. Cumulative walking distances ranging from 609m to 835m (longest tested) were achieved in three animals. In these three cats, the mean peak supportive force was 3.5±0.6N corresponding to full-weight-support of the hind legs, while the angular range of the hip, knee, and ankle joints were 23.1±2.0°, 29.1±0.2°, and 60.3±5.2°, respectively. To further demonstrate the viability of ISMS for future clinical use, a prototype implantable module was successfully implemented in a subset of trials and produced comparable walking performance.
Significance
By activating inherent locomotor networks within the lumbosacral spinal cord, ISMS was capable of producing bilaterally coordinated and functional over-ground walking with current amplitudes <100 µA. These exciting results suggest that ISMS may be an effective intervention for restoring functional walking after spinal cord injury.
Keywords: Spinal networks, functional electrical stimulation, locomotion, neural injury, patterned activation
INTRODUCTION
Spinal cord injuries (SCIs) often adversely and permanently affect motor and sensory systems, disrupting functions such as grasping, walking, breathing and bladder control, and reducing quality of life of the affected person and their family. Regaining the ability to walk is a high priority for people with paraplegia (Widerstrom-Noga et al., 1999, Brown-Triolo et al., 2002, Anderson, 2004). To date, interventions to restore functional walking after SCI in humans have had limited success (Fouad and Pearson, 2004, Boulenguez and Vinay, 2009). One of the most promising techniques involves the use of electrical stimulation for activating muscles and nerves in the periphery or neural networks in the spinal cord to restore movement (Creasey et al., 2000, Prochazka et al., 2001, Creasey et al., 2004, Stein and Mushahwar, 2005, Ethier et al., 2012, Peckham and Kilgore, 2013, Memberg et al., 2014). When applied to muscles and nerves, this technique, known as functional electrical stimulation (FES), produces large forces that are adequate to support the body while standing (e.g., (Triolo et al., 2012)). Alternating the activation between flexor and extensor muscles, often on command by the user through hand switches, produces walking aided by crutches or walkers (Kobetic et al., 1997, Graupe and Kohn, 1998, Brissot et al., 2000, Stein and Mushahwar, 2005, Mushahwar et al., 2007, Bulea et al., 2013). Nonetheless, the challenge of controlling the activation of separate muscles individually and the rapid rate of muscle fatigue have generally limited the distances of functional walking to less than 100m; thus hindering the clinical acceptance of these walking systems (Stein and Mushahwar, 2005, Thrasher and Popovic, 2008). More recently, epidural electrical stimulation of the dorsal surface of the spinal cord combined with intensive body-weight supported treadmill training was presented as a potential means of improving the locomotor rhythm in people with severe SCI (Herman et al., 2002, Carhart et al., 2004, Harkema et al., 2011, Tator et al., 2012). The intent of epidural stimulation is to enhance the basal activity level of neurons in the lumbar region of the spinal cord to facilitate better integration of afferent and residual descending input. In people with incomplete SCI who had undergone body-weight supported treadmill locomotor training, epidural stimulation reduced their sense of effort while walking over-ground and improved their walking distance (Herman et al., 2002, Carhart et al., 2004). In people with complete SCI, some augmentation of voluntary leg control was demonstrated by epidural stimulation (Angeli et al., 2014); however, walking could not be produced. Instead, locomotor-like patterns when the legs were fully unloaded were seen but sufficient weight-bearing and propulsive forces required for functional walking over-ground could not be obtained (Harkema et al., 2011), confirming findings from earlier work in cats with complete SCI (Iwahara et al., 1992).
We have pioneered an FES approach called intraspinal microstimulation (ISMS) for targeted activation of elements of the locomotor networks within the spinal cord. Unlike epidural stimulation, ISMS uses penetrating microwires (up to 50µm diameter) to activate specific regions in the ventral horn of the lumbosacral enlargement (Mushahwar and Horch, 2000b, Bamford et al., 2016). The lumbosacral enlargement extends 3 cm in cats (5 cm in human) and contains the cell bodies of motoneurons innervating all the muscles of the lower extremities (Romanes, 1964, Vanderhorst and Holstege, 1997). It also contains large proportions of the neuronal networks involved in locomotion (Noga et al., 1995, Kiehn and Kjaerulff, 1998, Jordan and Schmidt, 2002, Dai et al., 2005, Guevremont et al., 2006, Giszter, 2015). ISMS in this region taps into the “spinal centre” for locomotion while avoiding the use of wide spread implants and connecting leads, as commonly needed by FES approaches for muscle and peripheral nerve stimulation. Furthermore, by directly activating the ventral horn, ISMS provides more specific and modulated activation of critical elements of locomotion than that afforded by epidural stimulation.
Large forces and ranges of motion are generated with stimuli ≤100µA in amplitude delivered through individual ISMS electrodes (Mushahwar and Horch, 1998, Mushahwar et al., 2000, Mushahwar and Horch, 2000a). Even though the electrode tips are commonly located in lamina IX where the motoneurons reside, ISMS activates motoneurons trans-synaptically (Calixto et al., 2006, Gaunt et al., 2006, Calixto and Mushahwar, 2007). This results in the recruitment of motor units in a near normal physiological order (Bamford et al., 2005), which significantly reduces the rate of muscle fatigue (Lau et al., 2007). Although, for small wire electrodes the typical functional geometric limit of the ISMS current spread at 100µA is less than 0.5mm in radius (Jankowska and Roberts, 1972, Mushahwar and Horch, 1997), the effective limit can be more than 10mm from the stimulation site due to the activation of fibers in passage (Calixto et al., 2006, Gaunt et al., 2006, Calixto and Mushahwar, 2007). This activation explains the production of large forces with stimulus amplitudes that are 100 times lower than those used in peripheral FES systems and epidural stimulation (Mushahwar and Horch, 2000a, Snow et al., 2006, Lau et al., 2007). It also explains the activation of coordinated multi-joint synergies across the hip, knee and ankle joints whose motoneuronal pools lie tens of millimeters apart (Mushahwar et al., 2000, Saigal et al., 2004). Depending on the site of stimulation, upward flexion, downward extension, forward and backward propulsive movements can be produced.
Movements produced by ISMS remain stable for long periods of time (6 months, longest tested) (Mushahwar et al., 2000). Moreover, the electrode implantation and long-term stimulation produce negligible tissue damage (Prochazka et al., 2001, Guevremont and Mushahwar, 2008, Bamford et al., 2010, Bamford and Mushahwar, 2011). Using ISMS sites that produce extensor synergies, we demonstrated that long durations of standing (> 30 minutes) in the hind legs of cats with forces adequate for lifting the animals’ hindquarters can be produced (Lau et al., 2007). These durations were >5 times longer than those generated by direct stimulation of muscles and nerves (Davis et al., 2001, Lau et al., 2007, Mushahwar et al., 2007, Triolo et al., 2012). Also, by using as few as four ISMS electrodes in each side of the spinal cord (typically 8 per side), long episodes of weight-bearing and fatigue-resistant in-place stepping can be produced after chronic SCI (Saigal et al., 2004).
In this study, we demonstrate that ISMS can produce long-distances of propulsive walking over ground in anesthetized animals. Distances achieved were more than 10 times longer than those produced by peripheral FES (Mazurek et al., 2012). These findings suggest that ISMS may be a viable approach for restoring functional standing and walking after SCI.
MATERIALS AND METHODS
Overview of ISMS Implants
All experimental procedures were approved by the University of Alberta Animal Care and Use Committee. Five intact male cats (4.2–5.5kg) were used in acute, non-recovery experiments. The cats were anesthetized with sodium pentobarbital and a laminectomy was performed between vertebrae L4 to L6 to expose spinal cord segments L4–S1. A custom microwire electrode array was implanted bilaterally with 12 electrodes per side (e.g., figure 1D). The electrodes were Pt-Ir 80–20%, 50µm in diameter, insulated with 4µm polyimide except for the tip. The tip was deinsulated over a region of 200 – 400µm and beveled to ~15° (Bamford et al., 2016), resulting in an approximate surface area of 43,101µm2.
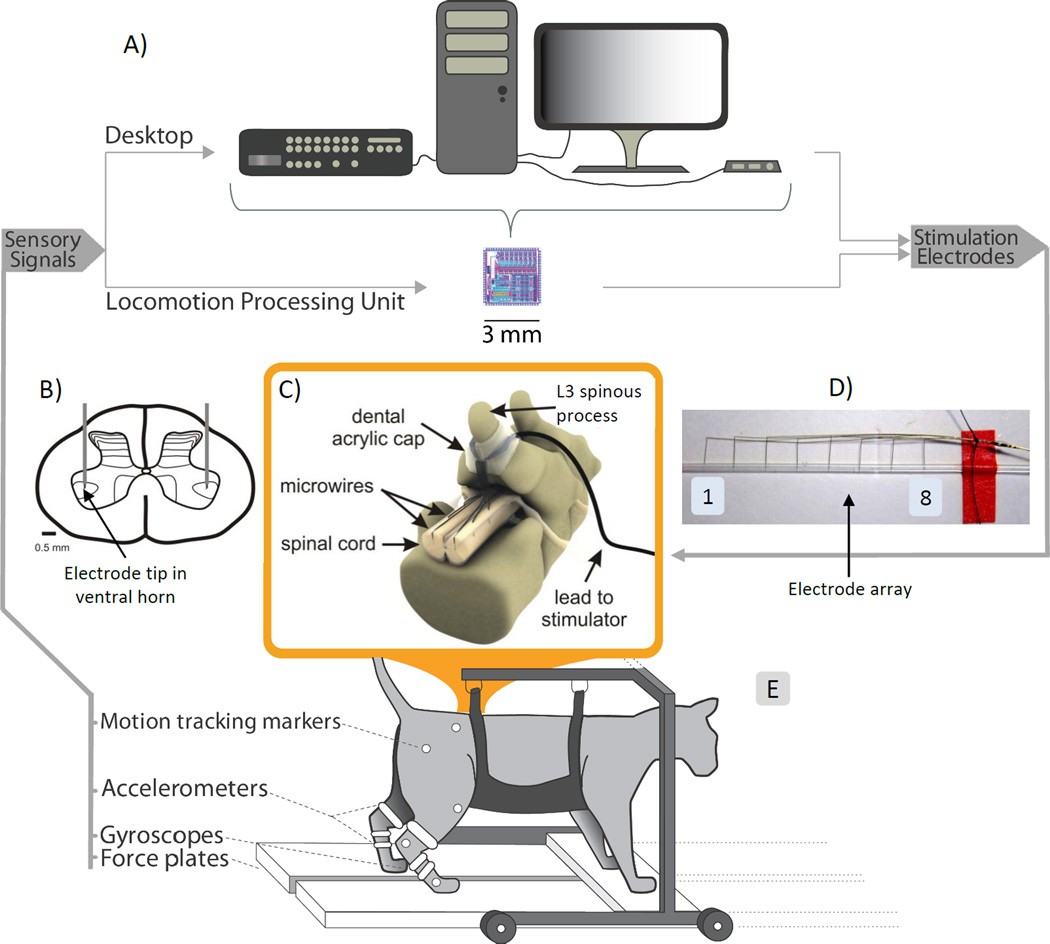
Figure 1. Schematic representation of the experimental setup.
(A) Sensory signals were converted from analog to digital and were used to control a rule-base in a desktop computer that also generated stimulation patterns to the ISMS implant. All these operations could be performed by a specially designed implantable 3mm×3mm chip (Locomotion Processing Unit). (B) Schematic of the target location of the ISMS electrode tips in the ventral horn of the spinal cord. (C) Schematic of the ISMS implant. (D) Picture of a microwire electrode array with 8 Pt/Ir electrodes. The electrode tips were inserted in a silastic tube prior to implantation to protect them from damage. (E) Experimental setup with sensor locations on the suspended cat.
Electrode implantation followed established techniques (Mushahwar et al., 2000, Bamford et al., 2016). Locations in the lumbosacral enlargement producing hip and ankle flexors, knee extensors, and a full limb backward extensor synergy with ISMS were targeted for each side based on previous maps of motoneuronal pools (Vanderhorst and Holstege, 1997, Mushahwar and Horch, 1998, 2000b, Saigal et al., 2004). The ventral horn locations in each side of the spinal cord (figure 1B) activating these four functional limb movements were used to produce a walking cycle in the ipsilateral limb.
While a walking cycle can be activated using a total of four ISMS electrodes per side, improved reliability can be achieved by implanting more than one electrode to produce a given function. In these experiments, 3 electrodes were typically implanted per function and the two electrodes that qualitatively elicited the strongest activations of the desired movement were used during the experiment (for a total of eight electrodes per side). The 16 channels of the stimulator dictated the total number of active electrodes.
Migration of the electrode leads was minimized during walking in two ways: 1) anchoring the tube containing the electrode leads to the L3 spinous process using dental acrylic (figure 1C) as was previously described (e.g., (Mushahwar et al., 2000, Bamford et al., 2016)); and 2) locking the lumbar spine in a hyperextended position using a stiff wire affixed to the L3 spinous process and the iliac crests bilaterally. The latter is a procedure only used in acute (terminal) studies as is the case of the experiments described in the present study.
ISMS Parameters
Stimuli consisted of asymmetric biphasic, charge-balanced pulses 290µs duration, and repeated at a rate of 62Hz. Stimulation amplitudes through each electrode were generally modulated between threshold (<10µA) and 50–75µA that produced the desired forces and ranges of motion necessary for generating propulsive, weight-bearing and natural looking walking movements. With these parameters, current density was 1,740 µA/mm2 and charge density 5.04 nC/cm2. Overall, the maximal level of stimulation did not exceed 125µA for any electrode.
Experimental Setup
After implantation of the ISMS array, the cats were transferred to an instrumented walkway (Guevremont et al., 2007, Mazurek et al., 2012) and partially suspended in a cart-mounted sling and maintained under anesthesia (figure 1). The cart-mounted sling supported the head, forelimbs, trunk, and abdomen while allowing free movement of the hind legs. Motion tracking markers were fixed to the iliac crest, hip, knee, ankle and metatarsophalangeal joints of the right hind leg to record kinematics with a camcorder (120fps, JVC Americas Corp., Wayne, NJ, USA). The camera was positioned 4.5m away from the midpoint of the walkway with the lens parallel to the walkway. Force transducers mounted under the walkway plates captured independent left and right supportive (vertical) and propulsive (horizontal) forces.
ISMS Pattern Generation and Pattern Formation
Walking in the hind legs was generated by stimulation patterns created by a control algorithm that mimicked the physiological control of walking as previously described (Guevremont et al., 2007, Mazurek et al., 2012). Briefly, the control strategy for producing over-ground walking with ISMS resembled a two layer central pattern generator (CPG): the pattern generation (clock) and pattern formation (synergies) layers (Rybak et al., 2006a, Rybak et al., 2006b, McCrea and Rybak, 2008). The pattern generation layer provided an intrinsic timer that oscillated between flexor and extensor phases of the step cycle using preset timing. The step cycle duration was 1.5s and had four sequential step states (one flexion, F; 3 extension E1, E2, E3 as classically defined (Goslow et al., 1973)). These states accounted for 20%, 20%, 20% and 40% of the cycle period, respectively (Mazurek et al., 2012). During each state, the group of electrodes that produced the best upward flexion (F), forward (E1), downward extension (E2) and backward propulsive (E3) movements were used for stimulation. The best movements were defined as movements that: 1) were predominantly in the sagittal plane; 2) generated strong ground reaction or propulsive force with minimal stimulation current; and 3) were graded with increases in stimulation amplitude.
The pattern formation layer delivered the ISMS stimuli through the group of electrodes producing the synergistic movements associated with each phase of the step cycle. Each synergy was produced by stimulating through combinations of ISMS electrodes that elicited the desired movements and force responses. Sensory modifications based on limb position (miniature accelerometers and gyroscopes placed on the foot of each hind leg) and loading (force plates for each hind leg) modified the timing of the rhythmic oscillations and corresponding flexor-extensor phase durations. Sensory input also modified the amplitude of the movement synergies (Rybak et al., 2006a, Rybak et al., 2006b, Guevremont et al., 2007, McCrea and Rybak, 2008, Mazurek et al., 2012).
This over-ground walking controller was implemented in two systems. The first was a desktop system that used a 16-channel bench-top, current-controlled stimulator to deliver trains of pulses through 8 electrodes per side. The second system consisted of a custom integrated circuit (3mm × 3mm), called the locomotor processing unit (LPU), and contained the control logic and 14 channels of on-chip stimulators. For both systems, the phases of the step cycle in the left and right hind legs were coordinated to be out of phase with a period of double-limb support. The timing of each phase of the step cycle and the amplitude of stimulation were modified in real-time by sensory signals indicating limb loading and position using the same control strategy implemented previously with intramuscular stimulation (Mazurek et al., 2012).
Locomotion Processing Unit
The LPU is a miniaturization of the components required for an ISMS-based neuroprosthesis (Mazurek et al., 2010, Mazurek and Etienne-Cummings, 2011, Mazurek et al., 2016). The prototype was a proof-of-concept towards an implantable version in the future. In this study, the LPU was connected to the electrode leads and rested on the cart supporting the cat’s body. The goal of the in vivo testing with the LPU was to demonstrate that walking with kinematic and kinetic properties similar to the desktop system could be produced using a single integrated circuit. The core decision-making component of the LPU contained a programmable, state-based realization of the walking cycle capable of implementing previously-developed control strategies (Guevremont et al., 2007, Mazurek et al., 2012). Similarly, state transitions occurred based on programmed times or sensory input. The LPU contained onboard stimulators that generated trains of biphasic, cathodic leading stimulation waveforms to produce walking. The LPU was used for a subset of trials in cat E.
The LPU was designed to produce a comparable control strategy and stimulation output to the desktop system. Its architecture contained state-based control logic and each state allowed for programming the stimulation amplitudes of each of the 14 output channels, along with the rules which would cause a state transition (Mazurek et al., 2010, Mazurek and Etienne-Cummings, 2011, Mazurek et al., 2016). State transitions could be programmed based on timing rules or sensory rules. This system allowed for realizing IF-THEN rules similar to those previously demonstrated to produce walking patterns (Mazurek and Etienne-Cummings, 2011). The outputs were cathodic-leading, biphasic (245µs per phase, up to 125µA in amplitude) stimulation pulses with a reset switch for charge balancing. The stimulation frequency was set using integrate-and-fire neuron circuits implemented on chip (Vogelstein et al., 2008, Joucla et al., 2016, Mazurek et al., 2016). The silicon neurons triggered the generation of an output stimulus waveform through each channel allowing all 14 channels to operate in parallel. Variability in the integrated circuit components (potentially due to fabrication mismatch when creating the integrated circuit), caused for slight variance in the inter-channel stimulation frequency, but the value was chosen to be as close to 60Hz as possible (range: 55–65Hz). Stimulation amplitudes were modified to match the movements generated by the desktop system.
The LPU was housed in a prototype custom designed printed circuit board which also recorded the kinematic and kinetic data through a microprocessor (further removing constraints of the desktop recording system). The average power (Pavg) of the LPU during the operation of an open loop walking cycle (1.5s walking period, 60Hz stimulation frequency, biphasic, 245us pulse width, 125µA on all channels) was estimated to be 0.62mW. This is based on the average amount of power consumed during state transitions and producing ISMS stimulation for the 8 state walking cycle (a full derivation can be found in (Mazurek et al., 2016)). As a comparison, if the same power analysis were performed for an intramuscular stimulation experiment using this same setup but with a maximal stimulating current set to 20mA per phase, the resulting power requirement would be 41.34mW (assuming 14 channels were active for the same duration as above). More detailed information about the design of the LPU can be found in Mazurek et al. (Mazurek et al., 2016).
ISMS Walking and Evaluation
During initial setup, appropriate stimulation amplitudes were established for each step state (F, E, E2, E3) in each hind leg. The initial stimulation parameters were adjusted through trial and error to produce a walking cycle with adequate force (ground reaction and propulsive) capable of lifting the hind legs above the sling and propel the cat forward. Once the ISMS parameters were established (during an average of 9 set up trials collected over ~10min), data collection began and the hind legs bilaterally propelled the cat and cart a distance of 2.9m (the walkable length of the walkway). For the majority of the trials, the mass of the cart and equipment on it was offset by a pulley system to reduce the effect of static friction (by pulling the cart forward with about 5–10% of the cat body mass). Because a main focus of the study was to evaluate the distances walked with ISMS, open-loop, timing-only control was used for most trials. In some trials which were dispersed randomly throughout the experiment, the controller processed incoming signals from external sensors (force transducers, accelerometers and gyroscopes) in real time with a moving average filter of 120ms using custom-written Matlab routines (MathWorks Inc., Natick, MA, USA). Detailed analysis of the control strategy has been previously reported (Mazurek et al., 2012). Experiments were terminated when the cat could no longer traverse the walkway or after a total count of 300 walking trials, whichever came first. The duration between the completion of one trial and the initiation of the next trial was ~1 min.
Across all experiments, an individual trial was considered successful if the cat traversed the entire length of the walkway (2.9m) within 90 seconds. To account for variations in stepping when walking was initiated and terminated within a trial, a 2.2m section in the middle of the walkway was used for analysis. Marker positions were digitized by custom Matlab software written by Dr. Douglas Weber (University of Pittsburgh, Pittsburgh, PA, USA). Each frame was calibrated with a known set of reference markers. All kinematic parameters were calculated from the marker set, and filtered using a 3Hz low pass Butterworth filter (10th degree).
The beginning of each step was indicated by touchdown on the force plate. Stepping parameters were determined for each step and averaged across a trial. Ground clearance was recalibrated for each step with respect to the walkway location to minimize tracking error. The coupling of movements between 2 joints was assessed using the angular component of the coefficient of correspondence (ACC), which was calculated according to methods described by Field-Fote & Tepavac (Field-Fote and Tepavac, 2002).
Throughout the experiments, stimulation amplitudes were adjusted to ensure successful traversing of the walkway. For cats C, D and E, the walking outcome variables were averaged for the first 10 and last 10 successful trials to evaluate walking performance over the course of the experiment. Stimulation amplitudes increased in ~5% increments throughout the experiment for flexors (mean: 49 to 74µA) and extensors (41 to 69µA). Minor changes in performance (peak ground clearance, stride length, supportive forces) were seen throughout the walking trials as increased stimulation amplitudes were applied to maintain weight bearing and propulsive walking.
At the end of the experiment, the cats were kept deeply anaesthetized and perfused intra-cardially with Formalin (4 % formaldehyde). The spinal cords were extracted with the implanted microwire electrode arrays in place and imaged using magnetic resonance imaging (MRI) to determine the location of the electrode tips in the ventral horn. The images of the spinal cords were acquired using a 4.7 Tesla magnet at the Peter S. Allen MR Research Centre of the University of Alberta. A 3D gradient echo MRI sequence with 0.2mm × 0.2mm × 0.2mm resolution was used and the electrode tips were located based on the tissue-electrode contrast of the acquired images. The electrodes caused perturbations of the magnetic field resulting in a signal dropout in the corresponding areas of the images. The electrodes were then removed and the spinal cord was sectioned in 50µm transverse slices and histologically processed. Transverse histological slices containing electrode tracks were compared to the corresponding MRI slices (figure 2, top). The comparison between the two sets of images was made possible by taking advantage of three features: 1) the shape of the gray and white matter which changes distinctively depending on the location in the lumbosacral enlargement; 2) the angle of electrode insertion in the cord (MRI) or electrode path (histology); and 3) the distance of a given slice from the start or end of the imaged/processed block of tissue. Using these features, we were successful in matching MR and histological images for all electrodes. The tip of an electrode was identified as the ventral edge of the signal loss in the MRI slice. The histological analysis helped discard the possibility that electrodes may have moved during animal perfusion or spinal cord extraction. The tip locations of all electrodes used for ISMS across experiments were collated and transposed on a series of representative cross-sections throughout the lumbar enlargement.
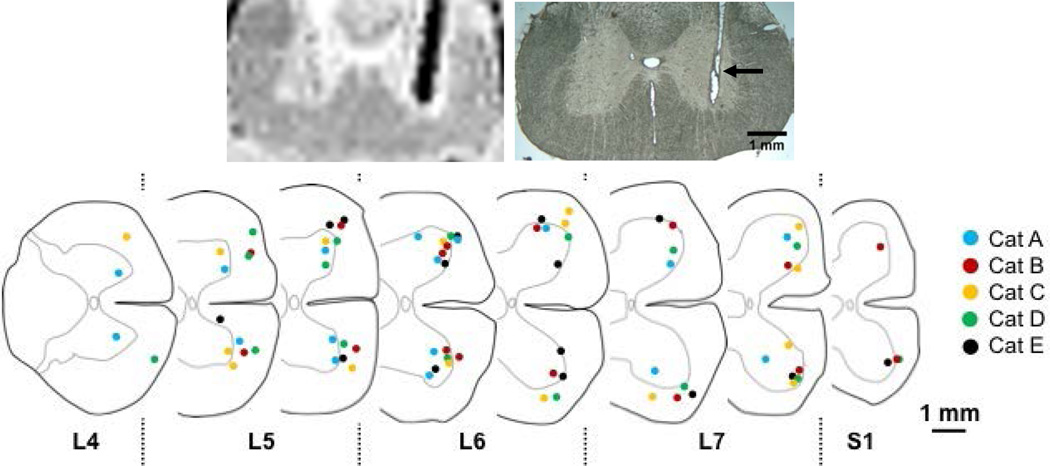
Figure 2. Location of electrode tips in the ventral region of the lumbosacral enlargement.
Top left: a typical 0.2mm thick MRI slice showing the location of a microwire in situ. The location is indicated by the signal loss (dark black line). The signal loss is ~400 µm, 8 times larger than the diameter of the Pt-Ir ISMS microwire electrode. Top right: a 50µm cross section of the spinal cord centered on the same electrode shown in the MRI slice. The location of the electrode track is indicated by the arrow. Using MRI slices and subsequent sectioning of the extracted spinal cord, the tip locations of the electrodes used for ISMS in each experiment were identified. Bottom: Summary of the electrode tip locations for each of the five experiments; 60.7% of the electrode tips were located in the gray matter.
Statistical Analysis
Student’s t-test was used to test the differences between group means with α = 0.05. Statistical significance was achieved with p ≤ 0.05. A squared Pearson correlation coefficient (r2) was used to measure the linear relationship between variables where appropriate. Results are reported as mean ± standard deviation (unless otherwise indicated).
RESULTS
Intraspinal Activation Sites
The tip locations of the electrodes selected for stimulation were determined and are summarized in figure 2 (bottom) from all cats. Across all experiments, 48 out of the 79 electrode tips were positioned in the gray matter (60.7%). For cats A–E, the percentage of the tip locations in the gray matter was 94%, 56%, 50%, 50%, and 53%, respectively. Stimulation amplitudes at the beginning of the experiments were, on average, higher for evoking satisfactory flexor movements (range of movement) than extensor movements (range of movement and force). The mean stimulus amplitude for flexor movements was 69.1±28µA and that for extensor movements was 52.6±18µA. The mean stimulus amplitudes for the flexor and extensor movements were not significantly different from each other (p=0.064).
ISMS-Produced Over-ground Walking
The goal of each experiment was to produce walking capable of propelling the cat a cumulative distance of 870 m (300 trials). The walking produced by ISMS was evaluated by comparing kinematics and kinetics to published literature on freely walking cats (e.g., (Halbertsma, 1983)). Unless otherwise stated, results are from trials that used the desktop system to produce walking.
Results from one trial in which open-loop, timing-only control was used are shown in figure 3 after the cat (cat E) had already performed 281 successful trials (cumulative walking distance of 815m). This trial tested the maximal force output of the cat hind legs by increasing the rolling resistance of the cart and the stimulation amplitudes. The rolling resistance was increased by removing the unloading that offset the weight of the suspension cart and associated equipment. The amplitudes across all electrodes were ~30% higher (average current per electrode: 59µA) than during the previous trials. Stick figure representations of the right hind leg at 125ms intervals are shown in figure 3A. The stride length ranged from 123mm to 179mm depending on the variable resistance between the walkway railings and suspension cart during a given trial. The average stride length for the trial (153±15mm) was less than the average value previously reported for freely waking cats (200mm) (Halbertsma, 1983), although statistical comparisons were not performed. This difference could result from the slower average walking speed (0.12m/s vs. 0.20m/s) since stride length increases with walking speed (Halbertsma, 1983), and the lack of participation of the forelimbs in translating the body forward. The average peak ground clearance of the paw was 10.3 ± 3.6mm which was less than the 16mm clearance reported for natural walking, but still provided adequate clearance (Lavoie et al., 1995). The limb angle (vector from hip to metatarsophalangeal joint) in figure 3B–C reached a maximal backward excursion (from forward horizontal, measured clockwise) of 127°, which was comparable to the 130° reference value reported for natural walking (figure 2c in Shen & Poppele (Shen and Poppele, 1995)).
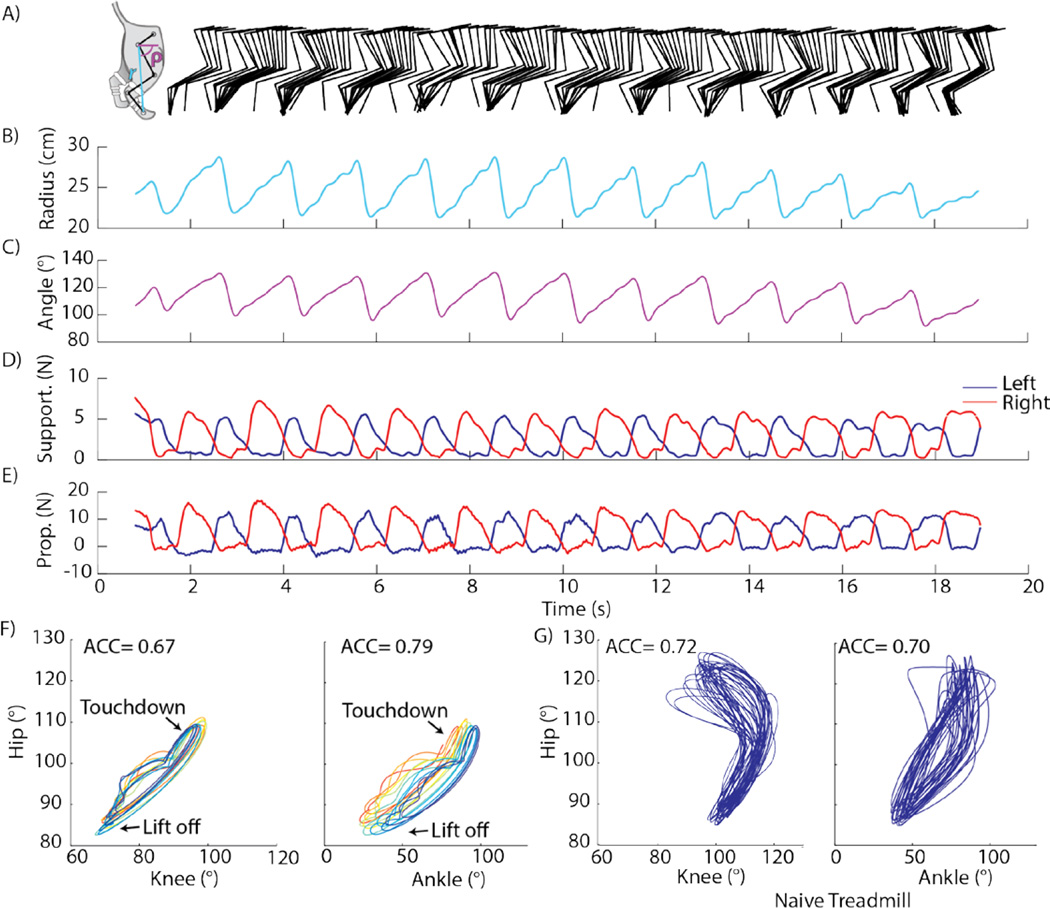
Figure 3. Results from an ISMS walking trial with maximal force production (cat E).
(A) Stick figure representation of the right hind limb from video motion capture. Cartoon insert shows angular convention for kinematic parameters. (B) Distance between the hip marker and metatarsophalangeal (MTP) joint. (C) Angle of the vector described in (B) with respect to forward horizontal, measured clockwise. (D) Supportive (vertical) forces. (E) Propulsive forces (parallel to walking direction). (F) Angle-angle plots of hip, knee, and ankle joints with each step shown in a different color. (G) Similar to (F) for walking on a treadmill by a normal (naïve) cat.
Successful over-ground walking also requires sufficient supportive and propulsive forces. Figure 3D depicts traces of the left and right supportive forces (vertical). For this trial, the average peak supportive force during the stance phase was 5.2N and 6.0N for the left and right hind legs, respectively, and the mean supportive force was 3.0N and 3.7N, respectively. These peak stance forces corresponded to 12.1% and 13.9% body weight (BW), and were comparable to a peak step force of 17.1% BW produced using peripheral muscle stimulation (Mazurek et al., 2012). The supportive forces reflect the weight bearing of the hind legs (excluding the abdomen which is supported by the sling). While they are lower than the weight normally supported by the hind quarters of a cat which include the abdomen (46% of BW during stance (Macpherson, 1988)), these supportive forces produced full weight-bearing of the hind legs during stance. The peak propulsive forces (figure 3E) averaged across all steps within this trial reached 12.0N and 14.4N for the left and right legs, respectively. When considering the combination of the propulsive and supportive forces, the magnitude of the resulting force vector was 12.9N for the left leg and 15.5N for the right leg (corresponding to 30% BW and 36% BW, respectively). Thus, ISMS produced sufficient resultant force for the cat to propel itself and the cart across the walkway.
Regions of double stance (where both limbs are on the ground) facilitate safe transitions between the swing and stance states for each limb when walking at slow to moderate speeds. The average duration of double stance was 22% of the walking cycle compared to 20% of the walking cycle reported for naturally walking cats at similar speeds (Lavoie et al., 1995). During these regions of double stance, the mean total supportive force was 4.8N (11.2% BW).
The consistency of the step cycle throughout a trial was evaluated by measuring the intralimb coordination using the angular component of the coefficient of correspondence (ACC) (Field-Fote and Tepavac, 2002). The ACC has a range from 0 to 1, where 0 implies no consistency and 1 is complete consistency between the joint movements in multiple step cycles. We calculated the ACC using joint angles measured from video recordings of the right hind limb. Plots of hip-knee and hip-ankle angles are shown in figure 3F with each step shown in a different color. For comparison, angle-angle plots from a cat walking freely on a treadmill are shown in figure 3G (“naïve treadmill”). The ACC for the hip-knee relationship was 0.67 (compared to a normal ACC of 0.72) and for the hip-ankle relationship was 0.79 (compared to a normal ACC of 0.70). The area of the angle-angle cyclogram represented the conjoint range of movements and was 0.49 (normal=1.47) for the hip-knee and 1.41 (normal=1.12) for the hipankle (Goswami, 1998). The deviations from normal data are due to a decreased hip range (imposed by the setup) and increased ankle range. Taken collectively, these results demonstrate that ISMS successfully produced walking kinematics and kinetics comparable to those reported for a healthy walking cat.
ISMS Produced Fatigue-Resistant Walking
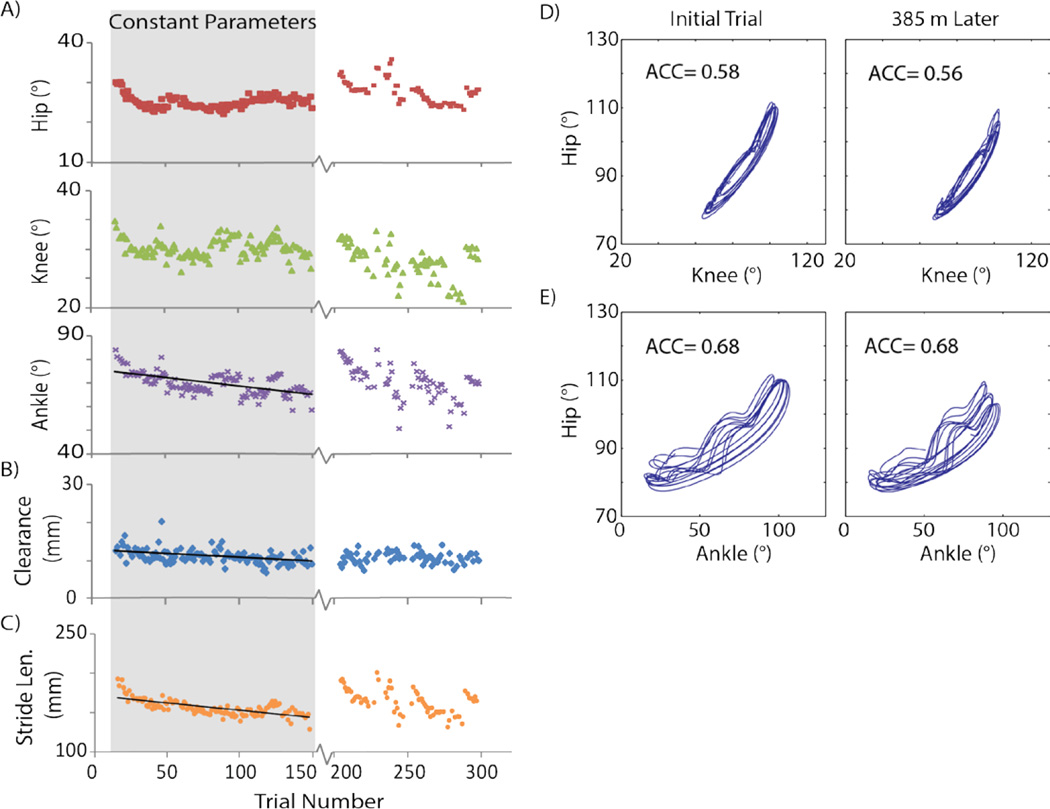
To be clinically useful, an intervention for restoring walking has to provide the ability to walk for functionally long distances. Therefore, the walking produced by ISMS in this study was analyzed over 385m to measure the sustainability of movements. During this subset of trials (133 trials from cat E), stimulation parameters (amplitudes, timing patterns) were held constant to test for effects of fatigue, and sensory-driven modulations were not used. All successful walking trials obtained using the desktop system (LPU trials not included) in cat E are shown in figure 4; however, the gray region highlights the subset of 133 trials with constant stimulation parameters (290µs pulse width, 62Hz frequency, 34.5µA mean amplitude to produce extensor movements, 49.5µA mean amplitude to produce flexor movements). A linear regression was performed to determine if there was a significant relationship between trial number and the outcome walking variables. The top, middle, and bottom panels of figure 4A show the average ranges of the hip, knee and ankle angles per walking trial, respectively. During these 133 trials, the hip and knee joint ranges did not show a significant correlation with trial number (i.e., distance walked) suggesting that there was no decay in these parameters. Nonetheless, there was a significant negative relationship between trial number and ankle joint range (p<0.001, r= −0.60), ground clearance (figure 4B, p<0.001, r= −0.43), and stride length (figure 4C, p<0.001, r2= −0.70), indicating that these variables decreased in value over walking distance. Over the course of these trials, the ankle joint range decreased from 74.8° to 65.0° (a difference of 13%), the average ground clearance changed from 12.6mm to 9.75 mm (23%), and average stride length decreased from 192mm to 161mm (16%).
Figure 4. Kinematics of ISMS-produced walking in cat E using the desktop system.
(A) Range of hip, knee, or ankle joints across all trials in experiment. (B) Average peak ground clearance of the paw. (C) Average stride length. (D) Angle-angle plot demonstrating consistency of walking at the beginning and end of the trials with constant stimulation parameters (first 133 trials) for the hip-knee relationship. (E) Similar to (D) for the hip-ankle relationship. Data from a total of 239 trials are shown (133 open-loop trials with constant parameters; 23 open-loop and 83 closed-loop trials with varying parameters, randomly interspersed). Trials using the LPU (between 150 and 200) are not shown.
The angle-angle plots, shown in figure 4D, compare a trial near the beginning with one near the end of the trials with constant stimulation parameters to evaluate the changes in intralimb coordination. The first panel shows the hip-knee plot with an ACC of 0.58 for the early trial. In the second panel, after walking 325m, the ACC was 0.56. Similarly, in figure 4E angle-angle plots are shown for the hip-ankle relationship with an ACC of 0.68 for both trials. Overall, movements produced by ISMS showed little degradation in multi-joint coordination over extended periods of walking when all control and stimulation parameters were held constant.
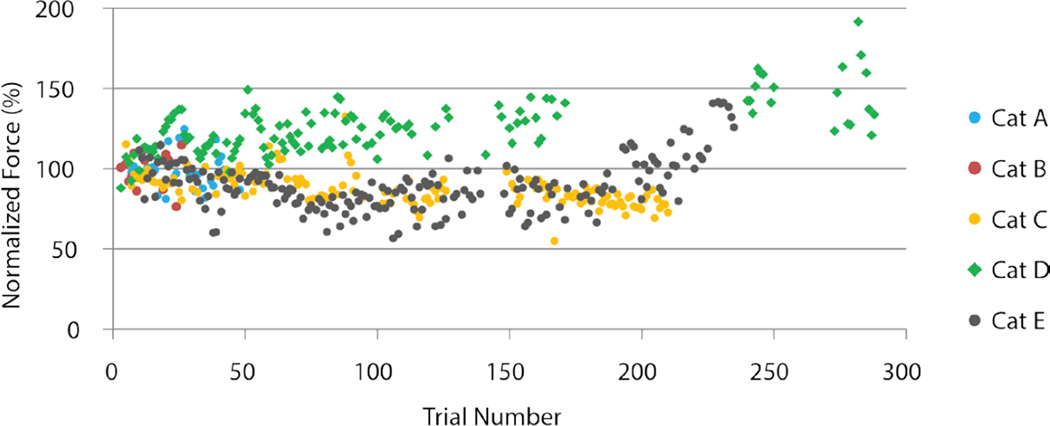
In figure 5, peak left and right supportive forces for each trial were normalized to the average of the first five trials and plotted for the entire experiment. This experiment (cat E) was divided into three sections: the first 133 trials with the desktop system where the stimulation parameters were held constant (open-loop), the 42 trials where the LPU was implemented with parameters adjusted as needed (open-loop), and the remaining trials using the desktop system where the stimulation parameters were also adjusted as needed (23 open-loop and 83 closed-loop randomly interspersed). During the constant stimulation trials, the supportive forces showed a significant negative relationship with trial number (Left, r= −0.41, p<0.001; Right, r= −0.40, p<0.001). Predicted forces showed a 4.8% decrease (from 93.6% to 88.8%) for the left leg, and a 5.7% decrease (from 94.3% to 88.6%) for the right leg with respect to the initial values over a distance of 385m (133 trials). Small increases in stimulation amplitudes using the LPU and the desktop system restored the force production back to the initial values as shown in figure 5 (trials 150 and higher).
Figure 5. Supportive forces produced during ISMS walking for cat E including both systems (desktop, LPU).
Average peak supportive forces for the left and right hind legs are shown per trial. Forces were normalized to the average produced during the first 5 trials. Linear trend lines were fitted for each limb to the trials with constant stimulation parameters. The forces produced by the LPU (dark shaded region) were similar to those produced by the desktop system. Stimulation amplitudes and control parameters were modified during the desktop system trials performed after the LPU trials. Data from a total of 281 trials are shown (133 open-loop trials with constant parameters; 42 open-loop LPU trials with varying parameters; 23 open-loop and 83 closed-loop trials with varying parameters, randomly interspersed).
A summary of the supportive forces per walking trial from all cats is shown in figure 6. The average of the peak force produced in each trial was normalized to the average peak force produced in the first successful trial. Trials that were produced with open-loop, timing-only control and with the desktop system are shown. With the exception of cats A and B after which the stimulation paradigm was modified, ISMS produced well supported over-ground walking for long distances (i.e., large number of trials). Minor reductions in the supportive force were corrected by minor increases in the stimulus amplitude.
Figure 6. Changes in the level of supportive force during walking.
Shown are the average peak supportive forces (ground reaction) per walking trial for all animals as a function of trial number (walking distance). The average peak supportive force obtained during each walking trial was normalized to the average peak supportive force obtained during the first walking trial for each cat.
For comparison, in walking experiments using intramuscular stimulation and the same experimental setup and control strategy, an average of only 18±7 trials (52.2±20.3m) could be obtained before the cats could no longer traverse the walkway due to muscle fatigue (Mazurek et al., 2012). Walking was ISMS was maintained for a total of 300 trials (the longest tested) resulting in up to 835m of successful cumulative walking with only minor modifications to the stimulation amplitudes. Moreover, walking with ISMS required an average stimulation amplitude across all electrodes of 44.3±15.0µA while that with intramuscular stimulation required 7.3±0.2mA.
Implementation in an Integrated Circuit
The LPU was tested in cat E. With it, the cat successfully completed 42 trials (most tested) equaling a walking distance of 121.8m. The mean stimulation levels of the flexor and extension groups were 47.1±17.3µA and 30.0±3.4µA, respectively. This was qualitatively similar to the corresponding mean stimulation levels with the desktop system (51.3±20.3µA and 37.1±10.2µA, respectively). Overall, the LPU produced ranges of movement for the hip, knee, and ankle of 23.0±1.9°, 29.4±2.3°, and 59.4±4.5°, respectively, which were qualitatively similar to those produced by the desktop system for the remaining trials in the same experiment (26.7±2.5°, 29.4±2.5° and 70.0±5.7°, respectively). The mean supportive forces for walking with the LPU were 2.0±0.4N compared with 1.7±0.4N with the desktop system. Walking speeds of 0.13±0.03m/s and 0.16±0.02m/s were obtained by the LPU and desktop system, respectively.
Consistency of Walking Movements
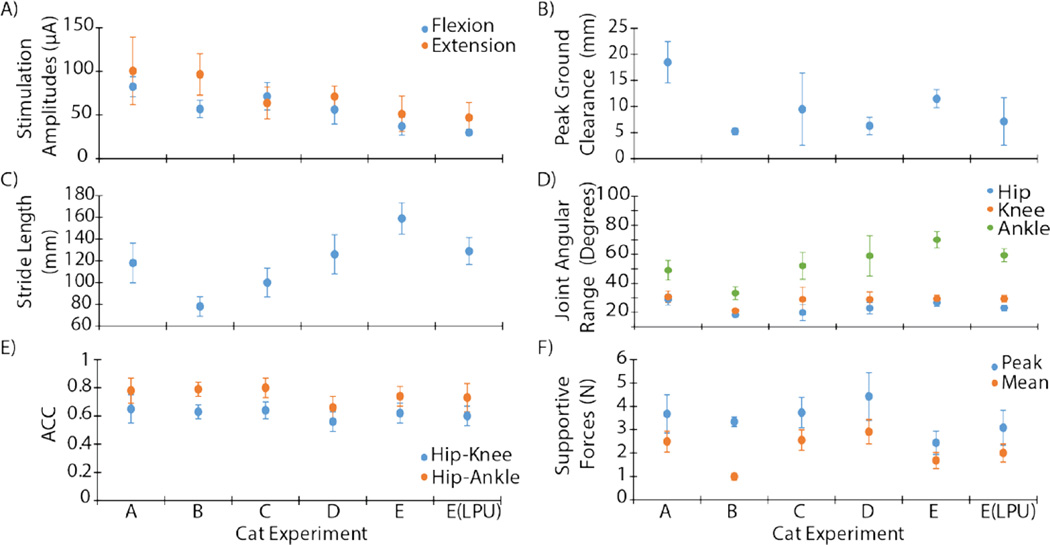
Comparative walking metrics for 5 cats are shown in figure 7. In the first two cats (A and B), the electrodes produced weaker movements requiring higher stimulation levels to elicit the desired response (figure 7A). The remaining 3 cats (C, D, and E) each walked a total of 300 trials across the walkway (maximum tested) of which at least 210 trials were considered successful during post-hoc analyses. These three cats successfully walked between 609m and 835m and their results are averaged in table 1. Walking distances, kinematics and force production were similar across these cats. The peak ground clearance and stride lengths for these experiments were 9.1±1.5mm and 128.3±17.1mm (mean ± SEM), respectively. The angular joint ranges for the hip, knee, and ankle for these experiments were 23.1±2.0°, 29.1±0.2°, and 60.3±5.2° (mean ± SEM), respectively. The average ACC value across these experiments was also computed for the Hip-Knee and Hip-Ankle relationships as 0.6±0.02 and 0.7±0.04 (mean ± SEM).
Figure 7. Comparison of the different parameters and walking metrics between experiments (cats A–E).
(A) Stimulation amplitudes for producing flexor and extensor movements. (B) Peak ground clearance. (C) Stride length. (D) Joint angular range for hips, knees, and ankles. (E) ACC Coefficient for hip-knee and hip-ankle joint pairs. (F) Average peak and supportive forces.
In terms of overall steps taken during these three experiments, the variation is correlated to the range in overall walking distance. The average number of steps taken per experiment was 4384.0±837.0. The average kinetic data are also shown for peak supportive force and mean supportive force during stance as 3.5±0.6N and 2.4±0.4N, respectively, with an average force duration time of 0.8±0.1s.
In addition, walking metrics were compared at the beginning and end of each experiment to determine the effect of fatigue. An average of the first 10 successful trials was compared to an average of the last 10 successful trials for cats C, D, and E. Peak ground clearance and stride length decreased from 11 to 7mm and 140 to 126mm, respectively. In addition, the supportive forces increased from 3.5 to 4.0N suggesting that by increasing stimulation amplitudes to compensate for changing kinematics, forces were subsequently increased as well. Collectively, these results demonstrate that ISMS can consistently produce functional over-ground walking for long distances.
DISCUSSION
The goal of this study was to investigate the ability of ISMS to produce over-ground walking. We reported in the past that ISMS can produce weight-bearing in-place stepping after chronic SCI (Saigal et al., 2004). In the present study, we demonstrated, for the first time, that weight-bearing and propulsive over-ground walking is achievable with ISMS. We also demonstrated that this ISMS system can be implemented in a small chip that is suitable for implantation in the future. Walking over ground requires both supportive and propulsive forces to propel the body and achieve forward progression, a combination of forces that is currently not achieved in human FES systems. Weight-bearing and propulsive over-ground walking was achieved with ISMS by eliciting coordinated, multi-joint synergies in the hind legs through methodical activation of spinal networks related to locomotion.
Characteristics of walking achieved with ISMS
Long distances of successful over-ground walking were produced by ISMS with minor reductions over time in some walking metrics. Reductions in supportive and propulsive forces or changes in walking kinematics were corrected by small increases in ISMS stimulus amplitudes. Even with such increases, the total amplitude of stimulation per ISMS electrode remained below 125µA, the maximal level for safe charge injection through the electrodes used in this study. The results were reproducible over multiple cats and suggest that variations in anatomy and physiology between subjects do not affect the reproducibility of fatigue-resistant walking.
Walking produced by ISMS was highly responsive to stimulation onset and offset. Unlike stepping movements evoked by epidural stimulation, transitions between flexor and extensor phases with ISMS were predictable and well-modulated based on sensory input. Moreover, walking produced by ISMS did not depend on the administration of various excitatory pharmacological agents or long-durations of treadmill locomotor training (van den Brand et al., 2012). Walking was also not reflex-driven as is the case when stepping on the moving belt of a treadmill (Bouyer and Rossignol, 2003, Rossignol et al., 2004) or by stimulating rostro-dorsal regions of the lumbar cord (Barthelemy et al., 2007). Instead, walking with ISMS in this study was consistently and purposefully driven by a feed-forward system that is essential for functional ambulation. Feedback modulation of the feed-forward system ensured responsiveness to changes in the walking environment.
The control strategies used to modulate the electrical stimulus between various electrodes plays a critical role in any FES system. For the restoration of walking, these control systems ensure the production of safe and predictable walking. Epidural stimulation depends mostly on an ON-OFF tonic train of non-specific stimulation to the dorsal surface of the spinal cord to produce rhythmic locomotor-like patterns, or assist in enhancing walking capacity after incomplete SCI. While this approach appears to be suitable for incomplete SCI where residual voluntary drive is present (Herman et al., 2002, Carhart et al., 2004), it is inadequate for producing functional weight-bearing stepping or walking over ground after complete SCI. Phasic stimulation patterns with appropriate modulation of current amplitudes through targeted electrodes produces more reliable functional walking movements. For this reason, current FES systems using peripheral nerve or muscle stimulation for the restoration of walking utilize user-operated switches to control stimulation patterns that produce transitions between flexor and extensor phases of walking. Nonetheless, the resulting walking using this strategy is very slow and energetically costly (Popovic et al., 2003). In the present experiments, we used a physiologically-based control strategy that allowed for safe automatic transitions between the flexor and extensor phases of walking, while also modulating stimulus amplitude in response to fatigue, and phase durations in response to environmental perturbations (Guevremont et al., 2007, Mazurek et al., 2012). We anticipate that similar control strategies would be capable of producing safe and more energetically efficient walking in future FES applications in humans (Popovic et al., 2003).
A single, slow walking speed (cycle duration of 1.5 s) was used in this study and varying walking speeds were not tested. Faster walking speeds can be achieved with ISMS by modifying the duration of the gait cycle in the controller. This in turn would automatically change the time allocation for each of the four phases of walking (F, E1, E2 and E3) and their corresponding synergies.
Mechanisms of Action of ISMS Relative to Other FES Interventions
The use of electrical stimulation for restoring function after SCI is primarily based on the activation of neural elements that remain intact below the level of injury. Regardless of the site of stimulation (e.g., dorsal surface of the spinal cord, inside the spinal cord, peripheral nerve or muscle), the principles of neural excitation with electrical stimulation are similar. Electrical stimulation activates axons more easily than cell bodies, and axons with larger diameters are more excitable with electrical stimulation than those with smaller diameters. Moreover, the environment surrounding the neural elements plays an important role in distributing the electrical currents. These biophysical principles underlie the outcomes of electrical stimulation applied in the periphery, to the dorsal surface of the spinal cord and inside the spinal cord.
When applied to peripheral nerves or muscles, an environment where only axons are present, electrical simulation activates the largest myelinated axons first, depending on the distance between the axons and the electrodes. Because the largest motor axons innervate large, fast-fatigable muscle fibers, large forceful movements are produced with electrical stimulation, followed by rapid fatigue. This in turn plays an important factor in limiting the distances of weight-bearing walking that can be produced. Moreover, due to the preferential activation of large axons, the movements generated by peripheral FES cannot be easily graded; instead, large changes in force are obtained with small changes in stimulus amplitude. The lack of gradation in muscle force increases the challenges in controlling the generation of movement (Grahn et al., 2014). This was the case in our work with intramuscular stimulation for producing standing and over-ground walking, where extensive time and effort was invested in adjusting the parameters of stimulation in each experiment (Guevremont et al., 2007, Lau et al., 2007, Mazurek et al., 2012).
When applied to the epidural surface of the spinal cord, the environment near the electrodes is comprised of various materials such as meninges, cerebrospinal fluid (CSF), epidural fat, spinal cord white matter, dorsal rootlets and spinal cord gray mater. Among all elements, the CSF has significantly higher electrical conductivity (~3× the longitudinal conductivity of white matter, ~7× the conductivity of the gray matter and ~21× the transverse conductivity of white matter) (Sin and Coburn, 1983, Struijk et al., 1991, Barolat et al., 1993). Therefore, most of the electrical current flows through the CSF. Nonetheless, analysis of the exact current pathway is complex due to variables such as geometry and volume of the anatomical elements, position and spacing of electrodes, number of cathodes/anodes and frequency dependence of the electrical properties of biological tissue. Therefore, extensive two- and three-dimensional models of the thoracic spine and spinal cord were developed and simulations using finite element analysis of epidural stimulation were conducted (Coburn, 1980, 1985, Coburn and Sin, 1985, Holsheimer et al., 1991, Struijk et al., 1991, Struijk et al., 1993a, Struijk et al., 1993b, Holsheimer, 1998). The results suggest that the following elements are activated using epidural stimulation: dorsal root fibers, 0.2 – 0.3 mm depth of the dorsal and dorsolateral white matter of the spinal cord and posterior spinocerebellar tracts. Collectively, these elements do not directly activate the locomotor networks in the ventral region of the spinal cord, making the control of such networks for the purpose of walking after complete SCI rather difficult and unachievable in the near future (http://www.nibib.nih.gov/2015-nibib-consortium-report).
Intraspinal microstimulation directly targets the ventral regions of the spinal cord in the lumbar enlargement. In addition to the motoneuronal pools, this region contains large proportions of the neural networks involved in locomotion (Kiehn, 2006). Within this region, ISMS activates fibers in passage composed of afferent projections in the ventral horn and propriospinal neurons. These in turn activate motor neurons trans-synaptically; thus recruiting them in a near normal physiological order that leads to graded production of force with increases in stimulus amplitude. Moreover, these fibers functionally connect the motoneuronal pools of synergistic muscles together while inhibiting antagonistic pools. This leads to the generation of coordinated, multi-joint synergies through inherently built networks; thus reducing the challenge of coordinating the activity of individual muscles faced by peripheral FES approaches. Because of the direct activation of ventral neural elements related to locomotion, ISMS produces predictable movements that are weight-bearing, and not dominated by flexor withdrawal-like responses that are commonly seen in movements elicited with epidural stimulation (e.g., (van den Brand et al., 2012)) or ISMS in the dorsal regions of the cord (Barthelemy et al., 2007).
Implications of Findings to Humans with SCI
While the ISMS results in this study were demonstrated in anesthetized animals, we anticipate that they may be applicable in humans for several reasons. First, ISMS was able to generate weight-bearing, in-place stepping in animals with chronic, complete SCI (Saigal et al., 2004). The locations in the spinal cord and the stimulation thresholds producing the various gait-related synergies with ISMS in the animals with chronic SCI (Saigal et al., 2004) were similar to the locations and thresholds seen in this study, as well as the locations and thresholds seen in chronically-implanted, intact, awake animals (Mushahwar et al., 2000). The primary difference between the anesthetized animals in this study and the unanesthetized animals in Saigal et al. (Saigal et al., 2004) and Mushahwar et al. (Mushahwar et al., 2000) is the strength of movements generated with ISMS: larger supportive forces were generally produced in the unanesthetized animals. Second, the organization of motoneuronal pools in the lumbar enlargement of the spinal cord is largely preserved between species and is expected to be similar in humans (Sharrard, 1955, Romanes, 1964, Sharrard, 1964, Nicolopoulos-Stournaras and Iles, 1983, Portal et al., 1991, Vanderhorst and Holstege, 1997). Therefore, similar movements of the legs may be produced in humans, although exploratory testing in a limited number of people with SCI demonstrating this outcome is needed.
The stimulation amplitudes with ISMS were at least two orders of magnitude lower than those used in other FES techniques (Kobetic et al., 1997, Sharma et al., 1998, Kobetic et al., 1999, Brissot et al., 2000, Davis et al., 2001, Stein et al., 2005, Lau et al., 2007, Mushahwar et al., 2007, Mazurek et al., 2012, Triolo et al., 2012). Although the differences in current amplitudes could partly be explained by the different surface areas between ISMS and intramuscular electrodes, the lower stimulation currents allow a portable device to consume less power and operate for longer durations between battery recharges. The average power consumption per walking cycle produced by the prototype LPU was less than 0.62mW, while that for the same system integrating intramuscular stimulation is 41.34mW.
Future work will focus on designing an ISMS implant for human use, optimizing power consumption to incorporate wireless operation, and verifying the performance of ISMS over rougher terrain. Moreover, the use of volitional intent signals for intuitive control of ISMS paradigms for standing and walking will also be utilized (Mushahwar et al., 2006, Moritz et al., 2008, Ethier et al., 2012, Shahdoost et al., 2014, Shahdoost et al., 2015). ISMS may also be used as a therapeutic approach to promote targeted plasticity and long-term recovery after incomplete SCI (Mondello et al., 2014, McPherson et al., 2015). The use of the approach can also be extended to the restoration of hand function after SCI (Zimmermann and Jackson, 2014), where hand and arm-specific synergies may be produced.
Study Limitations
Although a model with complete chronic SCI and no anesthesia would be the ultimate demonstration for clinical relevance, the goal of this work was to produce long distance walking, defined as 300 trials of over-ground walking or until walking could no longer be achieved due to muscle fatigue (whichever occurred first). Achieving this goal in an awake model is not feasible. Anesthetized cats were specifically used in order to assess the distances of walking that could be produced with ISMS without interruptions during these long experiments (~8 hours of testing). Other than humans who would have the patience and understanding of the purpose of such experiments, no awake animal models would be a suitable substitute in the present work. In previous work, acute ISMS implants in animal models with chronic SCI and no anesthesia demonstrated that weight-bearing, fatigue-resistant stepping can be produced (Saigal et al., 2004). The present work expanded those findings by demonstrating that ISMS can produce long distances of over-ground walking with appropriate supportive and propulsive force production and movement kinematics.
Of the 74 electrodes used in this study, 39% of the tips overshot their target location and were placed in the white matter. Depending on the amount by which a target is overshot (e.g., if the electrode tips are close to ventral rootlets), the force produced resembles what is commonly seen with peripheral nerve stimulation; namely, the force increases steeply with small increases in stimulus amplitude. This results in a strong and almost ballistic movement that is difficult to modulate. For an electrode tip within the target in the gray matter or very close vicinity in the white matter, as was the case in this study, the force increases gradually with increases in stimulus amplitude. Nonetheless, better targeting techniques have now been incorporated to reduce targeting errors (Bamford et al., 2016). These include pre-operative magnetic resonance imaging to allow for preparation of ISMS electrode arrays with subject-specific dimensions, and the use of an electrode insertion guide to reduce off-vertical insertions of the electrodes in the cord (e.g., figure 2 top). Grahn et al., developed an image-guided implantation system for ISMS that may also improve targeting accuracy (Grahn et al., 2016).
Delays (periods of ~ 1min) between trials may have affected the rate of muscle fatigue. These delays resulted from the time required for the cart supporting the cat to be manually returned to the beginning of the walkway and the time required for compression of the video from a completed trial to finish. In the future, these delays will be eliminated by using a continuous instrumented walkway and a continuously streaming motion analysis system.
It remains unknown how long cats can actually walk with ISMS. In accordance with the experimental protocol, experiments concluded after 300 trials of walking were completed even though additional walking trials could still be produced due to the low rate of muscle fatigue. Post-hoc analysis identified successful trials by choosing only trials in which coordinated, bilateral walking across the 2.9m was completed, and in a duration ≤ 90 s. This resulted in 210 to 288 successful walking trials with equivalent walking distances of 609 to 835m in cats C–E. Immediate, on-line exclusion of unsuccessful trials would have increased the achieved walking distances in this project. Nonetheless, these over-ground walking distances far exceed those achieved by peripheral FES approaches. For example, the walking distances obtained with ISMS in this study were >10× longer than those achieved with intramuscular stimulation using the same set up, control strategy and time delays between trials (Mazurek et al., 2012). In the intramuscular stimulation study, experiments were terminated once walking could no longer be produced due to muscle fatigue (Mazurek et al., 2012). The walking distances achieved in this study also appeared to be a 10 fold improvement over walking systems utilizing muscle and peripheral nerve stimulation in humans (Kobetic et al., 1997, Sharma et al., 1998, Kobetic et al., 1999, Stein et al., 2005). Importantly, the achieved walking distances in the present experiments were highly functional. This is exemplified by the average of 4384 steps taken per experiment (table 1), which is equivalent to walking distances of two miles for the average person.
Acknowledgments
The authors thank Robert Rolf and Rodney Gramlich for technical assistance, and Dr. Douglas Weber (University of Pittsburgh) for MotionTracker2D. This work was funded by the National Institutes of Health (NIH-NINDS NS44225), the Canadian Institutes of Health Research, and Alberta Innovates-Health Solutions (AIHS) awarded to VKM. KAM was supported by NIH-NINDS under award F31NS074828, AMLO was supported by an AIHS Post-doctoral Fellowship, AT was supported by an AIHS Graduate Studentship and a Vanier Canada Graduate Scholarship, and VKM was an Alberta Heritage Foundation for Medical Research Senior Scholar.
References
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain : a journal of neurology. 2014 doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Lebel RM, Parseyan K, Mushahwar VK. The fabrication, implantation and stability of intraspinal microwire arrays in the spinal cord of cat and rat. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2016 doi: 10.1109/TNSRE.2016.2555959. In Press. [DOI] [PubMed] [Google Scholar]
- Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Progress in brain research. 2011;194:227–239. doi: 10.1016/B978-0-444-53815-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Putman CT, Mushahwar VK. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. Journal of Physiology. 2005;569:873–884. doi: 10.1113/jphysiol.2005.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Todd KG, Mushahwar VK. The effects of intraspinal microstimulation on spinal cord tissue in the rat. Biomaterials. 2010;31:5552–5563. doi: 10.1016/j.biomaterials.2010.03.051. doi: 5510.1016/j.biomaterials.2010.5503.5051. Epub 2010 Apr 5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolat G, Massaro F, He J, Zeme S, Ketcik B. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78:233–239. doi: 10.3171/jns.1993.78.2.0233. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Leblond H, Rossignol S. Characteristics and mechanisms of locomotion induced by intraspinal microstimulation and dorsal root stimulation in spinal cats. J Neurophysiol. 2007;97:1986–2000. doi: 10.1152/jn.00818.2006. Epub 2007 Jan 1910. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Vinay L. Strategies to restore motor functions after spinal cord injury. Curr Opin Neurobiol. 2009;19:587–600. doi: 10.1016/j.conb.2009.10.005. doi: 510.1016/j.conb.2009.1010.1005. Epub 2009 Nov 1015. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. Journal of neurophysiology. 2003;90:3640–3653. doi: 10.1152/jn.00497.2003. [DOI] [PubMed] [Google Scholar]
- Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, Dassonville J. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine. 2000;25:501–508. doi: 10.1097/00007632-200002150-00018. [DOI] [PubMed] [Google Scholar]
- Brown-Triolo DL, Roach MJ, Nelson K, Triolo RJ. Consumer perspectives on mobility: implications for neuroprosthesis design. Journal of rehabilitation research and development. 2002;39:659–669. [PubMed] [Google Scholar]
- Bulea TC, Kobetic R, Audu ML, Triolo RJ. Stance controlled knee flexion improves stimulation driven walking after spinal cord injury. Journal of neuroengineering and rehabilitation. 2013;10:68. doi: 10.1186/1743-0003-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto R, Mushahwar VK. Understanding the Mechanisms and Sites of Action of Intraspinal Microstimulation. 12th Annual Conference of the International Functional Electrical Stimulation Society; Philadelphia, Pennsylvania, USA. 2007. [Google Scholar]
- Calixto R, Musienko P, Norton JA, Everaert DG, Mushahwar VK. Modulation of single cell activity by intraspinal microstimulation in the lumbosacral enlargement of the cat spinal cord. Society for Neuroscience 36th Annual Meeting; Atlanta, Georgia, USA. 2006. [Google Scholar]
- Carhart MR, He J, Herman R, D'Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2004;12:32–42. doi: 10.1109/TNSRE.2003.822763. [DOI] [PubMed] [Google Scholar]
- Coburn B. Electrical stimulation of the spinal cord: two-dimensional finite element analysis with particular reference to epidural electrodes. Med Biol Eng Comput. 1980;18:573–584. doi: 10.1007/BF02443129. [DOI] [PubMed] [Google Scholar]
- Coburn B. A theoretical study of epidural electrical stimulation of the spinal cord--Part II. Effects on long myelinated fibers. IEEE Trans Biomed Eng. 1985;32:978–986. doi: 10.1109/TBME.1985.325649. [DOI] [PubMed] [Google Scholar]
- Coburn B, Sin WK. A theoretical study of epidural electrical stimulation of the spinal cord--Part I: Finite element analysis of stimulus fields. IEEE Trans Biomed Eng. 1985;32:971–977. doi: 10.1109/tbme.1985.325648. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Ho CH, Triolo RJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. Clinical applications of electrical stimulation after spinal cord injury. Journal of Spinal Cord Medicine. 2004;27:365–375. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- Creasey GH, Kilgore KL, Brown-Triolo DL, Dahlberg JE, Peckham PH, Keith MW. Reduction of costs of disability using neuroprostheses. Assist Technol. 2000;12:67–75. doi: 10.1080/10400435.2000.10132010. [DOI] [PubMed] [Google Scholar]
- Dai X, Noga BR, Douglas JR, Jordan LM. Localization of spinal neurons activated during locomotion using the c-fos immunohistochemical method. Journal of neurophysiology. 2005;93:3442–3452. doi: 10.1152/jn.00578.2004. [DOI] [PubMed] [Google Scholar]
- Davis JA, Jr, Triolo RJ, Uhlir J, Bieri C, Rohde L, Lissy D, Kukke S. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers--where do we stand? Journal of Rehabilitation Research & Development. 2001;38:609–617. [PubMed] [Google Scholar]
- Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Physical therapy. 2002;82:707–715. [PubMed] [Google Scholar]
- Fouad K, Pearson K. Restoring walking after spinal cord injury. Progress in neurobiology. 2004;73:107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol. 2006;96:2995–3005. doi: 10.1152/jn.00061.2006. Epub 2006 Aug 2930. [DOI] [PubMed] [Google Scholar]
- Giszter SF. Spinal primitives and intra-spinal micro-stimulation (ISMS) based prostheses: a neurobiological perspective on the "known unknowns" in ISMS and future prospects. Front Neurosci. 2015;9:72. doi: 10.3389/fnins.2015.00072. eCollection 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow GE, Reinking RM, Stuart DG. The cat step cycle: Hind limb joint angles and muscle lengths during unrestrained locomotion. J Morph. 1973;141:1–42. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Goswami A. A new gait parameterization technique by means of cyclogram moments: Application to human slope walking. Gait & posture. 1998;8:15–36. doi: 10.1016/s0966-6362(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Grahn PJ, Goerss SJ, Lujan JL, Mallory GW, Kall BA, Mendez AA, Trevathan JK, Felmlee JP, Bennet KE, Lee KH. MRI-Guided Stereotactic System for Delivery of Intraspinal Microstimulation. Spine (Phila Pa. 2016;41:E806–E813. doi: 10.1097/BRS.0000000000001397. doi: 810.1097/BRS.0000000000001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn PJ, Mallory GW, Berry BM, Hachmann JT, Lobel DA, Lujan JL. Restoration of motor function following spinal cord injury via optimal control of intraspinal microstimulation: toward a next generation closed-loop neural prosthesis. Front Neurosci. 2014;8:2. doi: 10.3389/fnins.2014.00296. eCollection 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupe D, Kohn KH. Functional neuromuscular stimulator for short-distance ambulation by certain thoracic-level spinal-cord-injured paraplegics. Surgical Neurology. 1998;50:202–207. doi: 10.1016/s0090-3019(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Guevremont L, Mushahwar VK. Tapping into the spinal cord for restoring function after spinal cord injury. In: Bronzino Da., editor. Neural Engineering: Research, Industry and the Clinical Perspective. CRC Press; 2008. p. 19-11-26. [Google Scholar]
- Guevremont L, Norton JA, Mushahwar VK. Physiologically based controller for generating overground locomotion using functional electrical stimulation. Journal of neurophysiology. 2007;97:2499–2510. doi: 10.1152/jn.01177.2006. [DOI] [PubMed] [Google Scholar]
- Guevremont L, Renzi CG, Norton JA, Kowalczewski J, Saigal R, Mushahwar VK. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2006;14:266–272. doi: 10.1109/TNSRE.2006.881592. [DOI] [PubMed] [Google Scholar]
- Halbertsma JM. The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiologica Scandinavica Supplementum. 1983;521:1–75. [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. doi: 1910.1016/S0140-6736(1911)60547-60543. Epub 62011 May 60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R, He J, D'Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–68. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531–540. doi: 10.1038/sj.sc.3100717. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Struijk JJ, Rijkhoff NJ. Contact combinations in epidural spinal cord stimulation. A comparison by computer modeling. Stereotact Funct Neurosurg. 1991;56:220–233. doi: 10.1159/000099409. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Research Bulletin. 1992;28:99–105. doi: 10.1016/0361-9230(92)90235-p. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Roberts WJ. An electrophysiological demonstration of the axonal projections of single spinal interneurons in the cat. Journal of Physiology. 1972;222:597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Progress in Brain Research. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Joucla S, Ambroise M, Levi T, Lafon T, Chauvet P, Saighi S, Bornat Y, Lewis N, Renaud S, Yvert B. Generation of Locomotor-Like Activity in the Isolated Rat Spinal Cord Using Intraspinal Electrical Microstimulation Driven by a Digital Neuromorphic CPG. Front Neurosci. 2016;10:67. doi: 10.3389/fnins.2016.00067. eCollection 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Annals of the New York Academy of Sciences. 1998;860:110–129. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kobetic R, Triolo RJ, Marsolais EB. Muscle selection and walking performance of multichannel FES systems for ambulation in paraplegia. IEEE transactions on rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 1997;5:23–29. doi: 10.1109/86.559346. [DOI] [PubMed] [Google Scholar]
- Kobetic R, Triolo RJ, Uhlir JP, Bieri C, Wibowo M, Polando G, Marsolais EB, Davis JA, Jr, Ferguson KA. Implanted functional electrical stimulation system for mobility in paraplegia: a follow-up case report. IEEE Transactions on Rehabilitation Engineering. 1999;7:390–398. doi: 10.1109/86.808942. [DOI] [PubMed] [Google Scholar]
- Lau B, Guevremont L, Mushahwar VK. Strategies for generating prolonged functional standing using intramuscular stimulation or intraspinal microstimulation. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2007;15:273–285. doi: 10.1109/TNSRE.2007.897030. [DOI] [PubMed] [Google Scholar]
- Lavoie S, McFadyen B, Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Experimental brain research. 1995;106:39–56. doi: 10.1007/BF00241355. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. Journal of neurophysiology. 1988;60:204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- Mazurek K, Holinski BJ, Everaert DG, Stein RB, Mushahwar VK, Etienne-Cummings R. IEEE Biomedical Circuits and Systems Conference (BioCAS) Cyprus: Paphos; 2010. Locomotion Processing Unit; pp. 286–289. [Google Scholar]
- Mazurek KA, Etienne-Cummings R. IEEE Biomedical Circuits and Systems Conference (BioCAS) San Diego, California, USA: 2011. Implementation of functional components of the Locomotion Processing Unit. [Google Scholar]
- Mazurek KA, Holinski BJ, Everaert DG, Mushahwar VK, Etienne-Cummings R. A Mixed-Signal VLSI System for Producing Temporally Adapting Intraspinal Microstimulation Patterns for Locomotion. IEEE transactions on biomedical circuits and systems. 2016;9:9. doi: 10.1109/TBCAS.2015.2501419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek KA, Holinski BJ, Everaert DG, Stein RB, Etienne-Cummings R, Mushahwar VK. Feed forward and feedback control for over-ground locomotion in anaesthetized cats. J Neural Eng. 2012;9 doi: 10.1088/1741-2560/9/2/026003. 026003. doi: 026010.021088/021741-022560/026009/026002/026003. Epub 022012 Feb 026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain research reviews. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JG, Miller RR, Perlmutter SI. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:12193–12198. doi: 10.1073/pnas.1505383112. doi: 12110.11073/pnas.1505383112. Epub 1505382015 Sep 1505383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memberg WD, Polasek KH, Hart RL, Bryden AM, Kilgore KL, Nemunaitis GA, Hoyen HA, Keith MW, Kirsch RF. Implanted Neuroprosthesis for Restoring Arm and Hand Function in People With High Level Tetraplegia. Archives of physical medicine and rehabilitation. 2014 doi: 10.1016/j.apmr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello SE, Kasten MR, Horner PJ, Moritz CT. Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Frontiers in neuroscience. 2014;8:21. doi: 10.3389/fnins.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. doi: 610.1038/nature07418. Epub 02008 Oct 07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Experimental Neurology. 2000;163:422–429. doi: 10.1006/exnr.2000.7381. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Guevremont L, Saigal R. Could cortical signals control intraspinal stimulators? A theoretical evaluation. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2006;14:198–201. doi: 10.1109/TNSRE.2006.875532. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Proposed specifications for a lumbar spinal cord electrode array for control of lower extremities in paraplegia. IEEE Transactions on Rehabilitation Engineering. 1997;15:237–243. doi: 10.1109/86.623015. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Annals of the New York Acadamy of Science. 1998;860:531–535. doi: 10.1111/j.1749-6632.1998.tb09096.x. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Muscle recruitment through electrical stimulation of the lumbo-sacral spinal cord. IEEE Transactions on Rehabilitation Engineering. 2000a;8:22–29. doi: 10.1109/86.830945. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Transactions on Rehabilitation Engineering. 2000b;8:11–21. doi: 10.1109/86.830944. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Jacobs PL, Normann RA, Triolo RJ, Kleitman N. New functional electrical stimulation approaches to standing and walking. Journal of neural engineering. 2007;4:S181–S197. doi: 10.1088/1741-2560/4/3/S05. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Noga BR, Fortier PA, Kriellaars DJ, Dai X, Detillieux GR, Jordan LM. Field potential mapping of neurons in the lumbar spinal cord activated following stimulation of the mesencephalic locomotor region. Journal of Neuroscience. 1995;15:2203–2217. doi: 10.1523/JNEUROSCI.15-03-02203.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham PH, Kilgore KL. Challenges and opportunities in restoring function after paralysis. IEEE transactions on bio-medical engineering. 2013;60:602–609. doi: 10.1109/TBME.2013.2245128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic D, Radulovic M, Schwirtlich L, Jaukovic N. Automatic vs hand-controlled walking of paraplegics. Med Eng Phys. 2003;25:63–73. doi: 10.1016/s1350-4533(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Portal J-J, Corio M, Viala D. Localization of the lumbar pools of motoneurones which provide hindlimb muscles in the rabbit. Neuroscience Letters. 1991;124:105–107. doi: 10.1016/0304-3940(91)90832-e. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Mushahwar VK, McCreery DB. Neural prostheses. Journal of Physiology. 2001;533:99–109. doi: 10.1111/j.1469-7793.2001.0099b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanes GJ. The motor pools of the spinal cord. Progress in brain research. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Langlet C, Barthelemy D, Chau C, Giroux N, Brustein E, Marcoux J, Leblond H, Reader TA. Determinants of locomotor recovery after spinal injury in the cat. Progress in brain research. 2004;143:163–172. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. The Journal of physiology. 2006a;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. The Journal of physiology. 2006b;577:641–658. doi: 10.1113/jphysiol.2006.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Transactions on Neural Systems & Rehabilitation Engineering. 2004;12:430–440. doi: 10.1109/TNSRE.2004.837754. [DOI] [PubMed] [Google Scholar]
- Shahdoost S, Frost S, Dunham C, DeJong S, Barbay S, Nudo R, Mohseni P. Cortical control of intraspinal microstimulation: Toward a new approach for restoration of function after spinal cord injury. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2159–2162. doi: 10.1109/EMBC.2015.7318817. [DOI] [PubMed] [Google Scholar]
- Shahdoost S, Frost S, Van Acker G, DeJong S, Dunham C, Barbay S, Nudo R, Mohseni P. Towards a miniaturized brain-machine-spinal cord interface (BMSI) for restoration of function after spinal cord injury. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:486–489. doi: 10.1109/EMBC.2014.6943634. [DOI] [PubMed] [Google Scholar]
- Sharma M, Marsolais EB, Polando G, Triolo RJ, Davis JA, Jr, Bhadra N, Uhlir JP. Implantation of a 16- channel functional electrical stimulation walking system. Clinical Orthopaedics & Related Research. 1998:236–242. [PubMed] [Google Scholar]
- Sharrard WJ. The Segmental Innervation of the Lower Limb Muscles in Man. Ann R Coll Surg Engl. 1964;35:106–122. [PMC free article] [PubMed] [Google Scholar]
- Sharrard WJW. The distribution of the permanent paralysis in the lower limb in poliomyelitis: a clinical and pathological study. Journal of Bone and Joint Surgery. 1955;37B:540–558. doi: 10.1302/0301-620X.37B4.540. [DOI] [PubMed] [Google Scholar]
- Shen L, Poppele RE. Kinematic analysis of cat hindlimb stepping. Journal of neurophysiology. 1995;74:2266–2280. doi: 10.1152/jn.1995.74.6.2266. [DOI] [PubMed] [Google Scholar]
- Sin WK, Coburn B. Electrical stimulation of the spinal cord: a further analysis relating to anatomical factors and tissue properties. Med Biol Eng Comput. 1983;21:264–269. doi: 10.1007/BF02478492. [DOI] [PubMed] [Google Scholar]
- Snow S, Horch KW, Mushahwar VK. Intraspinal microstimulation using cylindrical multielectrodes. IEEE Transactions on Biomedical Engineering. 2006;53:311–319. doi: 10.1109/TBME.2005.857638. [DOI] [PubMed] [Google Scholar]
- Stein RB, Hayday F, Chong S, Thompson AK, Rolf R, James KB, Bell G. Speed and efficiency in walking and wheeling with novel stimulation and bracing systems after spinal cord injury: a case study. Neuromodulation : journal of the International Neuromodulation Society. 2005;8:264–271. doi: 10.1111/j.1525-1403.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- Stein RB, Mushahwar V. Reanimating limbs after injury or disease. Trends in Neurosciences. 2005;28:518–524. doi: 10.1016/j.tins.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Struijk JJ, Holsheimer J, Barolat G, He J, Boom HBK. Paresthesia thresholds in spinal cord stimulation: a comparison of theoretical results with clinical data. IEEE Trans Rehabil Eng. 1993a;1:101–108. [Google Scholar]
- Struijk JJ, Holsheimer J, Boom HB. Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans Biomed Eng. 1993b;40:632–639. doi: 10.1109/10.237693. [DOI] [PubMed] [Google Scholar]
- Struijk JJ, Holsheimer J, van Veen BK, Boom HBK. Epidural spinal cord stimulation: calculation of field potentials with special reference to dorsal column nerve fibers. IEEE Transactions on Biomedical Engineering. 1991;38:104–110. doi: 10.1109/10.68217. [DOI] [PubMed] [Google Scholar]
- Tator CH, Minassian K, Mushahwar VK. Spinal cord stimulation: therapeutic benefits and movement generation after spinal cord injury. Handb Clin Neurol. 2012;109:283–296. doi: 10.1016/B978-0-444-52137-8.00018-8. [DOI] [PubMed] [Google Scholar]
- Thrasher TA, Popovic MR. Functional electrical stimulation of walking: function, exercise and rehabilitation. Annales de readaptation et de medecine physique : revue scientifique de la Societe francaise de reeducation fonctionnelle de readaptation et de medecine physique. 2008;51:452–460. doi: 10.1016/j.annrmp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Triolo RJ, Bailey SN, Miller ME, Rohde LM, Anderson JS, Davis JA, Jr, Abbas JJ, DiPonio LA, Forrest GP, Gater DR, Jr, Yang LJ. Longitudinal performance of a surgically implanted neuroprosthesis for lower-extremity exercise, standing, and transfers after spinal cord injury. Archives of physical medicine and rehabilitation. 2012;93:896–904. doi: 10.1016/j.apmr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. doi: 1110.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Vogelstein RJ, Tenore F, Guevremont L, Etienne-Cummings R, Mushahwar VK. A silicon central pattern generator controls locomotion in vivo. IEEE transactions on biomedical circuits and systems. 2008;2:212–222. doi: 10.1109/TBCAS.2008.2001867. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yezierski RP. Perceived difficulty in dealing with consequences of spinal cord injury. Archives of physical medicine and rehabilitation. 1999;80:580–586. doi: 10.1016/s0003-9993(99)90203-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann JB, Jackson A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front Neurosci. 2014;8:87. doi: 10.3389/fnins.2014.00087. eCollection 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]