Abstract
Purpose
Postoperative pain is a major challenge for patients undergoing breast reconstruction after surgical treatment of breast cancer, resulting in prolonged hospitalizations and additional resource utilization. Evidence on the efficacy of techniques to minimize postoperative pain in autologous breast reconstruction is lacking. The purpose of this study is to determine if preoperative paravertebral block, a regional anesthetic technique, impacts postoperative pain control and length of stay in patients undergoing autologous breast reconstruction.
Methods
Consecutive patients undergoing postmastectomy autologous breast reconstruction between 2012 and 2015 were identified from a prospectively-collected database to compare those who received PVB to those who did not. Primary outcomes included self-reported pain score, time to oral-only narcotic usage (TTON), and hospital length of stay (LOS). Sample differences were compared using Wilcoxon rank-sum and Chi-squared for continuous and categorical variables. Kaplan-Meier analysis was used to evaluate TTON and LOS, with Mantel-Cox test used to compare groups.
Results
Of 78 patients, 39 received PVB and 39 did not. Study groups did not differ regarding age, BMI, ASA class, mastectomy type, flap type, or cancer stage (p>0.05). Patients in the PVB group reported significantly lower postoperative pain at 2 (p<0.01) and 24 hours (p<0.01) and shorter median TTON (66 vs. 76 hours, p<0.01). Importantly, median LOS was reduced for patients receiving a PVB in both hours (95 vs. 116, p<0.01) and hospital nights (4 vs. 5, p=0.05).
Conclusions
Preoperative PVB is associated with improved postoperative pain control and shorter hospitalizations for patients with breast cancer undergoing postmastectomy autologous reconstruction.
BACKGROUND
In the United States, an estimated 246,660 new cases of invasive breast cancer will be diagnosed in 2016.1 Improvements in the comprehensive treatment of breast cancer and the implementation of the Women’s Health and Cancer Rights Act in 1998 mandating universal coverage for postmastectomy breast reconstruction have led to steady increases in the national rates of reconstruction utilization after mastectomy.2,3 This trend corroborates evidence demonstrating women undergoing breast reconstruction after mastectomy have significantly improved health-related quality of life compared to women who do not undergo postmastectomy breast reconstruction, including greater satisfaction with appearance and higher physical, psychosocial, and sexual well-being.4–7
A major challenge for patients undergoing postmastectomy breast reconstruction is postoperative pain, which results in prolonged hospitalizations and additional resource utilization. In the last several years, strategies to alleviate postoperative pain in patients with breast cancer undergoing surgical treatment have been increasingly expounded in the literature. Particularly promising among these is the regional anesthesia technique of preoperative paravertebral block administration in additional to general anesthesia, which has consistently been demonstrated to improve postoperative pain control and decrease length of stay in patients with breast cancer undergoing mastectomy and/or prosthetic breast reconstruction.8–12 However, to date, there are no studies that evaluate the potential benefit of a paravertebral block in patients with breast cancer undergoing autologous microvascular breast reconstruction after mastectomy. Given that patients undergoing autologous reconstruction often require extended hospital stays and narcotic usage, we hypothesize that paravertebral block administration can have substantial benefits for this patient population. The purpose of this study is to determine if a preoperative paravertebral block impacts postoperative pain control and length of stay in patients with breast cancer undergoing autologous microvascular breast reconstruction after mastectomy.
METHODS
Study Design and Patient Population
This is a cohort analysis comparing patients who received preoperative paravertebral block, in addition to general anesthesia, versus patients who only received general anesthesia prior to undergoing autologous, abdominally-based, microvascular breast reconstruction after mastectomy for breast cancer. Consecutive patients undergoing autologous reconstruction after mastectomy by a single surgeon at Siteman Cancer Center, Washington University School of Medicine, from June, 2012 to June, 2015 comprised the study population and were identified from a prospectively collected database with Institutional Review Board approval (#201404004). Autologous reconstruction was defined as a microvascular free flap transfer of the patient’s abdominal tissue (autologous) to their breast and included muscle-sparing Transverse Rectus Abdominis Myocutaneous (ms-TRAM) flaps and Deep Inferior Epigastric Perforator (DIEP) flaps. Patients were ineligible for inclusion if they underwent prosthetic (tissue expander or implant-based) breast reconstruction, a synchronous non-breast procedure at time of reconstruction, non-abdominally based microvascular reconstruction (ex. superior gluteal artery perforator flap), or pedicled, non-microvascular, autologous reconstruction (ex. latissimus dorsi flap or pedicled TRAM flap).
Pertinent covariates were tabulated for each patient. Clinical variables included age, body-mass index (BMI), preoperative opioid use, American Society of Anesthesiology (ASA) classification, history of postoperative nausea/vomiting (PONV), and comorbidities. Pathologic variables included history of adjuvant therapy, mastectomy type, mastectomy location, cancer stage, and cancer laterality. Reconstructive variables included location (unilateral vs. bilateral), timing after mastectomy (immediate vs. delayed), and flap type.
Paravertebral Block and Operative Procedure
The main exposure variable was paravertebral block (PVB) administration, with block failure (inadequate anesthetic response) and block complications (complications resulting from block administration) tabulated. All patients scheduled to have autologous reconstruction following mastectomy were offered a PVB by a dedicated regional anesthesia service directed by a fellowship trained anesthesiologist. All blocks were performed in the preoperative area prior to surgery, with unilateral blocks placed for patients undergoing unilateral surgery and bilateral blocks placed for patients undergoing bilateral surgery. Appropriate ASA standards were followed with full cardiovascular monitoring and supplemental oxygen provided throughout administration. In all cases, time-out was performed and sterile technique was employed to administer the anesthetic using a 20- or 21-gauge needle under ultrasound guidance (Sonosite S-nerve). Incremental injection technique was performed, which composed of 15mL of 0.5% bupivacaine (per side, 30mL total in bilateral cases), in the paravertebral space at the T2-T4 levels. All patients had surgery under general anesthesia. No patients received an implantable local anesthetic device in the abdominal portion of the procedure. Intraoperative antiemetics were administered at the discretion of the anesthesia provider and included a combination of: scopolamine transdermal patch, intravenous metoclopramide, intravenous ondansetron, and/or intravenous dexamethasone. Postoperatively, patients were taken to the postanesthesia care unit (PACU) and subsequently admitted to a specialty care hospital floor.
Outcome Measures and Data Collection
The primary outcome variables of interest were: self-reported pain score at 2 hours, self-reported pain score at 24 hours, time to usage of oral-only narcotics, length of stay in hours, and length of stay in hospital nights. Nursing staff, unaware of the study hypothesis, recorded patients’ self-reported pain scores at 2 hours and 24 hours on a verbal numeric rating scale (VNRS) from 0–10, with 0 indicating “no pain” and 10 indicating “worst pain imaginable.” Time to oral-only narcotic usage was calculated, in hours, as the time from PACU admission to the time of first oral narcotic use without concomitant intravenous narcotic use. Length of stay was counted from the end of the surgical procedure, defined as the time of PACU admission, to the time of hospital discharge and was measured in hours and hospital nights. The decision to discharge patients was made by the surgical team and reflected a consistent criteria based on patients: tolerating a regular oral diet without discomfort, ambulating, having pain control with oral-only medications, and affirming their comfort with continuing care at home. Secondary outcomes of interest included postoperative nausea, defined as the need for an oral or intravenous antiemetic, and, postoperative emesis. Any patient experiencing a thrombotic flap complication requiring operative takeback during initial hospital admission was excluded from this study. The rationale behind excluding these patients is a takeback would prolong length of stay and, potentially, postoperative pain and, therefore, confound the relationship between the exposure variable and outcome variables. Each of these cases was reviewed to ensure that the reason for operative takeback was not related to PVB. Patients with unsuccessful blocks were not excluded from analysis.
Statistical Analysis
Established methods were used to characterize sample distributions, with Wilcoxon rank-sum used to compare sample continuous variables and Chi-squared analysis to compare sample categorical variables. Density distributions were used to estimate self-reported pain score at 2 hours and at 24 hours post-operatively, with Wilcoxon rank-sum used to compare samples for this outcome. Kaplan-Meier analysis was used to estimate time to oral-only narcotic usage, length of stay in hours, and length of stay in hospital nights. Use of oral-only narcotic pain medication was censored in calculating time to oral-only narcotic usage, while hospital discharge was censored in calculating length of stay in hours and length of stay in hospital nights. Sample differences in these outcomes were tested with Mantel-Cox log-rank test. Two-sided α = 0.05 was set a priori to detect significance and all statistical analyses were conducted using commercially available software (SAS 9.4, SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Patient Population
A total of 86 patients with breast cancer undergoing autologous, abdominally-based microvascular breast reconstruction after mastectomy were consecutively enrolled in this study. From this initial cohort, 8 patients (3 in the PVB group and 5 in the non-PVB group) experienced a postoperative complication requiring an immediate return to the operating room; therefore, these patients were excluded from analysis, leaving a total study population of 78 patients. Of these, 39 patients received a PVB and 39 patients did not. There were no significant differences in patient and treatment characteristics between the two groups, including age, BMI, ASA score, and medical comorbidities, as demonstrated in Table 1.
Table 1.
Patient Clinical, Pathological & Operative Characteristics
| Non-Paravertebral Block Group (N = 39) | Paravertebral Block Group (N = 39) | P-Value | |
|---|---|---|---|
|
| |||
| Age (years) | 48 (41–55) | 49 (40–55) | 0.67α |
|
| |||
| BMI (kg/m2) | 29 (24–32) | 29 (26–33) | 0.78α |
|
| |||
| Smoking | No 32 (82%) | No 37 (95%) | 0.08β |
| Yes 7 (18%) | Yes 2 (5%) | ||
|
| |||
| Preoperative Opioid Use | No 37 (95%) | No 37 (95%) | 1.00β |
| Yes 2 (5%) | Yes 2 (5%) | ||
|
| |||
| ASA class | I 5 (13%) | I 6 (15%) | 0.78β |
| II 33 (85%) | II 31 (79%) | ||
| III 1 (3%) | III 2 (5%) | ||
|
| |||
| History of PONV | None 28 (72%) | None 29 (74%) | 0.80β |
| Yes 11 (28%) | Yes 10 (26%) | ||
|
| |||
| Medical Comorbiditiesδ | No 20 (51%) | No 15 (39%) | 0.26β |
| Yes 19 (49%) | Yes 24 (62%) | ||
|
| |||
| Chemotherapy | No 11 (28%) | No 13 (33%) | 0.62β |
| Yes 28 (72%) | Yes 26 (67%) | ||
|
| |||
| Radiation | No 17 (44%) | No 15 (39%) | 0.65β |
| Yes 22 (56%) | Yes 24 (62%) | ||
|
| |||
| Breast Cancer Stage | Prophylactic 4 (10%) | Prophylactic 0 (0%) | 0.15β |
| I 10 (26%) | I 8 (21%) | ||
| II 16 (41%) | II 24 (62%) | ||
| III 6 (15%) | III 6 (16%) | ||
| IV 3 (8%) | IV 1 (3%) | ||
|
| |||
| Breast Cancer Side | Left 16 (41%) | Left 17 (44%) | 0.11β |
| Right 19 (49%) | Right 20 (51%) | ||
| Bilateral 0 (0%) | Bilateral 2 (5%) | ||
|
| |||
| Mastectomy Type | Bilateral Simple 4 (10%) | Bilateral Simple 5 (13%) | 0.11β |
| Unilateral Skin-Sparing 8 (21%) | Unilateral Skin-Sparing 6 (15%) | ||
| Bilateral Skin-Sparing 14 (36%) | Bilateral Skin-Sparing 7 (18%) | ||
| Unilateral Modified Radical 9 (23%) | Unilateral Modified Radical 5 (13%) | ||
| Simple and Modified Radical 1 (3%) | Simple and Modified Radical 5 (13%) | ||
| Skin-Sparing and Modified Radical 3 (8%) | Skin-Sparing and Modified Radical 5 (13%) | ||
| Unilateral Simple 0 (0%) | Unilateral Simple 3 (8%) | ||
| Unilateral Nipple-Sparing 0 (0%) | Unilateral Nipple-Sparing 2 (5%) | ||
| Skin-Sparing and Simple 0 (0%) | Skin-Sparing and Simple 1 (3%) | ||
|
| |||
| Mastectomy Location | Unilateral 17 (44%) | Unilateral 16 (41%) | 0.82β |
| Bilateral 22 (56%) | Bilateral 23 (59%) | ||
|
| |||
| Mastectomy Timing | Immediate 10 (26%) | Immediate 7 (18%) | 0.41β |
| Delayed 29 (74%) | Delayed 32 (82%) | ||
|
| |||
| Reconstruction Location | Unilateral 19 (48%) | Unilateral 18 (46%) | 0.82β |
| Bilateral 20 (51%) | Bilateral 21 (54%) | ||
|
| |||
| Flap Type | Unilateral DIEP 13 (33%) | Unilateral DIEP 16 (41%) | 0.40β |
| Unilateral ms-TRAM 6 (15%) | Unilateral ms-TRAM 2 (5%) | ||
| Bilateral DIEP 9 (23%) | Bilateral DIEP 11 (28%) | ||
| Bilateral ms-TRAM 11 (29%) | Bilateral ms-TRAM 10 (26%) | ||
Wilcoxon rank-sum test, samples summarized as median (IQR)
Chi-squared test
Comorbidities included asthma, hypothyroidism, anemia, depression, atrial fibrillation, hypertension, hyperlipidemia, diverticulitis, diabetes, bronchitis, and liver disease
Surgical Procedures
A total of 119 autologous reconstructions were performed after 123 mastectomy procedures in the 78 study patients (21 bilateral reconstructions in the PVB group vs. 20 bilateral reconstructions in the non-PVB group, P = 0.82) (Table 1). For patients receiving a PVB, autologous reconstruction was performed utilizing a unilateral DIEP flap in 16 (41%) cases, a ms-TRAM flap in 2 (5%) cases, bilateral DIEP flaps in 11 (28%) cases, and bilateral ms-TRAM flaps in 10 (26%) cases. Flap utilization for patients not receiving a PVB did not differ significantly from the aforementioned (P = 0.40) (Table 1). Axillary lymph node dissection was performed in 15 (39%) patients in the PVB group and 13 (33%) patients in the non-PVB group (P = 0.11). There was no significant difference regarding the timing of reconstruction after mastectomy (82% delayed in PVB group vs. 74% in non-PVB group, P = 0.41).
Clinical Outcomes and Resource Utilization
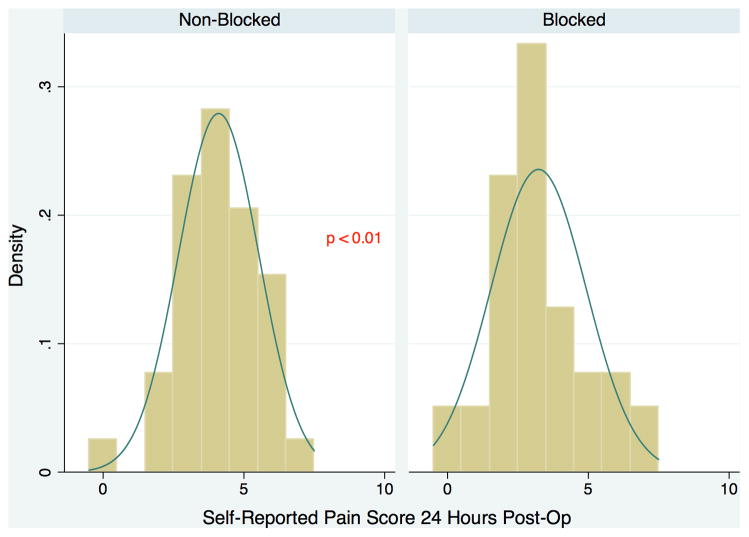
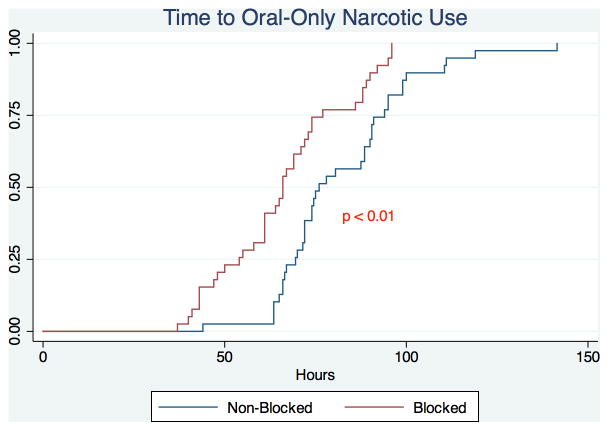
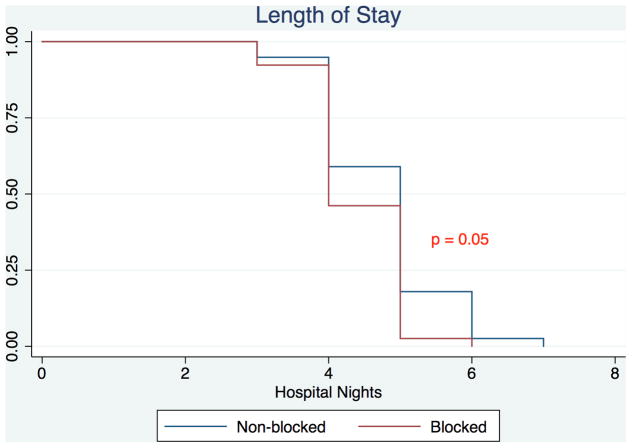
Patients treated with a PVB versus standard anesthesia alone had significantly reduced median postoperative pain scores at 2 hours (3 vs. 5, P < 0.01) and 24 hours (3 vs. 4, P < 0.01) (Figure 1). Patients receiving a PVB exhibited significantly shorter median time to oral-only narcotic usage compared to patients who did not receive a PVB (66 vs. 76 hours, P < 0.01) (Figure 2). Median hospital length of stay for the PVB group was 95 hours (interquartile range 89–114), significantly shorter than the median hospital length of stay of 116 hours (interquartile range 95–121) for the non-PVB group (P < 0.01). This significance remained when analyzing the difference in length of stay in hospital nights, with patients in the PVB group staying a median of 4 nights compared to a median of 5 nights for patients in the non-PVB group (P = 0.05) (Figure 3). Outcomes of interest for the study groups are presented in Table 2. On evaluation of secondary outcomes, there was no significant difference between groups regarding the proportion of patients who developed PONV (P = 0.26). There were no complications related to block administration. However, one patient had an inadequate anesthetic response to the PVB; this patient was not excluded from analysis.
Figure 1.
Self-Reported Pain Score at 24 Hours Post-Operative for Patients undergoing Autologous Breast Reconstruction with a Paravertebral Block (Blocked) vs. Patients undergoing Autologous Breast Reconstruction without a Paravertebral Block (Non-Blocked).
Figure 2.
Kaplan-Meier Analysis of Time to Oral-Only Narcotic Use of Patients undergoing Autologous Breast Reconstruction with a Paravertebral Block (Blocked) vs. Patients undergoing Autologous Breast Reconstruction without a Paravertebral Block (Non-Blocked).
Figure 3.
Kaplan-Meier Analysis of Length of Stay of Patients undergoing Autologous Breast Reconstruction with a Paravertebral Block vs. Patients undergoing Autologous Breast Reconstruction without a Paravertebral Block.
Table 2.
Outcomes: Postoperative Pain, Postoperative Nausea & Vomiting, and Resource Utilization
| Non-Paravertebral Block Group (N = 39) | Paravertebral Block Group (N = 39) | P-Value | |
|---|---|---|---|
|
| |||
| Pain Score at 2 Hours | 5 (4–6) | 3 (2–5) | <0.01α |
|
| |||
| Pain Score at 24 Hours | 4 (3–5) | 3 (2–4) | <0.01α |
|
| |||
| Time to Oral-Only Narcotics (hours) | 76 (70–94) | 66 (54–77) | <0.01γ |
|
| |||
| Postoperative Vomiting | No 29 (74%) | No 33 (85%) | 0.26β |
| Yes 10 (26%) | Yes 6 (15%) | ||
|
| |||
| LOS (hospital nights) | 5 (4–5) | 4 (4–5) | 0.05γ |
|
| |||
| LOS (hours) | 116 (95–121) | 95 (89–114) | <0.01γ |
Wilcoxon rank-sum test, samples summarized as median (IQR)
Chi-squared test
Mantel-Cox log-rank test, samples summarized as median (IQR)
DISCUSSION
This is the first study to evaluate paravertebral blocks (PVB) in patients with breast cancer undergoing autologous microvascular breast reconstruction after mastectomy. This study demonstrates that preoperative PVB administration significantly reduces acute postoperative pain and hospital length of stay for patients undergoing postmastectomy autologous breast reconstruction. Patients receiving a PVB experienced significantly improved pain control at 2 hours and 24 hours postoperatively and were less dependent on intravenous opioids compared to patients who did not receive a PVB. Furthermore, hospital length of stay was significantly reduced in both hours and days for patients receiving a PVB compared to patients who did not receive a PVB.
The findings from this study corroborate the existing literature that demonstrates PVB improves postoperative pain control in patients with breast cancer undergoing surgical treatment.10,11,13 However, whereas all previous published studies regarding the role of PVB in breast reconstruction have only included patients undergoing prosthetic (implant/expander) reconstruction, this study expands our current understanding of the benefits of PVB to patients undergoing autologous breast reconstruction. This is an important distinction as there is an evolving body of literature that demonstrates autologous tissue reconstruction, compared to prosthetic (tissue expander/implant) reconstruction, is a cost-effective strategy in the long-term and is associated with improved patient-reported quality of life and satisfaction with cosmetic outcomes for patients pursuing postmastectomy breast reconstruction.6,14 Furthermore, in patients requiring radiation therapy in addition to mastectomy for breast cancer treatment, autologous reconstruction is associated with higher patient satisfaction and lower complication rates when compared to prosthetic reconstruction.15 However, despite its many advantages, one of the major challenges with autologous reconstruction is that it is a more invasive procedure compared to prosthetic reconstruction, and therefore associated with increased acute postoperative pain.16–18 Increased acute postoperative pain is a significant predictor of persistent postsurgical pain, which negatively impacts quality of life and affects nearly 50% of patients with breast cancer.19–21 Furthermore, acute postoperative pain is associated with increased narcotic requirements and prolonged hospitalizations, contributing to additional resource utilization.17,18 Autologous microvascular breast reconstruction requires extensive dissection of the flap recipient site (chest wall) and usually involves rib resection for access to recipient vessels for flap anastomosis. Hospital length of stays are reported to be as high as 7–10 days following autologous reconstruction, contributing significantly to resource utilization.17,18,22 For large-volume hospitals functioning at maximum capacity with operative waiting lists, prolonged hospitalizations represent lost financial revenue and potential treatment delays as they limit the ability to perform additional operations requiring inpatient hospitalization. Compounding this is the initiation of pay for performance programs and bundled care packages, where length of postoperative hospital stay is an increasingly emphasized quality metric that may affect financial reimbursement and provider reputation.
Therefore, improving acute postoperative pain control and recovery has significant implications for patients by enhancing physical, psychological, and social well-being and hospitals by improving resource utilization. This study demonstrates the ability of a PVB to improve the quality of care and enhance recovery for patients undergoing autologous breast reconstruction, resulting in superior patient-reported pain outcomes and improved resource utilization with shorter hospitalizations. Prior studies by Coopey et al. and Fahy et al. separately demonstrated decreased length of stay for patients receiving a PVB prior to prosthetic breast reconstruction.10,12 However, a limitation of these studies is that hospital length of stay was only reported in hours. While this is valuable, a majority of hospital costs outside of the operating room are related to overnight hospitalizations or nights, not hours, spent in the hospital. We demonstrated that PVB administration also reduces length of stay in hospital nights for patients undergoing autologous reconstruction, which could represent substantial cost savings for the health care system.
This study does have a few limitations. Patients were not randomized to the exposure group in this cohort study. A potential selection and provider bias may exist in that certain anesthesiologists who were early adopters of the technique more aggressively advocated for a PVB compared to delayed adopters. Thus, if the potential benefits of a PVB were more clearly delineated to certain patients, these patients may have been more likely to opt for the intervention. A series of univariate comparisons was done between the groups along known confounders and the groups were found to be similar with respect to each of these, reducing the likelihood that these variables confound the results; however, the potential for unknown confounding still exists. Further limitations are that we did not collect data on intraoperative narcotic administration and did not perform a long-term evaluation of postoperative pain as we did not have adequate long-term follow-up on all patients to conduct this analysis; when 1 and 2 year follow-up data is available on all patients, this analysis will be performed in a supplementary study.
In conclusion, preoperative PVB administration was associated with significant improvements in postoperative pain control, shorter time to oral-only narcotic usage, and reduced hospital length of stay for patients with breast cancer undergoing autologous breast reconstruction after mastectomy. Further studies are needed to evaluate if increased adoption of this regional anesthesia technique has an impact on long-term health-related quality of life and will result in substantial cost savings across the health-care system.
Synopsis.
The management of postoperative pain remains a substantial challenge in patients undergoing autologous breast reconstruction after mastectomy for breast cancer. This study demonstrates preoperative paravertebral blocks significantly reduce postoperative pain and length of stay in this patient population.
Acknowledgments
RPP is supported by a National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant, T32CA190194 (PI: Colditz), by the Foundation for Barnes-Jewish Hospital, and by Siteman Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Disclosures: Dr. Myckatyn receives research grant and consulting fees from LifeCell, Allergan, and Andrew Technologies; none of these relationships relate to, or had an impact on, this study. All other authors have nothing to disclose. No financial or material support was provided for this study.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society, Inc; 2016. [Google Scholar]
- 2.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plastic and reconstructive surgery. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 3.Sisco M, Du H, Warner JP, Howard MA, Winchester DP, Yao K. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. Journal of the American College of Surgeons. 2012;215(5):658–666. doi: 10.1016/j.jamcollsurg.2012.07.008. discussion 666. [DOI] [PubMed] [Google Scholar]
- 4.Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet (London, England) 1983;1(8322):459–462. doi: 10.1016/s0140-6736(83)91452-6. [DOI] [PubMed] [Google Scholar]
- 5.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery. 2013;132(2):201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 6.Jagsi R, Li Y, Morrow M, et al. Patient-reported Quality of Life and Satisfaction With Cosmetic Outcomes After Breast Conservation and Mastectomy With and Without Reconstruction: Results of a Survey of Breast Cancer Survivors. Annals of surgery. 2015;261(6):1198–1206. doi: 10.1097/SLA.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winters ZE, Benson JR, Pusic AL. A systematic review of the clinical evidence to guide treatment recommendations in breast reconstruction based on patient- reported outcome measures and health-related quality of life. Annals of surgery. 2010;252(6):929–942. doi: 10.1097/SLA.0b013e3181e623db. [DOI] [PubMed] [Google Scholar]
- 8.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. British journal of anaesthesia. 2013;111(5):711–720. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coveney E, Weltz CR, Greengrass R, et al. Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases. Annals of surgery. 1998;227(4):496–501. doi: 10.1097/00000658-199804000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahy AS, Jakub JW, Dy BM, et al. Paravertebral blocks in patients undergoing mastectomy with or without immediate reconstruction provides improved pain control and decreased postoperative nausea and vomiting. Annals of surgical oncology. 2014;21(10):3284–3289. doi: 10.1245/s10434-014-3923-z. [DOI] [PubMed] [Google Scholar]
- 11.Shah A, Rowlands M, Krishnan N, Patel A, Ott-Young A. Thoracic Intercostal Nerve Blocks Reduce Opioid Consumption and Length of Stay in Patients Undergoing Implant-Based Breast Reconstruction. Plastic and reconstructive surgery. 2015;136(5):584e–591e. doi: 10.1097/PRS.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 12.Coopey SB, Specht MC, Warren L, Smith BL, Winograd JM, Fleischmann K. Use of preoperative paravertebral block decreases length of stay in patients undergoing mastectomy plus immediate reconstruction. Annals of surgical oncology. 2013;20(4):1282–1286. doi: 10.1245/s10434-012-2678-7. [DOI] [PubMed] [Google Scholar]
- 13.Wolf O, Clemens MW, Purugganan RV, et al. A Prospective, Randomized, Controlled Trial of Paravertebral Block versus General Anesthesia Alone for Prosthetic Breast Reconstruction. Plastic and reconstructive surgery. 2016;137(4):660e–666e. doi: 10.1097/01.prs.0000481070.79186.0d. [DOI] [PubMed] [Google Scholar]
- 14.Matros E, Albornoz CR, Razdan SN, et al. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plastic and reconstructive surgery. 2015;135(4):937–946. doi: 10.1097/PRS.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 15.Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: A meta-analysis. Journal of surgical oncology. 2015;112(5):468–475. doi: 10.1002/jso.24032. [DOI] [PubMed] [Google Scholar]
- 16.Atisha D, Alderman AK. A systematic review of abdominal wall function following abdominal flaps for postmastectomy breast reconstruction. Annals of plastic surgery. 2009;63(2):222–230. doi: 10.1097/SAP.0b013e31818c4a9e. [DOI] [PubMed] [Google Scholar]
- 17.Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. Journal of the American College of Surgeons. 2013;216(2):229–238. doi: 10.1016/j.jamcollsurg.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Jagsi R, Jiang J, Momoh AO, et al. Complications After Mastectomy and Immediate Breast Reconstruction for Breast Cancer: A Claims-Based Analysis. Annals of surgery. 2016;263(2):219–227. doi: 10.1097/SLA.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. Jama. 2009;302(18):1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 20.Hickey OT, Burke SM, Hafeez P, Mudrakouski AL, Hayes ID, Shorten GD. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. The Clinical journal of pain. 2010;26(7):556–560. doi: 10.1097/AJP.0b013e3181dee988. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. British journal of cancer. 2005;92(2):225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight MA, Nguyen DTt, Kobayashi MR, Evans GR. Institutional review of free TRAM flap breast reconstruction. Annals of plastic surgery. 2006;56(6):593–598. doi: 10.1097/01.sap.0000202226.92967.f0. [DOI] [PubMed] [Google Scholar]