Abstract
Expression of the transglutaminase TG2 has been linked to constitutive activation of NF-kB and chemotherapy resistance in mantle cell lymphoma (MCL) cells. TG2 forms complexes with NF-kB components, but mechanistic insights that could be used to leverage therapeutic responses has been lacking. In the present study, we address this issue with the discovery of an unexpected role for TG2 in triggering autophagy in drug-resistant MCL cells through induction of IL-6. CRISPR-mediated silencing of TG2 delayed apoptosis while overexpressing TG2 enhanced tumor progression. Under stress, TG2 and IL-6 mediate enhanced autophagy formation to promote MCL cell survival. Interestingly, the autophagy product ATG5 involved in autophagosome elongation positively regulated TG2/NF-kB/IL-6 signaling, suggesting a positive feedback loop. Our results uncover an interconnected network of TG2/NF-kB and IL-6/STAT3 signaling with autophagy regulation in MCL cells, the disruption of which may offer a promising therapeutic strategy.
Keywords: Mantle cell lymphoma, TG2, NF-kB, IL-6, Autophagy
Introduction
Mantle cell lymphoma (MCL) is an aggressive B-cell lymphoma that accounts for approximately 6%–8% of non-Hodgkin lymphomas. Despite improved therapeutic responses with frontline therapies (1,2), MCL patients often relapse, which results in inevitable chemoresistance and poor clinical outcomes (3,4).
TG2, encoded by the TGM2 gene, is an 80-kDa enzyme. It has multiple physiologic functions and is associated with cancer cell survival, metastatic behavior and drug resistance (5–9). TG2 has been proposed as a promising therapeutic target in the treatment of human cancers (10,11). Increasing evidence has demonstrated that TG2 is closely associated with constitutive nuclear factor-kappa B (NF-kB) expression in cancer cells (12,13). Our previous study has shown that TG2 forms complexes with NF-kB components, which drives the translocation of NF-kB to the nucleus and constitutive expression of NF-kB (11). Moreover, TG2 and NF-kB are highly expressed in MCL cells that are stem-like, suggesting that TG2/NF-kB signaling plays a critical role in MCL progression (11).
Signaling pathways such as NF-kB, Janus kinase / signal transducer and activator of transcription (JAK/STAT), and mitogen-activated protein kinases (MAPK) signaling are linked to the upregulation of cytokines, such as interleukin-6 (IL-6), IL-2 or IL-10 (14,15). The JAK/STAT inhibitor degrasyn inhibits MCL cell growth, and this inhibition correlates with the down-regulation of constitutive NF-kB signaling and STAT3 phosphorylation (16). A major upstream activator of STAT3 is IL-6, which binds its receptor and activates JAK, which in turn phosphorylates and activates STAT3. However, it remains unclear whether these events are connected to TG2 signaling and whether the drug resistance of MCL is dependent on the IL-6 expression mediated by TG2/NF-kB signaling.
Autophagy is a highly conserved homoeostatic mechanism for the lysosomal degradation of cytosolic constituents (17). It can be induced by different conditions, including nutrient deprivation/starvation, oxidative stress, hypoxia, and chemotherapeutic drugs (17–20). Autophagy also plays an important role in innate and adaptive immunity and can be regulated by different cytokines, such as transforming growth factor beta (TGF-β) or IL-6 (17,21–24). TGM2 is considered to be a stress-responsive gene, and TG2 activity is upregulated by various stressors (13,25). Given that both autophagy and TG2 activity can be induced under cellular stress and various cytokines are involved in autophagy regulation, we hypothesized that autophagy could be regulated by either the TG2/NF-kB signaling pathway or its downstream cytokine IL-6.
In the present study, we discovered that up-regulated TGM2 is correlated with a poor prognosis in MCL patients; increased TG2 levels promote tumor progression in vivo. Silencing TG2 by CRISPR-Cas9 using a lentiviral system significantly affected NF-kB activities and IL-6/STAT3 signaling. Notably, our data are the first to indicate an interconnected network among TG2/NF-kB, IL-6/STAT3 signaling and autophagy regulation in MCL cells and how this network regulates MCL survival under stress. Connecting this signaling network may help in the design of targeted therapies and the identification of novel therapeutic targets in the future.
Materials and Methods
Cells and cell culture
The human MCL cell line Jeko was obtained from the American Type Culture Collection (Manassas, VA). The human MCL cell line SP53 was a kind gift from MD Anderson Cancer Center (MDACC). HS5 and HS27a bone marrow stromal cells (BMSCs) were a kind gift from Dr. B. Torok-Storb (Fred Huchinson Cancer Research Center, Seattle, WA). Cell lines were authenticated using short tandem repeats in Characterized Cell Line Core Facility at MDACC. Cells were maintained under 5% CO2 at 37°C and cultured in complete RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, 100≤μg/ml streptomycin and 100 U.I. /ml penicillin.
Human MCL samples
Peripheral blood (PB) and bone marrow (BM) samples from MCL patients were obtained after informed consent, as approved by MDACC as well as by the University of Texas Health Science Center (UT-HSC) Institutional Review Boards. Mononuclear cells were isolated from MCL patient apheresis blood or BM using standard Ficoll gradient separation methods. CD19+ B cells from MCL patients or healthy donors were isolated as previously described (26). Primary cells were cultured in complete RPMI1640 medium for further analysis.
Generation of lentivirus constructs and infection
The MIT CRISPR design software was used for the design of sgRNAs (http://crispr.mit.edu). To clone individual sgRNAs, 25-bp oligonucleotides containing the sgRNA sequences were synthesized (Sigma). Details for generation of lentivirus constructs and lentivirus infection can be found in supplementary methods.
ELISA assay
Cells were counted using Trypan Blue Solution before each assay. The DNA-binding activities of NF-kB components, TG2 enzymatic activity and IL-6 levels were determined by ELISA assays, as previously described (11,26).
Cell viability assay and IC50
Cytotoxicity was assessed with fluorimetric cell viability assay using CellTiter-Blue® as previously described (27). The Hill-Slope logistic model is used to calculate IC50 using CompuSyn software.
Quantitative real-time PCR (qRT-PCR)
Procedures for qRT-PCR were performed as previously described (27). The involved primers are provided in supplementary methods. The relative expression level of each gene was normalized to the GAPDH by the method of 2−ΔΔCt.
Immunoblotting and semi-quantitative analysis
The STAT3 pathway was detected as previously described (26). Total harvested cells were lysed to perform immunoblots as previously reported (27). Immunoblotting was subjected to semi-quantitative analysis using ImageJ software.
MethoCult colony assay
MCL cells (5 × 103) were suspended in 1 ml of complete MethoCult medium (see supplementary methods for detailed components) and plated onto 35mm petri dishes. Cells were co-cultured with HS5 BMSCs, HS5 conditioned media (HS5-CM) or HS5-CM plus IL-6 neutralizing antibodies (1 μg/ml). Colonies were maintained at 37°C, 5% CO2 with 95% humidity for 5 days, and were counted and photographed at day 5 using an Olympus IX70 microscope. Only colonies consisting of 50 or more cells were considered for analysis.
Tumor xenograft studies
Immuno-deficient NOD/SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained under barrier conditions. All animal procedures were approved by the UT-HSC Animal Care Committee. Manipulated SP53 MCL cells (3.5 × 106) were subcutaneously injected into NOD/SCID mice (n=5, male) and tumor growth was monitored weekly. Mice were sacrificed four weeks post injection and tumors, spleens and bone marrows were isolated for further analysis. The volumes of tumors and spleens were measured as previously described (26).
Results
TG2/NF-kB signaling axis is critical for MCL survival
Many cancer cells constitutively express NF-kB components and show elevated levels of phosphorylated STAT3 (p-STAT3) due to the upregulation of cytokines such as IL-6 or IL-10 (15). To determine the downstream events of TG2 and NF-kB, we silenced TG2 (TG2KO) in MCL cells using a lentiviral CRISPR-Cas9-mediated knockout system. The construct of TG2-sgRNA-LentiCRISPR plasmid was validated by DNA sequencing, and the knockout efficiency of TG2 in MCL cells was evaluated by immunoblotting, which indicated complete silencing (Supplementary Fig. 1). We then utilized TG2KO and TG2-overexpressed cells (TG2OE) to evaluate the effects of TG2 on MCL survival, respectively. The empty vector-transfected MCL cells were used as controls. The proliferation rates of TG2KO cells were largely reduced compared with controls, while TG2OE cells showed higher proliferation rate than the control (Fig. 1A), indicating a potential oncogenic role of TG2 in the pathogenesis of MCL. Notably, TG2KO cells underwent a delayed apoptosis after a 10-day incubation, suggesting that TG2 is critical in maintaining MCL survival (Supplementary Fig. 2A).
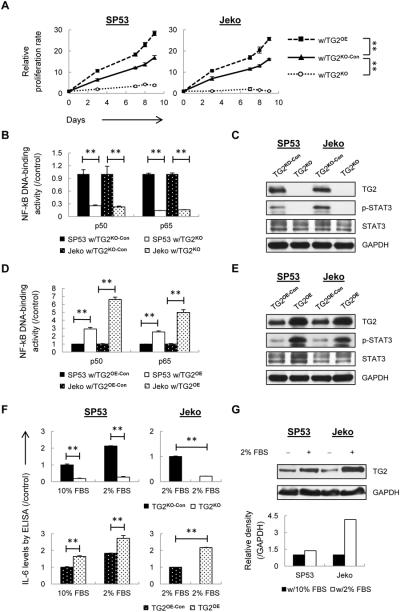
Figure 1. IL-6/STAT3 signaling is dependent on TG2/NF-kB signaling axis in MCL.
(A) Cell proliferation of TG2KO and TG2OE cells were determined using Trypan Blue and MTT assays. The controls were mock-transfected cells. (B) The DNA binding activities of p50 and p65 were measured using nuclear extracts from TG2KO or control (TG2KO-Con) cells. The colorimetric values in TG2KO cells were normalized to the controls. (C) STAT3 and p-STAT3 (Tyr705) were measured using immunoblotting with GAPDH as a loading control. (D) The DNA binding activities of p50 and p65 were measured using nuclear extract from TG2OE or control (TG2OE-Con) cells. The colorimetric values in TG2OE cells were normalized to the controls. (E) STAT3 and p-STAT3 were measured using immunoblotting with GAPDH as a loading control. (F) ELISA analysis of IL-6 using conditioned media from TG2KO and TG2OE cells under normal conditions (10% FBS) or low-serum conditions (2% FBS). (G) TG2 levels were analyzed using immunoblotting and the relative intensity of the bands is shown compared with cells in normal conditions. The data are shown as the mean ± S.D. **p < 0.01.
To elucidate the mechanisms involving reduced proliferation of TG2KO cells, cell cycle and related factors were analyzed. In SP53 MCL cells, the diminished proliferation in TG2KO cells was associated with increased G0/G1 phase (Supplementary Fig. 2B). TG2KO cells contained increased p53, p21 and p27 levels and decreased cyclin genes such as CDK4, CDK6 and CDC2, indicating a cell cycle arrest (Supplementary Fig. 2C). TG2KO cells had decreased levels of anti-apoptotic genes BCL-XL and BCL-2, and increased expression of pro-apoptotic genes BAX, BAK, and NOXA. Apoptotic indicators, such as cleaved Caspase 3/9 and cleaved PARP were observed after one-week incubation. Collectively, these data support TG2KO cells have a delayed apoptosis (Supplementary Fig. 2D). Interestingly, silencing TG2 reduced BCL-XL and BCL-2, but not MCL-1 levels. Similar to what was reported (28–30), accumulation of MCL-1 did not prevent apoptosis caused by TG2 silencing; instead, it may slow down an apoptotic induction. Since TG2KO cells have delayed apoptosis, we were able to use viable TG2KO cells for all subsequent experiments.
In order to investigate the effects of TG2 signaling on the downstream events, we examined NF-kB levels in MCL cells using an ELISA-based assay, which is an alternative way to measure NF-kB binding instead of a radioactivity-based assay (31). NF-kB p50 and p65 DNA-binding activities as well as NF-kB downstream target gene such as IL-8 (32), were dramatically decreased in TG2KO cells (Fig. 1B and Supplementary Fig. 3A). p-STAT3 expression was also significantly reduced in TG2KO MCL cells (Fig. 1C). Correspondingly, overexpressed TG2 resulted in higher NF-kB and STAT3 signaling activities (Fig. 1D and E; Supplementary Fig. 3B).
A major upstream activator of STAT3 signaling is IL-6. We then measured IL-6 basal levels in both TG2KO and TG2OE SP53 cells using ELISA, because Jeko cells do not produce IL-6 under normal cell culture conditions (33). IL-6 levels were significantly reduced after TG2 silencing while enhanced in TG2OE cells (Fig. 1F). We further cultured cells in low-serum conditions (2% FBS), which induces IL-6 secretion in both SP53 and Jeko MCL cells (33). Compared with the control cells in low serum, TG2OE cells induced more IL-6, while TG2KO cells failed to increase IL-6 (Fig. 1F). Interestingly, MCL cells in low-serum conditions not only increased IL-6 levels but also enhanced TG2 expression (Fig. 1G), suggesting that TG2 may be a part of the stress-induced survival mechanisms for MCL.
TG2 has been proposed as a promising therapeutic target in the treatment of human cancers due to its association with drug resistance (6,8,10,11). We then investigated whether TG2 expression contributes to chemotherapy resistance in MCL. Cells were treated with chemotherapeutic drugs for 24 h. Compared with the controls, TG2 silencing exhibited sensitivity of MCL cells to chemotherapeutic drugs, while TG2 overexpressing cells showed resistant to the drugs with higher IC50 values (Supplementary Fig. 4A and B). Longer incubation time with drugs in TG2OE cells also showed chemoresistance compared to the controls (Supplementary Fig. 4C). These data provide strong evidence that TG2 confers drug resistance properties to MCL.
TG2/NF-kB downstream signaling molecule IL-6 regulates colony formation in MCL cells
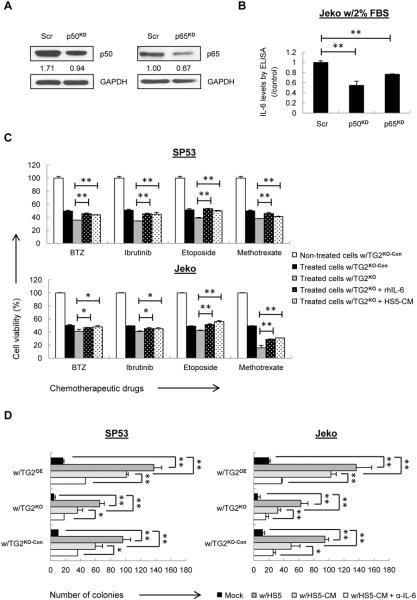
To further investigate the roles of the TG2/NF-kB downstream cytokine IL-6 in MCL, we generated p50-knock-down (p50KD) and p65-knock-down (p65KD) MCL cells using shRNA-mediated lentivirus, because NF-kB knockout leads to rapid apoptosis in MCL. It showed efficient knock-down of p50 or p65 expression in GFP+ Jeko cells (Fig. 2A and Supplementary Fig. 5). We cultured Jeko under low-serum conditions, as reported, to ensure IL-6 secretion (33). IL-6 levels were significantly reduced in both p50KD and p65KD cells compared with the control under low-serum conditions (Fig. 2B). Together with the result shown in Figure 1F, these findings suggest that the increased levels of IL-6 in MCL cells are mediated by TG2/NF-kB signaling.
Figure 2. IL-6 regulates colony formation in MCL cells mediated by TG2/NF-kB signaling.
(A) The p50 and p65 levels were analyzed using immunoblotting with a scrambled shRNA plasmid (Scr) as a negative control. (B) ELISA analysis of IL-6 using conditioned media from p50KD or p65KD Jeko cells under low-serum conditions. The colorimetric values of p50KD or p65KD cells were normalized to the controls. (C) TG2KO and control cells were treated with each drug at IC50 values with or without rhIL-6 or HS5-CM for 24 h. Cell viability was determined using MTT assays, which normalized to non-treated control cells. (D) TG2KO and TG2OE cells were co-cultured with HS5 cells, HS5-CM, and HS5-CM plus IL-6 neutralizing antibody (α-IL-6). The average number of colonies in SP53 or Jeko cells from three parallel assays in each group is shown in the diagram. The data are shown as the mean ± S.D. *p < 0.05; **p < 0.01.
Studies have reported the association of IL-6 expression with tumor progression, poor prognosis and chemotherapeutic resistance (34–36). Targeting IL-6 has been regarded as an adjuvant therapy for human cancers (37,38). We next investigated whether IL-6 can protect MCL cells from chemotherapy-induced cell death. MCL cells were co-cultured with HS5 BMSCs or HS5-CM and treated with chemotherapeutic drugs. HS5 cells have been reported to produce large amounts of IL-6 (39). MCL cells co-cultured with HS5 cells or HS5-CM exhibited higher cell viability compared with the cells cultured under standard conditions (Supplementary Fig. 6). Addition of human recombinant IL-6 (rhIL-6) or HS5-CM was able to partially reverse the effects of TG2 depletion on increased drug sensitivity (Fig. 2C), suggesting that IL-6 contributes to the drug resistant properties of TG2. Furthermore, MCL cells co-cultured with HS5 cells or HS5-CM also significantly increased colony formation in MethoCult medium, while blocking IL-6 using neutralizing antibodies dramatically reduced the colony-forming ability of the MCL cells (Fig. 2D; Supplementary Table 1 and Supplementary Fig. 7). Additionally, TG2 expression also contributes to the colony formation compared with control cells, suggesting that TG2 is involved in the oncogenic mechanism in MCL (Fig. 2D; Supplementary Table 1 and Supplementary Fig. 7).
Reciprocal interactions between TG2/NF-kB signaling and autophagy in MCL cells
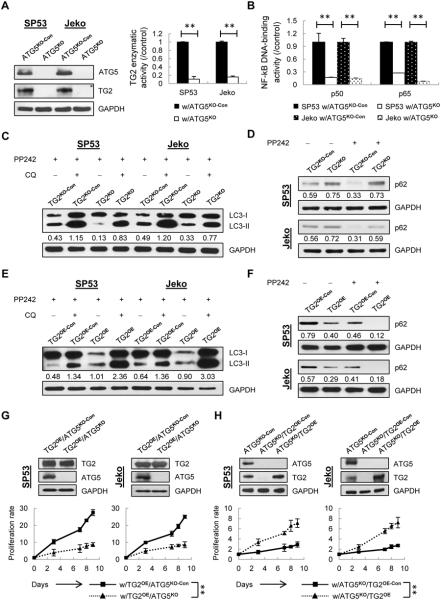
Autophagy plays a dual role in different stages of cancer development. At a very early stage, autophagy prevents cancer initiation by eliminating oncogenic protein substrates; once a tumor forms and develops, autophagy is activated and plays a cytoprotective role (40,41). Many autophagy-related (ATG) genes are involved in autophagosome initiation and maturation (17,18,40–43). To elucidate how autophagy and TG2/NF-kB signaling are connected, we generated ATG5-knockout (ATG5KO) MCL cells by lentivirus-mediated CRISPR-Cas9 using a same schematic protocol described above. The knockout efficiency of ATG5 in MCL cells was evaluated by immunoblotting, which showed complete silencing (Supplementary Fig. 8). ATG5KO cells showed a reduced proliferation rate compared with the controls (Supplementary Fig. 9A) due to the impaired autophagosome and autolysosome formation (Supplementary Fig. 9B and C), suggesting autophagy may serve as an important survival mechanism in MCL cells. Interestingly, ATG5KO cells showed increased apoptosis similar to TG2KO cells (Supplementary Fig. 9D), implying the potential interactions between TG2 and autophagy.
To investigate roles of TG2/NF-kB signaling in autophagy, we examined TG2 expression and activity in ATG5KO cells using immunoblotting and ELISA assays. TG2 expression levels and enzymatic activities were decreased dramatically compared with the controls (Fig. 3A). Silencing ATG5 also remarkably inhibited NF-kB activation (Fig. 3B), indicating that TG2/NF-kB signaling pathway is involved in autophagy in MCL.
Figure 3. Interactions between TG2/NF-kB signaling and autophagy in MCL cells.
(A) TG2 was measured using immunoblotting with GAPDH as a loading control. TG2 enzymatic activities in ATG5KO cells were normalized to controls. (B) The DNA-binding activities of p50 and p65 in the nuclear extracts of ATG5KO cells were measured using ELISA. (C) Cells were treated with the autophagy inducer pp242 (1 μM) with or without lysosomal inhibitor CQ (20 μM) for 24 h. The indicated values of LC3-II under each lane were normalized to GAPDH. (D) p62 was measured using immunoblotting with or without pp242 for 24 h. The normalized values (p62/GAPDH) in each lane are indicated. (E) Autolysosome and autophagosome formation were measured upon TG2 overexpression with normalized values (LC3-II/GAPDH) indicated under each lane. (F) p62 was measured in TG2OE MCL cells with or without pp242 treatment. The p62/GAPDH values in each lane are indicated. (G) Knockout efficiency of ATG5 in TG2OE cells (TG2OE/ATG5KO) was validated. The controls were mock-transfected cells (TG2OE/ATG5KO-Con). Cell proliferation was determined using Trypan Blue. (H) Enhanced TG2 levels in ATG5KO cells (ATG5KO/TG2OE) were validated. Mock-transfected cells (ATG5KO/TG2OE-Con) were used as controls. Cell proliferation was determined using Trypan Blue. The data are shown as the mean ± S.D. **p < 0.01.
To confirm whether TG2 signaling in turn regulates autophagy, we first determined the basal levels of autophagy in TG2KO and TG2OE cells. Both autophagosome and autolysosome formation were inhibited in TG2KO cells but enhanced in TG2OE cells compared with the controls (Supplementary Fig. 10A and B). We then treated cells with the autophagy inducer PP242. As shown in Figure 3C, TG2KO cells exhibited decreased LC3-II formation after PP242 treatment compared with the controls. Autophagic flux assays that measure autolysosome formation also showed decreased LC3-II levels in TG2KO cells in the presence of lysosomal inhibitor chloroquine (CQ), indicating that autophagy formation was impaired upon TG2 silencing. These data were further validated using additional detection of autophagy marker p62 (Fig. 3D). Conversely, TG2OE cells showed enhanced LC3-II formation after PP242 treatment; further CQ treatment caused more LC3-II accumulation in TG2OE cells compared with that in control cells, indicating the autophagic flux was increased upon TG2 overexpression (Fig. 3E). Consistently, a significant reduction of p62 was observed in TG2OE cells compared with the controls (Fig. 3F).
To better understand the reciprocal relationship between TG2 and autophagy, we further silenced ATG5 in TG2OE MCL cells (TG2OE/ATG5KO) using a CRISPR-Cas9 system (Fig. 3G). Compared to controls, TG2OE/ATG5KO cells have reduced proliferation rate and decreased chemoresistance (Fig. 3G; Supplementary Fig. 11A). The combination of chemotherapeutic drugs with 3-MA, an autophagy inhibitor upstream of ATG5, also decreased drug resistance of TG2OE cells (Supplementary Fig. 11B), indicating that upregulated autophagy may be responsible for drug resistance in TG2OE MCL. Correspondingly, overexpression of TG2 led to a partial increase of proliferation in ATG5KO cells (Fig. 3H), suggesting that decreased TG2 levels could be a causative factor for reduced survival of ATG5KO cells.
Targeting mTOR pathway has been a promising therapy for human cancers; however, mTOR inhibition induces autophagy, which can counteract the effect of the inhibitors (44,45). We then investigated whether there is an association between TG2 expression levels and responses to mTOR inhibitors. Similar to what was observed, TG2 deletion sensitized MCL cells to PP242, a potent mTOR inhibitor, whereas TG2OE cells displayed lower sensitivity to PP242 (Supplementary Fig. 12A). Our data support important roles of autophagy in cancer drug resistance, because autophagy inhibitors 3-MA or CQ increased cytotoxicity of MCL cells to mTOR inhibitors (Supplementary Fig. 12B). Taken together, our results support autophagy is one of the important processes involved in the therapeutic resistance and oncogenic survival mechanism in TG2OE MCL cells.
Autocrine and paracrine cytokine IL-6 regulates autophagy in MCL cells
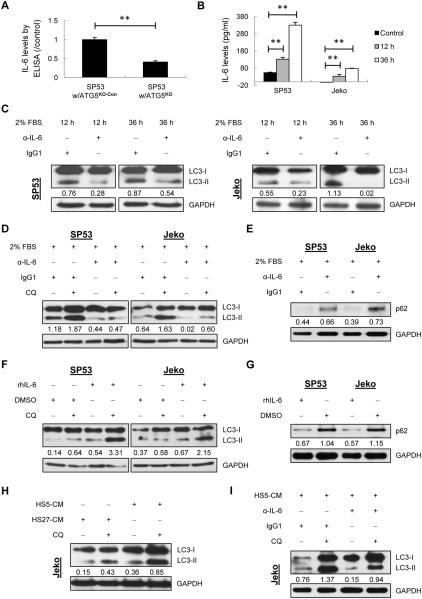
Given that TG2/NF-kB signaling axis is involved in autophagy induction, it is important to delineate the downstream signaling pathway. We first measured IL-6 levels in ATG5KO cells using ELISA. IL-6 levels were significantly inhibited in ATG5KO cells (Fig. 4A), indicating that impaired ATG5 expression affects IL-6 signaling in MCL cells.
Figure 4. Autocrine and paracrine IL-6 stimulates autophagy in MCL cells.
(A) ELISA analysis of IL-6 using conditioned media from ATG5KO SP53 cells under normal conditions. The data were normalized to the control. (B) IL-6 levels were measured using conditioned media from MCL cells under 2% FBS or 10% FBS (control) for 12 h and 36 h, respectively. (C) LC3-II levels were analyzed in SP53 and Jeko cells using immunoblotting with α-IL-6 (1 μg/ml) or IgG1 antibody (control). The LC3-II/GAPDH values are indicated. (D) Cells were cultured in 2% FBS with α-IL-6 or IgG1. Autophagic flux was determined with or without CQ treatment (20 μM). (E) MCL cells were cultured in 2% FBS with α-IL-6 or IgG1. p62 was measured with normalized values under each lane. (F) Cells were treated with rhIL-6 (50 ng/ml) or DMSO with or without CQ. The LC3-II/GAPDH values in each lane are indicated. (G) MCL cells were treated with rhIL-6 or DMSO. p62 was measured with normalized values under each lane. (H) Jeko cells were cultured in HS5-CM or HS27a-CM with or without CQ. The LC3-II/GAPDH values are indicated. (I) Jeko cells were cultured in HS5-CM with α-IL-6 or IgG1 in the presence or absence of CQ.
We then analyzed whether the autocrine IL-6 produced by MCL cells plays an important role in autophagy formation. MCL cells were challenged with low FBS (2%) to stimulate autocrine IL-6 (Fig. 4B; Supplementary Fig. 13A and B). Autophagy was induced by autocrine IL-6, while neutralization of IL-6 resulted in largely decreased LC3-II formation (Fig. 4C). Autophagic flux assays showed a decreased LC3-II level in the cells that are blocked by neutralizing IL-6 (Fig. 4D). Blocking IL-6 also led to increased p62 levels (Fig. 4E). These data indicated that IL-6 signaling is critical for starvation-induced autophagy formation.
Because starvation-induced IL-6 can complicate the interpretation of the autophagy formation, we studied the effects of paracrine IL-6 on autophagy using either rhIL-6 or HS5-CM. rhIL-6 resulted in enhanced autophagy formation and increased autophagic flux; rhIL-6 treatment also led to a significant reduction of p62 levels compared to the controls (Fig. 4F and G). To further verify this finding, Jeko MCL cells, which are not able to secrete IL-6 under normal conditions (Fig. 4B and Supplementary Fig. 13B), were cultured in HS5-CM for 36 h, and autophagy formation was evaluated. HS27a cells, which have been reported to produce stromal cell-derived factor 1 (SDF-1) but not IL-6 (39), were used as a control. The results showed that paracrine IL-6 secreted by BMSCs led to an increase in LC3-II formation; further CQ treatment caused more LC3-II accumulation under HS5-CM compared with that under HS27a-CM (Fig. 4H). We then blocked IL-6 in HS5-CM using neutralizing antibodies. LC3-II levels were decreased after the neutralization of IL-6 in either the presence or absence of CQ (Fig. 4I), indicating that the autophagy formation was induced by paracrine IL-6. Taken together, these data suggest IL-6 is an important mediator for autophagy signaling in MCL.
TG2 overexpression promotes tumor progression in xenograft mice
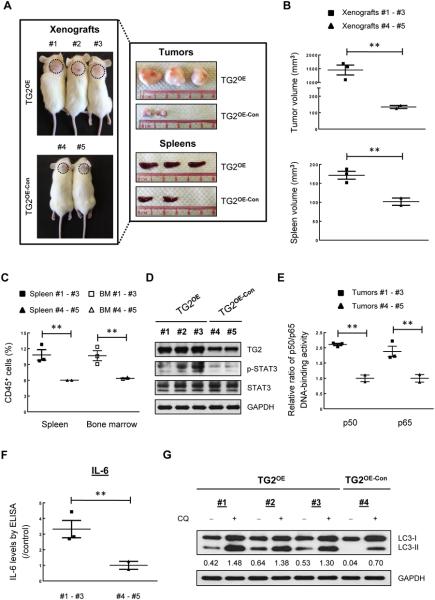
To verify our findings using in vivo models, we subcutaneously transplanted TG2OE SP53 MCL cells or control cells. Compared with controls, TG2OE xenograft mice developed larger tumors and enlarged spleens (Fig. 5A and B). Higher numbers of CD45+ cells were found in the spleens and bone marrows from TG2OE xenograft mice, indicating increased tumor dispersal (Fig. 5C). Immunoblotting analysis using tumor samples revealed increased levels of p-STAT3 in TG2OE mice (Fig. 5D). Overexpressed TG2 in mice also resulted in enhanced NF-kB p50 and p65 DNA-binding activities and increased IL-6 levels (Fig. 5E and F). Furthermore, we found increased autophagy formation and autophagic flux in tumor samples of TG2OE xenografts (Fig. 5G). These data strongly support the oncogenic ability of TG2 in MCL, and further validate an interconnected network among TG2/NF-kB/IL-6 signaling and autophagy regulation in MCL.
Figure 5. TG2 overexpression enhances tumor progression in xenograft mice.
(A) TG2OE or TG2OE-Con MCL cells were subcutaneously injected into NOD/SCID mice (n=5). Xenograft mice were sacrificed 4 weeks post injection. Dotted circles represent area of subcutaneous tumors. Tumors and spleens were isolated and photographed against a centimeter ruler. (B) The size of tumors and spleens were measured. (C) CD45+ human cells were isolated from spleens and bone marrows using CD45-MicroBeads, and the numbers of CD45+ cells were counted using Trypan Blue. (D) Immunoblots of tumor samples for TG2, p-STAT3, and STAT3 with GAPDH as a loading control. (E) The DNA binding activities of p50 and p65 in the nuclear extracts of xenograft tumors. The colorimetric values in TG2OE xenografts were normalized to the controls. (F) Xenograft tumor cells were cultured in complete RPMI1640 medium for 48 h. IL-6 expression was measured using ELISA. The data were normalized to the controls. (G) Xenograft tumor cells were cultured in complete RPMI1640 medium with or without CQ treatment (20 μM) for 24 h. The LC3-II/GAPDH values in each lane are indicated. The data are shown as the mean ± S.D. **p < 0.01.
Increased TG2 levels are associated with a poor prognosis in MCL patients
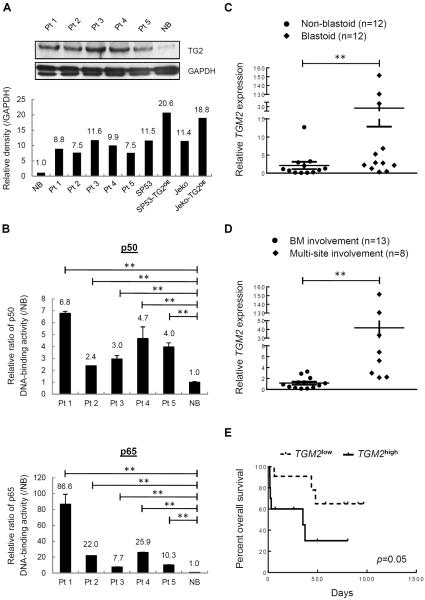
To determine the clinical relevance of our findings, we firstly measured TG2 protein levels in MCL patient cells using immunoblots. B cells of PB from healthy donors were used as a control. Elevated TG2 levels were noted in MCL samples compared with normal B (NB) cells (Fig. 6A). MCL cells isolated from different patients also showed elevated NF-kB activity levels compared with NB cells (Fig. 6B). We then investigated the correlation of TG2 expression with patient prognosis. TGM2 gene expression levels were compared in CD19+ B cells from MCL patients or NB cells from healthy donors. TGM2 mRNA was elevated in malignant B cells compared with NB cells; interestingly, 3 of the 24 MCL cases displayed a significant elevation of TGM2 levels, with approximately 30-fold, 130-fold and 150-fold increases compared with the NB cells (Supplementary Fig. 14). These patients are a blastoid type of MCL, which is a highly aggressive subtype form of MCL that has a worse prognosis compared with classical MCL (Supplementary Table 2). Further analysis of samples revealed that blastoid subtypes displayed significantly higher TGM2 levels than non-blastoid cases (Fig. 6C). TGM2 levels of MCL patients that have a record of multi-site infiltrations (e.g., gastrointestinal, spleen, liver or central nervous system) besides BM were higher compared to the patients that only had BM involvement (Fig. 6D). The overall survival of patients with TG2high was also significantly lower than that of patients with TG2low (Fig. 6E). Our data support that TG2/NF-kB signaling is involved in not only the pathogenesis of MCL cell lines but also of MCL patients.
Figure 6. Expression patterns of TG2 and NF-kB in MCL patients.
(A) TG2 levels were measured in MCL patients (n=5) and normal B (NB) cells (n=1). The intensity of the bands in MCL patients (Figure 6A) and cell lines (Figure 1E) were analyzed using ImageJ software and normalized to GAPDH. The values compared to NB cells are indicated. (B) Nuclear extracts from MCL patients (n=5) and NB cells (n=1) were subjected to ELISA assays to evaluate p50 and p65 DNA-binding activities. The colorimetric values in MCL samples were normalized to the NB cells. (C) TGM2 mRNA levels were measured in CD19+ B cells isolated from MCL blastoid patients (n=12) or non-blastoid patients (n=12). (D) TGM2 mRNA levels of MCL patients with multi-site infiltrations besides BM involvement (n=8) were compared to those with solely BM involvement (n=13). (E) Overall survival of MCL patients (n=24) (p=0.05, Mantel-Cox curve analysis). TGM2high and TGM2low refer to the upper and lower 50% of the TGM2 levels in MCL patients, respectively. The results are shown as the mean ± S.D. **p < 0.01.
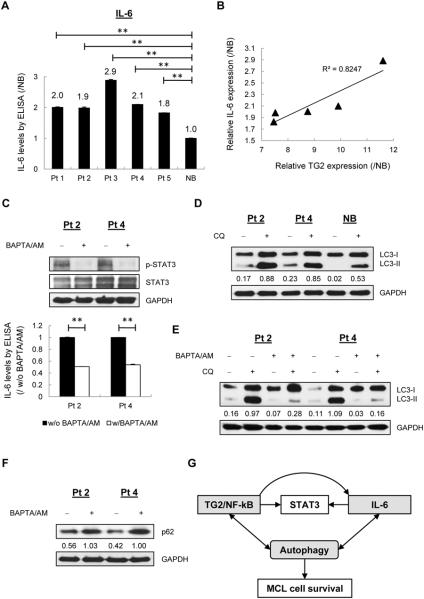
We next investigated the roles of the TG2/NF-kB downstream IL-6 in MCL patients. Cells from MCL patients demonstrated 1.8-fold to 2.9-fold increases in IL-6 expression compared with NB cells (Fig. 7A), suggesting constitutive expression. Interestingly, the calculation of coefficient of determination (R2) based on relative values revealed a linear correlation between IL-6 expression and TG2 expression, which further supports the possibility that IL-6 is downstream of TG2/NF-kB signaling (Fig. 7B).
Figure 7. Calcium blockers suppressed IL-6/STAT3 signaling and autophagy formation in MCL patients.
(A) ELISA analysis of IL-6 using conditioned media from MCL patients cells (n=5) cultured in complete RPMI1640 medium for 48 h. The values compared to NB cells are indicated. **p < 0.01. (B) Linear regression analysis of relative TG2 expression (Figure 6A) and relative IL-6 expression (Figure 7A) based on densitometry analyses of immunoblots. R-square (R2) value is 0.8247. (C) Primary MCL cells were treated with or without BAPTA/AM (60 μM) for 24 h for p-STAT3 immunoblotting and 48 h for IL-6 analysis. (D) Primary MCL cells and NB cells were treated with or without CQ for 24 h. The LC3-II/GAPDH values in each lane are indicated. (E) Autolysosome and autophagosome formation were measured in MCL patients upon BAPTA/AM treatment with or without of CQ for 24 h. (F) p62 was measured in MCL patients with or without BAPTA/AM treatment. (G) Interactions among TG2/NF-kB, IL-6/STAT3 signaling and autophagy in MCL cells. TG2 affects NF-kB activity and STAT3 signaling. IL-6, the upstream activator of STAT3, is stimulated by TG2/NF-kB signaling. Both TG2/NF-kB signaling and IL-6 contribute to the progression of autophagy, which in turn regulates TG2/NF-kB signaling and IL-6 secretion, suggesting a potential feedback loop underlying the survival mechanism in MCL cells.
To further determine the clinical relevance of TG2 in regulating IL-6/STAT3 signaling and autophagy formation, we inhibited TG2 enzymatic activities using a calcium chelator, 1,2-bis (o-aminophenoxy) ethane-N, N, N', N'-tetraacetic acid-acetoxymethyl ester (BAPTA/AM) in MCL primary cells (11). IL-6 levels and p-STAT3 expression were significantly reduced after treatment of BAPTA/AM (Fig. 7C). Compared with NB cells, primary cells from MCL patients showed upregulated autophagy formation (Fig. 7D). However, LC3 conversion and autophagic flux were decreased upon BAPTA/AM treatment in MCL primary cells (Fig. 7E), which was in line with the further detection of p62 levels (Fig. 7F). Collectively, these findings support that up-regulated TGM2 is a potential prognosis marker to predict survival of advanced MCL patients; TG2 may serve as an important signaling event in MCL that utilized NF-kB/IL-6/STAT3 signaling pathway and autophagy formation to promote MCL cell survival (Fig. 7G).
Discussion
Although intensive chemotherapies combined with newly approved drugs have improved MCL patient survival, highly refractory and relapsed cases remain major obstacles to improving the overall survival rate (1–4). In the present study, we demonstrated that TG2 and NF-kB were uniformly expressed at high levels in MCL patient cells compared with NB cells; overexpressed TG2 contributes to chemotherapy resistance in MCL. Notably, we found that silencing TG2 displayed a lower proliferation rate and short-term survival time, which emphasizes the important biological functions of TG2 in MCL cells. Accumulated evidence has indicated that the inhibition of TG2 can induce apoptosis (9,46), and the same is true in our results. Interestingly, we found a delayed onset of apoptosis in TG2KO cells. One possible reason may be due to the sustained expression of MCL-1, which is able to delay apoptosis (29), and plays an essential survival role in human myeloma cells rather than BCL-2 or BCL-XL (30). Although downregulation of BCL-2 and BCL-XL failed to induce rapid apoptosis in TG2KO cells, they sensitize these cells to different types of chemotherapeutic drugs, suggesting that silencing TG2 lowers the threshold for drug-induced apoptosis.
Studies have shown that the constitutive NF-kB signaling is associated with STAT3 phosphorylation in hematological malignancies (16,47,48). Being a major upstream activator of STAT3, IL-6 became a target of interest with regard to its role in MCL cells. In this study, we found that IL-6 was highly expressed in MCL patient cells. Silencing TG2 or NF-kB components led to abolishment of both IL-6 and p-STAT3 expression, which can be reversed by TG2 overexpression, indicating that IL-6/STAT3 signaling is mediated by the TG2/NF-kB signaling axis. Interestingly, we found that IL-6 plays an important role in regulating colony formation of MCL and contributes to drug resistance, suggesting that it may serve as a survival gene in MCL. Nevertheless, the potential survival mechanism underlying TG2/NF-kB/IL-6 remains unclear. To elucidate this situation, we introduced a major survival mechanism, autophagy, into our study.
Autophagy is well-accepted as a protective mechanism for cancer cells under different conditions (17–20). In the present study, we found that silencing ATG5 led to undetectable TG2 levels and reduced NF-kB expression; while TG2 in turn regulated autophagy formation and flux. What's more, the protective effects of TG2 overexpression can be reversed by inhibiting autophagy, revealing a novel mechanism of TG2-mediated autophagy in MCL cells. It is noteworthy that ATG5KO cells exhibited a similar cell fate as TG2KO cells in MCL. Apart from persistent loss of autophagy, reduced TG2 levels in ATG5KO cells might be another possible reason, since TG2 overexpression could partially rescue the phenotype of ATG5KO cells. Thus, dual targeting TG2 and autophagy may be one promising strategy to reduce chemoresistance in MCL.
IL-6 has been shown to regulate autophagy via activation of AMPK/mTOR pathway (24), and ATG5 depletion inhibits IL-6 production (49). Here we discovered that both autocrine and paracrine IL-6 can positively regulate autophagy. This finding partially explains why MCL cells display higher cell viability and colony formation in an IL-6-enriched co-culture setting. More interestingly, we found that autophagy, as well as being regulated by IL-6, can itself influence IL-6 secretion. Combined with our results that IL-6 levels are enriched in MCL patient samples and bone microenvironment, we herein conclude that MCL cells could elicit IL-6-mediated autophagy as an important way to survive and migrate to the BM. Nevertheless, HS27a-CM can also induce autophagy based on our result, although the autophagy induction by HS27a-CM was not as `strong' as that by HS5-CM. To some extent, we believe that the MCL cells are exposed to multiple paracrine signals from BMSCs that would modify MCL cell behavior and ultimately favor cancer progression.
Taken together, our data support a model where the MCL cells exploit both TG2/NF-kB signaling and IL-6 to enhance a cytoprotective autophagy response, and autophagy in turn regulates TG2/NF-kB signaling and IL-6 secretion, implying a potential feedback loop underlying the survival mechanism in MCL cells. Nonetheless, there are many unanswered questions, such as whether ATG5 genes directly associate with TG2/NF-kB and whether other inducers are involved in the regulatory network of TG2/IL-6/autophagy. Further studies will be needed in the future. Collectively, this study provides valuable insights into the survival mechanism of MCL cells. Disruption of TG2/IL-6/autophagy network may be a promising therapeutic target in the treatment of MCL patients and will introduce novel strategies to overcome chemoresistance in MCL.
Supplementary Material
Acknowledgements
This work is funded by NCI/NIH grant (5R01CA181319) given to NM.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. The New England journal of medicine. 2015;372(10):944–53. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 2.Kritharis A, Coyle M, Sharma J, Evens AM. Lenalidomide in non-Hodgkin lymphoma: biological perspectives and therapeutic opportunities. Blood. 2015;125(16):2471–76. doi: 10.1182/blood-2014-11-567792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maddocks K, Blum KA. Treatment strategies in mantle cell lymphoma. Cancer treatment and research. 2015;165:251–70. doi: 10.1007/978-3-319-13150-4_10. [DOI] [PubMed] [Google Scholar]
- 4.Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48–55. doi: 10.1182/blood-2014-05-521898. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ML, Keillor JW, Xu W, Eckert RL, Kerr C. Transglutaminase Is Required for Epidermal Squamous Cell Carcinoma Stem Cell Survival. Molecular cancer research : MCR. 2015 doi: 10.1158/1541-7786.MCR-14-0685-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(23):8068–76. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 7.D C, K P, S Q, H P, Jc S, Gj T, et al. MiR-19-mediated inhibition of Transglutaminase-2 leads to enhanced invasion and metastasis in colorectal cancer. Molecular cancer research : MCR. 2015 doi: 10.1158/1541-7786.MCR-14-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer research. 2006;66(21):10525–33. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 9.Yuan L, Choi K, Khosla C, Zheng X, Higashikubo R, Chicoine MR, et al. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Molecular cancer therapeutics. 2005;4(9):1293–302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]
- 10.Budillon A, Carbone C, Di Gennaro E. Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino acids. 2013;44(1):63–72. doi: 10.1007/s00726-011-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung HJ, Chen Z, Wang M, Fayad L, Romaguera J, Kwak LW, et al. Calcium blockers decrease the bortezomib resistance in mantle cell lymphoma via manipulation of tissue transglutaminase activities. Blood. 2012;119(11):2568–78. doi: 10.1182/blood-2011-09-377598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown KD. Transglutaminase 2 and NF-kappaB: an odd couple that shapes breast cancer phenotype. Breast cancer research and treatment. 2013;137(2):329–36. doi: 10.1007/s10549-012-2351-7. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Mehta K. Tissue transglutaminase constitutively activates HIF-1alpha promoter and nuclear factor-kappaB via a non-canonical pathway. PloS one. 2012;7(11):e49321. doi: 10.1371/journal.pone.0049321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parnsamut C, Brimson S. Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-alpha production via MAPK pathway in leukemic cell lines. Genetics and molecular research : GMR. 2015;14(2):3650–68. doi: 10.4238/2015.April.17.15. [DOI] [PubMed] [Google Scholar]
- 15.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer research. 1996;56(23):5499–505. [PubMed] [Google Scholar]
- 16.Pham LV, Tamayo AT, Li C, Bornmann W, Priebe W, Ford RJ. Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications. Molecular cancer therapeutics. 2010;9(7):2026–36. doi: 10.1158/1535-7163.MCT-10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris J. Autophagy and cytokines. Cytokine. 2011;56(2):140–4. doi: 10.1016/j.cyto.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kongara S, Karantza V. The interplay between autophagy and ROS in tumorigenesis. Frontiers in oncology. 2012;2:171. doi: 10.3389/fonc.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Teo A, McCarty N. ROS induced CXCR4 signaling regulates mantle cell lymphoma (MCL) cell survival and drug resistance in the bone marrow microenvironment via autophagy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR, et al. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells. Cancer research. 2009;69(23):8844–52. doi: 10.1158/0008-5472.CAN-08-4401. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki HI, Kiyono K, Miyazono K. Regulation of autophagy by transforming growth factor-beta (TGF-beta) signaling. Autophagy. 2010;6(5):645–7. doi: 10.4161/auto.6.5.12046. [DOI] [PubMed] [Google Scholar]
- 23.Delk NA, Farach-Carson MC. Interleukin-6: a bone marrow stromal cell paracrine signal that induces neuroendocrine differentiation and modulates autophagy in bone metastatic PCa cells. Autophagy. 2012;8(4):650–63. doi: 10.4161/auto.19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang PC, Wang TY, Chang YT, Chu CY, Lee CL, Hsu HW, et al. Autophagy pathway is required for IL-6 induced neuroendocrine differentiation and chemoresistance of prostate cancer LNCaP cells. PloS one. 2014;9(2):e88556. doi: 10.1371/journal.pone.0088556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochemical pharmacology. 2010;80(12):1921–9. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Teo AE, Chen Z, Miranda RN, McDonnell T, Medeiros LJ, McCarty N. Differential PAX5 levels promote malignant B-cell infiltration, progression and drug resistance, and predict a poor prognosis in MCL patients independent of CCND1. Leukemia. 2015 doi: 10.1038/leu.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Chen Z, Neelapu SS, Romaguera J, McCarty N. Hedgehog inhibitors selectively target cell migration and adhesion of mantle cell lymphoma in bone marrow microenvironment. Oncotarget. 2016 doi: 10.18632/oncotarget.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Xu X, Bai L, Chen W, Lin Y. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. The Journal of biological chemistry. 2011;286(24):21164–72. doi: 10.1074/jbc.M110.207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A, et al. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer research. 1994;54(24):6348–52. [PubMed] [Google Scholar]
- 30.Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100(1):194–9. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 31.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462(7269):104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung HJ, Chen Z, McCarty N. Synergistic anticancer effects of arsenic trioxide with bortezomib in mantle cell lymphoma. American journal of hematology. 2012;87(12):1057–64. doi: 10.1002/ajh.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Yang J, Qian J, Li H, Romaguera JE, Kwak LW, et al. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120(18):3783–92. doi: 10.1182/blood-2012-04-424630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamagiwa Y, Marienfeld C, Meng F, Holcik M, Patel T. Translational regulation of x-linked inhibitor of apoptosis protein by interleukin-6: a novel mechanism of tumor cell survival. Cancer research. 2004;64(4):1293–8. doi: 10.1158/0008-5472.can-03-2517. [DOI] [PubMed] [Google Scholar]
- 35.Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang YH, Wang YN, et al. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer science. 2014;105(9):1220–7. doi: 10.1111/cas.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jinno T, Kawano S, Maruse Y, Matsubara R, Goto Y, Sakamoto T, et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncology reports. 2015;33(5):2161–8. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorhees PM, Chen Q, Kuhn DJ, Small GW, Hunsucker SA, Strader JS, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(21):6469–78. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 38.Braconi C, Swenson E, Kogure T, Huang N, Patel T. Targeting the IL-6 dependent phenotype can identify novel therapies for cholangiocarcinoma. PloS one. 2010;5(12):e15195. doi: 10.1371/journal.pone.0015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf L, Iwata M, Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a. Blood. 2002;100(4):1509–11. doi: 10.1182/blood-2002-03-0844. [DOI] [PubMed] [Google Scholar]
- 40.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nature reviews Cancer. 2012;12(6):401–10. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang P, Mizushima N. Autophagy and human diseases. Cell research. 2014;24(1):69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. The Journal of cell biology. 2001;152(4):657–68. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosich L, Xargay-Torrent S, Lopez-Guerra M, Campo E, Colomer D, Roue G. Counteracting autophagy overcomes resistance to everolimus in mantle cell lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(19):5278–89. doi: 10.1158/1078-0432.CCR-12-0351. [DOI] [PubMed] [Google Scholar]
- 45.Rosich L, Colomer D, Roue G. Autophagy controls everolimus (RAD001) activity in mantle cell lymphoma. Autophagy. 2013;9(1):115–7. doi: 10.4161/auto.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang GY, Jeon JH, Cho SY, Shin DM, Kim CW, Jeong EM, et al. Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells. Oncogene. 2010;29(3):356–67. doi: 10.1038/onc.2009.342. [DOI] [PubMed] [Google Scholar]
- 47.Manni S, Brancalion A, Mandato E, Tubi LQ, Colpo A, Pizzi M, et al. Protein kinase CK2 inhibition down modulates the NF-kappaB and STAT3 survival pathways, enhances the cellular proteotoxic stress and synergistically boosts the cytotoxic effect of bortezomib on multiple myeloma and mantle cell lymphoma cells. PloS one. 2013;8(9):e75280. doi: 10.1371/journal.pone.0075280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scuto A, Kirschbaum M, Buettner R, Kujawski M, Cermak JM, Atadja P, et al. SIRT1 activation enhances HDAC inhibition-mediated upregulation of GADD45G by repressing the binding of NF-kappaB/STAT3 complex to its promoter in malignant lymphoid cells. Cell death & disease. 2013;4:e635. doi: 10.1038/cddis.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo MX, Wong SH, Chan MT, Yu L, Yu SS, Wu F, et al. Autophagy Mediates HBx-Induced Nuclear Factor-kappaB Activation and Release of IL-6, IL-8 and CXCL2 in Hepatocytes. Journal of cellular physiology. 2015 doi: 10.1002/jcp.24967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.