Abstract
Purpose
EGFR is highly overexpressed on several cancers and two targeted anti-EGFR antibodies which differ by isotype are FDA approved for clinical use. Cetuximab (IgG1 isotype) inhibits downstream signaling of EGFR and activates anti-tumor, cellular immune mechanisms. As panitumumab (IgG2 isotype) may inhibit downstream EGFR signaling similar to cetuximab, it might also induce adaptive immunity.
Experimental Design
We measured in vitro activation of cellular components of the innate and adaptive immune system. We also studied the in vivo activation of components of the adaptive immune system in patient specimens from two recent clinical trials using cetuximab or panitumumab.
Results
Both mAb primarily activate NK cells, although cetuximab is significantly more potent than panitumumab. Cetuximab-activated neutrophils mediate ADCC against HNSCC tumor cells, and interestingly, this effect was FcγRIIa and FcγRIIIa genotype dependent. Panitumumab may activate monocytes through CD32 (FcγRIIa), however monocytes activated by either mAb are not able to mediate ADCC. Cetuximab enhanced DC maturation to a greater extent than panitumumab, which was associated with improved tumor antigen cross presentation by cetuximab compared with panitumumab. This correlated with increased EGFR-specific cytotoxic CD8+ T cells in patients treated with cetuximab compared to those treated with panitumumab.
Conclusions
Although panitumumab effectively inhibits EGFR signaling to a similar extent as cetuximab, it is less effective at triggering anti-tumor, cellular immune mechanisms which may be crucial for effective therapy of HNSCC.
Keywords: Head and neck cancer, EGFR, Monoclonal antibody, Immunotherapy
INTRODUCTION
The epidermal growth factor receptor (EGFR) remains an important therapeutic target in many solid tumors, including head and neck squamous cell carcinomas (HNSCC) (1–4). In 2006, the anti-EGFR, murine-human chimeric, IgG1 monoclonal antibody (mAb), cetuximab, was approved as combination or single agent therapy for HNSCC. The same year, the anti-EGFR targeted, fully-human, IgG2 mAb, panitumumab, was approved for use in EGFR-expressing metastatic colorectal carcinoma (CRC). As HNSCC has one of the highest prevalence of EGFR overexpression of all solid tumors (80–90%) (5), panitumumab has been investigated as a second therapeutic anti-EGFR mAb. Recent studies looking at the addition of panitumumab to standard chemoradiation therapy for HNSCC indicate no improvement to loco-regional control or overall survival, (6, 7) while others suggest it may modestly improve progression-free survival (8). However, few studies have investigated the differential biology underlying clinical outcomes of HNSCC patients treated with either cetuximab or panitumumab.
We previously demonstrated that both monoclonal antibodies (mAbs) bind to EGFR and inhibit its subsequent phosphorylation and signaling (9). Aside from inhibiting downstream signaling of EGFR, cetuximab is known to enhance anti-tumor immunity (10–12). Although the biological features of IgG1 and IgG2 antibodies are known to differ, little comparative clinical data from treated cancer patients exist, and previous reports indicate that both cetuximab and panitumumab-treated PBMC were capable of mediating ADCC (9, 13). Based on these findings, we sought to identify and compare which cell type(s) are activated by cetuximab or panitumumab and to compare the extent to which they mediate cellular immunity. To determine whether these effects resulted in enhanced adaptive immune responses, we analyzed lymphocytes from patients on two recent clinical trials, of cetuximab or panitumumab. We measured activation of NK, neutrophils, DC and EGFR-specific cytotoxic CD8+ T lymphocytes (CTL). Taken together, our results suggest that although panitumumab effectively inhibits EGFR signaling to a similar extent as cetuximab, it is much less effective at mediating anti-tumor, cellular immune mechanisms; this finding may explain its lower clinical activity in HNSCC.
MATERIALS AND METHODS
Patient blood samples and lymphocyte isolation from PBMC
Following approval by the University of Pittsburgh Institutional Review Board (IRB #99-06), written informed consent was obtained for all patients. Peripheral venous blood samples were obtained from healthy donors from the Western Pennsylvania Blood Bank and HNSCC patients on two separate clinical trials. HNSCC patients with stage III/IVA disease receiving chemoradiotherapy combined with cetuximab (400mg/m2 followed by 250mg/m2 weekly) on a prospective phase II clinical trial (UPCI # 08-013, NCT 01218048) and HNSCC patients with stage III/IV disease treated with chemoradiotherapy in combination with panitumumab (2.5 mg/Kg) (UPCI 06-120, NCT00798655) (Supplementary Figure 1). Cetuximab treated patients also received 3–4 doses of preoperative treatment. Peripheral venous blood samples were drawn immediately before, and again after chemoradiotherapy in combination with either cetuximab or panitumumab. Lymphocytes were purified by Ficoll-Paque PLUS centrifugation (Amersham Biosciences) and either used in experiments on the same day or stored frozen at −80°C until further use. CD56+ and CD14+ cells were purified from lymphocytes using immunomagnetic positive selection, EasySep kits (Stem cell technologies). Neutrophils were isolated by immunomagnetic negative selection from whole blood using EasySep kits (Stem cell technologies). The percentage purity for CD14+ cells was approximately 93%, the percentage purity for CD56+ cells was approximately 92% and the percentage purity for neutrophils was approximately 94% as measured by flow cytometry (Supplementary Figure 2).
Dendritic cell Induction and Maturation
Fresh peripheral blood mononuclear cells (PBMCs) were obtained from the Western Pennsylvania Blood Bank and lymphocytes were purified by Ficoll-Paque PLUS centrifugation. Monocytes were isolated from PBMC by plastic adherence for 2 hours at 37°C. Plastic adherent cells were incubated at 37°C using Iscove modified Dulbecco medium (IMDM) media, supplemented with 10% fetal bovine serum, GM-CSF (1000 IU/mL) and IL-4 (1000 IU/mL). On day 3 of the culture, GM-CSF, and IL-4 were replenished to a final concentration of 2000 and 1000 IU/mL, respectively. Day 8 monocyte-derived, mature (CD11c+) DCs were harvested with trypsin EDTA.
Reagents and antibodies
Recombinant epidermal growth factor, Zombie Aqua™ Fixable Viability Kit, Alexa Fluor 488-EGFR, PerCP-Cy 5.5-CD3, PerCP-Cy 5.5-CD32 and PE-Cy5-CD86 flow antibodies were purchased from Biolegend (San Diego, CA). Cetuximab and panitumumab were purchased from the manufacturers, Bristol-Myers Squibb (New York, NY), and Amgen, (Thousand Oaks, California) respectively. FITC-CD69 and PE-Texas Red-CD8 flow antibody were purchased from Life Technologies (Grand Island, NY). PE-CD107a, PE-Cy5-CD137, PE-Cy7-CD16, APC-CD56, PE-CD32, APC-H7-HLA-A2 and FITC-CD80 flow antibodies were purchased from BD Biosciences (San Jose, CA). 12B6 antibody was produced by Dr. Ferrone (Harvard Medical School) and has been previously validated (14). PE-labeled HLA-A*0201-EGFR853-861 tetramer was provided by the NIH tetramer core facility and used for identification of EGFR-specific CTL (15). PE-Labeled HLA-A*02:01 HIV-tetramer was purchased from MBL International.
Flow cytometry
Flow cytometry for EGFR-specific CTL was performed as follows. The PE-labeled HLA-A*0201-EGFR853-861 tetramer was obtained from the Tetramer Facility of the National Institute of Allergy and Infectious Disease (Atlanta, GA). Specificity was confirmed by the lack of staining of HLA-A2− PBMC obtained from normal donors and a non-specific PE-HLA-A*02:01HIV-Tetramer. PBMC from patients on both trials were typed for HLA-A2* status. 7 out of 16 patients (43%) on the panitumumab trial and 15 of the 32 patients (46%) on the cetuximab trial were found to be HLA-A2+. HLA-A2+ PBMC were harvested and washed once in PBS, then resuspended in 100uL of PBS. Fluorophore conjugated tetramer was added at a 1:300 dilution incubated for 30 minutes at 25°C and washed twice by sequential centrifugation at 1400 RPM with FACS buffer. Then, flow cytometry for cell surface proteins was performed as follows. Cells were harvested and washed once in PBS, then resuspended in 100uL of fluorescence-activated cell sorting (FACS buffer). Fluorophore conjugated antibodies were added at 1:100 dilution, incubated for 30 minutes at 4°C, washed twice by sequential centrifugation at 1400 RPM with FACS buffer and resuspended in 2% PFA solution until analyzed in the flow cytometer. Isotype control antibody staining was added for each condition and each mAb. Each experiment was repeated at least three times and mean and standard error of the mean (SEM) was calculated and plotted using GraphPad PRISM software version 6. Statistical analysis of the data included ANOVA (two tailed) with Tukey test when more than 2 different group means were compared or student’s t test when 2 means were compared.
Tumor cell lines
JHU-029 (HLA-A2−, EGFRhi and MAGE-3+) was a kind gift of Dr. James Rocco (The Ohio State University). PCI-15B (HLA-A2−, EGFRhi and MAGE-3−) cells were isolated and cultured from a patient at the University of Pittsburgh through the explant/culture method, authenticated, and validated as unique using STR profiling and HLA genotyping every 6 months (16, 17). EGFR expression is higher in JHU-029 cells compared with PCI 15B cells (Supplementary Figure 3). All cell lines were routinely tested and found to be free of mycoplasma. Cells were grown/passaged in 10% FBS Dulbecco-modified eagle medium (Mediatech, Herndon, VA), supplemented with 2% L-glutamine and 1% penicillin/streptomycin (Invitrogen Corp, Carlsbad, CA) at 37°C in a 5% CO2 atmosphere at 95% humidity.
Cytotoxicity assays
51Cr release assay was used to determine cytotoxicity. Target cells were incubated in 100μL of media with 25μCi of Na251CrO4 (Perkin Elmer, Boston MA) for 60 min at 37°C and resuspended in RPMI 1640 medium supplemented with 25mM HEPES. Cells were thoroughly washed in PBS and plated at various effector:target (E:T) ratios in 96-well plates. Cetuximab, panitumumab, human IgG1control or human IgG2 control (10 ng/mL) was incubated with effector cells added at the specified E:T ratios. Plates were incubated at 37°C for 4 h in a 5% CO2 atmosphere. Controls for spontaneous (cells only) and maximal lysis (cells treated with 1% Triton-X) and specific mAb control (human IgG1 or IgG2 isotype) were included. Each reaction was done in triplicate and repeated three times. The supernatants were collected and analyzed with a Perkin Elmer 96-well plate gamma counter. Results were normalized with the formula lysis = (experimental lysis − spontaneous lysis)/(maximal lysis − spontaneous lysis) × 100 and results were plotted on a graph.
RESULTS
Cetuximab and panitumumab bind to EGFR similarly
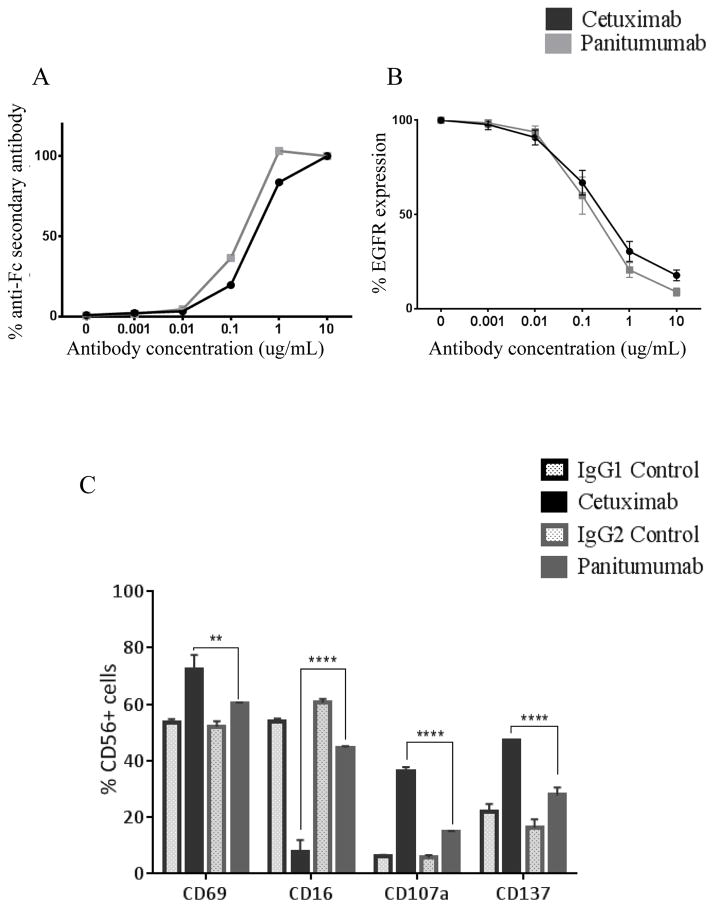
Previous studies indicated that panitumumab, a fully-human mAb may bind to the EGFR with greater affinity than the mouse-human chimeric mAb cetuximab (dissociation constant Kd = 0.12nM vs 0.31nM, respectively) (18). To directly compare the binding of cetuximab and panitumumab to EGFR on HNSCC cells, we incubated EGFR high JHU-029 HNSCC cells with either mAb, at increasing concentrations (0.001–10 μg/mL) for 30 min at 4°C, then stained these cells with either FITC-labeled Fc-specific mAb (Figure 1A) or commercial anti-EGFR antibody (Figure 1B) then analyzed these cells by flow cytometry. The results shown in Figure 1A demonstrate that binding of both mAbs to EGFR are similar at all concentrations of mAb tested (p>0.05). Binding of both panitumumab and cetuximab to EGFR resulted in a similar extent of blocking of the active EGFR binding site at all the mAb concentrations tested (Figure 1B).
Figure 1. Cetuximab and panitumumab bind EGF receptor similarly but cetuximab activates PBMC to a greater extent than panitumumab.
(A–B) Binding of cetuximab (IgG1) and panitumumab (IgG2) specific monoclonal antibodies (mAbs) to JHU-029 HNSCC cells. Cells were treated with increasing concentrations of either cetuximab or panitumumab (0.001–10 μg/mL) for 30 minutes at 4°C then stained with either FITC–labeled Fc-specific Ab, or EGFR Ab and analyzed by flow cytometry. Graphs show the percentage of FITC positive cells obtained at each concentration of mAb. (C) Whole PBMC from healthy donors were co-cultured with JHU-029 cells and treated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) for 24h then surface expression of activation markers, CD69, CD16, CD107a and CD137 were measured by flow cytometry. PBMC treated with cetuximab express significantly higher activation markers compared to cells treated with either control antibodies or panitumumab. Data are mean + SEM, **p<0.01, ****p<0.0001 cetuximab compared with panitumumab.
Whole PBMC are activated to a greater extent by cetuximab than by panitumumab
Aside from inhibiting EGFR signaling, anti-EGFR mAbs play an important role in activating cellular components of the immune system, in particular CD16/FcγRIIIa bearing NK cells. We investigated whether there were differences in activation of CD3−CD56+ NK cells incubated with cetuximab or panitumumab. After isolating PBMC from healthy donors, NK cells were purified by positive selection from PBMCs and co-cultured with JHU-029 HNSCC cells plus cetuximab, IgG1 isotype control Ab, panitumumab or IgG2 isotype control Ab (10 μg/mL) for 24h at 37°C. We measured NK cell activation markers, CD69, CD16, CD107a and CD137 on CD56+CD3− NK cells by flow cytometry. The activation markers CD69, CD107a and CD137 were upregulated by both mAbs (Figure 1C). CD16 activation, as indicated by CD16 internalization and downregulation of surface expression was also enhanced by both mAb over isotype controls. Interestingly, cetuximab significantly activated all NK cell surface markers and downregulated CD16 to a greater extent than panitumumab (p<0.01).
ADCC mediated by whole PBMC and NK cells is significantly greater with cetuximab than panitumumab
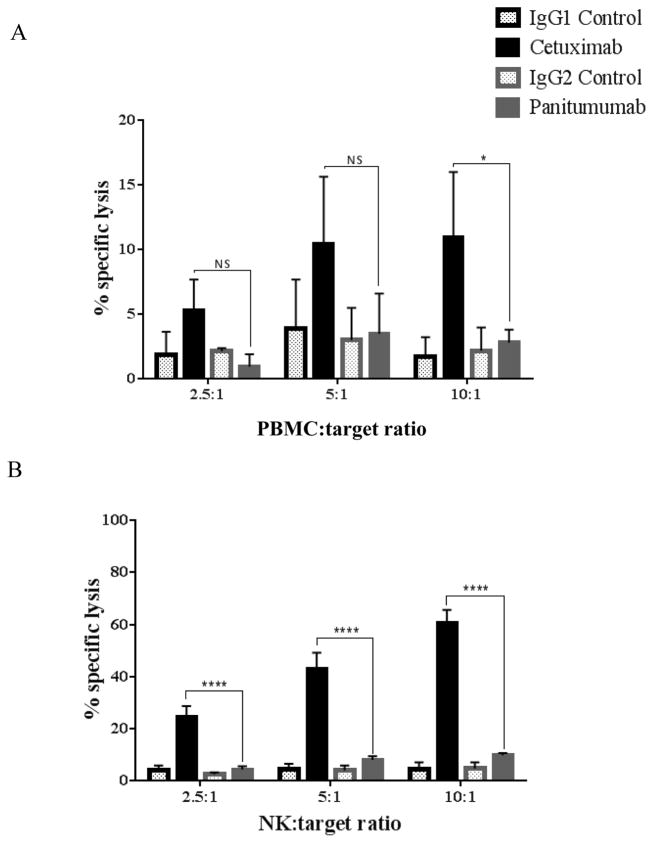
Previous studies demonstrate that cetuximab activation of NK cells, and subsequent antibody dependent cellular cytotoxicity (ADCC) is an important feature of anti-tumor therapy (10, 19). We sought to establish whether panitumumab-activated NK cells could function in antibody-dependent cell cytotoxicity (ADCC) to the same extent as cetuximab-activated NK cells. Whole PBMC isolated from healthy donors were co-cultured for 4h with 51Cr labeled JHU-029 HNSCC cells coated with cetuximab, panitumumab or (IgG1 or IgG2) isotype control mAbs (10 μg/mL) at different E:T ratios (2.5:1, 5:1 and 10:1). Panitumumab-activated PBMC did not appear to induce ADCC above isotype control. Conversely, cetuximab significantly enhanced ADCC compared to panitumumab at E:T ratio of 10:1 (p<0.05) (Figure 2A). As NK cells are thought to be the primary mediators of cetuximab-mediated ADCC, we repeated similar 51Cr-release assays using positively-isolated CD56+ cells (Figure 2B). Again, panitumumab-activated NK cells did not appear to induce ADCC above isotype control. Indeed, cetuximab-activated NK cells significantly induced ADCC to a greater extent than panitumumab at all E:T ratios tested.
Figure 2. Greater triggering by cetuximab than by panitumumab of PBMC or NK cell-dependent ADCC against HNSCC cells.
(A) Whole PBMC co-cultured for 4h with 51Cr labeled JHU-029 HNSCC cells coated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) at different E:T ratios (2.5:1, 5:1 and 10:1). Cetuximab significantly enhanced ADCC compared to panitumumab at an E:T ratio of 10:1. When this experiment was repeated using isolated NK cells (B), cetuximab significantly enhanced ADCC in comparison with panitumumab at all E:T ratios. Data are mean + SEM,*p<0.05, ****p< 0.0001 cetuximab compared with panitumumab.
Cetuximab-activated neutrophil mediated ADCC is enhanced in donors who are homozygous for FcγIIIa VV genotype and FcγIIa HH genotype
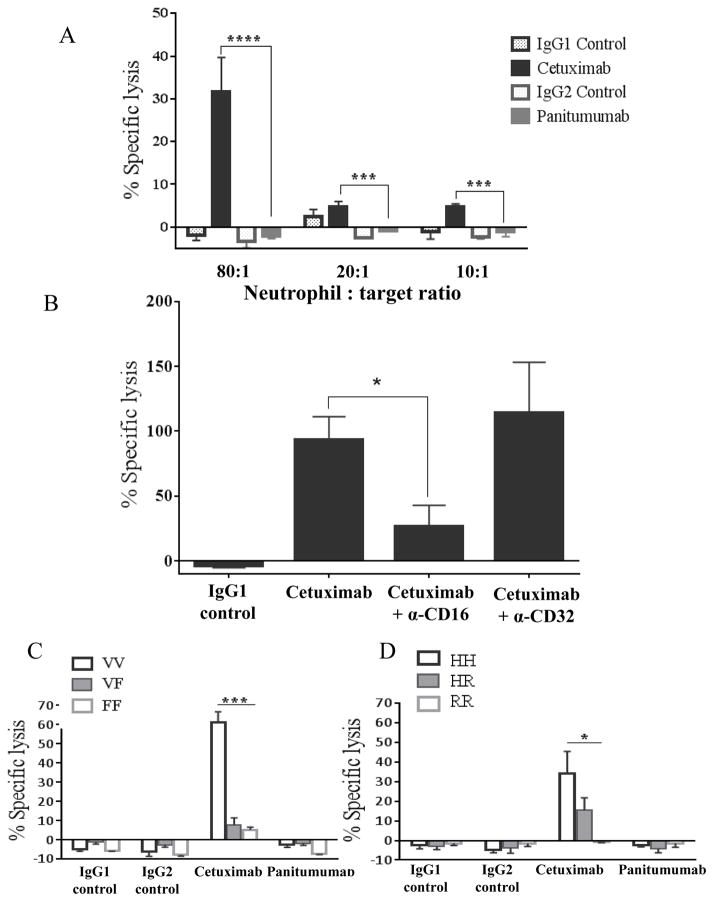
Although panitumumab did not appear to activate NK cell mediated ADCC, it has been suggested that it may be effective in mediating ADCC by myeloid or granulocytic effector cells (13). To determine whether mAb-activated neutrophils could mediate ADCC against HNSCC cells, we co-cultured negatively selected neutrophils with 51Cr labeled JHU-029 HNSCC cells coated with cetuximab, panitumumab (10 μg/mL) or (IgG1 or IgG2) isotype control mAb for 4h at different E:T ratios (10:1, 20:1 and 80:1). Cetuximab-activated neutrophils significantly induced ADCC over isotype controls at all E:T ratios (p = 0.0004) (Figure 3A). Panitumumab-activated neutrophils did not mediate ADCC over isotype control. This effect was dependent on CD16 but not on CD32, as demonstrated by significant reduction of cetuximab-activated neutrophil mediated ADCC only when a CD16-blocking mAb was used (Figure 3B). By genotyping the neutrophils used in the ADCC assays at the FcγRIIa and FcγRIIIa loci, we found that neutrophils from donors homozygous for FcγRIIIa VV demonstrated significantly enhanced ADCC activity than VF and FF donors (Figure 3C). A similar trend was noted in patients homozygous for FcγRIIa HH genotype compared to HR and RR donors (Figure 3D).
Figure 3. Cetuximab-activated neutrophil mediated ADCC is enhanced in donors who are homozygous for FcγIIIa VV genotype and FcγIIa HH genotype.
(A) Negatively isolated neutrophil co-cultured for 4h with 51Cr labeled JHU-029 HNSCC cells coated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) at different E:T ratios (10:1, 20:1 and 80:1). Cetuximab-activated neutrophils mediate ADCC above isotype controls whereas panitumumab-activated neutrophils do not. (B) JHU-029 HNSCC cells pre-treated with either CD16 or CD32 blocking antibodies for 30 minutes, washed once, then labeled with 51Cr, then co-cultured with negatively isolated neutrophils for 4h in the presence of cetuximab or IgG1 control antibody (10 μg/mL) at an 80:1 E:T ratio. Cells pre-treated with CD16 blocking antibody show a reduction in cetuximab-activated neutrophil mediated ADCC compared with non-pretreated cells and cells pre-treated with CD32 blocking antibody. (C) Neutrophils from donors separated by FcγRIIIa and FcγRIIa genotype co-cultured for 4h with 51Cr labeled JHU-029 HNSCC cells coated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) at 80:1 E:T ratio. Neutrophils from FcγRIIIa VV donors demonstrate significantly enhanced ADCC activity compared with VF and FF donors. (D) Neutrophils from FcγRIIa HH donors mediate enhanced ADCC compared with HR and RR donors. Data are mean + SEM,*p<0.05, ***p< 0.001, ****p< 0.0001.
Panitumumab activates CD32 receptors on monocytes to a greater degree than cetuximab but does not induce ADCC against HNSCC cells
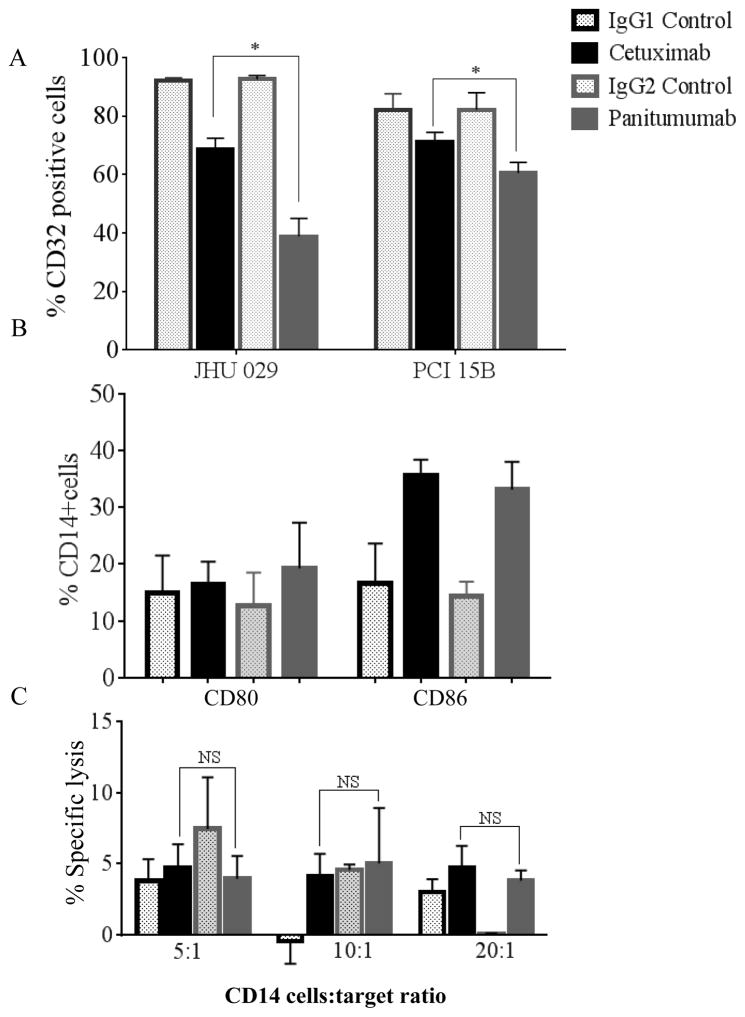
We next sought to determine whether CD32 activation on CD14+ monocytes, as measured by internalization of CD32, could be enhanced by either cetuximab or panitumumab. We co-cultured CD14+ monocytes with HNSCC cell lines treated with cetuximab, panitumumab or isotype control Ab, then measured surface CD32 expression by flow cytometry (Figure 4A). As a measure of activation, CD32 internalization in monocytes co-cultured with JHU-029 cells occurred to a greater extent with panitumumab, as compared to that with cetuximab (p<0.05). Positively isolated CD14+ cells were again co-cultured with JHU-029 cells and incubated with cetuximab, panitumumab or isotype control mAbs for 72h at 37°C. Then, CD80 and CD86 surface expression was measured by flow cytometry (Figure 4B). We observed no significant difference in the expression of CD80 or CD86 on cells treated with either cetuximab or panitumumab.
Figure 4. Panitumumab activates CD32 receptors on monocytes to a greater degree than cetuximab but does not induce ADCC against HNSCC cells.
Surface activation markers, CD32 (A) and CD80, CD86 (B) of isolated monocytes (CD14+ positive selection) co-cultured with JHU029 and PCI15B HNSCC cells and treated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) for 72h were measured by flow cytometry. Monocytes treated with panitumumab activate surface CD32 to a greater extent than those treated with cetuximab as demonstrated by downregulation of surface CD32. There is no significant difference in CD80 or CD86 expression on monocytes treated with cetuximab or panitumumab. (C) CD14+ cells co-cultured for 4h with 51Cr labeled JHU-029 HNSCC cells coated with 10 μg/mL of cetuximab, panitumumab or isotype controls (IgG1 or IgG2) at different E:T ratios (5:1, 10:1 and 20:1). Results demonstrate that monocytes did not mediate ADCC above isotype controls in the presence of either cetuximab or panitumumab. Data are mean + SEM,*p<0.05 cetuximab compared with panitumumab.
To determine if mAb-activated CD14+ cells could mediate ADCC, we co-cultured CD14+ cells with 51Cr labeled JHU-029 HNSCC cells coated with cetuximab, panitumumab (10 μg/mL) or (IgG1 or IgG2) isotype control mAb for 4h at different E:T ratios (5:1, 10:1 and 20:1). We found that CD14+ cells did not mediate ADCC above isotype control mAb in the presence of either cetuximab or panitumumab (Figure 4C).
Cetuximab enhances cross-presentation and adaptive immune responses to a greater extent than panitumumab
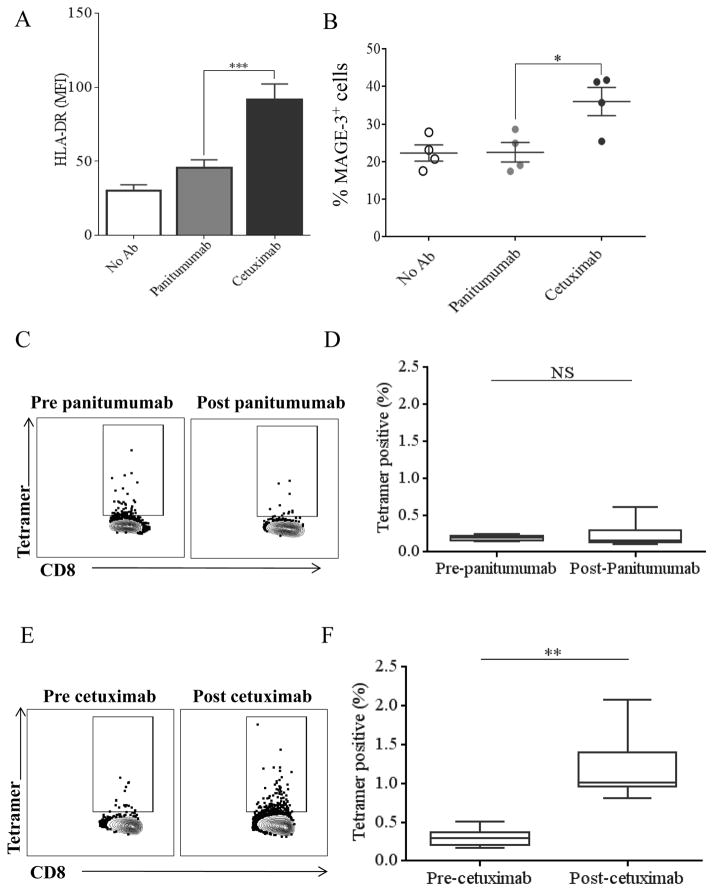
We next investigated the effect of these two mAbs on adaptive cellular immunity. Although we had established that cetuximab activates NK cells to a greater extent than panitumumab, we wished to compare their ability to enhance NK cell-mediated DC maturation. NK cells were co-cultured with CD11c+ DC and either cetuximab or panitumumab for 48h, then HLA-DR expression on the DC was analyzed by flow cytometry (Figure 5A). Cetuximab-treated NK cells mediated significantly higher HLA-DR expression on DC than panitumumab (p<0.001).
Figure 5. Cetuximab enhances adaptive cellular immune responses to a greater extent than panitumumab.
(A) HLA-DR expression on CD11c+ DC co-cultured with NK cells and cetuximab or panitumumab for 48, measured by flow cytometry. HLA-DR expression on DC co-cultured with cetuximab is significantly higher than on DC co-cultured with panitumumab. (B) Mature DC, NK cells and JHU-029 (MAGE-3+) tumor cells were co-cultured in the presence of no antibody, cetuximab (10 μg/mL) or panitumumab (10 μg/mL) at a 1:1:1 ratio for 48 hours. DC were then stained using 12B6 antibody and expression of MAGE-3 was analyzed by flow cytometry. MAGE-3 expression on DCs co-cultured in the presence of cetuximab was significantly higher than those co-cultured in the presence of panitumumab. EGFR-specific CTL frequencies of PBMC from patients on two clinical trials employing chemoradiotherapy combined with either cetuximab or panitumumab measured by flow cytometry. (C) Representative plots illustrating the frequency of EGFR-tetramer-positive CD8+ T cells in patient PBMC pre and post panitumumab. (D) The percentage of EGFR-specific CTL in patients treated with panitumumab did not significantly change post treatment. (E) Representative plots illustrating the frequency of EGFR-tetramer-positive CD8+ T cells in patient PBMC pre and post cetuximab. (E) Patients treated with cetuximab demonstrate significantly greater percentage of EGFR-specific CTL post treatment. Data are mean + SEM,*p<0.05, **p<0.01 ***p<0.001.
Next, we investigated whether increased DC maturation corresponded with improved presentation of tumor antigens in the presence of cetuximab or panitumumab. NK cells, DC and JHU-029 cells were co-cultured with cetuximab, panitumumab or isotype control mAbs. Using a novel antibody (12B6) we measured the expression of HLA-A2:MAGE-3271-279 complex on mature DC (mDC) to compare efficiency of antigen processing and presentation by the DC (Figure 5B). NK cells treated with cetuximab induced significantly higher surface MAGE-33271-279 presentation on DC compared to those treated with panitumumab (p<0.05) indicating that cetuximab improves cross-presentation of endogenous tumor antigen compared with panitumumab.
We then assessed whether there were differences in EGFR-specific CTL frequencies in PBMC from two prospective chemoradiotherapy clinical trials, combined with either cetuximab (UPCI # 08-013, NCT 01218048) or panitumumab (UPCI 06-120, NCT00798655). Using paired PBMC from HLA-A2+ HNSCC patients treated on these two clinical trials, we measured EGFR-specific CTL by flow cytometry, as a measure of adaptive immunity due to cross-presentation induced by the respective mAbs (10, 11). There was no increase in EGFR-specific CTL frequencies in patients post-panitumumab treatment (n=7) (Figure 5C, 5D). Interestingly, in all the tested paired samples from the cetuximab clinical trial, (n=8) we observed that EGFR-specific CTL frequencies increased significantly following cetuximab therapy (p=0.003) (Figure 5E, 5F).
DISCUSSION
EGFR overexpression and subsequent downstream signaling activates tumor proliferative and pro-survival pathways in HNSCC (20). Despite near-universal EGFR expression, two FDA approved mAb have modest activity, and cetuximab appears more clinically effective for unclear reasons. We hypothesized that mAb isotype may underlie this differential clinical activity since IgG1 isotype mAb such as cetuximab are more apt to induce cellular immunity than IgG2 isotype mAb like panitumumab. Cetuximab, an EGFR-targeted mAb has been shown to improve outcomes in patients with recurrent or metastatic HNSCC (21, 22). Studies using panitumumab in HNSCC have shown lower clinical activity compared to cetuximab (7, 8).
Inhibition of downstream EGFR signaling, by blocking ligand binding, is an important function of mAbs. Indeed, panitumumab has been shown to bind the EGFR with an 8-fold greater affinity than cetuximab (23). Based on our prior studies (9) and this study using HNSCC cells, we observed that both panitumumab and cetuximab bind to EGFR and HNSCC cells with similar dilution curves and, importantly, both mAbs appeared to inhibit EGFR internalization and ligand-induced activation at similar doses. Thus, EGFR binding affinity is of uncertain clinical significance.
The activation of Fcγ receptor (FcγR) bearing innate immune cells plays a role in effective anti-tumor responses generated by mAbs. Following cetuximab mediated ADCC, antibody-bound EGFR-positive tumor cells are recognized by immune cells via FcγR. This results in the release of lytic granules from the effector immune cell (24). NK cells are the most frequently studied facilitators of cetuximab-mediated ADCC through surface CD16/FcγRIIIa and there is some evidence correlating FcγRIIIa polymorphisms with clinical outcomes (25, 26). IgG2 isotype therapeutic mAbs are believed to be relatively inert in their Fc functions, and this isotype may even be preferred when Fc-mediated effector functions were not desired (27). The role of anti-EGFR mAbs on myeloid cells in the context of HNSCC is yet undetermined, some studies suggest that panitumumab may mediate ADCC through CD32/FcγRIIa expressed by monocytes (13). Furthermore, recent data suggest that cetuximab may ameliorate suppressive phenotypes of myeloid cells in HNSCC patients (28). However, no significant difference in activation markers on isolated CD14+ monocytes was noted between cetuximab and panitumumab. Interestingly, CD32 appeared to be partially activated and internalized by panitumumab as compared with cetuximab. However, neither cetuximab-activated nor panitumumab-activated CD14+ monocytes were effective mediators of ADCC. Schneider-Merck et al demonstrated that anti-EGFR IgG2 mAbs can effectively trigger neutrophil-mediated ADCC and this effect was enhanced in neutrophils from FcγRIIa HH donors (13). Interestingly, we found that cetuximab but not panitumumab-activated neutrophils were able to mediate ADCC against HNSCC cells. We additionally found that both functional FcγRIIa and FcγRIIIa polymorphisms may additionally affect cetuximab-activated neutrophil ADCC. In the clinical setting the significance of lymphoid versus myeloid-mediated ADCC is yet unknown, these data suggest that anti-EGFR mAbs may exert their effect through the combined enhancement of both innate and adaptive immune systems.
Enhanced adaptive cellular immunity is the ultimate goal of any therapeutic agent. Our prior in vitro studies show that through its immunological effects on NK cells and DC, which lead to enhanced DC maturation and tumor antigen processing, cetuximab therapy ultimately results in priming of T cell-based immunity (10, 11). Here, we compared the ability of cetuximab and panitumumab-activated NK cells to enhance DC maturation and NK:DC cross-talk. We found that panitumumab-activated NK cells showed modest increase in DC maturation. However, cetuximab-activated NK cells were significantly better at maturing DC and thus, cross presenting tumor antigen to T cells. In HNSCC patient samples, we noted increased EGFR-positive CTL following treatment with cetuximab, this was not seen in patients treated with panitumumab. Given that recently published data indicated that mDC phenotype and myeloid-derived suppressor cells induction correlated with clinical response to cetuximab, a potential mechanism of myeloid-DC priming may underlie this enhancement of EGFR-specific T cell expansion (28).
Taken together, our data show that, although panitumumab is capable of binding EGFR and inhibiting its activation to the same extent as cetuximab, its ability to activate innate immune cells and enhance cellular immune responses is inferior in HNSCC. Future studies examining other factors which may affect the efficacy of panitumumab in the clinical setting are warranted including the effect of genomic heterogeneity of HNSCC as well as novel combination immunotherapies available.
A recent phase III trial of single-agent panitumumab or cetuximab in chemotherapy-refractory, wild-type KRAS exon 2 metastatic colorectal cancers (CRC) determined that overall survival following panitumumab treatment was non-inferior to cetuximab (29), suggesting that CRC and HNSCC could manifest differences in therapeutic response to EGFR inhibition based on unique features of this pathway in each disease. Indeed, it has been established that in colorectal tumors the presence of activating KRAS mutations, specifically in exon 2 (codons 12 and 13), predicts resistance to anti-EGFR mAbs (30). The majority of HNSCC contain wild-type KRAS with less than 5% harboring KRAS mutations compared with 35%–45% of colorectal cancers (31, 32). In contrast, the HNSCC MAPK mutational pathway is primarily characterized by HRAS mutations (33). Therefore, it remains to be seen whether these mutations could impact clinical responses to panitumumab treatment in patients with head and neck cancer. Clinical trials examining the effect of cetuximab in combination with novel antibodies targeting immune checkpoint inhibitors and toll-like receptor agonists with various radiotherapy schedules in HNSCC are currently underway. The effect of panitumumab may be enhanced when given in similar combinations and this remains an area of future investigation.
Supplementary Material
Statement of Translational Relevance.
EGFR overexpression in head and neck cancers provides the basis to develop targeted monoclonal antibody-based therapy. Two antibodies, differing by isotype, cetuximab and panitumumab, have been approved for the clinic. Aside from inhibiting downstream EGFR signaling, cetuximab (IgG1) has been shown to enhance anti-tumor immunity. Panitumumab (IgG2) is known to bind EGFR and inhibit its phosphorylation to a similar extent as cetuximab. However, its effect on anti-tumor immunity has not been confirmed. Here we identify and compare the cell types that are activated by cetuximab or panitumumab and the extent to which they mediate cellular immunity. We measured in vitro activation of cellular components of the innate and adaptive immune system, and in vivo activation from patients on two recent clinical trials using cetuximab or panitumumab. We conclude that panitumumab is less effective at mediating anti-tumor cellular immunity. This finding may explain its lower clinical activity and guide therapeutic decisions.
Acknowledgments
Financial support: This work was supported by National Institute of Health grants R01 DE 019727, P50 CA097190, T32 CA060397 and used the University of Pittsburgh Cancer Institute award P30 CA047904.
Footnotes
Conflicts of Interest statement: Robert L. Ferris: consulting or advisory role: AstraZeneca, Bristol-Myers Squibb, Merck, ONO Pharmaceutical and Celgene. Research funding: Amgen (Inst), Bristol-Myers Squibb (Inst.) AstraZeneca (Inst.) and VentiRx (Inst.)
References
- 1.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 2.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. The New England journal of medicine. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:1204–13. [PubMed] [Google Scholar]
- 5.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nature reviews Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 6.Mesia R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. The Lancet Oncology. 2015;16:208–20. doi: 10.1016/S1470-2045(14)71198-2. [DOI] [PubMed] [Google Scholar]
- 7.Giralt J, Trigo J, Nuyts S, Ozsahin M, Skladowski K, Hatoum G, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. The Lancet Oncology. 2015;16:221–32. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. The Lancet Oncology. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Archives of otolaryngology--head & neck surgery. 2007;133:1277–81. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 10.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunologic research. 2011;50:248–54. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer immunology, immunotherapy : CII. 2009;58:1853–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–20. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-Induced HLA Class I Upregulation Enhances Immunogenicity and Clinical Response to Anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer immunology research. 2015;3:936–45. doi: 10.1158/2326-6066.CIR-15-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade Filho PA, Lopez-Albaitero A, Gooding W, Ferris RL. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 2010;33:83–91. doi: 10.1097/CJI.0b013e3181b8f421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7248–64. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo DS, Snyderman C, Gollin SM, Pan S, Walker E, Deka R, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer research. 1989;49:5167–75. [PubMed] [Google Scholar]
- 18.Day KE, Sweeny L, Kulbersh B, Zinn KR, Rosenthal EL. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2013;15:722–9. doi: 10.1007/s11307-013-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer research. 1993;53:3579–84. [PubMed] [Google Scholar]
- 21.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England journal of medicine. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 22.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 23.Kim GP, Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics : targets & therapy. 2008;2:223–8. doi: 10.2147/btt.s1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–40. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel D, Guo X, Ng S, Melchior M, Balderes P, Burtrum D, et al. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Human antibodies. 2010;19:89–99. doi: 10.3233/HAB-2010-0232. [DOI] [PubMed] [Google Scholar]
- 26.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 27.Carter PJ. Potent antibody therapeutics by design. Nature reviews Immunology. 2006;6:343–57. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. Journal for immunotherapy of cancer. 2015;3:54. doi: 10.1186/s40425-015-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. The Lancet Oncology. 2014;15:569–79. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 30.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 31.Langer CJ. Exploring biomarkers in head and neck cancer. Cancer. 2012;118:3882–92. doi: 10.1002/cncr.26718. [DOI] [PubMed] [Google Scholar]
- 32.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. British journal of cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer discovery. 2013;3:761–9. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.