Abstract
Mesenchymal stromal cells (MSC) have been shown to reverse radiation damage to marrow stem cells. We have evaluated the capacity of MSC-derived extracellular vesicles (MSC-EVs) to mitigate radiation injury to marrow stem cells at 4 hours to 7 days after irradiation. Significant restoration of marrow stem cell engraftment at 4, 24 and 168 hours post-irradiation by exposure to MSC-EVs was observed at 3 weeks to 9 months after transplant and further confirmed by secondary engraftment. Intravenous injection of MSC-EVs to 500cGy exposed mice led to partial recovery of peripheral blood counts and restoration of the engraftment of marrow. The murine hematopoietic cell line, FDC-P1 exposed to 500 cGy, showed reversal of growth inhibition, DNA damage and apoptosis on exposure to murine or human MSC-EVs. Both murine and human MSC-EVs reverse radiation damage to murine marrow cells and stimulate normal murine marrow stem cell/progenitors to proliferate. A preparation with both exosomes and microvesicles was found to be superior to either microvesicles or exosomes alone. Biologic activity was seen in freshly isolated vesicles and in vesicles stored for up to 6 months in 10% DMSO at −80°C. These studies indicate that MSC-EVs can reverse radiation damage to bone marrow stem cells.
Introduction
Radiation exposure results in different levels of tissue injury depending on dose, including the immune system, the hematopoietic system, gastrointestinal tract, kidney, skin and lung1, 2. Hematopoietic stem cells (HSC) are sensitive to radiation and exposure can result in bone marrow failure. Three months after exposure to 100 cGy whole body irradiation, the engraftment capacity of murine marrow was reduced to 49% of the non-irradiated control marrow3. A number of radiation mitigators such as cytokines and growth factors have been described which improve hematopoietic recovery from irradiation damage4–6. The transplantation of marrow can restore hematopoiesis in lethally irradiated subjects7, however, aside from transplantation, the efficacy of these treatments is relatively limited and temporally constrained.
The mesenchymal stromal cells (MSC) are multipotentent and play a critical role in microenvironmental support of HSC8, 9. The capacity of MSC for tissue repair has been reported in past decades. The repair mechanisms are believed to be related to either their differentiation capacity or to paracrine effects10, 11. Transplantation of MSC alone or with HSC has also been shown to enhance engraftment and improve bone marrow recovery from radiation injury12–18.
Extracellular vesicles (EVs) are the small spherical membrane particles released from cells, which contain mRNA, miRNA, non-coding RNA, protein, lipids and DNA. They have been shown to be involved in cell-to-cell communication and to affect the phenotype of target cells19–25. Recent studies have shown that MSC-EVs mediate reversal of different tissue injuries to kidney, brain and myocardium26–28. In this study, we evaluated whether marrow MSC-derived vesicles (MSC-EVs) could reverse irradiation damage to marrow stem/progenitor cells.
Materials and Methods
Cell and culture medium and reagents
FDC-P1 cell line (ATCC) was cultured in DMEM medium with 10%FBS/5%WEHI conditioned media. When preparing culture media for vesicle collection or vesicle-cell co-culture, vesicle depleted FBS (overnight ultracentrifugation at 100,000g) was used. Whole bone marrow cells (WBMC) and lineage-negative cells were cultured in DMEM medium with 15% FBS/1% Penicillin/Streptomycin (PS) containing 50ng/ml stem cell factor. Primary murine marrow-derived MSC were cultured in α-MEM medium with 10% FBS and 1%PS. All culture medium and related supplements were purchased from Life Technologies. The antibodies against TER119(#553669), B220(#553083), Gr-1(#553669), CD11b(#553307), CD4(#553726), CD8(#553026) and CD45(#553076) were purchased from BD Bioscience antibodies; The antibodies against CD 73 (#12-0731-81) CD44(#12-0441-82), CD29(#12-029-82), CD105(#12-1051-82), Sca-1(#11-5981-82), Ia(#12-5321-82), CD3(#112-0311-82), CD11b(#11-0112-82), CD45(#11-045-82), CD34(#11-0341-82), CD86 (#12-0861-82) and CD34(#14-0341-85) were purchased from eBioscience; ExoAb Antibody Kit (# EXOAB Kit-1)including antibodies against CD9, CD63 and CD81 were purchased from System Biosciences.
Experimental animals
Six- to eight-week-old male C57BL/6 or B6.SJL mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All mouse studies were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital. The mice were euthanized by using CO2 inhalation followed by cervical dislocation.
Isolation of WBMC
Cell preparation was performed as previously reported29, 30. To harvest WBMC, the marrow was flushed from tibiae, iliac crest and femurs into ice-cold PBS/5% heat-inactivated fetal calf serum (HIFCS)/1% PS by a syringe with a 22-gauge needle. For isolation of lineage-negative cells, bones were crushed with ice-cold PBS/5%HIFCS/1%PS by mortar and pestle, followed by filtration through a 40μm cell strainer (BD Biosciences). Mononuclear cells, were then isolated from WBM by density centrifugation using OptiPrep (Axis-Shield PoC.), and then depleted of lineage positive (Lin+) cells using magnetic Dynabeads (Life Technologies) and anti-TER119, B220, Gr-1, CD11b, CD4 and CD8 antibodies.
Culture of human/murine MSC
Human marrow-derived MSC (Donor #2002L), purchased from the Texas A&M University System Health Science Center, were cultured in α-MEM medium with 2–4mM L-glutamine, 15% FBS and 1% PS according to the manufacturer’s instructions.
The murine bone marrow-derived MSC and bone-derived MSC were isolated, cultured and characterized as per previous reports31, 32. The MSC were magnetically depleted of CD34+, CD45+ and CD11b+ cells. Cells were cultured for 7 days followed by vesicle collection. The 7 day conditioned medium from the murine bone-derived MSC and murine bone marrow-derived MSC were harvested and combined for vesicle isolation by differential ultracentrifugation.
The murine MSC phenotypes characterized by flow cytometry expressed CD44, CD29, CD105 and Sca-1 and did not express Ia, CD31, CD11b, CD45, CD34 and CD86 (Supplemental Figure 1A). The MSC differentiated into osteogenic, adipogenic and chondrogenic cells when cultured in the appropriate differentiative media purchased from Life Technologies (Supplemental Figure 1B).
EVs isolation and characterization
WBM or human/murine MSCs were cultured in medium with vesicle depleted FBS for 7 days. Only less than 8 passages of MSCs were used to produce MSC-EVs. The vesicles were isolated from culture medium using differential ultracentrifugation as previously described20. Unless otherwise noted, all vesicle separations in this study were by differential centrifugation at 300g for 10 minutes, 2,000g for 30 minutes, 10,000g for 1 hour and 100,000g for 1 hour with collection of the 100,000g pellet (exosomes). The vesicles were washed two times with PBS and either tested after storage for 1–7 days at 4°C or resuspended in PBS with 10%DMSO and stored at −80°C. EVs were used within one week after harvested for the in vivo studies. EV functional effects in vitro were maintained for up to 6 months when stored in 10% DMSO at −80°C.
Human and mouse marrow derived MSC-EVs and WBMC-EVs were analyzed by electron microscopy as previously described33. The pictures are shown in Supplemental Figure 2. Surface epitope protein expression (CD9, CD63 and CD81) in human and mouse marrow derived MSC-EVs and WBMC-EVs were analyzed by Western blot (Supplemental Figure 3 and Table 1). The number and size distribution of vesicles was determined on a NanoSight NS500 (Malvern Instruments, Malvern, UK) with a Syringe Pump (Supplemental Figure 4).
Transplantation
C57BL/6J or B6.SJL recipient mice received 200, 500 or 950 cGy based on experimental design 2–4 hours prior to cell infusion (Gammacell 40 Exactor, Cesium 137 source irradiator, 0.94–0.96 Gy/min). A 300–500 μl volume of WBM cells or lineage-negative stem cells were injected by tail vein. Donor chimerism in peripheral blood or marrow was determined by four-color flow cytometry (BD LSR II flow cytometer, BD Biosciences) using a cocktail of fluorescently tagged antibodies against CD45.1, CD45.2, B220, CD3, CD11b and GR-1. The percentage of engraftment was calculated as the ratio of CD45.1 (donor) cells to CD45.1 plus CD45.2. For secondary transplantation, WBM was collected from primary recipient mice, and infused into lethally irradiated mice with subsequent determination of ratios of original donor and recipient cells. For competitive engraftment, WBMC collected from donor mice were competed with an equal number of host WBMC into lethally irradiated mice.
Two basic models for engraftment of marrow stem cells were utilized. In one, we competed donor marrow versus residual host marrow in sublethally irradiated (200 cGy) mice. This is a model we have previously validated3, 34. In the other model, we competed donor cells (B6.SJL CD45.1) versus host cells (C57BL/6J CD45.2) in equal ratios.
EVs labeling procedure
EVs were directly labeled with 1 μM Vybrant Cell Tracers DiO or DiD(Life Technologies) by incubation for 30 minutes at 37°C and then washed twice by ultracentrifugation at 100,000g for 1 hour in 1X phosphate-buffered saline (PBS).
Fluorescence Molecular Tomography (FMT)
FMT was used to evaluate the EVs biodistribution in tissues of live animals. Mice were injected with 2×109 of DiD labeled human MSC-EVs by tail vein injection, after 24hrs post 500 cGy irradiation. The mice were sacrificed and the organs were dissected for fluorescent signal scanning by the FMT-4000 scanner (PerkinElmer, Waltham, MA) according to manufacturer’s recommended procedure at 6 hours post EVs injection. The quantification of the fluorescence data was performed with TrueQuant software (version 3.0, PerkinElmer, Waltham, MA). The EVs’ fluorescence intensity in dissected tissue was determined using Region of Interest (ROI) analysis.
Apoptosis assay
The histone-associated DNA fragmentation was detected in FDC-P1 cells by using Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals), according to the manufacturer’s instructions.
Western blot assay
Cells were harvested and lysed in RIPA lysis buffer (Thermo Scientific). Twenty μg of protein samples were separated on SDS-PAGE and transferred to a polyscreen PVDF membrane. PARP specific antibody and Phospho-H2A.X (Ser139) Antibody (Cell Signaling Technology) were used for immunoblotting. Amersham ECL Advance Western Blotting Detection Kit was used for detection of protein (GE Healthcare).
Cell proliferation assay and colony assay
FDC-P1 cells were seeded in 96-well plate at 1000–1500 cells/200μl/well and co-cultured with vesicles for 10–14 days. Cell proliferation was based on measurement of cellular DNA content via fluorescent dye binding using a CyQuant proliferation assay kit (Life Technologies) according to the manufacturer’s protocol or direct analysis by counting colony formation in each well of a 96-well plate using an inverted microscopy with 2.5× objectives. Methylcellulose-based reagents were used for murine bone marrow Colony Forming Cell Assay according to manufacturer’s protocol (STEMCELL Technologies Inc.).
miRNA RT-PCR analysis
RNA was isolated from WBMC with miRNeasy Micro kit (Qiagen) according the manufacturer’s protocol. RNA was measured by Nanodrop ND-1000 spectrophotometry (Fisher Scientific Inc.). 50ng of RNA was converted into cDNA using RT Primers and TaqMan microRNA RT Kit (Applied Biosystems), followed by cDNA preamplified using RT PreAmp Primers and Taqman PreAmp Master Mix (Applied Biosystems) according to the manufacturer’s protocol. 0.25 μl diluted PreAmp product was mixed with TaqMan Universal PCR Master Mix and miRNA primer and run using an Applied Biosystems 7900HT real-time PCR instrument (Applied Biosystems). The primers of miR125a-5p, miR210 and RNU6B were purchased from Applied Biosystems. RNU6B gene was used as the endogenous control. Results were analyzed using the δδCt method.
miRNA transfection
We purchased miRNA Mimics from Qiagen, including miR-221, miR-451a, miR654-3p, miR486-5p, miR142-5p, miR-466i-5p, miR106b, miR125a-5p, miR106b, miR210-5p, miR199, miR21-5p, and miR29a-3a. For miRNA overexpression experiments, 100 nM mimic RNA or control miRNA (control) in 100 μL “R buffer” was transfected into FDC-P1 cells using the Neon electroporation transfection system (Life Technologies) with an optimal program at 1,400 V with two 20 ms pulses.
Statistics
Non-parametric Mann–Whitney U tests and ANOVA with multi-comparison tests were used to determine statistical significance among the groups shown in each experiment (GraphPad Prism, Graphpad Software Inc.) and the level of statistical significance was set at 0.05. All P-values are two-sided.
Results
Murine and human marrow MSC-EVs reduce radiation damage to marrow stem cells at 4 hours to 7 days after irradiation
To determine whether murine marrow MSC-EVs could reverse hematopoietic radiation damage by in vitro exposure of hematopoietic cells to EVs, we investigated the reversal of radiation injury to marrow stem cells 7 days after irradiation (Figure 1A). Murine lineage-negative cells, harvested from B6.SJL mice 7 days post 100 cGy whole body irradiation, were cultured with 2×109/ml murine MSC-EVs or vehicle for 48 hours (Unless otherwise noted, PBS served as vehicle controls in the all experiments). The cells were then tail vein injected into 200 cGy exposed C57BL/6J mice and engraftment was analyzed at 3, 12, 24 and 36 weeks post-transplant. This is an engraftment model in which infused cells compete with residual host marrow cells3, 34. Vesicle exposure led to a statistically significant (p<0.05) increase in engraftment by the irradiated cells at 24 and 36 weeks, with the average percent donor engraftment equal to 8.8 ± 1.9% and 9.6 ± 2.1% for the vesicle treated groups compared to 3.7 ± 0.7% and 3.0 ± 0.8% for the non-vesicle treated groups at 24 weeks and 36 weeks, respectively (Figure 1B). We evaluated the capacity of marrow cells from the primary transplants to give rise to marrow repopulation in secondary transplants: WBMC were harvested 36 weeks post-transplant and these cells were transplanted into lethally irradiated B6.SJL mice. There was a significant increase (P<0.05%) in the engraftment rate by the irradiated cells treated with vesicles at 1 and 3 months after a secondary transplantation with 7.8 ± 0.7% and 8.0 ± 2.1% of donor chimerism compared to 1.7 ± 0.2% and 0.7 ± 0.2% for non-vesicle treated groups, respectively (Figure 1C).
Figure 1. Mesenchymal stem cell-derived EVs reverse radiation damage to marrow stem cells.
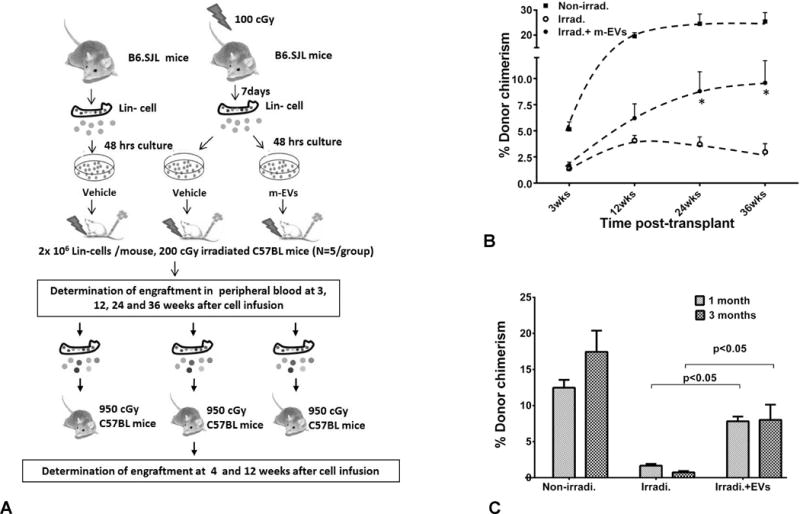
(A) Schematic diagram of the reversal of radiation injury to marrow stem cells 7 days after irradiation. (B) Effect of murine MSC-EVs on engraftment capacity of irradiated mouse marrow cells over time post cell infusion. The percentage of engraftment (donor chimerism) was calculated as the ratio of CD45.1 (donor) cells to CD45.1 plus CD45.2. Data are expressed as a mean ± SEM, N=5/group. (C) Vesicle effects on secondary engraftment. Data are expressed as a mean ± SEM, N=6/group.
In a similar experiment (Supplemental Figure 5A), murine lineage-negative cells were harvested 24 hours after 100 cGy whole body irradiation, and cultured with 2×109/ml of vesicles or vehicle for 24 hours. There was a statistically significant increase in donor engraftment by the lineage negative cells incubated with murine MSC-EVs at 6 and 8 months with engraftment rates at 3.0 ± 0.8% and 3.2 ± 0.9% for the vesicle treated groups compared to 0.7 ± 0.4 % and 0.7 ± 0.4% for the non-vesicle treated groups, respectively (Supplemental Figure 5B). Again, there was persistent enhanced engraftment with vesicle exposure in secondary transplantation with approximately 5-fold higher engraftment rate in vesicle treated cells compared to non-vesicle treated cells (Supplemental Figure 5C).
We further investigated whether human marrow MSC-EVs could rescue murine hematopoietic radiation damage in vitro (Supplemental Figure 6A). Murine WBMC, harvested 4 hours after 100 cGy whole body irradiation, were cultured with human MSC-EVs at 2×108, 1×109 and 2×109/ml respectively. Following 24 hours of co-culture, the cells were then competitively engrafted into 950 cGy-exposed C57BL/6J mice and chimerism was measured up to 5 months post-transplant. There was a trend of increased engraftment by cells treated with all three doses of vesicles compared to non-vesicle treated cells (Supplemental Figure 6B). There was a statistically significant increase in engraftment when the three vesicle treated groups were pooled together as the vesicle-treated group compared to the non-vesicle treated group (Supplemental Figure 6C).
Thus, our data indicate that MSC-EVs can reverse radiation damage to marrow when administered at 4 hours to 7 days after irradiation.
In vivo reversal of irradiation damage to marrow stem cells by human marrow MSC-EVs
We next evaluated the capacity of human MSC-EVs to reverse marrow radiation damage by in vivo administration. C57BL/6 mice were injected intravenously with 4×109 human MSC-EVs at 6, 24 and 72 hours after exposure to 500 cGy whole body irradiation (Figure 2A). We determined WBC, granulocyte, lymphocyte and monocyte counts by HemaTure Analyze (Heska Corporation) at one day before irradiation, and 1, 3 and 5 weeks after irradiation (Figure 2B). There was a significant WBC restoration with total WBC counts of 3.2 ± 0.2 ×106/ml in the vesicle treated group and 2.4 ± 0.2 ×106/ml in the non-vesicle treated group at 3 weeks after irradiation (p<0.05). The significant restoration was also seen at 5 weeks after irradiation with WBC counts of 5.0 ± 0.3 ×106/ml vs. 4.1 ± 0.2 ×106/ml (p<0.05). Granulocyte level was restored to 1.3 ± 0.1 ×106/ml at 3 weeks post irradiation in the vesicle treated group while in the non-vesicle treated group was 0.8 ± 0.1 ×106/ml (p<0.05). There were no other significant changes in peripheral blood cell types. Our data suggest that MSC-EVs can rescue radiation damage in bone marrow cells in vivo.
Figure 2. Mesenchymal stem cell-derived EVs promote WBC and granulocyte recovery after 500 cGy irradiation.
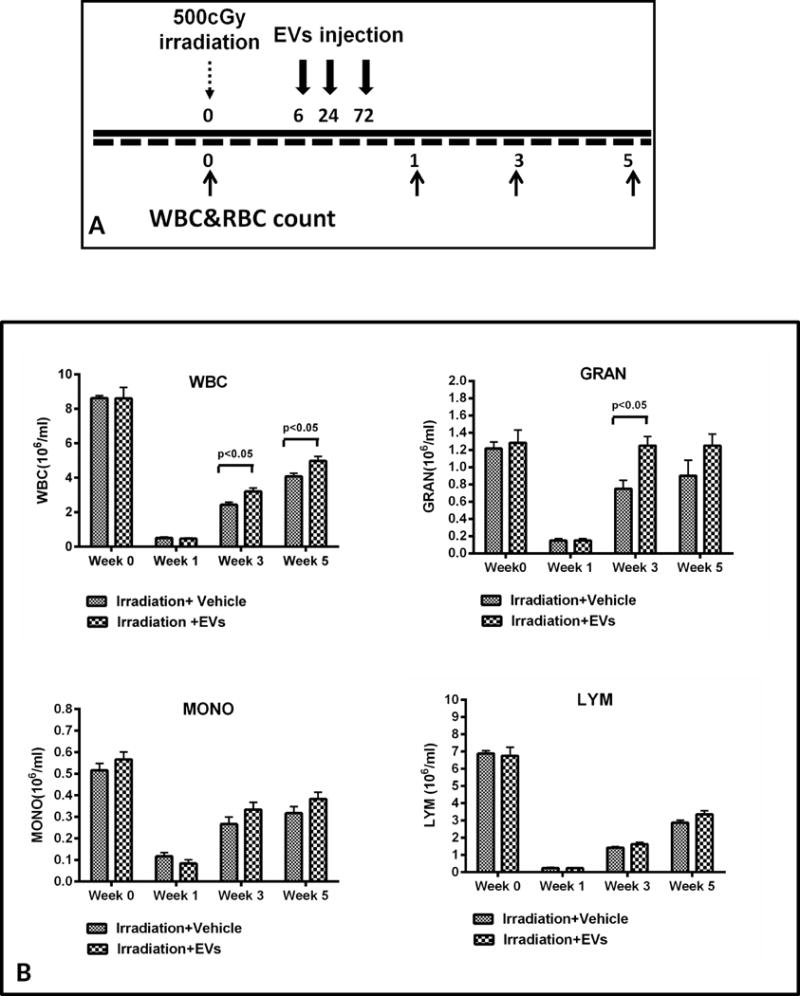
(A) Schematic diagram of experimental design. (B) The effect of human MSC-EVs on peripheral leukocyte counts after radiation injury. The WBC, granulocyte, lymphocyte and monocyte counts were used as indices of recovery (mean ± SEM, N=6/group).
The impact of murine MSC-EVs or WBMC-EVs on the in vitro growth of murine bone marrow or FDC-P1 hematopoietic cells
To determine if exposure of irradiated hematopoietic cells to vesicles could promote bone marrow stem cell proliferation, we next investigated the effect of murine MSC-EVs on murine WBM cell recovery from radiation damage. WBM cells, harvested from 0 or 200 cGy whole body irradiated mice, were cultured in DMEM medium with 50ng/ml stem cell factor(SCF) and 15% FBS with the presence or absence of 2×109 vesicles/ml murine MSC-EVs for 10 days and then cultured in methylcellulose medium per manufacturer’s instructions for another 10 days. Vesicle exposure induced a significant increase proliferation (Figure 3A) and colony formation (Figure 3B) in irradiated WBM cells.
Figure 3. Restoration of growth on hematopoietic cells by in vitro exposure to MSC/WBM-EVs.
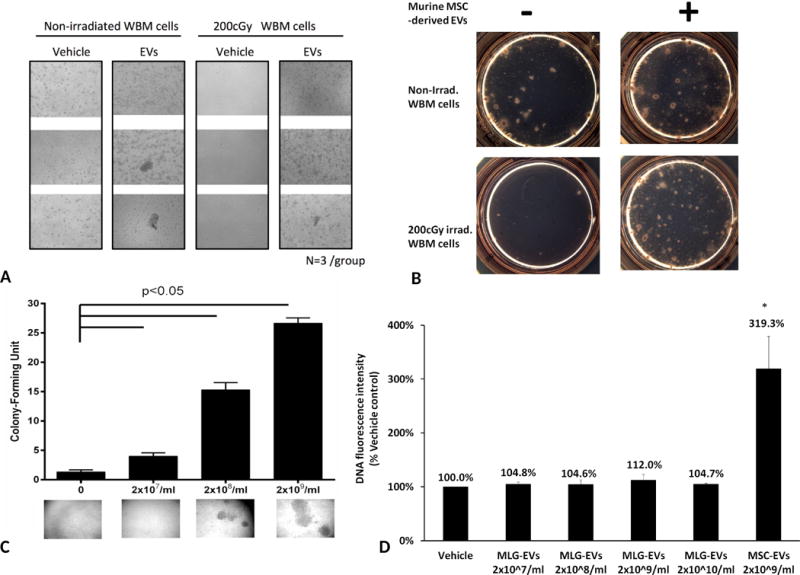
(A) Exposure to murine MSC-EVs led to a significant expansion of bone marrow cell (n=3/group). Cell growth images were taken under Zeiss Observer Z1 microscope (Carl Zeiss AS, Norway) with 2.5 × objectives (B) Murine MSC-EVs promoted colony formation on irradiated bone marrow cells. (C) Reversal of radiation toxicity to FDC-P1 cells by murine WBM-EVs. 500 cGy irradiated FDC-P1 cells were treated with 2×107, 2×108 and 2×109/ml of murine MSC-EVs for 10 days. Colony formation in the well of 96-well plate was determined by counting using a Zeiss Observer Z1 microscope (Carl Zeiss AS, Norway) and 2.5 × objectives. N=3/group; Colony: >50 cells/cluster. (D) Effect of MLG-EVs on recovery of growth on radiation damage FDC-P1 cells. 500 cGy irradiated FDC-P1 cells were treated with 2×107, 2×108, 2×109 and 2×1010/ml of MLG-EVs or 2×109/ml murine MSC-EVs for 10 days. The proliferation of radiation damaged FDC-P1 cells was determined by using CyQUANT NF Cell Proliferation Assay, with values normalized to the levels of vehicle control(mean ± SD, n=3/group). *: P<0.05, compared to vehicle control.
We also evaluated the effect of adding MSC-EVs and vesicles isolated directly from murine marrow to FDC-P1 murine hematopoietic cells in vitro. FDC-P1 cells were exposed to 500 cGy irradiation then cultured with murine MSC-EVs or WBM–EVs for 10 days. As expected, MSC-EVs exposure induced a significant increase proliferation in irradiated FDC-P1 cells (Data not shown). Interestingly, WBM-EVs exposure led to a dose-dependent restoration of cell growth (Figure 3C). To evaluate the specific capacity of MSC-EVs on recovery from radiation damage, four doses of EVs isolated from mouse lung fibroblast (MLG) cells were added to 500cGy irradiated FDC-P1 cells for 10 days and there were not significant improvement on cell proliferation(Figure 3D). This indicates that the restoration of proliferation after irradiation is specific for MSC-EVs.
EVs biodistribution in dissected organs and EVs intracellular uptake
To evaluate the biodistritubtion of EVs after injection, mice were injected with 2×109 of DiD labeled human MSC-EVs by tail vein, after 24hrs post 500 cGy irradiation. At 6 hours post EVs injection, the mice were sacrificed and the organs, including heart, lung, spleen, kidney, liver, tibia, femur and spinal were dissected for fluorescent signal scanning by FMT-4000 scanner (Figure 4A). There is a significantly strong fluorescent signal in liver and spleen, followed by a moderate signal in bone marrow in legs and a mild signal in spinal, but no signal in heart, kidney and lung was detected, compared to PBS treated group(Figure 4B). The fluorescence intensity in the spleen and bone marrow from legs with radiation exposure was significantly higher when compared to those organs from non-irradiation exposed mice after DiD labeled EVs injection (p<0.05, Figure 4B, C). This result suggests a specific accumulation of EVs at the site of injury.
Figure 4. EVs biodistribution in dissected organs from mice.
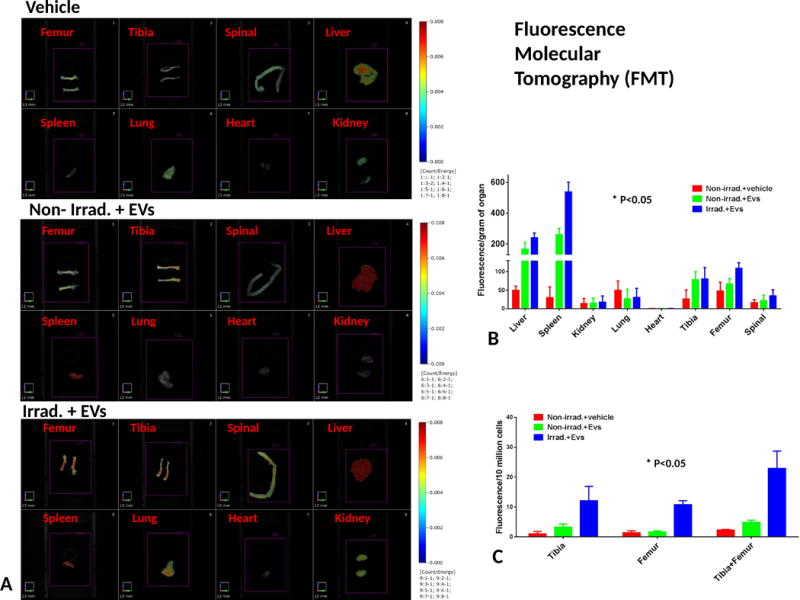
(A) Typical images of dissected organs from mice with 0 or 500cGy radiation exposure at 6hrs after DiD-EVs injection. N=3 mice/group. (B) EVs distribution quantitated with FMT. Fluorescence intensity of organs was normalized by organ weight. Fluorescence intensity is expressed as the average of Fluorescence intensity (count/energy) ± the standard error of the mean (SEM). ANOVA with multi-comparison test was performed. *p<0.05, Irrad. + EVs vs. Non-irrad. + EVs. (C) Bone marrow fluorescence quantification. The bone marrow cells were flushed out from 2 of tibia and 2 of femur and counted by hemocytometer after detection of fluorescence by FMT. Then the intensity of fluorescence was normalized by 10 million bone marrow cells, expressed as the average of Fluorescence intensity (count/energy) ± the standard error of the mean (SEM), The t test was performed. *p<0.05, Fluorescence intensity of femur, Irrad. + EVs vs. Non-irrad. + EVs.
We next examined the uptake rate of EVs by murine WBMC by flow cytometry. The fresh WBMCs harvested from the tibia and femur were cultured with DiD labeled human MSC-EVs for 24 hrs. There was a 31.73±1.55%uptake rate of WBMCs and this was further confirmed by confocal microscopy (Supplemental Figure 7A). A similar study also showed MSC-EVs intracelluarization in FDC-P1 cells (Supplemental Figure 7B).
We further evaluated if the miRNAs enriched in human MSC-EVs could be transferred or unregulated in murine bone marrow cells. Several miRNA candidates which were shown to be enriched in human MSC EVs miRNA profile through deep sequencing analysis (data not shown), were examined in murine WBMC after treatment with human MSC-EVs. We found that EVs treatment caused a 3.7±1.3 and 3.3±1.6-fold increase in miR-210 and miR-125a-5p expression(Supplemental Figure 7C), suggesting an upregulation of miRNA expression in target cells after EVs treatment might be due to horizontal transfer of the miRNA from EVs to target cells.
Our data indicates that EVs can accumulate in the injured bone marrow and alter the miRNA expression in bone marrow after EVs injection to mice.
MSC-EVs reverse radiation-induced DNA damage and apoptosis in FDC-P1 cells
We further investigated the expression of apoptosis, cleaved PARP and DNA damage, phosphorylated H2AX35, by Western blot analysis in FDC-P1 cells from 1 to 24 hours after 500 cGy irradiation. There was a significant increase of phosphorylated H2AX and cleaved PARP 18 hours after radiation. However, a significant increase of phosphorylated H2AX was detected at 1 hour after 1000 cGy irradiation and then phosphorylated H2AX rapidly decreased in 4 hours post-irradiation (Figure 5A, B). Our data indicated a time and dose dependent radiation damage in FDC-P1 cells. We next investigated if vesicle exposure could reverse radiation induced DNA damage and apoptosis. FDC-P1 cells were exposed to 500 cGy irradiation then cultured with or without murine MSC-EVs (2×109/ml) for 18 Hours. There was a significant increase in cleaved PARP and phosphorylated H2AX after radiation exposure, however, vesicle treatment significantly reduced the level of cleaved PARP and H2AX phosphorylation (Figure 5C), indicating that apoptosis in irradiated cells was reversed by exposure to vesicles. Similar results were also seen with human MSC-EVs or murine WBMC-EVs. In addition, DNA fragmentation, an indicator of apoptosis, was determined by the Cell Death Detection ELISA and further confirmed that murine MSC-EVs reverse radiation-induced apoptosis in FDC-P1 cells (Figure 5D).
Figure 5. Reversal of DNA damage and apoptosis of irradiated FDC-P1 cells in vitro by exposure to murine MSC-EVs.
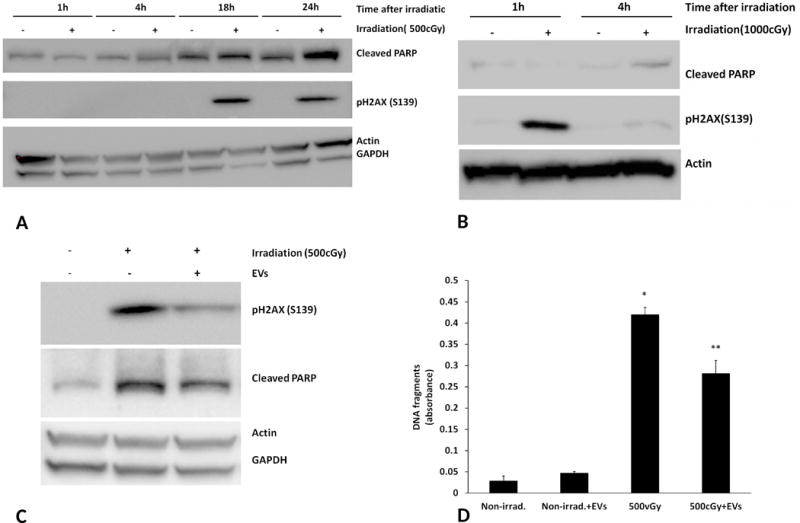
A, B: Phosphorylated H2AX and cleaved PARP levels in FDC-P1 cell. (A) Cells were harvested at 1, 4, 18 and 24 hours after 500 cGy irradiation. (B) Cells were harvested at 1 and 4 after 1000 cGy irradiation. Protein expression was assayed by Western blot analysis. (C) 500 cGy irradiated FDC-P1 cells were treated with 2×109/ml of murine MSC-EVs for 18 hours. Phosphorylated H2AX and cleaved PARP were measured by Western blot analysis. Actin or GAPDH was used as an internal control. The experiments were repeated three times. (D) DNA fragmentation in FDC-P1 cells 18 hours-post 500 cGy irradiation with murine MSC-EVs treatment was evaluated using a Cell Death Detection ELISA kit. The data are expressed as mean ± SEM of three separate experiments. *, P<0.05 compared to control cells, **, p<0.05 compared to 500 cGy irradiated group.
The effects of different EV populations derived from murine or human MSC on proliferation normal or irradiated FDC-P1 hematopoietic cells
Vesicles were isolated using differential ultracentrifugation steps (300g, 10,000g and then 100,000g). This is a classic method for preparation of exosomes. However, this separation isolates the smaller vesicles, discarding larger vesicles (microvesicles). We therefore, investigated the effects of 3 different preparations of vesicles on cell proliferation among the 10k pellet (large vesicles, microvesicles), the 100-10k pellet (small vesicles, exosomes) and the 100k pellet (no 10k spin, both small and large vesicles, exosomes and microvesicles).
Normal FDC-P1 cells were seeded in a 96-well plate at 1500 cells/well and cultured with three fractions of murine WBM-EVs (2×109/ml) for 7 days, followed by microscopic analysis of cell proliferation. With respect to inducing cellular proliferation, exosomes were clearly inferior compared to microvesicles and the combined exosome and microvesicle population (100k pellet) was superior (Figure 6A).
Figure 6. In vitro stimulation of the growth of FDC-P1 cells by different fractions of vesicles.
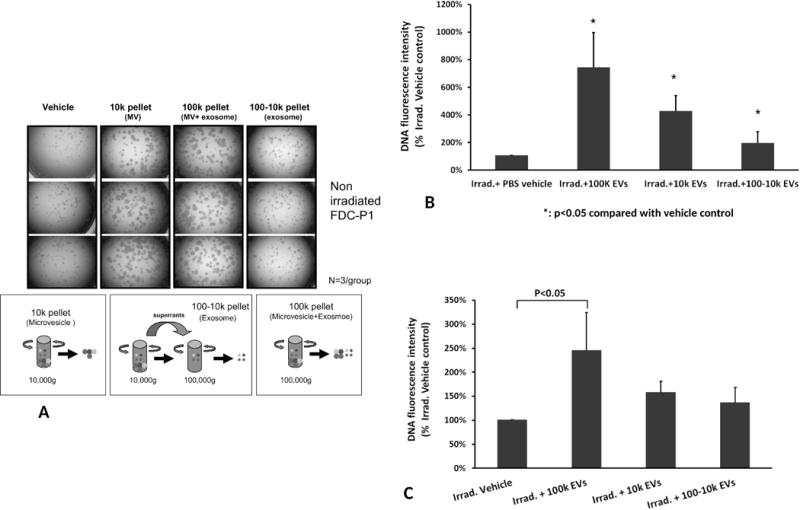
(A) The effects of different separative approaches on proliferation of non-irradiated FDC-P1 cells. Cell proliferation images were taken under microscopy with 2.5× objective (N=3/group). (B) Different fractions of WBM-EVs promoted radiated FDC-P1 cell proliferation. The proliferation of radiation damaged FDC-P1 cells was determined by using CyQUANT NF Cell Proliferation Assay, with values normalized to the levels of untreated cells. The cells were treated with three fractions of vesicles for 10 days (mean ± SD, n=3/group). *: P<0.05, compared to Irrad. + PBS vehicle control. (C) Effect of different human MSC-EVs fractions on irradiated FDC-P1 growth in vitro. FDC-P1 cells were co-cultured with 2×109/ml of EVs after 500 cGy exposure. The proliferation of cells was determined by using CyQUANT NF Cell Proliferation Assay after 10 days of MSC-EVs treatment (mean ± SD, n=3/group).
To evaluate the capacity of murine marrow-EVs to reverse radiation damage, FDC-P1 cells were co-cultured with 2×109/ml of WBM-EVs after 500 cGy exposure. The 100k pellet showed the largest effect on the reversal of radiation damage at 745 ± 252% of the non-vesicle treated control group by using the CyQUANT proliferation assay, while, the10k pellet treated group was determined to be 429 ± 111% and the 100-10k pellet was at 198 ± 80% of non-vesicles treated group (P<0.05) (Figure 6B). Again in the same experimental design, the combined fractions of human MSC-EVs were superior to the 10k and 100-10k fractions in the recovery of radiation damage in FDC-P1 cells (Figure 6C).
The effect of in vivo injection of human MSC-EVs on murine engraftable stem cells
We next tested the capacity of human MSC-EVs isolated from different fractions on reversal of murine bone marrow damage in vivo (Figure 7A). 4×109 vesicles of the three different fractions (10k, 100k-10k and 100k combined fractions) of human MSC-EVs were injected intravenously into B6.SJL mice 6, 24 and 72 hours after 500 cGy whole body irradiation. The mice were sacrificed to harvest WBM cells at week 5 post-irradiation. Two million of the WBM cells were competitively engrafted with the same amount of C57BL/6J WBM cells into lethally irradiated C57BL/6J mice and engraftment was analyzed at 1, 3 and 6 months post-transplant. The trend of increased engraftment in mice treated with three different fractions of vesicles compared to non-vesicle treated mice was shown at 1, 3 and 6 months post-transplant time points (data not shown). The 100k (combined fractions) fractions showed a significant increase in donor chimerism in bone marrow at 6 months post-transplant with 5 times the level of engraftment compared to the irradiation vehicle control. The 10k and 100-10k fractions showed intermediate recoveries (Figure 7B).
Figure 7. Evaluation of different fractions of human MSC-EVs for facilitating recovery from radiation injury in vivo.
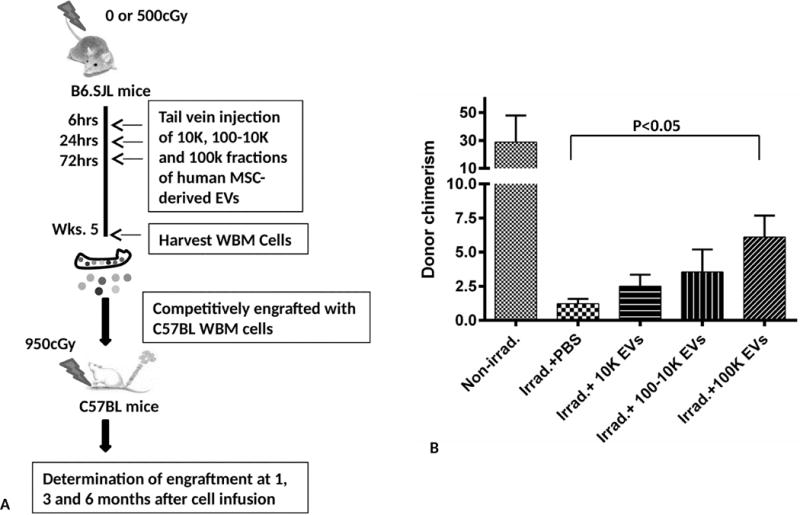
(A) Schematic diagram of experimental design. (B) Bone marrow was collected at 6 months post-transplant and analyzed for chimerism by FACS analysis. Each bar represents the average donor chimerism (mean ± SEM, 6 mice/group). *: P<0.05 (Irradiation + PBS vs. Irradiation+100K by Non-parametric Mann–Whitney u test).
Human MSC-EV associated miRNAs enhance reversal of radiation damage in FDC-P1 cells
A differential miRNA expression profile among 10k pellet (microvesicles), 100-10k pellet (exosomes) and 100k pellet (both exosomes and microvesicles) was determined using deep sequencing (data not shown). We focused on several of the most enriched miRNAs in 100k EVs, including miR-221, miR-451a, miR654-3p, miR486-5p, miR142-5p, miR106b-3p, miR125a-5p, miR155-5p, miR210-5p, miR199a-3p, miR21-5p, and miR29a-3p to further investigate the reversal of radiation damage in FDC-P1 cells. After exposure to 500 cGy, miRNAs were transfected into FDC-P1 cells using Neon electroporation transfection system. Cell proliferation assay by CyQuant proliferation assay and DNA fragmentation assay by the Cell Death Detection ELISA were performed 48 hours post-miRNA transfection. We found that overexpression of miR221, miR451a and miR654-3p in 500 cGy exposure FDC-P1 cells showed an increase growth effect of radiated cells at 124.8 ± 2.8%, 134.2 ± 6.4% and 139.7 ± 2.5% of the scramble miRNA treated control group, respectively (p<0.05, figure 8A). Overexpression of miR106b-3p, miR155-5p and miR210-5p showed inhibition of radiation induced apoptosis (DNA fragmentation formation) from 5.7± 0.6-fold increase to 3.5 ± 0.2, 3.7 ± 0.6 and 3.0 ± 0.8-fold change respectively compared to non-radiation exposed cells (P<0.05, Figure 8B).
Figure 8. Over expression of miRNA in FDC-P1 cells enhanced recovery from radiation.
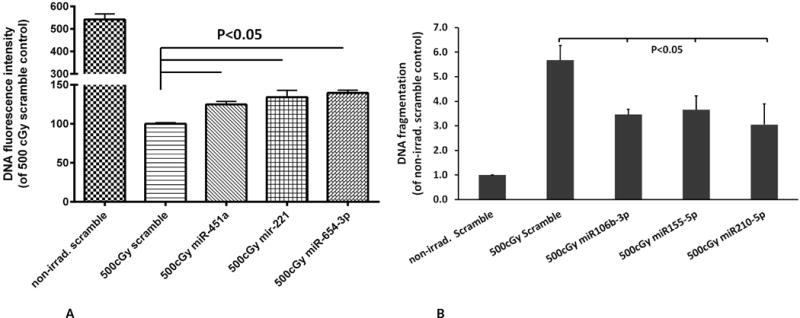
(A) miRNA enhances radiation injured FDC-P1 cell proliferation. (B) miRNA prevent radiation induced-apoptosis in FDC-P1 cell. (Mean ± SD, n=3/group).
Discussion
In this study, we have demonstrated that vesicles derived from murine or human marrow MSC or from murine whole bone marrow cells are able to reverse radiation injury to murine bone marrow in vivo and in vitro. In addition, our study showed that vesicles stimulate proliferation and reverse radiation induced DNA damage and apoptosis in FDC-P1 cells.
Many studies have demonstrated that the administration of MSC can protect and reverse radiation damage to bone marrow15–17 or other tissues36–42. However, the mechanisms underlining these beneficial effects are largely unknown and controversial. Yang and Lange reported that after intravenous infusion of MSC, there was only a small number of MSC found in the target organ, but there was a significant increase of hematopoietic recovery after irradiation15, 17. These data are consistent with studies on MSC reversal of kidney injury where MSC transiently accumulated in the injured kidney following intravenous infusion, but only few of these permanently engrafted within the kidney43–46. Recent studies showed that the conditioned medium or EVs from MSC had a similar effect on reversal of tissue injury26, 28, 47, 48, indicating that MSC engraftment or repopulation of target cells by MSC might not be required. In our study, we demonstrated that MSC-EVs clearly reverse the radiation damage to marrow hematopoietic cells both in vivo and in vitro, which supports the hypothesis that MSC acts as a paracrine mediator to repair injured target cells10, 11, 49.
Extracellular vesicles have the capacity to transfer biological information from parent cells to target cells. Several studies have provided evidence that vesicles could transfer mRNA to target cells21, 24, 25, 50. It was also demonstrated that vesicles from injured lung enter target marrow cells and induce lung-specific genes expression via both transfer of mRNA and a transcriptional modulator51. Work using mouse hybrid co-culture with RT-PCR primers specific for rat or mouse surfactant B and C indicated that if rat lung was cultured across from mouse marrow both rat and mouse surfactant B and C mRNA were detected early but only mouse mRNA persisted in cytokine supported cultures. Thus a stable epigenetic change was induced by lung vesicles in the target marrow cells. These effects appear to be variably RNAse sensitive and more recently, non-specific miRNA depletion of vesicles from Drosha knockdown-MSC was shown to abrogate the protective effect of vesicles in a kidney injury model52, indicating that the miRNA in the vesicles may play a critical transcriptional role in the healing capacity of MSC. Our data showed that overexpression of EV-enriched miRNA in FDC-P1 cells, could partially reverse the radiation damage. We found that overexpression of miR221, miR451 and miR654-3p stimulated cell growth and overexpression of miR210-5p, miR106b-3p and miR155-5p prevented radiation induced apoptosis. Interestingly, Grosso et al. indicated that overexpression of miR-210 increases radioresistance and promotes a more efficient DNA repair53. This suggests that vesicles mediate tissue repair via a noncoding RNA-induced epigenetic alteration in injured tissue. The underlying mechanisms need further investigation.
We demonstrated that different MSC-EV populations have varied effects on reversal of radiation damage on murine HSC. The combined fractions (microvesicles and exosomes) showed the greatest reversal effects. Microvesicles and exosomes are the two major types of EVs. However, there is large overlap between these vesicle populations: this led to the suggestion that it is appropriate to simply designate them as EVs and then define them further by the cell of origin and the context of specific experiments. The diversity of proteins and nucleotides between exosomes and microvesicles has been reported54, 55. We have determined vesicular protein or RNA per particle of vesicle from different subsets of human MSC-EVs. The amount of protein in the particle is variable among microviscle, exosome, and the combination of these two subsets is 4.6 ± 3.2, 1.9 ± 1.0 and 2.8 ± 2.32×10−6ng/particle respectively. The amount of RNA is 9.1 ± 4.1, 4.8 ± 2.9 and 5.4 ± 3.4×10−9ng/particle respectively. This is not related to huge difference of volume of EVs. Our data showed that different fractions of MSC-EVs facilitated recovery from radiation injury. This suggested that different populations of vesicles carry different information packets that might be selected and transferred to specific target cells.
Apoptosis and cell proliferation are critical for the maintenance of homeostasis in the hematopoietic system. DNA damage and apoptosis is an important cause of bone marrow irradiation injury56–58. The low dose radiation exposure is able to result in a long time injury of HSC3, 57. The immediate cellular response to DNA damage from radiation is cell cycle arrest. Here, we found that EVs from MSC or WBM could downgrade phosphorylation of H2AX after radiation exposure, indicating an acceleration of DNA repair efficiency or inhibition of DNA damage. We also showed that EV treatment could inhibit apoptosis and induce proliferation after radiation exposure in vitro. These are consistent with findings that MSC-derived EVs caused significant decrease of apoptosis in tubular cells of an acute kidney injury mice model59 and increased proliferation10. Thus, the mechanisms underlying recovery of hematopoietic cells by vesicles are not only involved in attenuating DNA damage and apoptosis but also stimulating proliferation.
Transplantation of MSC alone or with HSC has been shown to partially enhance engraftment and improve bone marrow recovery from radiation injury12–18. But it requires a period of time for preparation including a donor search before transplantation, therefore, limiting its clinical application, especially in the case of a radiation emergency. Our data shows that MSC-EVs partially reverse radiation damage to marrow stem cells. This EV-based, cell-free therapeutic strategy provides a convenient and safe therapeutic potential for rescuing marrow damage in patients treated with marrow toxic agents compared to MSC transplant. Early intervention is recommended in order to obtain the better long-term benefit. Some reports have shown that the best time to rescue the radiation injury is during the first 24–52 hours60. Here, we showed that MSC-EVs reverse the radiation damage from 4 hours to 1 week post-radiation exposure. Vesicles thus represent an interesting therapeutic strategy for the possible use in a radiation accident.
In summary, MSC-EVs reverse bone marrow cell radiation damage by accelerated HSC proliferation and differentiation and inhibition of DNA damage and apoptosis. EV reversal of marrow stem cell radiation damage suggests a unique new approach to radiation mitigation.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants 5UH2TR000880, 3UH3TR000880-03S1, 5R01HL103726, 5P20GM103468 and 5T32HL116249. We thank the Flow Cytometry Core at Division of Hematology/Oncology in Rhode Island Hospital, for providing excellent service. We also thank Rebecca Lynn, Research Administrator for her assistance on this project.
Footnotes
Conflict of interest statements: We have no conflict of interest to declare.
Authorship
This study was designed, supervised and coordinated by P.Q. The manuscript was written by S.W., revised by P.Q. and commented on by all authors. S.W. designed, performed experiments, collected, analyzed and interpreted data. M.D. designed and coordinated study. Y.C. and C.S performed experiments. E.P. and Y.D. performed flow cytometry analysis. M.D.T., M.P. and A.C. conducted bleeding animals for engraftment analysis. M.D., L.G. and J.A. contributed to mice transplantation, D.C., S.B. and F.C. provided technical advice, and G.C. gave conceptual advice and G.C and D.C edited the manuscript.
References
- 1.Arora R, Chawla R, Marwah R, Kumar V, Goel R, Arora P, et al. Medical radiation countermeasures for nuclear and radiological emergencies: Current status and future perspectives. Journal of pharmacy & bioallied sciences. 2010 Jul;2(3):202–212. doi: 10.4103/0975-7406.68502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiation research. 2010 Apr;173(4):557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart FM, Zhong S, Lambert JF, Colvin GA, Abedi M, Dooner MS, et al. Host marrow stem cell potential and engraftability at varying times after low-dose whole-body irradiation. Blood. 2001 Aug 15;98(4):1246–1251. doi: 10.1182/blood.v98.4.1246. [DOI] [PubMed] [Google Scholar]
- 4.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. The oncologist. 2010;15(4):360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature medicine. 2013 Mar;19(3):295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Experimental hematology. 2005 Oct;33(10):1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Asano S. Current status of hematopoietic stem cell transplantation for acute radiation syndromes. International journal of hematology. 2012 Mar;95(3):227–231. doi: 10.1007/s12185-012-1027-8. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974 Apr;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews Immunology. 2008 Sep;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 10.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 Aug;27(8):3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006 Aug 1;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 12.Qiao S, Ren H, Shi Y, Liu W. Allogeneic compact bone-derived mesenchymal stem cell transplantation increases survival of mice exposed to lethal total body irradiation: a potential immunological mechanism. Chinese medical journal. 2014;127(3):475–482. [PubMed] [Google Scholar]
- 13.Hiwase SD, Dyson PG, To LB, Lewis ID. Cotransplantation of placental mesenchymal stromal cells enhances single and double cord blood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem cells. 2009 Sep;27(9):2293–2300. doi: 10.1002/stem.157. [DOI] [PubMed] [Google Scholar]
- 14.Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L, et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010 Jun;16(6):838–847. doi: 10.1016/j.bbmt.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PloS one. 2011;6(1):e14486. doi: 10.1371/journal.pone.0014486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YZ, Lin F, Zhuang GB, Ren Y, Li PP. Protective effect of Renshen Yangrong Decoction () on bone marrow against radiation injury in mouse. Chinese journal of integrative medicine. 2011 Jun;17(6):453–458. doi: 10.1007/s11655-011-0634-1. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Balakrishnan I, Torok-Storb B, Pillai MM. Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion. Advances in hematology. 2012;2012:142530. doi: 10.1155/2012/142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Experimental hematology. 2003 May;31(5):413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 19.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010 Nov;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 20.Aliotta JM, Pereira M, Li M, Amaral A, Sorokina A, Dooner MS, et al. Stable cell fate changes in marrow cells induced by lung-derived microvesicles. Journal of extracellular vesicles. 2012:1. doi: 10.3402/jev.v1i0.18163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem cells. 2007 Sep;25(9):2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta. 1981 Jul 6;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 23.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008 Dec;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007 Jun;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006 May;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 26.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology: JASN. 2009 May;20(5):1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells. 2012 Jul;30(7):1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research. 2010 May;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg LR, Dooner MS, Johnson KW, Papa EF, Pereira MG, Del Tatto M, et al. The murine long-term multi-lineage renewal marrow stem cell is a cycling cell. Leukemia. 2014 Apr;28(4):813–822. doi: 10.1038/leu.2013.252. [DOI] [PubMed] [Google Scholar]
- 30.Becker PS, Nilsson SK, Li Z, Berrios VM, Dooner MS, Cooper CL, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Experimental hematology. 1999 Mar;27(3):533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 31.Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion. Methods in molecular biology. 2008;449:171–186. doi: 10.1007/978-1-60327-169-1_12. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature protocols. 2010 Mar;5(3):550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 33.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current protocols in cell biology/editorial board, Juan S Bonifacino [et al] 2006 Apr;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 34.Stewart FM, Zhong S, Wuu J, Hsieh C, Nilsson SK, Quesenberry PJ. Lymphohematopoietic engraftment in minimally myeloablated hosts. Blood. 1998 May 15;91(10):3681–3687. [PubMed] [Google Scholar]
- 35.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods in molecular biology. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Liao T, Wang H, Deng W, Yu D. Transplantation of bone marrow stromal cells overexpressing human vascular endothelial growth factor 165 enhances tissue repair in a rat model of radiation-induced injury. Chinese medical journal. 2014;127(6):1093–1099. [PubMed] [Google Scholar]
- 37.Lim JY, Ra JC, Shin IS, Jang YH, An HY, Choi JS, et al. Systemic transplantation of human adipose tissue-derived mesenchymal stem cells for the regeneration of irradiation-induced salivary gland damage. PloS one. 2013;8(8):e71167. doi: 10.1371/journal.pone.0071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, et al. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem cells. 2013 Oct;31(10):2231–2241. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shim S, Lee SB, Lee JG, Jang WS, Lee SJ, Park S, et al. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Experimental hematology. 2013 Apr;41(4):346–353 e342. doi: 10.1016/j.exphem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Drouet M, Mourcin F, Grenier N, Delaunay C, Mayol JF, Lataillade JJ, et al. Mesenchymal stem cells rescue CD34+ cells from radiation-induced apoptosis and sustain hematopoietic reconstitution after coculture and cografting in lethally irradiated baboons: is autologous stem cell therapy in nuclear accident settings hype or reality? Bone marrow transplantation. 2005 Jun;35(12):1201–1209. doi: 10.1038/sj.bmt.1704970. [DOI] [PubMed] [Google Scholar]
- 41.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PloS one. 2011;6(9):e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Chen XH, Si YJ, Li ZJ, Gao L, Gao L, et al. Reconstruction of hematopoietic inductive microenvironment after transplantation of VCAM-1-modified human umbilical cord blood stromal cells. PloS one. 2012;7(2):e31741. doi: 10.1371/journal.pone.0031741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, et al. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation. 2009 Jan 27;87(2):217–221. doi: 10.1097/TP.0b013e3181938998. [DOI] [PubMed] [Google Scholar]
- 44.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. The Journal of clinical investigation. 2005 Jul;115(7):1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. American journal of physiology Renal physiology. 2005 Jul;289(1):F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 46.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem cells. 2008 Aug;26(8):2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 47.Bi B, Guo J, Marlier A, Lin SR, Cantley LG. Erythropoietin expands a stromal cell population that can mediate renoprotection. American journal of physiology Renal physiology. 2008 Oct;295(4):F1017–1022. doi: 10.1152/ajprenal.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. Journal of cellular and molecular medicine. 2010 Jun;14(6B):1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruno S, Camussi G. Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatric nephrology. 2013 Dec;28(12):2249–2254. doi: 10.1007/s00467-013-2413-z. [DOI] [PubMed] [Google Scholar]
- 50.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013 Feb 18;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Experimental hematology. 2010 Mar;38(3):233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. Journal of the American Society of Nephrology: JASN. 2015 Apr 21; doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell death & disease. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015 Mar 15;31(6):933–939. doi: 10.1093/bioinformatics/btu741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes, metabolic syndrome and obesity: targets and therapy. 2012;5:247–282. doi: 10.2147/DMSO.S32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radford IR, Aldridge DR. Importance of DNA damage in the induction of apoptosis by ionizing radiation: effect of the scid mutation and DNA ploidy on the radiosensitivity of murine lymphoid cell lines. International journal of radiation biology. 1999 Feb;75(2):143–153. doi: 10.1080/095530099140591. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee D, Coates PJ, Rastogi S, Lorimore SA, Wright EG. Radiation-induced bone marrow apoptosis, inflammatory bystander-type signaling and tissue cytotoxicity. International journal of radiation biology. 2013 Mar;89(3):139–146. doi: 10.3109/09553002.2013.741280. [DOI] [PubMed] [Google Scholar]
- 58.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell and tissue research. 2000 Jul;301(1):133–142. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 59.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS one. 2012;7(3):e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovalenko OA, Azzam EI, Ende N. Human umbilical-cord-blood mononucleated cells enhance the survival of lethally irradiated mice: dosage and the window of time. Journal of radiation research. 2013 Nov 1;54(6):1010–1014. doi: 10.1093/jrr/rrt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


