Summary
Findings within the current issue indicate that treatment with IPH2101 when used as monotherapy in smoldering multiple myeloma, meant to enhance natural killer cell function through inhibitory KIR blockade, results in a surprising reduction of NK cell function mediated through monocyte trogocytosis. The significance of these findings is discussed.
In this issue of Clinical Cancer Research, Carlsten and colleagues discuss a mechanism that might explain limited efficacy of the anti-KIR2D blocking antibody IPH2101 tested in patients with smoldering multiple myeloma (MM) (1). This study focused on samples from a small phase II clinical trial that was closed due to lack of therapeutic benefit (2). Immune monitoring studies showed that treatment with IPH2101 lead to a profound and unexpected depletion of KIR2D+ NK cells. The decrease in KIR2D expression was not due to direct occupancy with IPH2101 as anticipated, but rather to absence of surface protein. The loss of KIR2D protein expression results from monocyte or neutrophil mediated trogocytosis, which is dependent on binding of FcγR1 to the Fc portion of the IgG4 IPH2101 antibody (Figure 1). While the IPH2101 antibody achieves generation of a mismatch between KIR and MHC, thus inducing ‘missing self’, in order to reduce KIR mediated inhibition, the authors show that the absence or blockade of KIR2D results in decreased functionality of NK cells due to absence of NK cell education. NK cell education, variations of which are also termed licensing, arming or tuning, is the process by which NK cells achieve functional maturation (3). This occurs through signaling of inhibitory receptors on NK cells, the Ly49 family in mice and KIR or NKG2A in humans, after ligation with cognate MHC class I ligand. NK cells without inhibitory receptors cannot be educated and remain hyporesponsive. Current data suggests that NK cell education is not hard wired but rather a dynamic process and requires constant signaling to be maintained, so the findings of the study indicating that IPH2101 impacts education fits current understanding of this process.
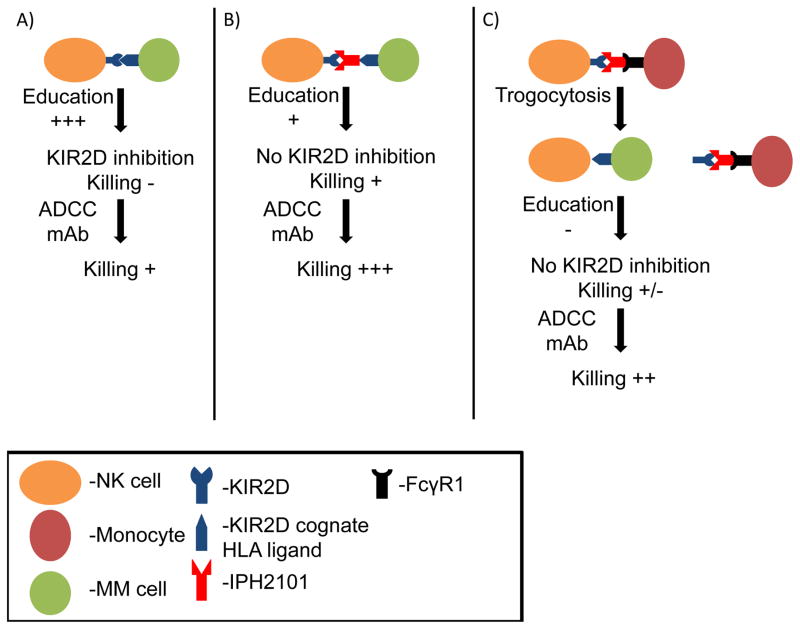
Figure 1. Proposed Model of anti-KIR (IPH2101) effects on NK cell responses to multiple myeloma (MM).
A) It is well established that engagement of NK cell inhibitory KIR2DL1/L2/L3 (labeled KIR2D in figure) to cognate class I HLA ligand results in protection from lysis of the HLA expressing cell but paradoxically also results in a robust education signal to acquire enhanced function (education). When KIR2D inhibitory signals are engaged, killing can be weakly induced with combination therapies that bypass education, such as therapeutic antibodies that mediate ADCC through a potent Fc receptor (CD16) or treatment with cytokines, or co-treatment with drugs like lenalidomide, thought to amplify NK cell function. B) The goal of anti-KIR (IPH2101) treatment is to block engagement of inhibitory KIR2D with its cognate HLA ligand resulting in abrogation of inhibitory signals hoped to lead to better tumor killing, but the same interruption can also reduce NK cell education. Without KIR2D inhibitory signals, NK cells can mediate only limited killing due to the lack of an educating signal, resulting in hyporesponsiveness. However, in a setting where inhibitory signals are not present, combination therapies that bypass NK cell education or enhance NK cell activation potently induce killing of tumor. C) Unexpectedly, binding of FcγR1 receptor on monocytes, macrophages, activated neutrophils and eosinophils to the Fc portion of IPH2101 can lead to trogocytosis of the KIR2DL1/L2/L3 molecules from the surface of the NK cell. This KIR2D negative NK cells cannot receive an education signal leading to hyporesponsiveness, which may be overcome by combination therapies.
So why might this have been missed in pre-clinical development? First, original in vitro studies testing IPH2101, or 1-7F9 as it used to be termed, did not thoroughly evaluate its impact on expression of inhibitory KIR2DL1/2/3 and activating KIR2DS1/2, to which the antibody also binds, on human NK cells (4, 5). In clinical studies (6–8), the noted KIR2D loss after treatment was interpreted to be IPH2101 receptor occupancy, but more detailed analysis in the current study shows this might not be the case. Preclinical in vitro assays were carried out in many cases with enriched NK cells, precluding monocyte driven trogocytosis. In addition, the short nature of preclinical assays used to test the impact of IPH2101 on NK cell function likely obscured the impact on NK cell education. Past studies evaluated the effect of IPH2101 on a polyclonal NK cell population while the current study evaluated function on the KIR2D single positive population, where the greatest effect on education is noted. The first in vivo studies suggesting inhibitory blockade has anti-tumor efficacy in mice used 5E6 F(ab′)2 against Ly49C/I rather than using full length antibodies, so trogocytosis would have been missed (5, 9). Xenogeneic studies using IPH2101 were done with adoptively transferred purified human NK cells, lacking the trogocytosis unless mouse monocytes and neutrophils can interact with human IgG4 (4). These complexities emphasize how studying a single immune component out of context from other components may not tell the full story.
In order to fully understand the implications of Carlsten’s findings, we need to acknowledge the clinical trial setting and how it differs with previous and ongoing studies. This was a single agent study on smoldering MM treated patients with six intravenous injections of 1 mg/kg of IPH2101 given every two months for one year. In the early disease defined by smoldering MM the patient’s immune system, including NK cells, is presumed to be intact (10), unlike relapsed/refractory MM and AML where IPH2101 has also been tested previously (6–8). Most of these more advanced disease patients in prior studies received previous therapy that could impact monocyte or neutrophil mediated trogocytosis. It should also be noted that in the relapsed/refractory MM trial were some objective responses were achieved IPH2101 was treated in conjunction with lenalidomide (6), whereas no objective responses were achieved in a similar trial without combination therapy with lenalidomide (7), raising the question of how lenalidomide might modify the IPH2101 response on NK cells themselves or on FcγR1 expressing cells. Previous trials also used lower doses of IPH2101, only reaching the 1 mg/kg dose in the highest treatment arm, and patients were dosed for a shorter period of time perhaps having less of an effect on education.
Although Carlsten and colleagues highlight cautions regarding efficacy of the IPH2101 in enhancing NK cell function against smoldering MM, it should be noted that in their last figure they do show KIR2D single positive NK cells from treated patients can degranulate better against MM targets when IPH2101 is present. This indicates that IPH2101 does induce “missing self” and increase NK cell function, even in NK cells from smoldering MM patients treated long term with IPH2101, where education has presumably been impacted. However, this study highlights unexpected mechanisms that may affect the success of IPH2101 in future studies. Decreased NK cell education may be overcome by combinational approaches. Therapeutic antibodies can bypass NK cell education by more potent activation through CD16 (FcRγIII), which can result in enhanced function of uneducated NK cells due to the concomitant absence of inhibitory signaling (11). Cytokines such as monomeric IL-15 or IL-15 trans-presentation complexes, currently being tested in the clinic for amplification of NK cell function and expansion, can bypass NK cell education(12). Although the exact mechanism by which lenalidomide amplifies NK cell function has not been described, there is promising data indicating that its combination with IPH2101 enhances its efficacy both preclinically and clinically (5, 6) and other derivatives of lenalidomide, like pomalidomide, may work in the same way to further enhance function. Another alternative to reduce the impact of IPH2101 on NK cell education may be to reduce dosing or abolish trogocytosis through Fc engineering. What is clear from the current study is the fact that IPH2101 alters NK cell function through dampening of NK cell education, a consideration that needs to be taken into account in future study designs. Interrupting immune checkpoints have the potential to back-fire in some settings and dampen immune function, as we have seen here. What is most important for this and any novel agent is proof of definitive clinical efficacy, which will take carefully designed clinical studies.
Footnotes
Conflict of interest statement: Dr. JS Miller serves on the Scientific Advisory Board of Celgene, Fate Therapeutics and Oxis Biotech and has received research funds and/or clinical trials support from these relationships. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices. These relationships had no role in this work.
Contributor Information
Martin Felices, Email: mfelices@umn.edu.
Jeffrey S. Miller, Email: mille011@umn.edu.
References
- 1.Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, et al. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK-cells in Patients with Myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korde N, Carlsten M, Lee MJ, Minter A, Tan E, Kwok M, et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99:e81–3. doi: 10.3324/haematol.2013.103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends in immunology. 2011;32:364–72. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson DM, Jr, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–91. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson DM, Jr, Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson DM, Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–33. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–23. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 9.Vahlne G, Lindholm K, Meier A, Wickstrom S, Lakshmikanth T, Brennan F, et al. In vivo tumor cell rejection induced by NK cell inhibitory receptor blockade: maintained tolerance to normal cells even in the presence of IL-2. European journal of immunology. 2010;40:813–23. doi: 10.1002/eji.200939755. [DOI] [PubMed] [Google Scholar]
- 10.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–30. doi: 10.1182/blood-2012-06-435503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. The Journal of clinical investigation. 2012;122:3260–70. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juelke K, Killig M, Thiel A, Dong J, Romagnani C. Education of hyporesponsive NK cells by cytokines. European journal of immunology. 2009;39:2548–55. doi: 10.1002/eji.200939307. [DOI] [PubMed] [Google Scholar]