Abstract
Objective
To identify genetic and environmental risk factors for otitis media in an indigenous Filipino population
Study Design
Cross-sectional study
Setting
Indigenous Filipino community
Subjects and Methods
Clinical history and information on breastfeeding, tobacco smoke exposure and swimming were obtained from community members. Heads of households were interviewed for family history and personal beliefs on ear health. Height and weight were measured. Otoscopic findings were described for presence and character of perforation or discharge. An A2ML1 duplication variant that confers otitis media susceptibility was Sanger-sequenced in all DNA samples. Co-occurrence of middle ear bacteria detected by 16S rRNA gene sequencing was determined according to A2ML1 genotype and social cluster.
Results
The indigenous Filipino population has a ~50% prevalence of otitis media. Young age was associated with otitis media (4 age strata; p=0.004), however age was non-significant as a bi-stratal or continuous variable. There was no association between otitis media and gender, body mass index, breastfeeding, tobacco exposure or deep swimming. In multivariate analyses, A2ML1 genotype is the strongest predictor of otitis media, with an odds ratio of 3.7 (95%CI: 1.3, 10.8; p=0.005). When otitis media diagnoses were plotted across ages, otitis media was observed within the first year of life and chronic otitis media persisted up to adulthood, particularly in A2ML1 variant carriers.
Conclusion
Among indigenous Filipinos, A2ML1 genotype is the primary risk factor for otitis media and main determinant of disease progression, although age, the middle ear microbiome and social clusters might modulate the effect of the A2ML1 genotype.
Introduction
Otitis media is the top reason for clinical consult and antibiotic use in US children, with an annual cost of >$5 billion.1–3 Worldwide, the incidence and prevalence of otitis media are highest in sub-Saharan Africa and Asia and in indigenous populations, which mostly bear the burden of hearing loss and health care use due to otitis media.4–6 Due to high prevalence of chronic suppurative otitis media and its complications in developing countries, it was recently proposed for chronic otitis media to be classified as a neglected tropical disease.7 Despite adequate vaccine coverage, otitis media prevalence can still be high in indigenous populations.8 To improve prevention and treatment regimens, better knowledge of otitis media pathophysiology is required, particularly in individuals with increased susceptibility to otitis media due to risk factors such as young age, crowded household, daycare attendance, ethnic differences or human mutation.9–10
An indigenous community in the Philippines was identified to have a very high prevalence of otitis media (Figure 1). Within this highly intermarried community, a large pedigree was used to identify a genetic variant, a duplication c. 2478_2485dupGGCTAAAT (p.(Ser829Trpfs*9)) within the A2ML1 gene that induces susceptibility to otitis media.10 A2ML1 encodes α-2 macroglobulin-like-1 protein or A2ML1, which is localized to middle ear mucosal epithelium and is very similar in sequence to the protease inhibitor α-2 macroglobulin or A2M. 10 It is predicted that A2ML1 and A2M have very similar structures and perform overlapping protective functions within the middle ear, e.g. trapping bacterial or host inflammatory proteases, such that dysfunctional A2ML1 can result in mucosal damage. 10 Audiometric testing in a few individuals from this population documented hearing loss due to otitis media.11 Microbiome studies on middle ear swabs from selected individuals with chronic otitis media revealed unique bacterial profiles according to carriage of the A2ML1 variant.12 In this report, environmental variables along with carriage of the A2ML1 variant and middle ear bacteria were analyzed as risk factors for otitis media within the indigenous Filipino population. Our findings suggest that A2ML1 genotype is the strongest predictor of otitis media occurrence within this population, with some evidence of modulation by age and social clusters in terms of disease onset, progression and carriage of specific bacteria within the middle ear.
Figure 1.
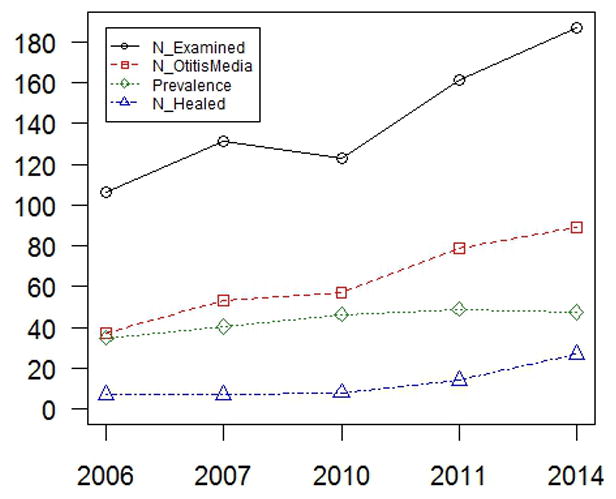
Otitis media prevalence within indigenous Filipino population, which was relatively flat over 8 years. However there was a 6% increase in healed otitis media rates between 2011 and 2014 vs. 0–2% change in previous years.
Methods
An indigenous island community within the central region of the Philippines was reported by community members to have a high prevalence of otitis media. The indigenous (Negrito) Filipinos are the original inhabitants of the island community but have suffered racial segregation over centuries due to their dark-colored skin, curly hair, flat noses and short stature. These physical features of Negritos are not disease-related and no other infectious, craniofacial, skeletal, cardiopulmonary, mental, genetic or immune diseases co-occur with nonsyndromic otitis media or are as prevalent within the community. Racial segregation has fostered intermarriage, and the majority of community members can be traced back to a few founders over six generations. The community was visited 5 times over a period of 8 years, with a relatively stable population size of 200. Ethical approval for conduct of the study was granted by the National Commission on Indigenous Peoples (NCIP), the University of the Philippines Manila Research Ethics Board, and the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals. In addition to community consent through the NCIP, informed consent was obtained individually from study participants.
For each individual who provided saliva using the Oragene DNA saliva kit (DNA Genotek, Ontario, Canada), DNA samples were isolated from saliva and genotyped for the A2ML1 variant using Sanger sequencing.10 For 16 individuals with chronic otitis media, carriage of specific bacterial taxa within the middle ear was determined using 16S rRNA gene profiling, as previously described.12 In brief, middle ear swabs were collected by rubbing sterile short polyester-tipped Pur-Wrap swabs (Puritan Medical, Guilford, ME, USA) against middle ear mucosa and edges of eardrum perforations, and by soaking the swab on discharge when present. The swab stem was cut with sterile scissors, placed in a tube and the tube closed, sealed and labeled. All samples were stored in a −20°C freezer until shipped on dry ice to the Baylor College of Medicine Alkek Center for Metagenomics and Microbiome Research. From each swab specimen, DNA was isolated using the PowerSoil DNA kit (MO BIO Laboratories, Carlsbad, CA, USA) and submitted for 16S rRNA gene sequencing. The 16S rRNA gene V4 region was amplified by PCR and sequenced in an Illumina MiSeq using the 2×250 bp paired-end protocol. Analysis was performed using an in-house pipeline that includes custom analytic packages which provide summary statistics and quality control measurements for validating built-in controls and characterizing microbial communities across large numbers of samples or groups. In this report, only the most significant, abundant or notable bacterial genera were selected for study.12
Participating subjects were interviewed for clinical history and personal information on other risk factors for otitis media such as breastfeeding in infancy, exposure to tobacco smoke, and swimming in deep water (i.e. ≥3 meters). To determine environmental factors within the community, a questionnaire was administered to the heads of households, including queries on household income, highest level of education within the household, water and sanitation facilities, and access to and personal medical beliefs on health care. Height and weight were measured. Body mass index (BMI) was calculated and weight was categorized based on BMI for age. Otoscopy was performed on both ears, and each ear was described for presence and character of perforation or discharge. If the two ears from the same individual had different diagnoses, otitis media status was determined according to the worse ear. Acute otitis media was defined as an episode of ear perforation with discharge, or intact but bulging eardrum with hyperemia and pain, usually in <2 weeks. Effusive otitis media was diagnosed for non-hyperemic, intact tympanic membranes with dullness, retracted position or poor movement with insufflation, with or without visible fluid behind the eardrum. Otitis media was considered chronic if persisting >3 months. Eardrums with signs of previous perforation or scarring but with no current perforation, discharge, hyperemia or fluid behind the eardrum were noted to have healed otitis media. For ears that were examined multiple times over the years, persistence, recurrence or resolution of otitis media were also noted, with the last ear exam as the basis of final diagnosis.
For assessment of the relation of different variables to otitis media status, standard statistical testing was performed using R.13 Fisher exact tests were performed to see the relation of otitis media status with carriage of the A2ML1 variant, age strata, gender, breastfeeding, BMI categories, tobacco exposure or deep swimming. Logistic regression was done to determine if otitis media status is dependent on BMI or age as continuous variables. For multivariate analysis, logistic regression was performed with otitis media status as dependent variable and including independent variables with p<0.2 in bivariate analyses, i.e. A2ML1 variant, household membership and interaction between age and sex.
Results
A total of 187 community members were examined by otoscopy, and 91 had current or previous otitis media (Table 1). Chronic otitis media was diagnosed in 37 individuals with eardrum perforations, mostly with mucoid discharge. Acute otitis media was identified in 13 and effusive otitis media in 10 individuals. Of those with perforated ears at last examination, about half were bilaterally affected (Table 1). Additionally 31 were documented to recover from active otitis media, had healed perforations or thickened/scarred eardrums. Young age was associated with otitis media if divided into 4 strata (Fisher’s exact p=0.004); however if age has only 2 strata (threshold 12.5 years) or treated as a continuous variable, age is non-significant (Table 1). There was no association between otitis media and gender, low or high BMI, breastfeeding in infancy, tobacco exposure or deep swimming. However for some of these variables, information was collected in only 50–80% of participants (Table 1) and non-significance may be due to small sample sizes.
Table 1.
Description of Indigenous Filipinos With or Without Otitis Media (OM)
| Variable | n (%) | Variable | n (%) |
|---|---|---|---|
| Population size (est. 2014) | ~200 | Body mass index | 150 (80.2) |
| Individuals examined | 187 (93.5) | - Underweight | 40 (26.7) |
| Individuals with OM | 91 (48.7) | - Overweight | 30 (20.0) |
| - Chronic OM | 37 (40.7) | Breastfed (n=127) | 104 (81.9) |
| - Acute OM | 13 (14.3) | Tobacco exposure (n=118) | 77 (65.3) |
| - Effusive OM | 10 (11.0) | Swimming in deep water (n=92) | 18 (19.6) |
| - Healed OM | 31 (34.1) | DNA samples obtained | 85 (45.5) |
| Perforated ears | 58 | - A2ML1 variant carrier | 50 (58.8) |
| - Bilateral perforations | 19 | --A2ML1 carrier with OM | 40 (80.0) |
| Age at last exam (years) | - Wildtype | 35 (41.2) | |
| - <5 | 41 (21.9) | --Wildtype with OM | 17 (48.6) |
| - 5–12 | 57 (30.5) | With ear swabs/microbiome data | 16 |
| - >25 | 48 (25.7) | Total households (2006 survey) | 43 |
| Female | 103 (55.1) | Average household members (2006) | 5 |
The indigenous Filipino population was identified to have a 48.7% prevalence of otitis media (Figure 1). Based on interviews of heads of 25 households, the environmental background of the different households was relatively homogeneous, with low income (average US$40 per month) and low educational attainment (most families do not have a high school graduate). In 2006, more than 90% of families lived in huts, and cooking was mostly done using firewood. They had access to piped water but had poor sanitary facilities, with three families on average sharing one toilet. When asked about beliefs regarding ear discharge, 56% of respondents believed that it is normal for children to have ear discharge, while 16% answered “maybe”. When asked if it is normal for a child to be hearing-impaired, 28% answered “yes”, 16% “a little bit”, and 12% “maybe”. A quarter of respondents never sought consult for ear symptoms, while half of respondents sought clinical consult sometimes, with reasons cited being ear discharge, ear pain and having medical missions in the area. Medical personnel and facilities are available on the island, but their personal medical beliefs reflect a lack of public health awareness about otitis media which, coupled with low income, results in a decreased personal value for continued medical care. In 2012, the community was granted a 2-hectare plot of protected land. The latest visit in 2014 revealed improvements in living conditions, including mixed brick and wood dwellings, a flush toilet for each family, stoves for cooking, and full access to piped water. Interestingly rates of healed otitis media slightly increased in 2014 compared to previous years, although overall otitis media prevalence was stable at 46.3–49.1% from 2010 to 2014 (Figure 1).
Previously the A2ML1 c. 2478_2485dupGGCTAAAT (p.(Ser829Trpfs*9)) duplication variant was shown to be associated with otitis media using two independent study groups, namely a large pedigree within the indigenous Filipino population and a case-control cohort of US children.10 Aside from 51 indigenous Filipinos who provided DNA samples in the original study, in this report an additional 34 individuals provided DNA samples. Out of 85 individuals who provided DNA samples, 50 (58.8%) were either heterozygous or homozygous for the A2ML1 variant while the rest were wildtype (Table 1). When multivariate regression was performed using A2ML1 genotype, household membership and interaction between age and sex as independent variables, only carriage of the A2ML1 variant was a significant predictor of otitis media occurrence (p=0.005). In addition, 80% of those who carry the A2ML1 variant developed otitis media, while less than half of those who are wildtype had current or previous otitis media (Table 1). The odds ratio for otitis media given the A2ML1 genotype was 3.7 (95%CI: 1.3, 10.8; p=0.005).
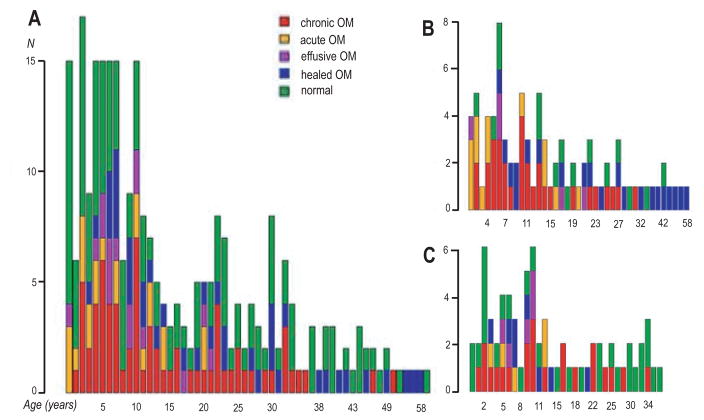
When all ear diagnoses were plotted within an age continuum (Figure 2A), diagnoses of otitis media started within the first months of life. The peak age for chronic otitis media was at 10 years, with a second peak at ages 2–5. Acute otitis media was more common at 0–4 years and effusive otitis media at ages 5–6 years. At 2–5 years, indigenous Filipino children may experience recurrent acute/effusive otitis media or proceed to chronic otitis media, then at ages 6–12 either they continue with chronic otitis media or heal. From teenagers to adults, the proportion of active otitis media progressively decreases. When comparing A2ML1 variant carriers vs. wildtype, carriers seem to have earlier age of otitis media onset at less than 1 year old, with peak age of active otitis media at 6 and 10 years (Figure 2B). In A2ML1 variant carriers, otitis media kept occurring up to ~30 years, with 81.6% of exams revealing active or healed otitis media rather than normal otoscopic findings (Figure 2B). In contrast, there is much less occurrence of active or healed otitis media (57.1%) in wildtype individuals, with more normal exams in infancy and in individuals >13 years old (Figure 2C). Overall these findings may imply that carriage of the A2ML1 variant affects otitis media onset and recovery.
Figure 2.
Ear findings by age in years in (A) overall population, (B) A2ML1 carriers, and (C) wildtype individuals.
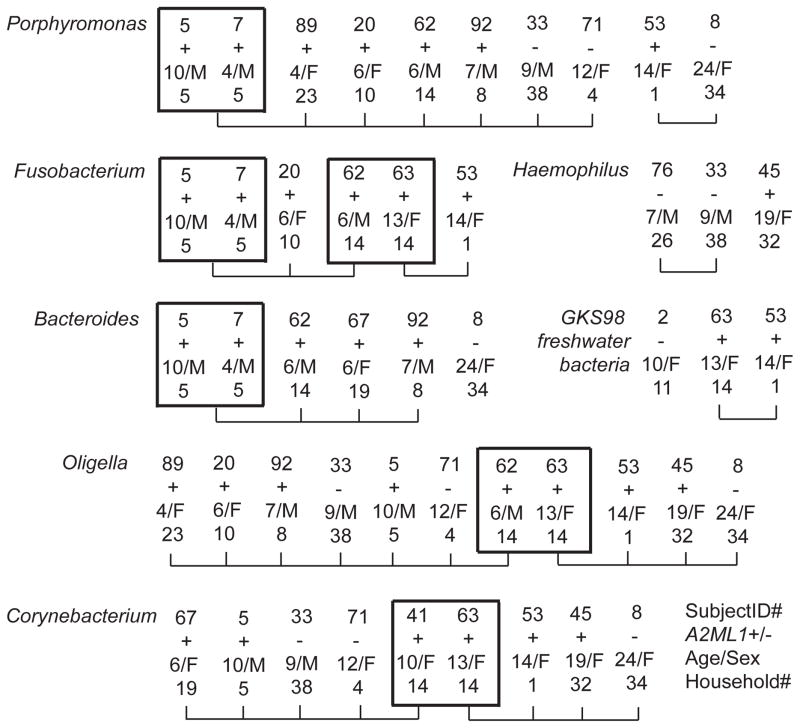
Our microbiome studies showed that: (a) Oligella and Corynebacterium were abundant in both A2ML1 variant carriers and non-carriers; (b) the phyla Bacteroidetes and Fusobacteria and genera Porphyromonas and Fusobacterium were more abundant in A2ML1 variant carriers; (c) Proteobacteria at phylum level and Haemophilus at genus level had higher relative abundance in wildtype individuals; and (d) GKS98 freshwater bacteria that was previously reported only in environmental samples14 was also detected in middle ears of indigenous Filipinos with chronic otitis media.12 When individuals with middle ear swabs were grouped according to A2ML1 genotype, age, sex and household, there seemed to be clustering of carriage of specific bacteria based on age and/or sex (Figure 3). All bacteria were detected at ≥5% abundance within the middle ear. Individuals with bilateral otitis media (ID 5, 53, 63, 92) had similar abundance levels of bacteria on both ears. The assumption is that male and female children play with each other and consist a social cluster. Teenage and older females are also clustered. Two caveats here are: (1) the sample size is limited by occurrence of eardrum perforations, and (2) diagnosis of chronic otitis media peaks at ages 5–10 years thus more than half of individuals with microbial samples are within this age group (Figure 3). Taken together, these findings might imply that although A2ML1 genotype is the primary risk factor for otitis media diagnosis and determinant of disease progression, age and social clusters (e.g. household membership, cluster by age and sex) may modulate the effect of the A2ML1 genotype on carriage of specific bacterial taxa within the middle ear.
Figure 3.
Indigenous Filipinos with 7 middle ear bacteria. For A2ML1: +, variant carriers; −, wildtype. Siblings from the same household are boxed. Connecting lines indicate individuals from the same age group, with threshold at 12.5 years.
Discussion
Of all the variables studied, A2ML1 genotype is the strongest predictor of otitis media within the indigenous Filipino population, increasing the risk of all forms of otitis media in A2ML1 variant carriers almost four-fold. Although age and household membership were not significant in multivariate analysis, there is indication that both age and social clusters play a role in carriage of specific bacterial taxa according to A2ML1 genotype. Additionally, onset of and recovery from otitis media were different in A2ML1 variant carriers vs. non-carriers and were highly dependent on age. The main strength of the study is the relative homogeneity of environmental background within the indigenous Filipino community, which allows for study of otitis media according to only a few variables. Nevertheless this study shows that in spite of the strong association between the A2ML1 variant and otitis media, A2ML1-related otitis media remains a complex trait that is shaped by multiple factors including age, social clusters and middle ear bacteria.
In our previous study, we identified abundant middle ear bacteria according to A2ML1 genotype and also bacteria that were rarely or never reported in the middle ear, namely Oligella and GKS98 freshwater bacteria.12 Among the top bacterial genera identified, Oligella and Corynebacterium were abundant in both A2ML1 variant carriers and non-carriers (Figure 3). Corynebacterium is commonly isolated from middle ear fluid cultures,15 but Oligella was reported only once in ear discharge.16 In wildtype individuals, Proteobacteria at phylum level and Haemophilus at genus level were more abundant compared to A2ML1 variant carriers.12 Non-typeable Haemophilus influenzae (ntHI) causes acute otitis media especially in indigenous children,8,17 so this finding is not surprising. In contrast, the phyla Bacteroidetes and Fusobacteria and genera Porphyromonas and Fusobacterium were more abundant in A2ML1 variant carriers (Figure 3).12 Although these two genera were previously isolated from culture of ear discharge from chronic otitis media, they are much less common than Proteus, Pseudomonas and Staphylococcus that are usually isolated in chronic otitis media patients worldwide and in the Philippines.18–19 The uniqueness of the microbial ear profiles of indigenous Filipinos can be attributed to the occurrence of the A2ML1 variant and also probably to the social structures within the community.
The diagnosis of otitis media in indigenous Filipino infants is consistent with findings in indigenous Australian children in whom acute otitis media was observed within the first 3 months of life.6 While indigenous Australian children have mostly acute otitis media, indigenous Filipino children have chronic otitis media as early as 2 years of age, particularly among A2ML1 variant carriers (Figure 2A). Carriers of the A2ML1 variant appear to have not just earlier onset but also a more protracted course of otitis media (Figure 2). Some wildtype individuals do have persistently chronic otitis media beyond 13 years old (Figure 2C), and for these specific individuals the possibility of a second otitis media susceptibility variant is currently being investigated. Thus among indigenous populations where socio-economic background may favor an increase in prevalence of otitis media,5–6 the indigenous Filipino population is unique in having at least one rare mutation that confers susceptibility to otitis media and favors carriage of specific middle ear pathogens,10,12 and which also influences disease patterns including chronicity and onset.
Whether less crowded conditions and better sanitation within the indigenous Filipino community are related to changes in rates of otitis media is unknown. There is however some indication that there was an increase in rates of healed otitis media after improvement in living conditions, and longer surveillance should help elucidate if better hygiene alleviates the burden of otitis media within the community. Additionally a more complete survey on A2ML1 genotype, middle ear bacteria, and household or social clusters should further illuminate the contribution of each of these factors to the increased otitis media prevalence within the community. More importantly the lack of access to health care is a systemic issue that needs to be addressed through advocacy. PHiD-CV10 vaccine confers additional protection against ntHI compared to other pneumococcal vaccines to which decrease of otitis media incidence has been attributed.17 PHiD-CV10 was recently included in the Philippine immunization program; to our knowledge at the time of study none of the indigenous community members were vaccinated with PHiD-CV10 or any pneumococcal vaccine. Through scientific study of the interplay of genetic and environmental factors that lead to otitis media, it is our hope that these results may be useful in supporting special appropriations towards the institution and maintenance of public health measures (e.g. antibiotic and surgical treatment, vaccination) for otitis media within the indigenous Filipino community.
Taken together, our findings suggest that among indigenous Filipinos, A2ML1 genotype is the primary risk factor for otitis media and main determinant of disease progression, although age, the middle ear microbiome and social clusters might modulate the effect of the A2ML1 genotype.
Acknowledgments
We are very thankful to the members of the indigenous Filipino community who participated in this study. We also thank surgical residents and technical assistants who assisted data collection, in particular M.C. Garcia, P.J. Labra, K. Fellizar, D. Roldan, C. Espina, D. Vanguardia, M. Pedro, S.M. Lagrana and V. Ostan. We are grateful to Tulin Ayvaz from the CMMR for her work in sample processing, and Ginger Metcalf, Donna Muzny and Richard Gibbs from the Human Genome Sequencing Center at Baylor College of Medicine, for their support in sequencing.
Footnotes
Meeting Presentation: This work was presented in part on September 28, 2015 at the AAO-HNSF 2015 Annual Meeting in Dallas, Texas.
Author Contributions
Regie Lyn P. Santos-Cortez, conception of study design, data collection, analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work; Ma. Rina T. Reyes-Quintos, conception of study design, data collection, manuscript writing, final approval, accountable for all aspects of the work; Ma. Leah V. Tantoco, data collection, manuscript writing, final approval, accountable for all aspects of the work; Izoduwa Abbe, data collection, manuscript writing, final approval, accountable for all aspects of the work; Erasmo Gonzalo d.V. Llanes, data collection, manuscript writing, final approval, accountable for all aspects of the work; Nadim Jose Ajami, data analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work; Diane S. Hutchinson, data analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work; Joseph F. Petrosino, data analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work; Carmencita D. Padilla, conception of study design, manuscript writing, final approval, accountable for all aspects of the work; Romeo L. Villarta, Jr., conception of study design, manuscript writing, final approval, accountable for all aspects of the work; Teresa Luisa Gloria-Cruz, data collection, manuscript writing, final approval, accountable for all aspects of the work; Abner L. Chan, data collection, manuscript writing, final approval, accountable for all aspects of the work; Eva Maria Cutiongco-de la Paz, data collection, manuscript writing, final approval, accountable for all aspects of the work; Charlotte M. Chiong, conception of study design, data collection, manuscript writing, final approval, accountable for all aspects of the work; Suzanne M. Leal, conception of study design, analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work; Generoso T. Abes, conception of study design, data collection, analysis and interpretation, manuscript writing, final approval, accountable for all aspects of the work.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: This study was funded by grants from the University of the Philippines Manila – National Institutes of Health (to G.T.A.); the Albert and Margaret Alkek Foundation (to J.F.P.); the National Institute on Deafness and Other Communication Disorders at the United States National Institutes of Health (grants R01 DC011651 and R01 DC003594 to S.M.L. and R01 DC015004 to R.L.P.S.C.); and the National Organization for Hearing Research, Action for Hearing Loss and the Hearing Health Foundation (to R.L.P.S.C.).
References
- 1.Gidengil C, Mangione-Smith R, Bailey LC, et al. Using Medicaid and CHIP claims data to support pediatric quality measurement: lessons from 3 centers of excellence in measure development. Acad Pediatr. 2014;14:S76–81. doi: 10.1016/j.acap.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Casey JR, Pichichero ME. Payment analysis of two diagnosis and management approaches of acute otitis media. Clin Pediatr (Phila) 2014;53:865–873. doi: 10.1177/0009922814533592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133:375–385. doi: 10.1542/peds.2013-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen RG, Koch A, Homøe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2013;77:1530–1535. doi: 10.1016/j.ijporl.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Jervis-Bardy J, Sanchez L, Carney AS. Otitis media in Indigenous Australian children: review of epidemiology and risk factors. J Laryngol Otol. 2014;128(Suppl 1):S16–27. doi: 10.1017/S0022215113003083. [DOI] [PubMed] [Google Scholar]
- 7.Li MG, Hotez PJ, Vrabec JT, Donovan DT. Is chronic suppurative otitis media a neglected tropical disease? PLoS Negl Trop Dis. 2015;9:e0003485. doi: 10.1371/journal.pntd.0003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leach AJ, Wigger C, Andrews R, Chatfield M, Smith-Vaughan H, Morris PS. Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules. BMC Pediatr. 2014;14:200. doi: 10.1186/1471-2431-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby P, Carville KS, Hall G, et al. Crowding and other strong predictors of upper respiratory tract carriage of otitis media-related bacteria in Australian Aboriginal and non-Aboriginal children. Pediatr Infect Dis J. 2011;30:480–485. doi: 10.1097/INF.0b013e318217dc6e. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Cortez RLP, Chiong CM, Reyes-Quintos MRT, et al. Rare A2ML1 variants confer susceptibility to otitis media. Nat Genet. 2015;47:917–920. doi: 10.1038/ng.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes-Quintos MRT, Santos-Cortez RLP, Tantoco MLC, et al. Otoscopic and audiologic findings in an Ati community in Boracay. Philipp J Otolaryngol Head Neck Surg. 2007;22:19–21. [Google Scholar]
- 12.Santos-Cortez RLP, Ajami NJ, Reyes-Quintos MRT, et al. Carriage of an A2ML1 duplication variant that confers susceptibility to otitis media influences the middle ear microbiome. Abstracts of the 39th Annual Midwinter Meeting of the Association for Research in Otolaryngology; 2016. [Accessed February 17, 2016]. p. 297. http://aro2016mwm.conferencespot.org/abstract-book-1.2856633. [Google Scholar]
- 13.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. http://www.R-project.org. [Google Scholar]
- 14.Wu L, Ge G, Zhu G, Gong S, Li S, Wan J. Diversity and composition of the bacterial community of Poyang Lake (China) as determined by 16S rRNA gene sequence analysis. World J Microbiol Biotechnol. 2012;28:233–244. doi: 10.1007/s11274-011-0812-5. [DOI] [PubMed] [Google Scholar]
- 15.Poetker DM, Lindstrom DR, Edmiston CE, Krepel CJ, Link TR, Kerschner JE. Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol. 2005;69:799–804. doi: 10.1016/j.ijporl.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Rossau R, Kersters K, Falsen E, et al. Oligella, a new genus including Oligella urethralis comb. nov. (formerly Moraxella urethralis) and Oligella ureolytica sp. nov. (formerly CDC Group IVe): relationship to Taylorella equigenitalis and related taxa. Int J Syst Bacteriol. 1987;37:198–210. [Google Scholar]
- 17.Leach AJ, Wigger C, Hare K, et al. Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine. BMC Pediatr. 2015;15:162. doi: 10.1186/s12887-015-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brook I. The role of anaerobic bacteria in chronic suppurative otitis media in children: implications for medical therapy. Anaerobe. 2008;14:297–300. doi: 10.1016/j.anaerobe.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.del Rosario R, Chiong CM, Chan AL, Yap EC, Jamir JC, Abes GT. Microbial flora in chronic otitis media: Value of ear aspirate culture studies. Philipp J Otolaryngol Head Neck Surg. 1990:58–66. [Google Scholar]