Abstract
Meta-analyses have demonstrated that low dose aspirin reduces the risk of developing adenocarcinoma metastasis, and when colon cancer is detected during aspirin treatment, there is a remarkable 83% reduction in risk of metastasis. As platelets participate in the metastatic process, the anti-platelet action of low dose aspirin likely contributes to its anti-metastatic effect. Cycloxooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) also contributes to metastasis, and we addressed the hypothesis that low dose aspirin also inhibits PGE2 biosynthesis. We show that low dose aspirin inhibits systemic PGE2 biosynthesis by 45% in healthy volunteers (p <0.0001). Aspirin is found to be more potent in colon adenocarcinoma cells than in the platelet, and in lung adenocarcinoma cells its inhibition is equivalent to that in the platelet. Inhibition of COX by aspirin in colon cancer cells is in the context of the metastasis of colon cancer primarily to the liver, the organ exposed to the same high concentrations of aspirin as the platelet. We find that the interaction of activated platelets with lung adenocarcinoma cells up-regulates COX-2 expression and PGE2 biosynthesis, and inhibition of platelet COX-1 by aspirin inhibits PGE2 production by the platelet-tumor cell aggregates. In conclusion, low dose aspirin has a significant effect on extraplatelet cyclooxygenase, and potently inhibits COX-2 in lung and colon adenocarcinoma cells. This supports a hypothesis that the remarkable prevention of metastasis from adenocarcinomas, and particularly from colon adenocarcinomas, by low dose aspirin results from its effect on platelet COX-1 combined with inhibition of PGE2 biosynthesis in metastasizing tumor cells.
Introduction
Both observational studies (1–5) and controlled clinical trials (6) support the concept that aspirin prevents mortality and metastasis from adenocarcinomas. Importantly, aspirin use reduced the development of metastases by 69% in patients who develop cancer while receiving aspirin (7). The anti-metastatic benefit of aspirin occurred even at low doses in the ranges used for its anti-platelet effect (75 to <300 mg daily) (7, 8).
A convincing body of evidence supports the concept thatplatelets are integral to the process of metastasis, and this forms the basis for considering that the anti-platelet effect of low dose aspirin contributes importantly to the prevention of metastasis (9–16).
In addition to the platelet, cyclooxygenase-2 (COX-2) and its product PGE2 are known to contribute to both tumorigenesis and metastasis. COX-2 inhibitors reduce the incidence of colorectal adenomas (15,16). PGE2 exerts multiple effects that promote tumorigenesis and metastasis (17–19), including induction of the transition from epithelial to mesenchymal phenotype (20), increased angiogenesis and cell growth (21, 22), invasiveness (23) and down regulation of the immune response (24–27).
Given these effects of the COX-PGE2 pathway on tumorigenesis and metastasis, an important question is whether low doses of aspirin can act directly on extra-platelet cyclooxygenases to inhibit PGE2 biosynthesis in humans. The strong inhibition of platelet COX-1 by low doses of aspirin results from at least 3 determinants. First, there is virtually no turnover of COX-1 in platelets, and daily administration of aspirin inhibits platelet COX-1 cumulatively over the platelet life span (28). Second, because of pre-systemic clearance of aspirin, exposure of platelets to the high concentrations of the drug in the portal circulation leads to greater effects on the platelets than on systemic targets (29). Finally, aspirin is particularly potent in inhibiting COX-1 in the platelet (30).
We previously discovered that there is cellular selectivity in the potency of aspirin. High concentrations of hydroperoxides in cells accelerate redox cycling in the peroxidase site of the enzyme to generate a protoporphyrin radical, and in this higher oxidative state, the acetylation of the COXs by aspirin is inhibited (31). Thus, COXs in cells with robust anti-oxidant defenses and low levels of peroxides, such as the platelet, are highly sensitive to inhibition by aspirin. Epithelial cells of the lung (32–35) and colon (36–38) also are protected from elevated levels of peroxides by extensive anti-oxidant systems. Accordingly, in the research reported here, we tested the hypothesis that aspirin would potently inhibit COX activity in epithelial cancer cells in vitro. The potency of aspirin as a cyclooxygenase inhibitor in cancer cells was found to be as great (lung adenocarcinoma) or greater (colon adenocarcinoma) than the potency in platelets.
The hypothesis that low dose aspirin also can inhibit the catalytic activity of extra-platelet cyclooxygenase in vivo was tested, demonstrating that aspirin 81 mg daily reduced the biosynthesis of PGE2 and prostacyclin significantly in healthy humans.
In addition to the action of aspirin to directly inhibit PGE2 biosynthesis in tumor cells, it is well established that aspirin also can reduce tumor cell PGE2 biosynthesis indirectly by blocking the PGE2 production that results from the interaction of platelets with colon cancer cells (16, 39). In those investigations, the interaction of platelets with colon cancer cells increased the expression of COX-2 and production of PGE2, and selective inhibition of the platelet cyclooxygenase was found to prevent the increase in PGE2 biosynthesis (16, 39). Further, a PGE2-dependent epithelial-to-mesenchymal transition of these platelet-activated colon cancer cells was demonstrated, supporting the importance of PGE2 in metastasis. To determine whether this effect of platelets to elicit PGE2 biosynthesis could be generalized to other tumor types, we evaluated the effect of the interaction of platelets with lung adenocarcinoma cells on PGE2 production. We found that, like colon cancer cells, PGE2 biosynthesis is increased by the exposure of lung adenocarcinoma cells to platelets. Moreover, the expression of COX-2 protein, while necessary, was not sufficient to account for the increased PGE2 production, and evidence is presented that is consistent with the hypothesis that platelet adherence initiates calcium-activated cPLA2 in the tumor cells.
Materials and Methods
Materials
Human A549, HCC827 and H2122 were obtained from American Type Culture Collection (ATCC, Manassas, VA). Human HCA-7 were a generous gift from Dr. Robert Coffey. Written informed consent was obtained from study participants and human blood was obtained following a protocol approved by the Vanderbilt Institutional Review Board. Hanks’ Balanced Salt Solution (HBSS), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), aspirin (acetyl salicylic acid), Sepharose 2B column, tris(hydroxymethyl) aminomethane (Tris), and IL-1β were purchased from Sigma-Aldrich (St. Louis, MO). Silica gel 60A thin layer chromatography plates were purchased from Whatman (Clifton, NJ). Antibiotic antimycotic solution was obtained from Life Technologies (Grand Island, NY). [2H8] Arachidonic acid (AA) was obtained from Perkin Elmer Life Sciences (Boston, MA). Anti COX-1 and COX-2 antibodies were obtained form Santa Cruz Biotechnologies (Dallas, TX) and anti GADPH antibodies were obtained from Ambion (Austin, TX).
Participant Blood Sample Procurement
Blood samples were obtained from healthy participants and collected from a peripheral vein using a 21-gauge needle into vacuum tubes containing 3.2% sodium citrate and centrifuged at 300 × g for 10 min at room temperature. The supernatant (platelet rich plasma, PRP) was used to prepare washed platelets as previously described (40). Volunteers were enrolled only if they could confirm lack of aspirin or non-steroidal inflammatory drug use for at least 10 days prior to sample procurement.
Preparation of washed platelets
PRP was acidified to pH 6.4 with 0.15 M citric acid, and centrifuged at 1000 × g for 10 min at room temperature. The resulting pellet was resuspended with 24.4 mM sodium phosphate buffer, containing 113 mM NaCl and 5.5 mM glucose, pH 6.4 and platelets were purified on a Sepharose 2B column equilibrated with the same buffer. Platelets were counted with a Coulter counter and diluted with suspension buffer (110 mM NaCl, 4 mM K2HPO4, 8 mM NaH2PO4 and 5 mM glucose, pH 7.2) to a final concentration of 300,000 platelets/µl.
Cell Culture
A549, HCC827 and H2122 non-small cell lung adenocarcinoma cells as well as HCA-7 and HT-29 colon cancer cells were cultured in 6-well plates at 37°C in a humid atmosphere containing 5% CO2. Cells were maintained in DMEM media supplemented with 10% (v/v) FBS and 1% (v/v) antibiotic-antimycotic solution. All cells except HCA-7 were activated with 1 ng/ml IL-1β once they were 90% confluent to induce expression of COX-2 for 24h prior to experiment of interest.
Relation of Aspirin Concentration to Inhibition of COX
To compare aspirin efficacy between cell types, washed platelets or activated adenocarcinoma cell lines, prostanoid production was assayed using similar experimental conditions: 1) all cells were incubated in albumin free media (41), 2) the same concentration of exogenous arachidonic acid was used for all experiments, 3) deuterated arachidonic acid was used to avoid confounding the results by contribution of prostanoids produced from endogenous arachidonic acid released during cell treatment, and 4) incubation time was the same for all cells. A detailed justification of these conditions is described in Supplemental Data. Washed platelets and activated adenocarcinoma cells were preincubated at 37 °C for 30 min with aspirin at different concentrations: 0, 10, 20, 30, 50 and 100 µM. Then, 2.0 µM [2H8] AA was added and incubated for 15 min. The medium was harvested for GC/NICI/MS analysis.
Participant Urine Sample Procurement
This study was approved by the Vanderbilt University Institutional Review Board and registered on ClinicalTrials.gov (NCT 00761891). Participants provided written informed consent. Healthy non-smoker volunteers over the age of 18 with no chronic medical illness or medications were enrolled. Exclusion criteria included concurrent use of other antiplatelet drugs, NSAIDs or COX-2 inhibitors, history of coronary artery disease, uncontrolled hypertension (systolic blood pressure > 180 mmHg), significant GI bleeding, hematocrit < 35%, or platelet count < 100,000/µL. Volunteers received a blister pack containing a 2-week supply of a daily dose of aspirin 81 mg (McNeil Pharmaceuticals) administered in the evening. The importance of strict adherence to therapy was emphasized and participants were contacted by the research coordinator mid-study to assess and encourage continued compliance. A pill count was performed at the conclusion of the study. Urine was collected for 24 hours starting after the first morning void on the day before the baseline and again prior to the 2-week visit.
GC/NICI/MS Analysis of PGE2 and TxB2
PGE2 and TxB2 (the stable rearrangement product of TxA2) were quantified by isotopic dilution by GC/NICI/MS monitoring selected ions as previously described (42). The signals for different molecules are: TxB2, m/z = 614; and internal standards [2H4] TxB2, m/z = 618; [2H4] PGE2, m/z = 528. To account for the deuterium-protium exchange at the position C12 of [2H8] PGE2, the summation of the signals obtained at m/z = 530, 531 and 532 was performed (43).
Urinary Metabolite Analysis
PGE-M, the major PGE2 urinary metabolite was quantified by isotopic dilution by LC/ESI/MS/MS as described previously (44). Detailed experimental protocol is described in Supplemental data. It should be noted that PGD2 is metabolized by a mechanism analogous to PGE2 and metabolites for this eicosanoid are detected using the same m/z transitions as PGE-M. The methoximated metabolites of PGE2 and PGD2 are chromatographically separated using this method (Supplemental Fig. S1).
PGI-M, the PGI2 urinary metabolite, was quantified by isotopic dilution using GC/NICI/MS as described previously (45).
Calculation of platelet-derived PGE2
To determine the effect of low dose aspirin on extra-platelet PGE2 biosynthesis, it is necessary to account for its effect on the very small amount of platelet-derived PGE2 production, assuming that this is inhibited almost completely by low dose aspirin. PGE2 is produced by the platelet in an amount that is approximately 6.4% that of TxB2 ((39), and Fig. 5). To estimate the fraction of PGE2 that is derived from the platelet, the production rates for total PGE2 and for platelet-derived PGE2 in humans have been calculated. The production rates for PGE2 in humans are 51 ng/mg creatinine in males and 22.7 ng/mg creatinine in females, where time is the amount of time in which 1 mg creatinine is excreted. This production rate is based on the determination that PGE-M represents 16% of infused tritium labelled PGE2 (46), and on the amount of PGE-M/mg Cr measured in this study.
Total PGE2 production rate ng/mg Cr = [PGE-M ng/mg Cr] /0.16
An estimate of platelet-derived PGE2 is based on the fraction: PGE2 produced by washed platelets/TxB2 produced by washed platelets, and on the evidence that ~80% TxM is derived from platelets. Thus:
Extra-platelet PGE2 production rate = (TxB2 production rate × 0.064) × 0.8/PGE2 production rate
where TxB2 production rate is based on the determination that the normal excretion rate of the TxB2 metabolite, 11-dehydrothromboxane B2 (0.369 ng/mg Cr)(47), represents 10.3% of the production rate (48). Thus, platelet-derived PGE2 constitutes 0.36% of total PGE-M in males and 0.8 % in females.
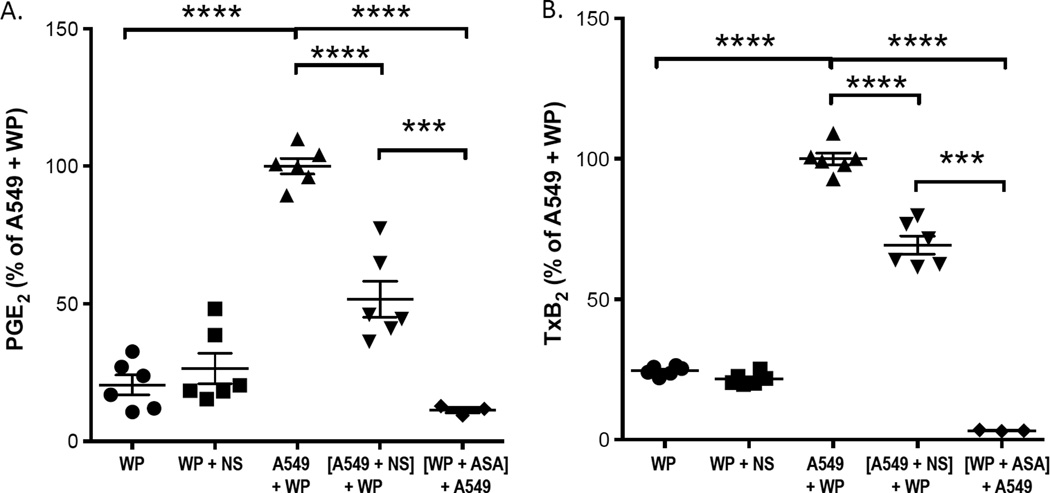
Figure 5.
Both platelet COX-1 and tumor cell COX-2 contribute to PGE2 and TxB2 production when platelets interact with the lung adenocarcinoma cells, A549. Washed platelets obtained from blood treated with aspirin ([WP + ASA]) or vehicle (WP) were activated with 50 µM ADP and subsequently incubated with A549 cells. PGE2 production (A), and TxB2 production (B) were measured by GC/NICI/MS. The COX-2 specific inhibitor NS-398 inhibited PGE2 production by 48% and platelet TxB2 production by 31%. NS-398 (100 nM) treatment of platelets in the absence of A549 cells did not inhibit TxB2 production (WP + NS). **** indicates p < 0.0001 and *** indicates p ≤ 0.001.
Cell Lysates and Western Blot Analysis
Adenocarcinoma cells lysates were obtained by washing cells with ice cold PBS containing a protease inhibitor (PI) cocktail and then scraping them off the well surface. Cells were pelleted by centrifugation at 300 × g for 10 min at room temperature. Cells were resuspended in 100 µl of PBS containing PI cocktail and protein were quantified using the BCA assay.
Proteins (80 µg) were separated by LDS-PAGE (NuPAGE) and transferred on nitrocellulose membranes for analysis by Western blot. Nonspecific binding sites were blocked with TBST buffer (50 mM Tris, 150 mM NaCl and 0.2% (v/v) Tween-20, pH 7.5) containing 5% (w/v) non-fat dry milk for 1 h at room temperature. After blocking, membranes were incubated overnight at 4°C with 1:1000 dilution of appropriate primary antibody: COX-2 goat polyclonal IgG (sc-1747, Santa Cruz), COX-1 (sc-1752, Santa Cruz) and GADPH goat polyclonal IgG (AM4300, Ambion). Bands of interest were detected using horseradish peroxidase conjugated donkey anti-goat IgG polyclonal antibodies (1:5000 dilution; sc-2020, Santa Cruz) and enhanced chemiluminescence kit. Quantification of signal in western blots was performed using Image J downloaded from NIH website.
A549 and Activated Platelets Studies
A549 cells were grown to 90% confluence and starved overnight in serum free DMEM. Two batches of washed platelets were prepared as follow. Whole blood was incubated with 100 µM aspirin (aspirin-treated) or not (no aspirin) for 40 min at room temperature. Washed platelets were then prepared as described separately from each batch as described above. Washed platelets (resuspended concentration of 600,000 platelets/µl) were activated with 50 µM ADP for 2 min at 37°C prior to addition to A-549 cells at a ratio of about 180 platelets per adenocarcinoma cell. Washed platelets were activated by ADP because we found that 1) activating platelets with ADP yielded much less variability than without activation, and 2) ADP does not directly activate A549 cells. Cell and platelet mixture were then incubated at 37°C for 2 hours with no addition of exogenous arachidonic acid. The medium was harvested for analysis of PGE2 by GC/NICI/MS as described above. The cells were then scraped from the wells, lysates obtained and protein quantified using BSA assay. Western blots were performed for COX-1, COX-2 and GADPH proteins as described above. Each experiment was performed in triplicates and a total of 3 experiments were performed (n = 9).
To confirm that the reduction in PGE2 production was mediated by the effect of aspirin to inhibit platelet COX-1 and not due to direct inhibition of COX-2 expressed by A549 cells by residual aspirin present in washed platelets, we performed an additional control experiment in which A549 cells were incubated with IL-1β overnight to induce COX-2 expression. A549 cells were then incubated for 2 h at 37°C with either PBS buffer, the supernatant from washed platelets or supernatant from washed platelets prepared from aspirin-treated whole blood. COX-2 catalytic activity in the platelet/A549 cell aggregates was assessed by measuring [2H8]-PGE2 production for 15 minutes following the addition of [2H8]-AA.
Fluorescence Microscopy
The ability of aspirin to block COX-2 active site has been further demonstrated using a fluorophore conjugated to indomethacin as previously described (49) In short, A549 cells were grown to 90% confluence and expression of COX-2 was induced as described above. Cells were incubated in 2.0 ml of a 1:1 dilution of HBSS and modified Tyrode’s buffer (20 mM HEPES, 10 mM glucose, 150 mM NaCl, 6 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, pH 7.4) containing 50 nM of the fluorescent probe, fluorocoxib A, for 30 min at 37°C. The cells were then washed briefly three times with PBS and a washout was performed by incubating the cells in HBSS/Tyrode’s solution for 30 min at 37°C. Following the washout period, the cells were imaged in 2.0 mL fresh HBSS/Tyrode’s on a Zeiss Axiovert 25 Microscope with the propidium iodide filter (0.5–1.0 s exposure, gain of 2). All treatments were performed in duplicate dishes in at least three separate experiments (n = 6). To block the COX-2 active site, the cells were preincubated with 10 µM NS-398 for 30 min at 37°C prior to the addition of fluorocoxib A. To test the ability of aspirin to block the COX-2 active site, the cells were pre-incubated with 100 µM aspirin for 1 hour at 37°C prior to the addition of fluorocoxib A.
Measurement of Diastolic Calcium
A549 cells were incubated for 2 hours at 37°C with washed platelets which were preincubated with ADP (50 µM) or aspirin (100 µM) or vehicle for 5 min. A549 cells were incubated with 2 µM Fura-2 acetoxymethyl ester (Fura-2 AM) for 8 min at room temperature to load the calcium indicator in the cytosol. A549 cells were washed twice for 10 min with Tyrode’s solution containing 250 µM probenecid to retain the indicator in the cytosol. A minimum of 30 min was allowed for de-esterification before the cells were imaged. All the experiments were conducted in Tyrode’s solution containing the following: 1 mM CaCl2, 134 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH adjusted to 7.4 with NaOH. The final concentration of Ca2+ was 1 mM. Baseline of Fura-2 fluorescence ratio were used as intracellular Ca2+ levels. Data were analyzed using commercially available data analysis software (IonWizard™, IonOptix, Milton, MA).
Statistical Analysis
Statistical analysis was performed using Prism 4 from GraphPad Software, Inc (San Diego, CA). Paired analyses with Wilcoxon signed ranked test were used to determine significant differences between PGI-M and PGE-M values before and after aspirin therapy. The significance of differences between PGE2 and COX-2 production in A549 cells that were untreated, treated with activated platelets and treated with activated platelets preincubated with aspirin, was determined using one-way ANOVA with Tuckey’s multiple comparisons test. Differences among the groups in diastolic calcium measurements were analyzed using one-way ANOVA. P<0.05 was considered statistically significant.
Results
Low dose aspirin inhibits non-platelet biosynthesis of prostaglandins
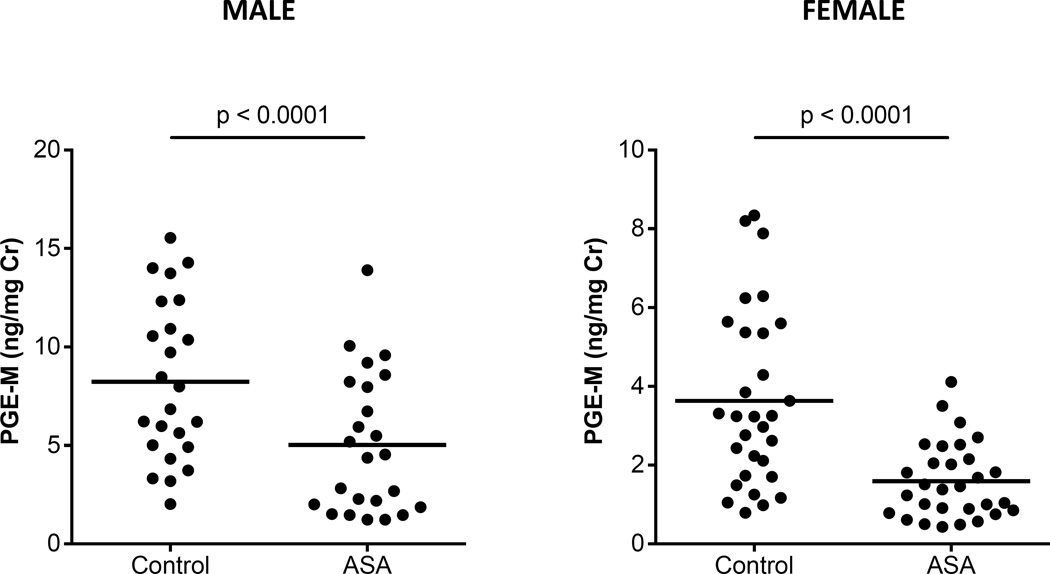
The effect of aspirin 81 mg daily for 2 weeks on urinary metabolites of PGE2 (PGE-M) and prostacyclin (PGI-M) was quantified in healthy volunteers who had no medical history of chronic inflammatory conditions that could predispose them to elevated prostaglandin levels. Detailed medication history was reviewed prior to enrollment to ensure that those did not interfere with the investigation. Demographics of study population are described in Table 1. Volunteers were given two weeks of aspirin 81 mg daily, and the urinary metabolites of PGE2 (11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid) was measured using LC/MS/MS. Because the average PGE-M excretion is greater in males (8.2 ng/mg creatinine) than in females (3.6 ng/mg creatinine), the effect of aspirin was determined separately by gender. Aspirin 81 mg daily was sufficient to reduce urinary PGE-M levels in healthy females by 56% and in healthy males by 39%. When correction is made for the estimated small contribution of platelet-derived PGE2 to total PGE2 biosynthesis (see Methods), the reduction in extra-platelet PGE2 production by aspirin is 55.4% in females and 38.6% in males (Fig. 1). The same aspirin dose also inhibited urinary PGI-M by 37% (p<0.0001, n=54) (Supplemental Fig. S2). Serum thromboxane B2 levels were reduced by 96.6%. These results demonstrate that low dose aspirin can inhibit non-platelet prostaglandin production.
Table 1.
Demographics of Study Population.
| All Subjects (N=52) |
|
|---|---|
| Age (y) | 68[61, 76] |
| Male sex | 36 (69%) |
| Race – White | 49 (94%) |
| Black | 2 (4%) |
| Other | 1 (2%) |
| Current or Former Smoker | 26 (50%) |
| BMI (kg/m2) | 26.2[23.7, 29.2] |
| Platelet count (×103/mL) | 184[168, 219] |
Values are median [IQR] or counts (%). BMI = body mass index;
Figure 1.
Daily low dose aspirin inhibits prostaglandin E2 biosynthesis in humans. 81 mg of aspirin was administered daily to healthy participants for 2 weeks. PGE-M, the urinary metabolite of prostaglandin E2, was measured by LC/MS before and after aspirin therapy. Data is presented for males and females. Paired analysis comparisons were performed using Wilcoxon-ranked tests. Low dose aspirin inhibited the biosynthesis of PGE2 by 45% (p<0.0001, n=54).
Aspirin is a potent inhibitor of PGE2 biosynthesis in adenocarcinoma cells
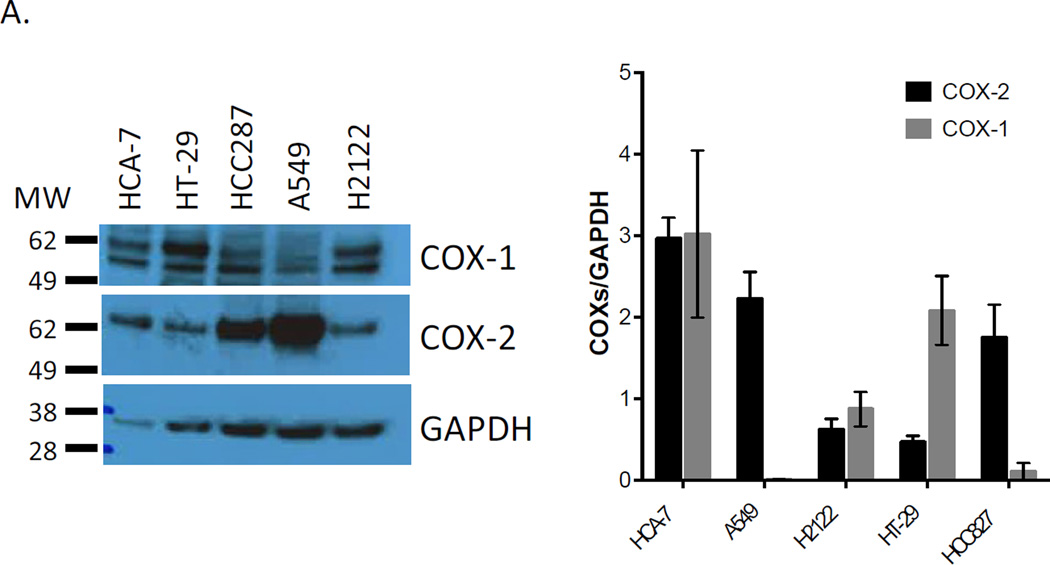
To examine the effect of aspirin on PGE2 biosynthesis in lung adenocarcinoma cells, we tested a range of concentrations of aspirin on PGE2 biosynthesis in the non-small cell lung adenocarcinoma cell lines, H2122, HCC827 and A549. As shown in figure 2A, all three adenocarcinoma cell lines express COX-2 after overnight stimulation with IL-1β, as well as various levels of COX-1. We compared the effect of the same concentrations of aspirin on COX-1 derived biosynthesis of thromboxane A2 in human washed platelets. Platelets were utilized as a comparator in this in vitro study, because the dose range designated as “low dose aspirin” in humans is defined as the lowest dose of aspirin that nearly completely inhibits platelet COX-1.
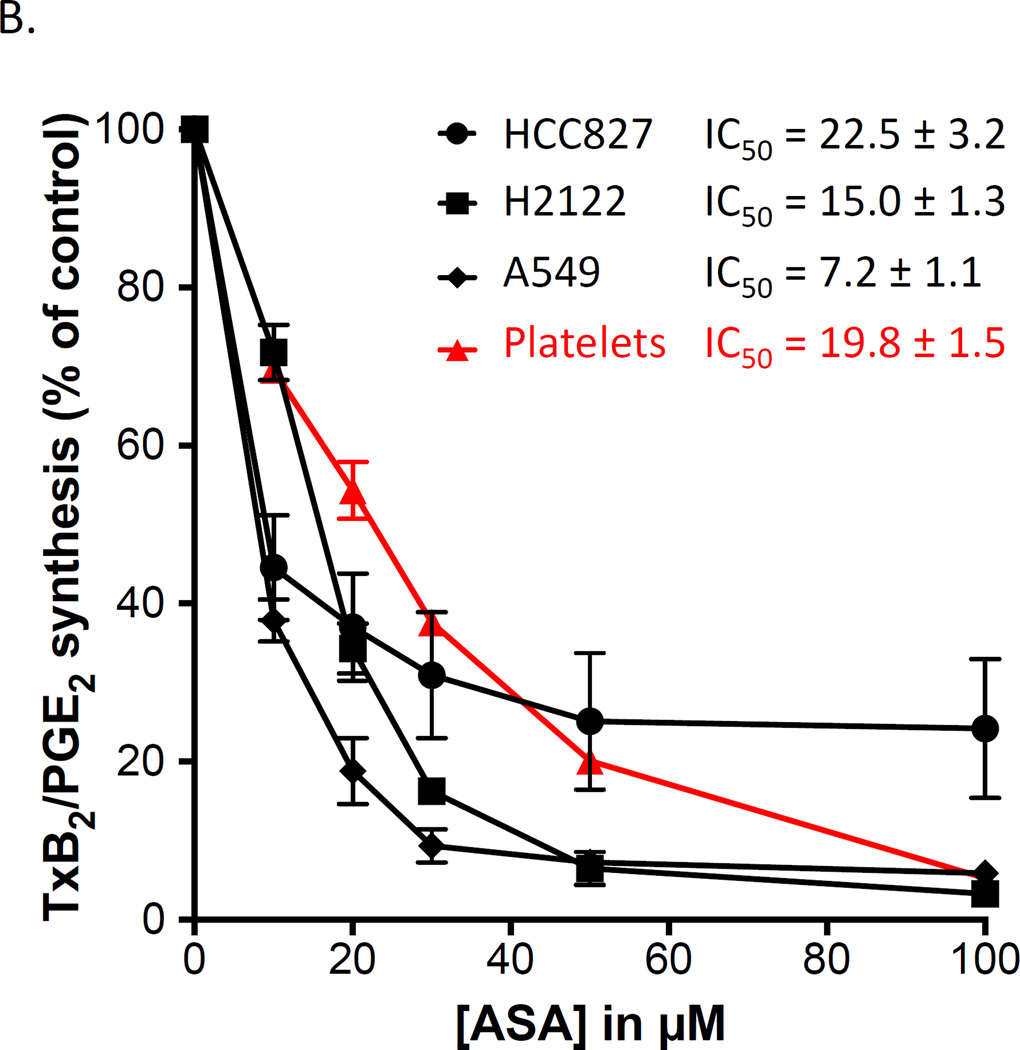
Figure 2.
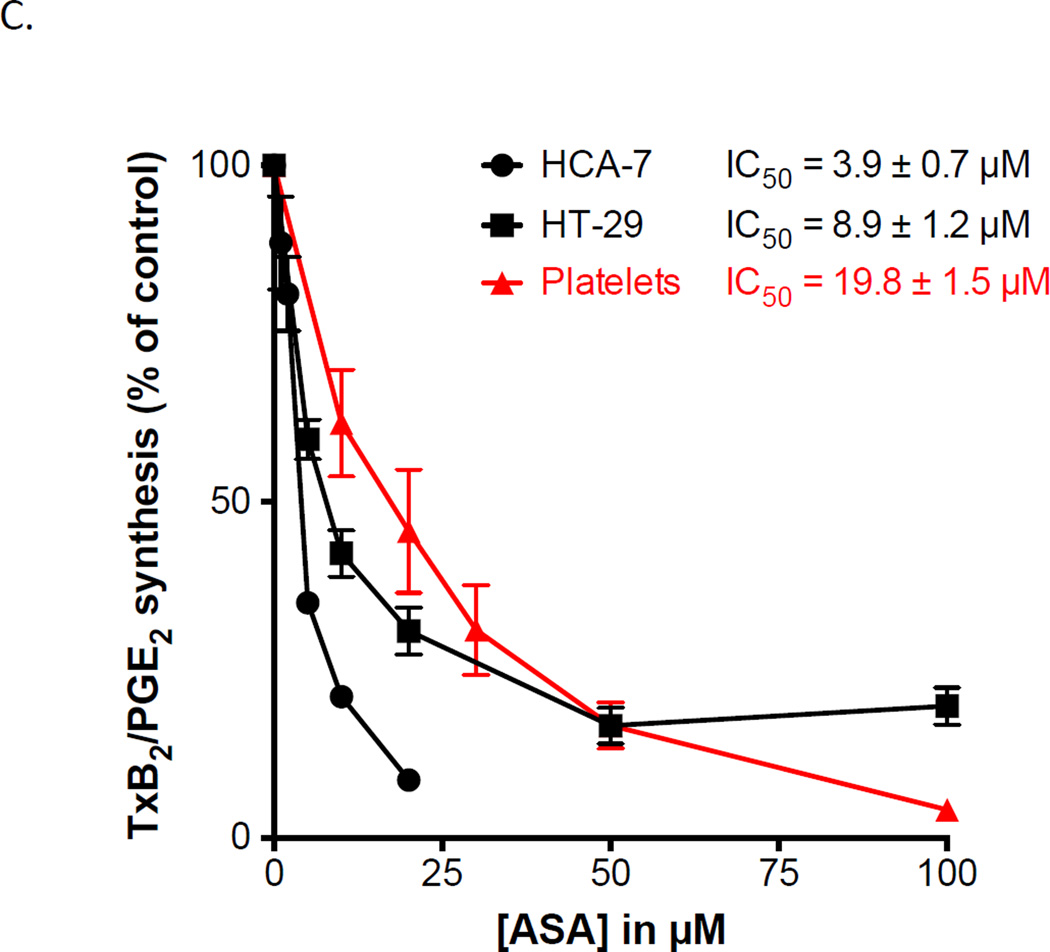
Aspirin inhibits COX-derived PGE2 in adenocarcinoma cells as potently as it inhibits COX-1-dependent TxB2 in washed platelets. Three lung and two colon adenocarcinoma cell lines were used to examine the potency of aspirin to inhibit PGE2 synthesis. (A) Western blots analysis of COX-1 and COX-2 expression in H2122, HCC827, A549 and HT 29 cells after cell activation with IL-1β ng/ml. HCA=7 was not activated prior analysis. Protein expressions are expressed as a ratio against housekeeping protein GAPDH. n = 3 (B) Washed platelet biosynthesis of TxB2 and lung adenocarcinoma cell line biosynthesis of PGE2 were measured by GC/MS. (C) Washed platelet biosynthesis of TxB2 and biosynthesis of PGE2 in the colon adenocarcinoma cell lines, HCA-7 and HT-29, were measured by GC/MS. Aspirin IC50 for the different cell types are indicated. Values represent means ± S.E.M. n = 6.
In these experiments, the same concentration of isotopically labelled arachidonic acid (2µM) was added to all cells as substrate and the correspondingly labelled product analyzed. In all three cell lines, PGE2 biosynthesis was markedly inhibited by aspirin (IC50s are 22.5 ± 3.2 µM, 7.2 ± 1.1 µM, and 15.0 ± 1.3 µM for HCC827, A549 and H2122 cells, respectively). By comparison, the IC50 for aspirin inhibition of platelet TxB2 biosynthesis assayed in the same conditions was 19.8 ± 1.5 µM (31).
Based on this evidence that aspirin potently inhibits PGE2 biosynthesis in lung adenocarcinoma cells, its effect as a COX inhibitor in colon cancer cells also was determined (Figure 2C). The IC50 for inhibition of PGE2 biosynthesis in the colon cancer cells, HCA-7 and HT-29, was 3.9 ± 0.7 µM and 8.9 ± 1.2 respectively. This demonstrates that aspirin is more potent as an inhibitor of COX in cells from colon adenocarcinomas than in the platelet (p< 0.0001). Thus, the catalytic activity of the cyclooxygenases in this group of lung and colon adenocarcinoma cells is inhibited by aspirin to the same or greater extent than COX-1 in the platelet.
We have previously demonstrated that aspirin equally inhibits both COX isoforms (31). Interestingly our results show that each of the five adenocarcinoma cells express various levels of the two isoforms. A549 cells, for example, mainly express COX-2, whereas HT 29 mainly express COX-1. Our results show that aspirin effectively inhibits prostanoid synthesis in all these cell lines irrespective of the isoforms expressed demonstrating that it is not selective for platelet COX-1. To further confirm that aspirin is not isoform specific, we measured prostanoid production before and after preincubation with the COX-2 specific inhibitor valdecoxib. As shown in supplemental figure S4, 100 nM valdecoxib only inhibited 60% of PGE2 synthesis in HCA-7, a concentration that fully inhibited PGE2 synthesis in A549 and H2122. The same concentration of valdecoxib had no effect on TxB2 synthesis by washed platelets (Supp. Fig. S4).
Aspirin blocks the COX-2 binding site in the COX-2 expressing A549 cell line
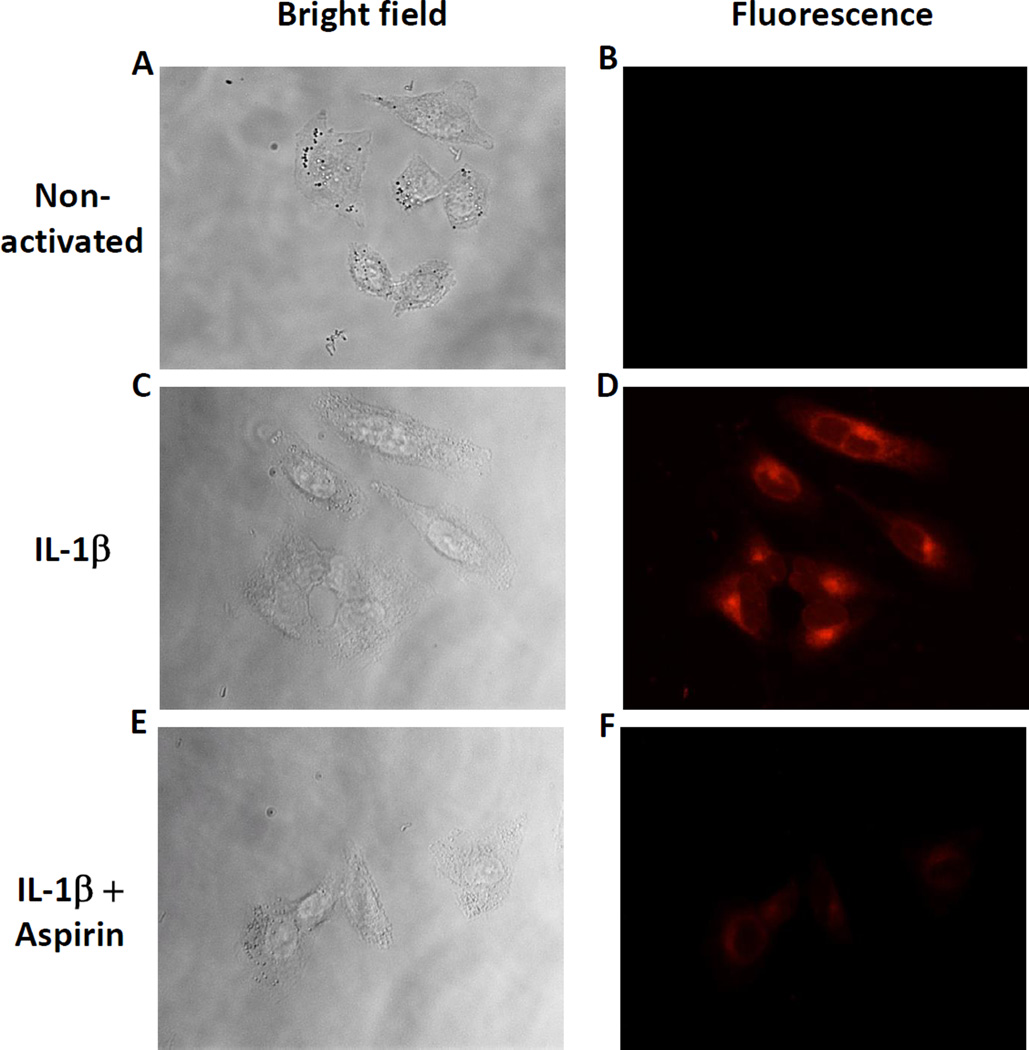
The experiments above examined aspirin’s inhibition of PGE2 biosynthesis in adenocarcinoma cells. To further support the hypothesis that aspirin can inhibit PGE2 production by blocking the COX-2 active site, we analyzed the effectiveness of aspirin at preventing the binding of a COX-2-specific fluorescent probe (49) using fluorescence microscopy. A549 cells were chosen because COX-1 expression is negligible (8 %) compared to that of COX-2 when activated with IL-1β (Fig. 2A and Sup. Fig. S3). A rhodamine conjugated-indomethacin probe, fluorocoxib A (49), was used to label the active site of COX-2 (Fig. 3). As expected, the probe did not label the non-activated A549 that are devoid of COX-2 (Fig. 3B) but strongly labelled IL-1β-activated cells (Fig. 3D). Importantly, aspirin effectively blocked COX-2 labeling by fluorocoxib A (Fig. 3F), providing direct evidence that aspirin blocks the COX-2 active site. These results support the concept that inhibition of PGE2 production by aspirin in A549 cells (Fig. 2) is caused by COX-2 inhibition and not by cyclooxygenases-independent effects of aspirin.
Figure 3.
Aspirin inhibits COX-2 labeling by fluorocoxib A in A549 lung adenocarcinoma cells. (A) (C) and (E) Bright field images of A549 cells. (B) Non-activated A549 cells were treated with 200 nM of rhodamine-conjugated indomethacin (fluorocoxib A) for 30 min. (D) IL-1β-activated A549 cells were treated with 200 nM of rhodamine-conjugated indomethacin (fluorocoxib A) for 30 min. (F) IL-1β-activated A549 cells were pretreated with 100 µM aspirin for 30 min before adding fluorocoxib A. Pretreatment with aspirin inhibited COX-2 associated fluorocoxib A fluorescence.
The interaction of platelets with lung adenocarcinoma cells induces PGE2 biosynthesis via a calcium-mediated mechanism
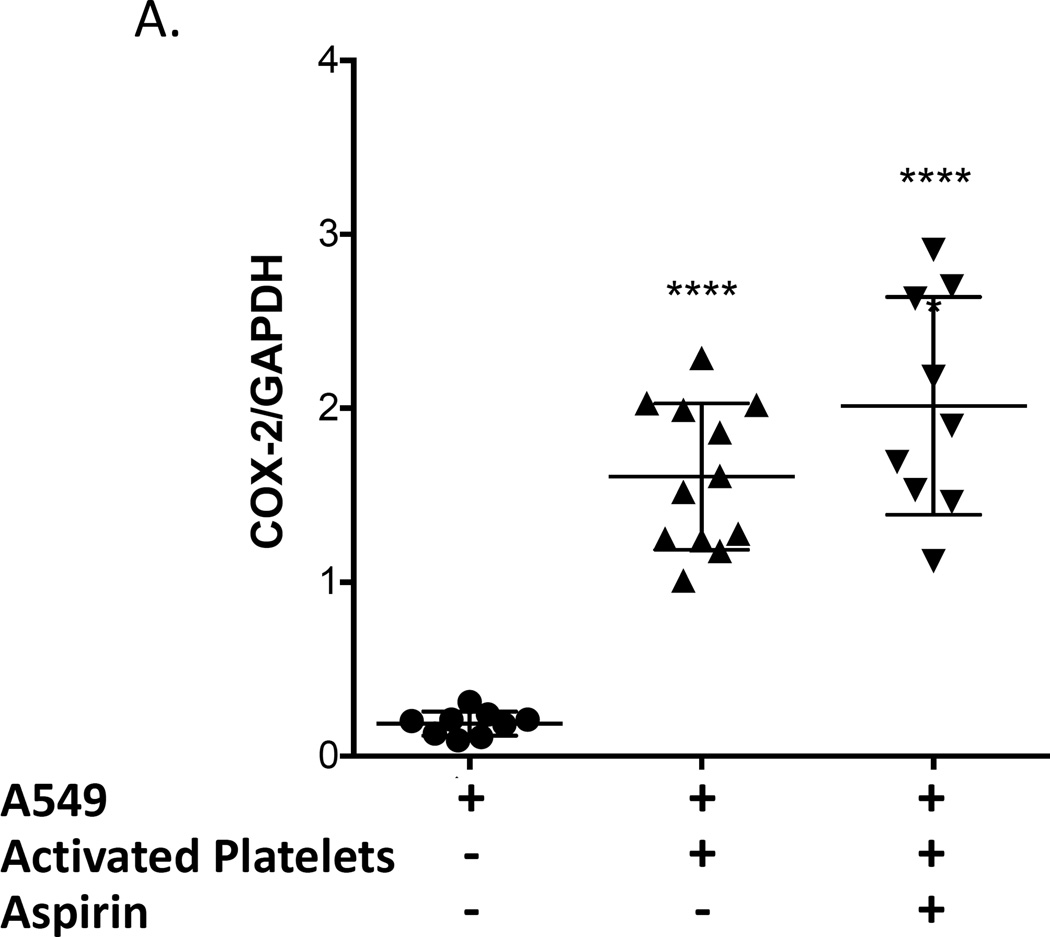
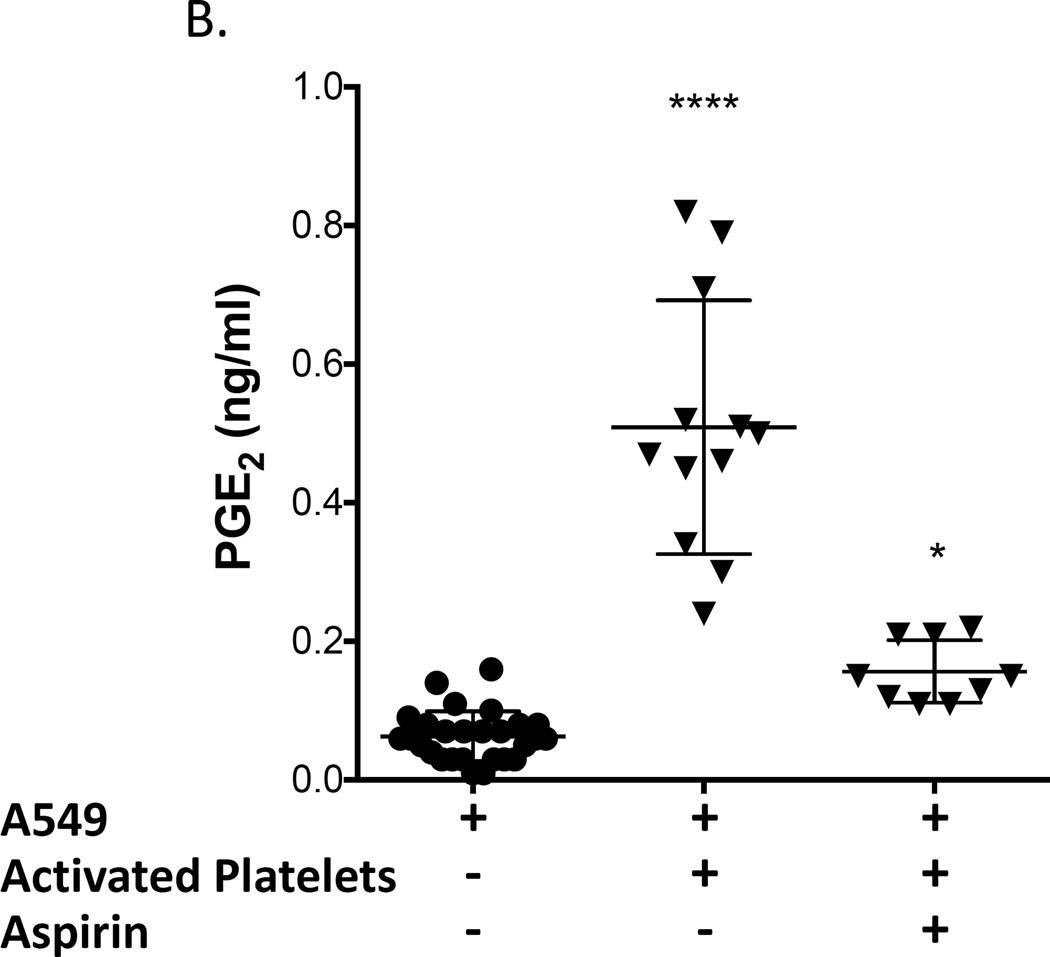
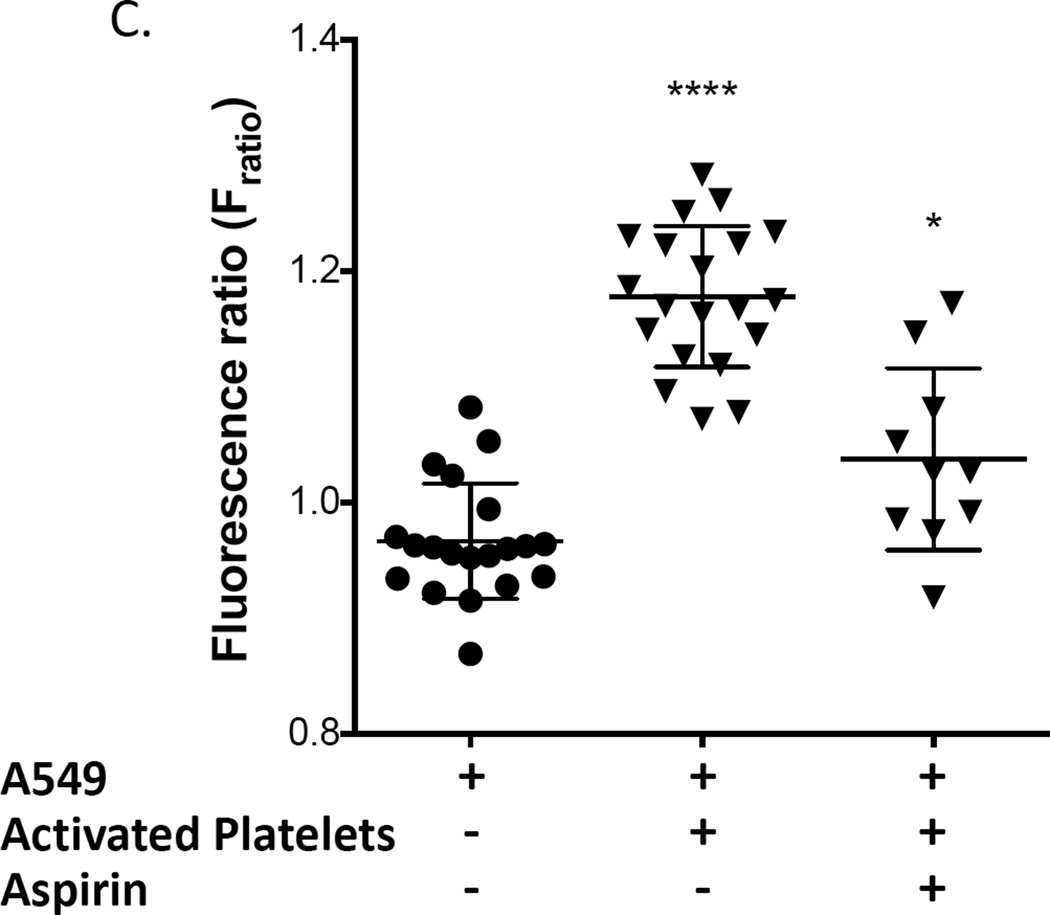
The effect of the interaction of platelets with adenocarcinoma cells on PGE2 production by COX-2 was evaluated in A549 cells because they produce low levels of PGE2 at baseline (Fig. 4). Washed platelets from a normal volunteer were activated with ADP and subsequently incubated with A549 cells, and COX-2 expression and PGE2 production were measured. Both COX-2 expression and PGE2 production were significantly increased (Fig. 4). We then determined whether inactivation of platelet COX-1 by aspirin would prevent COX-2 expression and/or PGE2 production by the platelet-A549 cell aggregates. We first incubated whole blood with 100 µM aspirin for 40 minutes and then prepared washed platelets from the aspirin-treated blood. This was done to ensure that all residual aspirin was removed from the platelet preparation and that no free aspirin was available for direct inhibition of A549’s COX-2. Platelets derived from aspirin-treated blood were activated using ADP and subsequently incubated with A549 cells. COX-2 protein expression in A549 cells was still upregulated by aspirin-inhibited platelets (Fig. 4A), but PGE2 production was significantly inhibited (Fig. 4B), suggesting that PGE2 synthesis is induced by activated platelets by a mechanism independent of the level of expression of COX-2.
Figure 4.
Activated platelets induce COX-2 expression and PGE2 production in lung adenocarcinoma cell line A549. Washed platelets were activated with 50 µM ADP and subsequently incubated with A549 cells. Incubation of A549 cells with activated platelets increased COX-2 protein expression (A), PGE2 production (B), and intracellular calcium mobilization (C) from baseline. Pretreatment of blood with aspirin (100 µM) prior to isolation of washed platelets and their activation with ADP effectively blocked PGE2 production by A549 cells (B) and intracellular calcium mobilization (C) but not COX-2 protein upregulation (B). (A) **** indicate p < 0.0001 versus A-549 alone, n ≥ 9; (B) and (C) **** indicate p < 0.0001 versus all other conditions, * indicates p < 0.05 vs A-549 alone. n ≥ 9.
To confirm that the reduction in PGE2 production was mediated by the effect of aspirin to inhibit platelet COX-1 and not due to direct inhibition of COX-2 expressed by A549 cells by residual aspirin present in washed platelets, we performed an additional control experiment in which COX-2 catalytic activity was measured in IL-1β-activated A549 cells incubated with either PBS buffer, the supernatant from washed platelets or supernatant from washed platelets prepared from aspirin-treated whole blood. PGE2 production was no different in all three groups (3.5 ± 0.2, 3.3 ± 0.2, and 3.3 ± 0.3 ng/ml, respectively; n=3). These results demonstrate that there is insufficient residual aspirin present in aspirin-treated platelets washed as described in our protocol to directly inhibit COX-2-mediated PGE2 biosynthesis by A549 cells.
Consideration was given to the finding that platelet COX-1 inhibition by aspirin inhibits PGE2 production without affecting COX-2 expression (Fig. 4). This could occur only if the increase in PGE2 elicited by the interaction of the A549 cells with platelets resulted from release of the COX substrate, arachidonic acid, from phospholipids by a phospholipase. In A549 cells, the phospholipase is cytosolic phospholipase A2 (cPLA2 or Group IVA PLA2) (50, 51). cPLA2 is activated by an increase in cytosolic calcium (52, 53), which binds to the C2 domain of the enzyme and increases its hydrophobicity, thereby initiating penetration of the lipid bilayer. In A549 cells, ionophore-induced increases in cytosolic calcium have been shown to increase production of PGE2 in a cPLA2 dependent manner (50). Accordingly, we measured cytosolic calcium in A549 cells in response to incubation with ADP-activated washed platelets inhibited or not by aspirin. Cytosolic calcium levels were quantified for the following conditions: A549 cells incubated with (a) vehicle, (b) incubated with ADP- activated platelets and (c) incubated with aspirin-pretreated ADP-activated platelets. Cytosolic calcium (expressed as fluorescence ratios) was increased by 21.9 % following incubation of A549 cells with ADP-activated platelets (Fig. 4C, n = 20) but this effect was abrogated when the platelets had been pre-incubated with aspirin (7.3 % increase, n = 10). These data indicate that platelets inhibited by aspirin are unable to trigger the mobilization of cytosolic calcium necessary for initiating PGE2 synthesis by A549 cells. Interestingly, this calcium signal does not appear to be necessary for COX-2 induction.
The cellular origin of the increased PGE2 in the platelet/A549 cell aggregates was investigated by employing the COX-2 inhibitor, NS-398, at 100 nM. We first demonstrated that this concentration of NS-398 that does not block the production of PGE2 or thromboxane B2 (TxB2) in washed platelets (Fig. 5B and Supplemental Figure S5) but fully blocks PGE2 production by IL-1β-activated A549 (Supplemental Figure S4). When washed platelets were incubated together with A549 cells, the increased PGE2 production was reduced by NS-398 by only 48%, indicating that approximately half of the increase in PGE2 in the platelet/A549 aggregates was derived from the platelets, either by increased synthesis of PGE2 in the platelet and/or intercellular transfer of PGH2 from the platelet to the A549 cells. Thus, when platelets adhere to the cancer cells, both the platelets and the cancer cells are activated and both contribute to the increased biosynthesis of PGE2.
Also, 31% of the increase in TxB2 that resulted from exposure of platelets to A549 cells was blocked by NS-398 (Fig. 5B). Because no TxB2 is formed by A549 cells in the absence of platelets, it may be inferred that intercellular transport of PGH2 from A549 cells to the platelet contributes to the increase in TxB2 that results from the interaction of platelets with A549 cells.
Discussion
We have demonstrated that aspirin 81 mg daily for 2 weeks inhibits the biosynthesis of PGE2 in humans by an average of 45%. Thus, even at this low dose, extra-platelet COX is inhibited significantly. To place this in the context of the effect of the non-steroidal anti-inflammatory drugs, our group found previously that ibuprofen 800 mg 4 times daily reduced PGE2 production by 63% (44).
Metastasis is facilitated by cyclooxygenase-derived PGE2 (54–57). PGE2 enhances the metastatic process via multiple mechanisms that in combination yield a strong pro-metastatic effect. The effects of PGE2 that promote metastasis include upregulation of the cell adhesion receptor, CD44 (56, 58, 59), induction of a transition from an epithelial to a mesenchymal phenotype (16, 20, 60), activation of matrix metalloproteinases (23, 54, 61), enhanced tumor cell migration (62), increased angiogenesis (63), and disruption of the immune surveillance of tumor cells (24, 26, 64–66).
Prostacyclin biosynthesis also reflects oxygenation of arachidonic acid by extra-platelet COX. Previous studies with small sample size (67, 68) suggested that low dose aspirin also can inhibit prostacyclin biosynthesls. Here we clearly demonstrate a 37% reduction in prostacyclin biosynthesis by aspirin 81 mg daily.
The potency of low dose aspirin as an inhibitor of COX-1 in platelets is one of the factors contributing to its anti-platelet efficacy even when administered at low doses. We found that aspirin inhibited COX isoforms expressed in lung adenocarcinoma cells as potently as its inhibition of platelet COX-1.
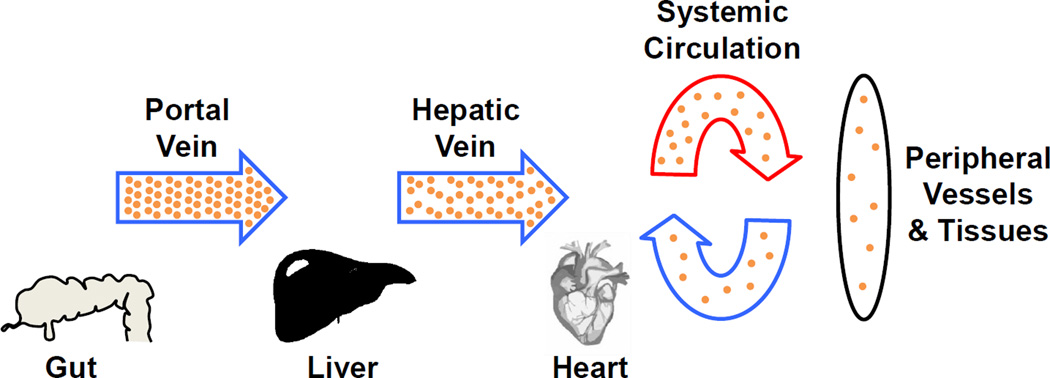
Inhibition of both COX isoforms expressed in cells from adenocarcinomas of the colon by aspirin is significantly greater than inhibition of platelet COX-1. Hepatic metastases from colon cancers derive from circulating tumor cells that lodge in the liver where they are exposed to the same high concentrations of aspirin that are so effective in inhibiting platelet COX-1 (Fig. 6), and are exposed to portal vein concentrations of aspirin constantly whereas circulating platelets are in the portal circulation only about 20–25% of the time. Our demonstration of the potency of aspirin in colon cancer cells, therefore, supports a hypothesis that low dose aspirin produces a high degree of inhibition of both COX isoforms in tumor cells from colon cancers that are sequestered in the hepatic portal vasculature. Given the evidence that PGE2 is pro-metastatic, substantial inhibition of the biosynthesis of PGE2 in tumor cells by low dose aspirin could be hypothesized to contribute to the anti-metastatic effect in cancers with venous drainage into the portal circulation. Indeed, the examination of metastasis in the meta-analysis of randomized controlled clinical trials of aspirin demonstrated exceptional prevention of metastasis from colon cancer. During the trials while randomized aspirin was being administered, if patients had no clinical evidence of metastasis at the time of diagnosis of colon cancers, the hazard ratio for later metastasis was 0.13 (95% CI 0.03–0.56, p=0.007) (7). For adenocarcinomas other than those of the colon, the hazard ratio was 0.47. The profound inhibition of metastasis from colon adenocarcinomas by low dose aspirin is consistent with its marked inhibition of the biosynthesis of PGE2 by COX-2 in the circulating tumor cells acting in concert with inhibition of platelet COX-1.
Figure 6.
Aspirin concentration in the different circulation compartments. Aspirin concentration is represented by the density of the yellow dots. Aspirin concentration decreases as it travels through the liver and is further diluted in the systemic circulation. The highest plasma concentrations are in the portal vein and in the liver where colon cancer metastases will be exposed to aspirin.
Circulating tumor cells from cancers with venous drainage into the systemic circulation would be exposed to lower concentrations of aspirin than cells released from colon adenocarcinomas. Consequently, less than complete inhibition of COX in these cells by low dose aspirin would be expected, as exemplified by the 45% reduction in total body PGE2 biosynthesis The potential biological importance of a partial reduction in PGE2 biosynthesis is contingent on whether the effect of PGE2 on its receptor approximates a log-linear function of the concentration of the agonist. The explosive release of TxA2 within seconds after platelet activation (69) produces concentrations of this agonist that are supramaximal relative to receptor response. In contrast to the platelet, PGE2 release progresses slowly from tumor cells (70). Inhibition of PGE2 biosynthesis by ibuprofen in humans by only 63% (44) occurs at doses that are associated with both efficacy and gastrointestinal bleeding, and an effect of aspirin on tumor volume in NNK-treated mice is observed even at doses inhibiting PGE2 levels by less than 50%. Accordingly, it is highly likely that a partial inhibition of PGE2 biosynthesis by aspirin would reduce receptor-mediated responses, and importantly, could be additive with the anti-platelet effects of aspirin on metastasis. However, adding an intervention that achieves a more complete and selective inhibition of PGE2 biosynthesis or action, e.g. an mPGES-1 inhibitor or an EP antagonist, conceivably could produce an anti-metastatic effect in non-colonic cancers that is superior to aspirin alone.
Adherence of platelets to circulating tumor cells (12, 13, 71) is important to the process of implantation at the metastatic site (14, 15) via interaction of platelet P-selectin with P-selectin glycoprotein ligand-1 on cancer cells (71, 72). The phenotype of tumor cells is reprogrammed by their interaction with platelets to a more metastatic phenotype (20). Recently, Paola Patrignani and her colleagues Dovizio et al. (39) and Guillem-Lobat (16) demonstrated that tumor cell-platelet interaction upregulates COX-2 expression and PGE2 release in the colon cancer cells, and provide convincing evidence that blockade of platelet COX-1 by aspirin inhibits the PGE2 release, inhibits EMT and also abrogates metastasis to the lung. We now provide evidence that adherence of platelets to lung adenocarcinoma cells also leads to PGE2 production, indicating that the phenomenon they describe in colon cancer cells is generalizable to other cell types. We further demonstrate in lung adenocarcinoma cells that the release of PGE2 is linked to a platelet-induced increase in cytosolic calcium in the tumor cells. Treatment of platelets with aspirin prevents calcium mobilization in the tumor cells and PGE2 release, but does not alter the expression of COX-2. We propose that interaction of activated platelets with lung adenocarcinoma cells leads to calcium-mediated activation of cPLA2 (52, 53), which yields the COX-2 substrate, arachidonic acid, from membrane phospholipids. Inhibition of platelet activation with aspirin reduces calcium mobilization in the tumor cells, thereby inhibiting formation of PGE2. The mechanism of thromboxane-dependent calcium mobilization in A549 remains to be characterized. However, it has been recently demonstrated that A549 cells express the thromboxane receptor (TP)(73). Activation of TP is well known to trigger calcium mobilization via Gq activation, a mechanism that could possibly be involved in the effective PGE2 production observed in our experiments.
In conclusion, low dose aspirin has a significant effect on extra-platelet cyclooxygenases. It inhibits systemic biosynthesis of PGE2 in humans by 45%, while blocking platelet TxB2 production by 96.6 %, and therefore is only relatively selective for the platelet. Aspirin inhibits COX catalytic activity and PGE2 biosynthesis in lung and colon adenocarcinoma cells with a potency as great or greater than that for COX-1 in platelets.
These findings provide a basis for considering that even partial inhibition of tumor COX activity by low dose aspirin could reduce the biosynthesis of PGE2 by these cells. In the context of the convincing body of data that PGE2 facilitates metastasis, it can be hypothesized that a reduction in tumor cell PGE2 could be additive with its anti-platelet effect, and thereby contribute to the reduction of mortality and metastasis from adenocarcinomas. Low dose aspirin is likely to produce a high degree of inhibition of COX in tumor cells arising from colon cancer that are sequestered in the portal circulation where they are exposed to the same high concentration of aspirin as the platelet. This forms the basis for a hypothesis that the remarkable prevention of metastasis from colon cancer by low dose aspirin results from its well recognized effect on platelet COX-1 combined with strong inhibition of PGE2 biosynthesis in potentially metastatic tumor cells sequestered in the liver. A conclusion that PGE2 contributes to colon cancer metastasis also implies that the metastatic potential of cancer cells that are not sequestered in the portal circulation could be further inhibited by combining aspirin with an inhibitor of PGE2 production or action.
Supplementary Material
Acknowledgments
Financial Support
P.P. Massion was supported by grants from the National Cancer Institute CA 090949, CA 152662, CA 102353, and by the Department of Defense W81XWH-11-2-0161. J.A. Oates was supported by grants from the National Cancer Institute, CA 89450; from the National Heart, Lung and Blood Institute P50 HL 081009, and by the Thomas F. Frist, Sr. Chair in Medicine. I.R. Sosa has been supported by grants from the National Institute for General Medical Sciences T32 GM007569; and from the American Heart Association National Fellow to Faculty Award 13FTF17400011. H.S. Hwang has been supported by a Scientist Development Grant from the American Heart Association (12SDG12050597). B. C. Knollmann was supported by grants from the National Heart, Lung and Blood Institute HL 088635. L. J. Marnett was supported by a grant from the National Institute for General Medical Sciences GM15431.
Footnotes
Disclosure of potential conflict of interest
The authors disclose no potential conflicts of interest.
Clinical Trial Registration
This investigation has been registered at www.clinicaltrials.gov (identifier NCT 00761891).
References
- 1.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan AT, Giovannucci EL, Schernhammer ES, Colditz GA, Hunter DJ, Willett WC, et al. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med. 2004;140:157–166. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Burn J, Mathers J, Bishop DT. Lynch syndrome: history, causes, diagnosis, treatment and prevention (CAPP2 trial) Dig Dis. 2012;(30 Suppl 2):39–47. doi: 10.1159/000341892. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 5.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 9.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–269. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong L, Mi HJ, Zhu H, Zhou X, Yang H. P-selectin-mediated platelet activation promotes adhesion of non-small cell lung carcinoma cells on vascular endothelial cells under flow. Mol Med Rep. 2012;5:935–942. doi: 10.3892/mmr.2012.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varki A, Varki NM, Borsig L. Molecular basis of metastasis. N Engl J Med. 2009;360:1678–1679. doi: 10.1056/NEJMc090143. author reply 9–80. [DOI] [PubMed] [Google Scholar]
- 13.Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72:4662–4671. doi: 10.1158/0008-5472.CAN-11-4010. [DOI] [PubMed] [Google Scholar]
- 14.Warren BA, Vales O. The adhesion of thromboplastic tumour emboli to vessel walls in vivo. Br J Exp Pathol. 1972;53:301–313. [PMC free article] [PubMed] [Google Scholar]
- 15.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115:3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016 doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedl K, Krysan K, Pold M, Dalwadi H, Heuze-Vourc’h N, Dohadwala M, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat. 2004;7:169–184. doi: 10.1016/j.drup.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- 20.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 23.Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 25.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 28.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984;311:1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 30.Majerus PW, Stanford N. Comparative effects of aspirin and diflunisal on prostaglandin synthetase from human platelets and sheep seminal vesicles. Br J Clin Pharmacol. 1977;(4 Suppl 1):15S–18S. doi: 10.1111/j.1365-2125.1977.tb04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bala M, Chin CN, Logan AT, Amin T, Marnett LJ, Boutaud O, et al. Acetylation of prostaglandin H2 synthases by aspirin is inhibited by redox cycling of the peroxidase. Biochem Pharmacol. 2008;75:1472–1481. doi: 10.1016/j.bcp.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury I, Mo Y, Gao L, Kazi A, Fisher AB, Feinstein SI. Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Free Radic Biol Med. 2009;46:146–153. doi: 10.1016/reeradbiomed.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehtonen ST, Svensk AM, Soini Y, Paakko P, Hirvikoski P, Kang SW, et al. Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- 34.Kinnula VL, Lehtonen S, Kaarteenaho-Wiik R, Lakari E, Paakko P, Kang SW, et al. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax. 2002;57:157–164. doi: 10.1136/thorax.57.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soini Y, Kahlos K, Napankangas U, Kaarteenaho-Wiik R, Saily M, Koistinen P, et al. Widespread expression of thioredoxin and thioredoxin reductase in non-small cell lung carcinoma. Clin Cancer Res. 2001;7:1750–1757. [PubMed] [Google Scholar]
- 36.Chu FF, Esworthy RS. The expression of an intestinal form of glutathione peroxidase (GSHPx-GI) in rat intestinal epithelium. Arch Biochem Biophys. 1995;323:288–294. doi: 10.1006/abbi.1995.9962. [DOI] [PubMed] [Google Scholar]
- 37.Rozell B, Hansson HA, Luthman M, Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol. 1985;38:79–86. [PubMed] [Google Scholar]
- 38.Zhang B, Su YP, Ai GP, Liu XH, Wang FC, Cheng TM. Differentially expressed proteins of gamma-ray irradiated mouse intestinal epithelial cells by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. World J Gastroenterol. 2003;9:2726–2731. doi: 10.3748/wjg.v9.i12.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84:25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc Natl Acad Sci U S A. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine KM, Ashbrook PC, Brigden LP, Maldonado JE, Didishelm P. Gel-filtered human platelets. Ultrastructure, function, and role of proteins in inhibition of aggregation by aspirin. Am J Pathol. 1976;84:11–24. [PMC free article] [PubMed] [Google Scholar]
- 42.Boutaud O, Brame CJ, Chaurand P, Li J, Rowlinson SW, Crews BC, et al. Characterization of the lysyl adducts of prostaglandin H-synthases that are derived from oxygenation of arachidonic acid. Biochemistry. 2001;40:6948–6955. doi: 10.1021/bi002629k. [DOI] [PubMed] [Google Scholar]
- 43.Roberts LJ, 2nd, Lewis RA, Oates JA, Austen KF. Prostaglandin thromboxane, and 12-hydroxy-5,8,10,14-eicosatetraenoic acid production by ionophore-stimulated rat serosal mast cells. Biochim Biophys Acta. 1979;575:185–192. doi: 10.1016/0005-2760(79)90020-1. [DOI] [PubMed] [Google Scholar]
- 44.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Daniel VC, Minton TA, Brown NJ, Nadeau JH, Morrow JD. Simplified assay for the quantification of 2,3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass spectrometry. Journal of Chromatography B: Biomedical Applications. 1994;653:117–122. doi: 10.1016/0378-4347(93)e0432-p. [DOI] [PubMed] [Google Scholar]
- 46.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem. 1971;246:6713–6721. [PubMed] [Google Scholar]
- 47.Adler DH, Cogan JD, Phillips JA, 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts LJ, 2nd, Sweetman BJ, Oates JA. Metabolism of thromboxane B2 in man. Identification of twenty urinary metabolites. J Biol Chem. 1981;256:8384–8393. [PubMed] [Google Scholar]
- 49.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, et al. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010;70:3618–3627. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders MA, Belvisi MG, Cirino G, Barnes PJ, Warner TD, Mitchell JA. Mechanisms of prostaglandin E2 release by intact cells expressing cyclooxygenase-2: evidence for a ‘two-component’ model. J Pharmacol Exp Ther. 1999;288:1101–1106. [PubMed] [Google Scholar]
- 51.Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997;272:14501–14504. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 52.Leslie CC. Cytosolic phospholipase A(2): physiological function and role in disease. J Lipid Res. 2015;56:1386–1402. doi: 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 54.Kamei D, Murakami M, Sasaki Y, Nakatani Y, Majima M, Ishikawa Y, et al. Microsomal prostaglandin E synthase-1 in both cancer cells and hosts contributes to tumour growth, invasion and metastasis. Biochem J. 2009;425:361–371. doi: 10.1042/BJ20090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krysan K, Reckamp KL, Sharma S, Dubinett SM. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med Chem. 2006;6:209–220. doi: 10.2174/187152006776930882. [DOI] [PubMed] [Google Scholar]
- 56.Backhus LM, Sievers E, Lin GY, Castanos R, Bart RD, Starnes VA, et al. Perioperative cyclooxygenase 2 inhibition to reduce tumor cell adhesion and metastatic potential of circulating tumor cells in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2006;132:297–303. doi: 10.1016/j.jtcvs.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 57.Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91:894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 58.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adhim Z, Matsuoka T, Bito T, Shigemura K, Lee KM, Kawabata M, et al. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br J Cancer. 2011;105:393–402. doi: 10.1038/bjc.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, et al. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829–841. doi: 10.2353/ajpath.2009.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang SF, Chen MK, Hsieh YS, Chung TT, Hsieh YH, Lin CW, et al. Prostaglandin E2/EP1 signaling pathway enhances intercellular adhesion molecule 1 (ICAM-1) expression and cell motility in oral cancer cells. J Biol Chem. 2010;285:29808–298016. doi: 10.1074/jbc.M110.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 64.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008;66:208–217. doi: 10.1016/j.critrevonc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leadbeater PD, Kirkby NS, Thomas S, Dhanji AR, Tucker AT, Milne GL, et al. Aspirin has little additional anti-platelet effect in healthy volunteers receiving prasugrel. J Thromb Haemost. 2011;9:2050–2056. doi: 10.1111/j.1538-7836.2011.04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA, et al. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol. 2011;31:435–442. doi: 10.1161/ATVBAHA.110.219527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rioux N, Castonguay A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 71.Gong L, Cai Y, Zhou X, Yang H. Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol Oncol Res. 2012;18:989–996. doi: 10.1007/s12253-012-9531-y. [DOI] [PubMed] [Google Scholar]
- 72.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park MK, Choi JK, Kim HJ, Nakahata N, Lim KM, Kim SY, et al. Novel inhibitory effects of cardamonin on thromboxane A2-induced scratching response: Blocking of Gh/transglutaminase-2 binding to thromboxane A2 receptor. Pharmacol Biochem Behav. 2014;126:131–135. doi: 10.1016/j.pbb.2014.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.