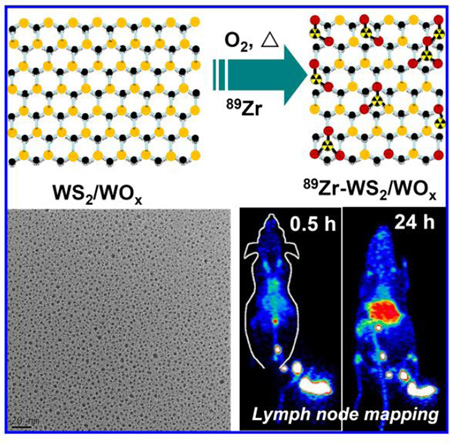
Abstract
While position emission tomography (PET) is an important molecular imaging technique for both preclinical research and clinical disease diagnosis/prognosis, chelator-free radiolabeling has emerged as a promising alternative approach to label biomolecules or nanoprobes in a facile way. Herein, starting from bottom-up synthesized WS2 nanoflakes, we fabricate a unique type of WS2/WOx nanodots, which could function as inherent hard oxygen donor for stable radio-labeling with Zirconium-89 isotope (89Zr). Upon simply mixing, 89Zr can be anchored on the surface of polyethylene glycol (PEG) modified WS2/WOx (WS2/WOx-PEG) nanodots via a chelator-free method with surprisingly high labeling yield and great stability. A higher degree of oxidation in the WS2/WOx-PEG sample (WS2/WOx (0.4)) produces more electron pairs, which would be beneficial for chelator-free labeling of 89Zr with higher yields, suggesting the importance of surface chemistry and particle composition to the efficiency of chelator-free radiolabeling. Such 89Zr-WS2/WOx (0.4)-PEG nanodots are found to be an excellent PET contrast agent for in vivo imaging of tumors upon intravenous administration, or mapping of draining lymph nodes after local injection.
Keywords: WS2/WOx nanodots, 89Zr-Chelator-free, Stability, In vivo PET imaging, Lymph node imaging
Graphical Abstract
Ultra-small WS2/WOx nanodots are fabricated and used as inherent hard oxygen donors for stable radio-labeling with Zirconium-89 (89Zr). Such 89Zr-WS2/WOx-PEG nanodots are found to be an excellent PET contrast agent for in vivo imaging of tumors upon intravenous administration, or mapping of deep-seated draining lymph node network after local injection.
Introduction
Molecular imaging accelerates translational preclinical research to clinical uses by providing physiological, anatomic, and metabolic information through interrogating certain targets.[1] Among various imaging technologies, positron emission tomography (PET) is a noninvasive, highly sensitive imaging modality suitable for whole body imaging.[2] In addition to the extensive applications of PET as a molecular imaging technique for diagnosis and prognosis of diseases such as cancers, radiolabeling of nanoparticles for PET imaging has also been widely explored not only to track the in vivo translocation of nanoparticles, but also to develop unique nanoprobes for imaging of specific targets of interests.[3] To label biomolecules or nanoparticles, chelator molecules such as NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) or DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraaceticacid) are often conjugated to their surface to allow chelation of radioisotope ions such as copper-64 (64Cu2+). Such a strategy has been applied for radioactive labeling of various types of nano-agents such as carbon nanotubes,[4] quantum dots,[5] superparamagnetic iron oxide nanoparticles (SPIONs),[6] and porous silica nanoparticles to enable PET imaging.[7]
However, chelator-based radiolabeling has several well-known limitations.[8] For example, the coordination chemistry of different isotopes varies greatly and there is no molecular chelator that can effectively bind many radioisotopes interchangeably. Secondly, even isotopes are stably chelated during radiolabeling, transchelation with endogenous proteins or detachment of the surface-bound molecular chelators can strip the nanoparticles of their radiolabels, yielding images that do not reflect the real biodistribution information of those nanoparticles or biomolecules. Thirdly, the introduction of new groups of chelators also would affect the surface properties of molecules or nanoparticles. Recently, chelator-free radiolabeling has emerged as a promising alternative approach to label nanoparticles in a facile way.[8b] By using this strategy, the labeled nanoparticles can preserve their surface coating chemistry as well as native pharmacokinetic profiles, and in the meantime leaving their surface functional groups intact for further modification or bioconjugation. While chelator-free radiolabeling of several different types of nanoparticles have been reported in the recent few years,[8a, 9] how to precisely control the surface chemistry/composition of nanoparticles to achieve the optimal radiolabeling has been relatively less explored to our best knowledge.
Recently, transition-metal dichalcogenides (TMDCs) such as MoS2, MoSe2, WS2 and WSe2 with two-dimensional (2D) structures have emerged as a unique type of functional nanomaterials attractive in many different fields including biomedicine.[10] TMDC nanosheets and their doped or composite nanostructures have shown great promises in nanomedicine for potential applications in biological sensing,[11] multimodal imaging,[12] drug delivery systems,[9c] cancer therapy,[10d, 12a] and tissue engineering.[13] It was also found that radioisotope ions such as 64Cu2+ could be firmly adsorbed on the surface of MoS2 nanosheets without the need of chelating molecules, to enable in vivo PET imaging.[14]
Compared to TMDC nanosheets, ultra-small dots of TMDCs have larger specific surface area with more edge atoms, and their photoluminescence properties are also interesting for optical imaging and detection.[15] In a few latest studies, MoS2 and WS2 dots were prepared by high-powered ultrasonication or high temperature hydrothermal processes,[15a, 15b, 16] during which the Mo (W) edge on the surface of the Mo(W)S2 nanosheets were slightly oxidized.[15a, 17] In this work, we for the first time report that the synthesized WS2/WOx nanodots could function as inherent hard oxygen donors for stable radiolabeling of 89Zr, which has a relatively long decay half-life (t1/2 = 78.4 h) and a low positron energy (β+ avg = 395.5 keV) particularly suitable for long-term tracking with PET imaging.[18] Those WS2/WOx nanodots with uniform ultra-small sizes but varied oxidation levels are prepared through solution treatment of WS2 nanoflakes synthesized by a bottom-up method. After functionalization with polyethylene glycol (PEG), the obtained WS2/WOx-PEG nanodots can be labeled with 89Zr via a chelator-free method upon simply mixing, yielding 89Zr-WS2/WOx-PEG with great labeling yield and high serum/ in vivo stability. Importantly, a higher degree of oxidation in the WS2/WOx-PEG sample produces more electron pairs, which would be beneficial for chelator-free labeling of 89Zr with higher yields, suggesting the importance of surface chemistry and particle composition to the efficiency of chelator-free radiolabeling. Interestingly, such 89Zr-WS2/WOx-PEG nanodots appears to be a great PET imaging probe not only for tumor imaging, but also for in vivo mapping of draining lymph nodes, after systemic or local injections, respectively.
Results and discussion
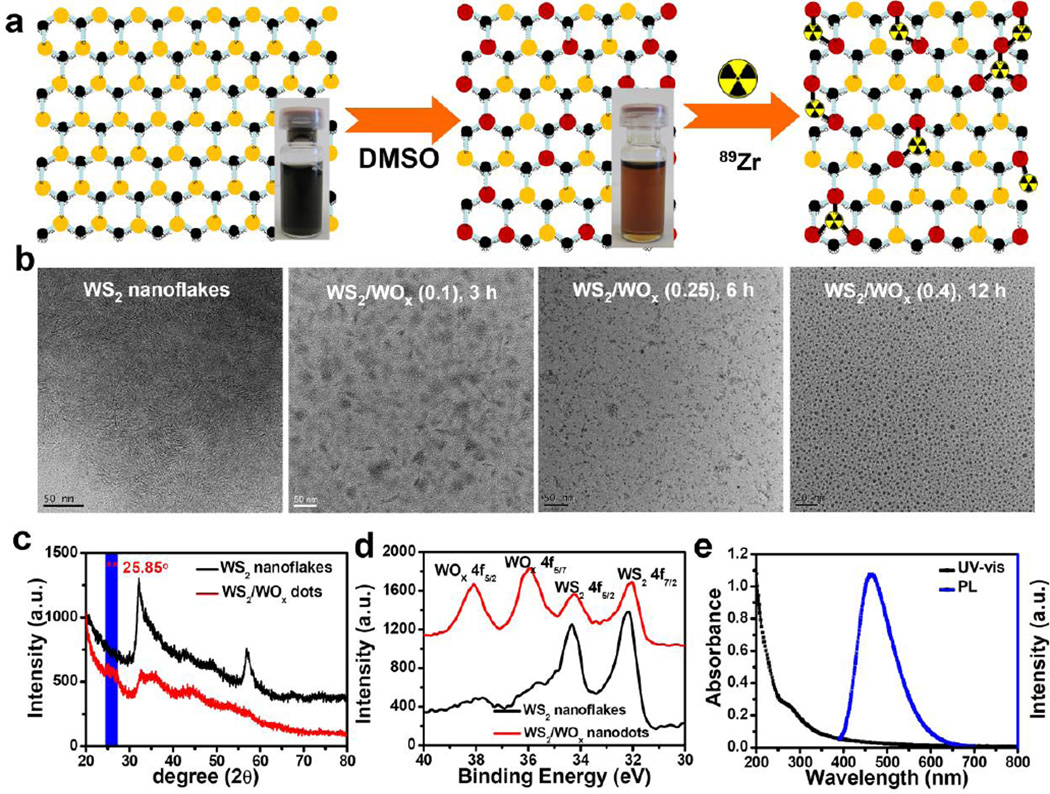
Ultra-small WS2/WOx nanodots were synthesized by a high-temperature solvent-thermal method and then broken in dimethyl sulfoxide (DMSO) under solution treatment to obtain small nanodots (Figure 1a). More detailed synthesis procedure could be found in the Experimental Section. Briefly, WS2 nanoflakes were obtained after adding sulfur/oleyamine (S/OM) into WCl6 in oleylamine (OM) and 1-octadecene (ODE) mixed solvent at 300 °C. As-synthesized WS2 showed flake-like structures with an average layer spacing of ~ 2 nm as observed under transmission electron microscope (TEM) (Figure 1b, left, Supporting Figure S1&S2). Under solvent-thermal treatment in DMSO within the air atmosphere, WS2 nanoflakes were broken into smaller sizes (Figure 1b, right). After treatment for 12 h, small WS2 dots with uniform size at ~5 nm were obtained as revealed by TEM imaging. X-ray diffraction (XRD) was employed to investigate the crystal phase and purity of the as fabricated nanodots (Figure 1c). The characteristics of hexagonal WS2 (JCPDS card no. 08-0237) were still observed, indicating that the crystal structure of WS2 nanodots was preserved during the solution treatment. However, after careful analysis the XRD spectrum of those nanodots, we observed a new peak at 2θ = 25.85°, which was attributed to orthorhombic tungsten oxide monohydrate (WOx·H2O, JCPDs card no. 43-0679).
Figure 1.
WS2/WOx nanodots synthesis and characterization. a) A schematic illustration of WS2/WOx nanodots synthesis and 89Zr chelator-free labeling. Inset: Photos of WS2 nanoflakes after ultrasonication in water (left) and WS2/WOx nanodots after solvent-thermal treatment in water (middle). b) TEM image of WS2 nanoflakes (left) and WS2/WOx nanodots (right) synthesized with different treatment time (3 h, 6 h, and 12 h). c) Powder XRD patterns of WS2 nanoflakes and WS2/WOx nanostructures. d) High-resolution XPS of as-obtained WS2 nanoflakes and WS2/WOx (0.4) nanodots at binding energies corresponding to W4f. e) UV-vis-NIR absorbance and photoluminescence emission spectra of WS2/WOx (0.4)-PEG nanodots in water.
To further investigate the chemical composition of the products obtained after solvent-thermal treatment, X-ray photoelectron spectroscopy (XPS) analysis of as-made WS2 nanoflakes and nanodots was performed. As shown in Figure 1d, the peaks located at 33.10 and 35.23 eV could be ascribed to W4f7/2 and W4f5/2 lines of W(IV), while those at 36.07 and 38.30 eV corresponded to W4f5/2 and W4f7/2 peaks of W(VI), the latter of which agreed well with that reported for WOx.[19] Moreover, we also found S and O signals in the spectra of WS2 nanodots (Supporting Figure S3). These spectroscopy characterizations unambiguously indicated that the obtained nanostructures should contain the complex of WS2 and WOx. Furthermore, with extended reaction time, the black color of the sample gradually changed to brown, indicating the further increase of oxidation degree (Figure 1a, Supporting Figure S4a). The approximate WOx: WS2 ratios in the WS2/WOx composite-nanostructures were measured to be 0.1 : 1, 0.25 : 1, and 0.4 : 1 prepared with the reaction time of 3 h, 6 h, and 12 h, respectively, as determined by XPS spectra of W4f. We thus named the synthesized samples to be WS2/WOx (0.1), WS2/WOx (0.25), and WS2/WOx (0.4) nanodots.
To understand the mechanism in the transformation of WS2 nanoflakes into WS2/WOx nanodots, we carefully studied several synthesis parameters affecting the formation of nanodots. Interestingly, we found that those WS2 nanoflakes showed little change under argon protection with the same reaction condition in DMSO, suggesting that the reaction atmosphere played an important role in the formation of WS2/WOx nanodots. Only under the air atmosphere condition, WS2 nanoflakes could be transformed into WS2/WOx composite nanodots, suggesting the role of oxygen in this reaction process (Equation 1). In addition, enhancing reaction temperature or increasing reaction time would be beneficial for higher degrees of oxidation.
| (1) |
In order to enhance the solubility of those WS2/WOx nanodots in physiological solutions, a PEG-grafted amphiphilic polymer (poly(maleic anhydride-alt-1-octadecen, C18PMH-PEG) was coated on the surface of as-prepared WS2/WOx nanodots.[20] After PEGylation, the obtained WS2/WOx (0.4)-PEG with an average hydrodynamic size at ~15 nm exhibited great stability in various physiological solutions including saline, cell culture medium, and fetal bovine serum (Supporting Figure S5). Moreover, with such biocompatible PEG coating, our WS2/WOx (0.4)-PEG showed no significant in vitro cytotoxicity to cells even at high concentrations (0.2 mg/mL, Supporting Figure S6). UV-vis-NIR spectra of PEGylated WS2/WOx (0.4) nanodots revealed a new peak (~277 nm) in the UV region, while its NIR absorbance was dramatically weakened compared with WS2 nanoflakes (Figure 1e, Supporting Figure S4b). The photoluminescence (PL) spectra of WS2/WOx (0.4) nanodots showed strong emission peaked at 470 nm under the excitation wavelength at 370 nm (Figure 1e, Supporting Figure S7). After 2 h of UV irradiation, WS2/WOx (0.4)-PEG nanodots showed excellent photostability (Supporting Figure S8).
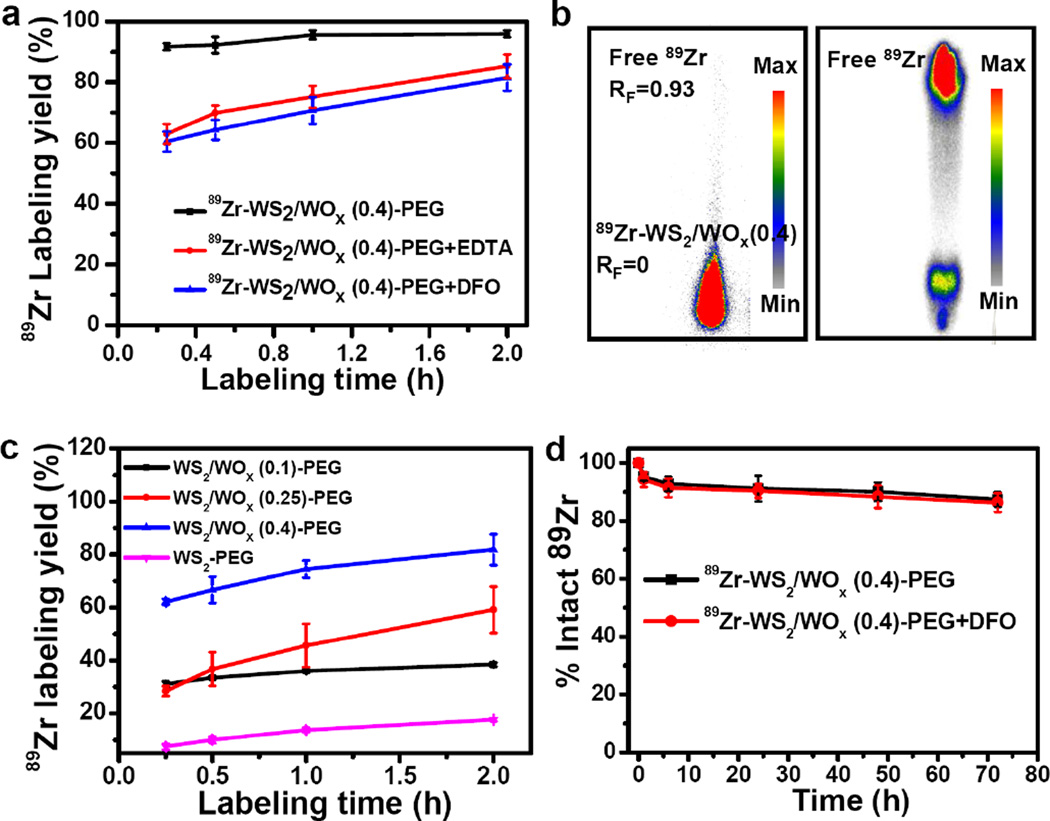
According to the special structure of the synthesized WS2/WOx nanodots, we hypothesized that abundant WOx groups (W-O-) on the surface of WS2/WOx nanodots may function as inherent hard oxygen donors for stable radio-labeling of 89Zr. 89Zr-oxalate was produced according to our previous procedures.[9d] After mixing 89Zr with water-soluble WS2/WOx -PEG nanodots in HEPES buffer (0.5 mM) at pH ~7 under 75 °C, which appeared to be the optimal condition for 89Zr-labeling in this system (Supporting Figure S9), 90% of 89Zr was strongly adsorbed on the WS2/WOx (0.4)-PEG nanodots surface after only 15 mins (Figure 2a&b), and the 89Zr labeling yield reached to as high as 95% after 2 h of incubation. In order to obtain accurate labeling yield, ethylenediaminetetraacetic acid (EDTA) and pisothiocyanatobenzyl desferrioxamine B (DFO) were used as simpler and stronger competitive chelation agents for the thin layer chromatography (TLC) analysis to remove any unstable 89Zr loosely associated with those nanodots. Though some weakly bound 89Zr was eliminated from the system, a high labeling yield > 80% was still obtained under the challenge condition (Figure 2a). Furthermore, some negative control experiments with the mixture of free 89Zr and polymer (C18PMH-PEG) showed nearly zero labeling yields, confirming the successful labeling of 89Zr on WS2/WOx (0.4)-PEG nanodots.
Figure 2.
89Zr chelator-free labeling. a) Time-dependent 89Zr labeling yields of WS2/WOx (0.4)-PEG nanodots without / with EDTA or DFO competitive reaction. b) Autoradiograph of TLC plates of 89Zr-WS2/WOx (0.4)-PEG nanodots (left) and free 89Zr (right). c) Time-dependent 89Zr labeling yields of WS2-PEG and different WS2/WOx-PEG nanodots (WS2/WOx (0.1), WS2/WOx (0.25), and WS2/WOx (0.4)) with EDTA competitive reaction. d) Serum stability study of 89Zr-WS2/WOx (0.4)-PEG nanodots incubated in whole mouse serum without or with DFO competitive reaction at 37 °C.
To understand how the composition of nanoparticles would affect their radiolabeling, WS2 and WS2/WOx nanostructures with different oxidation degrees were labeled with 89Zr using this chelator-free method (Figure 2c). With the oxidation degree enhanced, the labeling yield increased significantly: 81.8 % of WS2/WOx (0.4) nanodots > 59.1 % of WS2/WOx (0.25) nanodots > 38.5 % of WS2/WOx (0.1) nanodots >17.6 % of WS2 nanoflakes. This phenomenon is likely due to more electron pairs on the surface of WS2/WOx (0.4) nanodots than that for other samples. 89Zr4+ is a hard Lewis acid and thus prefers to bind with hard Lewis bases such as oxygen on WS2/WOx (0.4) nanodots that act as electron donors. Notably, we also synthesized WO3 nanorods following the previous method.[21] However, those WO3 nanostructures appeared to be rather unstable in aqueous buffers and would be quickly degraded, thus not suitable for radiolabeling and further imaging applications (Supporting Figure S10). 89Zr labeling on 89Zr-WS2/WOx (0.4)-PEG nanodots was also found to be highly stable in mouse serum for up to 72 h even in a DFO competitive situation (Figure 2d, Supporting Figure S11). Therefore, WS2/WOx (0.4)-PEG nanodots would be an ideal platform for chelator-free labeling with 89Zr with high labeling yield and excellent stability, promising for in vivo PET imaging.
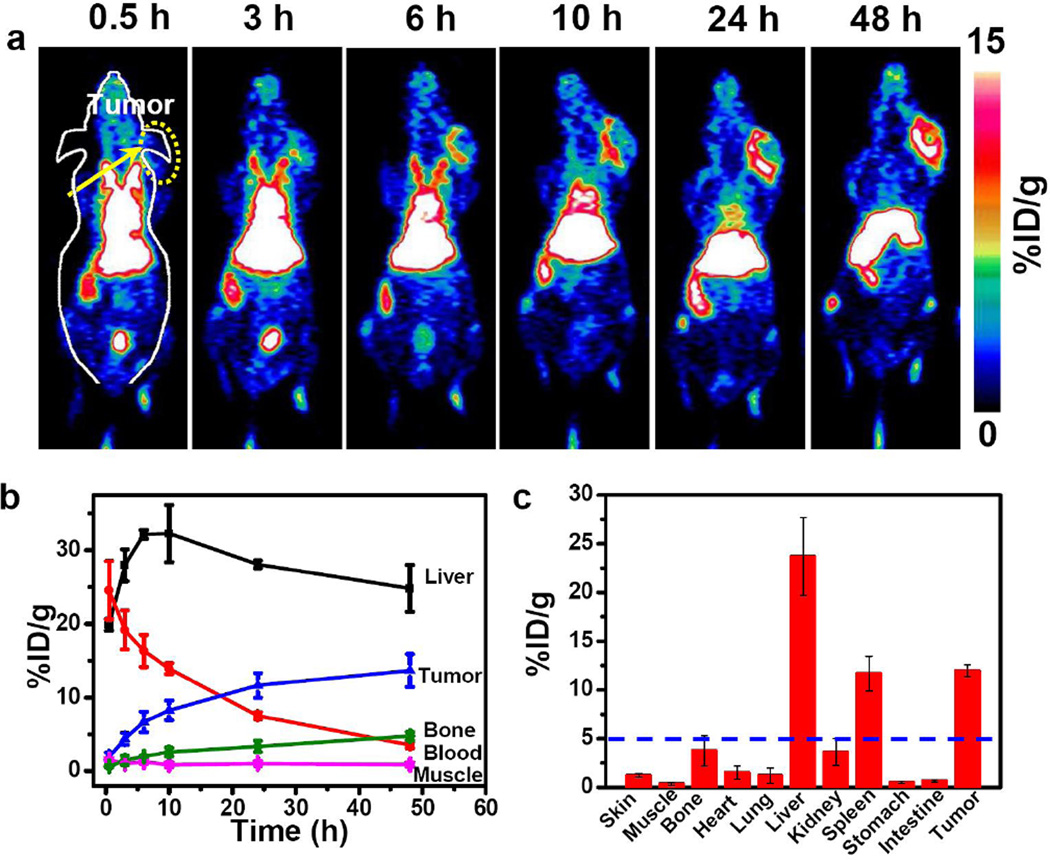
To demonstrate the feasibility of 89Zr-WS2/WOx-PEG nanodots for PET imaging and investigate their in vivo biodistribution, 89Zr-WS2/WOx (0.4)-PEG nanodots solution (200 µL, ~6.5 MBq, dose = 1 mg/kg) was intravenously (i. v.) injected into Balb/c mice bearing 4T1 tumors (Figure 3a). Interestingly, 89Zr-WS2/WOx (0.4)-PEG nanodots showed efficient tumor accumulation, which showed a time-dependent increase after injection. Quantitative data obtained from region-of-interest (ROI) analysis of these PET images revealed that the tumor uptake of 89Zr-WS2/WOx(0.4)-PEG nanodots were determined to be 4.4 ± 0.8, 6.7 ± 1.4, 8.3 ± 1.3, 11.6 ± 1.7, and 13.7 ± 2.2 %ID/g at 3 h, 6 h, 10 h, 24 h, and 48 h post-injection (p. i.), respectively (Figure 3b). Such an efficient passive tumor homing of nanodots could be owing to the enhanced permeability and retention (EPR) effect of 4T1 tumors. High PET signals in the heart even at later time points suggested long blood circulation time of those nanodots, which should be favorable for the EPR-derived tumor uptake of 89Zr-WS2/WOx (0.4)-PEG. Those results demonstrated the possibility of using those nanodots as a PET imaging probe for in vivo tumor imaging upon systemic administration.
Figure 3.
In vivo tumor imaging after systemic injection. a) In vivo PET images of 4T1 tumor-bearing mice taken at various time points (0.5, 3, 6, 10, 24, and, 48 h) post intravenous injection of 89Zr-WS2/WOx (0.4)-PEG nanodots. (b) Quantification of 89Zr-WS2/WOx (0.4)-PEG uptake in the liver, tumor, blood, bone, and muscle at various time points p. i. The unit is the percentage of injected dose per gram of tissue (%ID/g). c) Biodistribution of 89Zr-WS2/WOx (0.4)-PEG nanodots 48 h after intravenous injection into 4T1 tumor-bearing mice as determined by 89Zr radioactivity measurement in various organs (Bl: blood; Sk: Skin; Mu: Muscle; B: Bone; H: Heart;, Lu: Lung; L: Liver; K: Kidney; Sp: Spleen; Pa: Pancreas; St: Stomach; In: Intestine; T: Tumor; Ta: Tail; and Br: Brain). Error bars were based on the standard error of the mean (SEM) of quadruplicate samples.
Beyond high tumor uptake, significant accumulation of 89Zr-WS2/WOx (0.4)-PEG nanodots was also observed in the liver, in which the radioactive signals peaked at 12 h p. i. (32 ± 3.8 %ID/g), and slightly decreased to 23.8 ± 3.2 % ID/g at 48 h p. i. Radioactivity signals in the bladder at the first time point could likely be due to the small amount of free 89Zr that was not completely removed prior to injection and got concentrated in the bladder after intravenous injection. Biodistribution of WS2/WOx (0.4)-PEG nanodots 48 h after injection also showed efficient tumor uptake, together with its retention in reticuloendothelial systems (RES) including liver and spleen (Figure 3c). In marked contrast, free 89Zr exhibited rapid renal clearance and nearly no uptake in the tumor and liver, instead significant bone uptake was observed (Supporting Figure S12&S13). Surprisingly, mice injected with 89Zr-WS2/WOx (0.4)-PEG nanodots showed superb in vivo stability throughout 2 weeks, with less than 3 %ID/g bone uptake at day 14 p. i. (Supporting Figure S14). Such a difference in biodistribution patterns evidenced that high in vivo labeling stability of 89Zr-WS2/WOx (0.4)-PEG nanodots. Notably, after intravenous injection with a high dose of cold WS2/WOx (0.4)-PEG (40 mg/Kg), which was 40 times of the imaging dose, we did not find any significant toxicity for the treated mice based on H&E staining and standard biochemistry examination (Supporting Figure S15&16).
The metastatic spread of tumor cells is directly or indirectly responsible for more than 90% of cancer deaths[22]. During cancer lymphatic metastasis, sentinel draining lymph nodes connecting with the primary solid tumor are usually the primary target organs of metastasizing cancer cells[23]. Therefore, it is of great importance to identify the exact locations of draining lymph nodes so as to remove those nodes together with the primary solid tumor during surgery. To realize lymph node mapping in the clinic, colored dyes or carbon black are injected into the solid tumor, whose draining lymph nodes are then labeled with color to facilitate surgical resection. However, the sensitivity of this method is quite limited. Previous studies have shown that nanoparticles ≥ 4 nm tend to rapidly enter the lymphatic capillaries and may be retained within the lymphatic system, resulting in efficient labeling of the vessels and the sentinel lymph nodes[24]. Lastly, several studies have shown that effective lymph node accumulation of nanoparticles does not require the addition of active targeting agents[23, 25]. As a proof-of-concept, we herein demonstrated the possibility of using noninvasive PET imaging with 89Zr-WS2/WOx-PEG probe for highly-sensitive in vivo lymph node mapping.
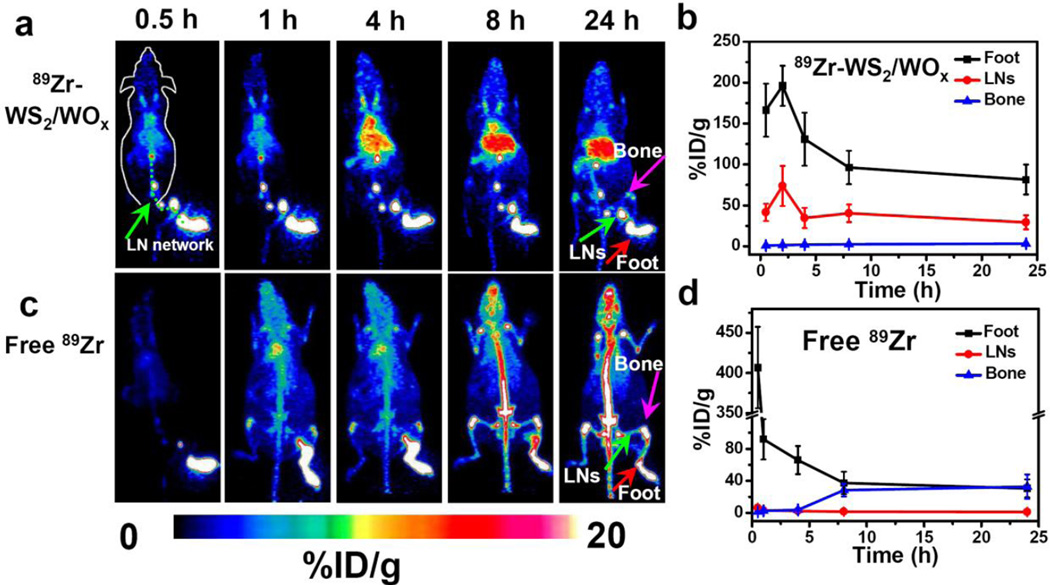
Upon local injection with 89Zr-WS2/WOx (0.4)-PEG nanodots (~30 µL, 50 µCi) into the right rear footpad of each mouse, serial PET scans were performed for the treated mice (Figure 4a&4b). An extensive draining lymphatic network containing multiple deep-seated lymph nodes was lightened up under PET imaging as early as 0.5 h post injection of 89Zr-WS2/WOx (0.4)-PEG. The signals in those lymph nodes remained to be rather strong 24 h after local injection of 89Zr-WS2/WOx(0.4)-PEG (green line in the Figure 4a). In marked contrast, after local injection with free 89Zr (~50 µCi in 30 µL PBS), we could only see PET signals in the first draining lymph node at the first time point (0.5 h p.i.). Later on, free 89Zr would gradually diffuse within the mouse body and showed domination uptake by the bone (Figure 4c&4d), similar to the in vivo distribution profile of free 89Zr after intravenous injection (Supporting Figure S12). Therefore, free 89Zr is not suitable for lymph node tracking. It is known that while small molecules could diffuse out of lymphatic vessels, nanoparticles with larger sizes may show limited transportation by lymphatic circulation and thus lower lymph node targeting ability.[26] Although various nanoparticles have been investigated for lymph node imaging, many of them are only capable of imaging a few draining lymph nodes nearby the injection sites.[23, 27] Our 89Zr-WS2/WOx (0.4)-PEG nanodots with ultra-small sizes thus appeared to be an ideal PET agent for mapping of the draining lymphatic network with deep-seated lymph nodes.
Figure 4.
In vivo lymph node mapping after local injection. a&c) In vivo lymph node imaging with PET after local injection of 89Zr-WS2/WOx (0.4)-PEG nanodots (a) and free 89Zr (c) into the right footpad of the mouse at different time points. b&d) Quantification of 89Zr-WS2/WOx (0.4)-PEG nanodots (b) and free 89Zr (d) uptake by the mouse foot palm, lymph nodes (LNs), and bone.
In addition to acting as an effective nano-probe for chelator-free 89Zr-labeling and PET imaging of tumors and lymphatic networks, such WS2/WOx nanodots are featured with several other interesting properties promising in nanomedicine: (i) This chelator-free radiolabeling strategy with WS2/WOx-PEG nanodots can be effectively utilized for other different kinds of isotopes (e.g. 64Cu2+) with high labeling yield and stability (Supporting Figure S17); (ii) As tungsten (W) processes large X-ray absorption coefficient, WS2/WOx-PEG nanodots could offer strong contrast under CT imaging (Supporting Figure S18), and may in the meanwhile be used as a radiosensitizer to enhance radiotherapy of cancer;[12b, 16] (iii) The functionalities of WS2/WOx nanodots could be further enriched by doping with different types of metal ions (e.g. Mn2+, Gd3+) to enable other modalities of imaging such as magnetic resonance imaging (MRI);[12b] (iv) Further optimizing the surface modification of WS2/WOx nanodots may be helpful not only to allow active targeting of specific biomolecules or lesions of interest, but also to realize renal clearance of those ultra-small nanodots.
Conclusion
In summary, we for the first time reported ultra-small WS2/WOx nanodots as inherent hard oxygen donors to label radioisotope 89Zr via a chelator-free manner for in vivo PET imaging. By a simple solvent-thermal treatment of WS2 nanoflakes, WS2/WOx nanodots with uniform ultra-small sizes were synthesized. After surface PEGylation, the obtained WS2/WOx-PEG nanodots could be labeled with 89Zr upon simple mixing with rather high labeling yield and stability. It was further found that a higher degree of oxidation would be beneficial for chelator-free labeling of 89Zr on WS2/WOx nanodots. Utilizing 89Zr-WS2/WOX-PEG as the nano-probe, in vivo PET imaging of tumors was demonstrated upon systemic administration, and in vivo mapping of deep-seated lymph nodes network was further realized with local injection of 89Zr-WS2/WOX-PEG. Our work here thus presents an interesting type of nano-probe that can be labeled with a number of different radioisotope ions via the chelator-free manner with high efficiency for PET imaging. The performance of this WS2/WOx-PEG nano-probe, while has already shown promises for passive targeting / imaging of tumors, as well as mapping of draining lymphatic network, may be further improved by optimization of its surface modification, or tuning of its elementary composition.
Experimental section
Synthesis of WS2 nanoflakes
A typical synthesis procedure is described as follows[12b, 28]: 1 mmol WCl6 was added into a mixed solvent of 20 ml of oleylamine (OM) and 10 ml of 1-octadecene (ODE) in a three-necked flask (50 ml) at room temperature. The solution was heated to 150 °C to remove water and oxygen under vigorous magnetic stirring in the presence of nitrogen for protection for ~30 min. Afterwards, the temperature of the solution was rapidly raised to 300 °C and kept there for another 30 min in the nitrogen atmosphere. A sulfur solution prepared by dissolving 2 mmol S powder in 5 ml OM was then injected into the flask at 300 °C within 10 min. The reaction was kept at 300 °C for 30 min. After the solution was cooled down to room temperature, WS2 nanoflakes were precipitated by adding anhydrous ethanol (~30 mL), collected by centrifugation, and washed repetitively with ethanol.
Synthesis of WO3 nanorods
To synthesize WO3 nanorods[21], tungsten ethoxide was decomposed in the presence of a mixture of trioctyl amine and oleic acid at a 1:1 ratio. The mixture was heated to 300 °C under inert atmosphere. The tungsten ethoxide solution was injected quickly into the flask at 300 °C, and kept at this temperature for another 5 min before removing the heating mantle. Immediately after injection, the color of the solution changed to dark blue. WO3 nanorods were precipitated by adding 15 mL of ethanol. The product was collected by centrifugation, and thoroughly washed with ethanol.
Synthesis of WS2/WOX nanodots
WS2 nanoflakes were first exfoliated to nanosheets by a solvent-thermal method. Typically, 500 mg of WS2 nanoflakes and 50 mL of DMSO were added in 100 ml serum bottle and kept under ultrasonication for 1 h to exfoliate WS2 nanoflakes by sonicator (KQ5200DB) with an output power at 400 W. Then the dispersion was decanted into flask and heated at 140 °C under vigorous stirring for different periods of time. Afterwards, the resulting suspensions were settled for several hours and centrifuged for 5 min at 14800 rpm to remove large aggregates. The light yellow supernatant was WS2/WOx nanodots.
Surface modification with C18PMH-PEG
C18PMH-PEG was synthesized following a literature procedure[20]. For the PEGylation of nanodots, a solution of 50 mg C18PMH-PEG polymer in 1 ml water was added into 5 ml stock solution of WS2/WOx (0.4) nanodots (5 mg/mL) in DMSO, and the mixture was stirred overnight before it was transferred into 5 ml water. WS2/WOx (0.4)-PEG nanodots were obtained after dialyzing the solution for 1 day in a dialysis bag (MWCO 14 kDa) to remove DMSO and other reagents. The resultant solution was centrifuged at speed of 5000 rpm to remove large aggregates (with supernatant collected). Excess PEG molecules were then removed by centrifugation at 14800 rpm (10 min) to collect the precipitate, which was then re-suspended in water for future use.
Characterization
The phase and crystallography of the WS2 nanoflakes and WS2/WOX nanodots were characterized by using a PANalytical X-ray diffractometer equipped with Cuka radiation (λ=0.15406 nm). A scanning rate at 0.05 °s−1 was applied to record the pattern in the 2θ range of 10–80°. Transmission electron microscopy (TEM) images of the nanoflakes were obtained using a FEI Tecnai F20 transmission electron microscope equipped with an energy dispersive spectroscope (EDX) at an acceleration voltage of 200 kV. X-ray photoelectron spectra (XPS) was performed on an SSI S-Probe XPS Spectrometer. UV-vis-NIR spectra were obtained with PerkinElmer Lambda 750 UV-vis-NIR spectrophotometer. Fluorescence spectra of different samples were obtained on a FluoroMax 4 luminescence spectrometer (HORIBA Jobin Yvon). The hydrodynamic diameters of WS2/WOX-PEG nanodots were determined by a Zetasizer Nano-ZS (Malvern Instruments, UK).
89Zr labeling
89Zr was produced with an onsite cyclotron (GE PETrace) in University of Wisconsin-Madison. 89ZrCl2 (150 MBq) was diluted in 300 µL of 1 mM HEPES solution (pH ~ 7) and mixed with 100 µL of WS2/WOx (0.4)-PEG nanodots (1 mg/mL). The reaction was conducted at 75 °C for 120 min with constant shaking. The labeling yield was determined by thin-layer chromatography (TLC) at different time points. In order to obtain accurate labeling yield, ethylenediaminetetraacetic acid (EDTA) and p-isothiocyanatobenzyl desferrioxamine B (DFO) were used as simpler and stronger competitive chelation agents in the TLC analysis to remove any unstable 89Zr loosely associated with those nanodots. Before TLC analysis, we added EDTA (0.5 mM, 25 µL) or DFO dissolved in DMSO solution (1 mM, 25 µL) was added into 25 µL 89Zr-WS2/WOx (0.4) solution and was shaken for 5 min at each time point. We also investigated the labeling yields under different pH values, temperatures, and different oxygen degrees of WS2/WOx nanocomposites. The resulting 89Zr-WS2/WOX-PEG was purified by a PD-10 column using PBS as the mobile phase.
Serum stability studies
For serum stability study, 89Zr-WS2/WOx (0.4)-PEG nanodots were incubated in complete mouse serum at 37 °C for up to 72 h. Portions of the mixture were sampled at different time points and filtered through 100 kDa cut-off filters. The filtrates were collected and their radioactivities were measured. The percentages of retained (i.e., intact) 89Zr on the WS2/WOx (0.4)-PEG nanodots were calculated using the following equation: (total radioactivity - radioactivity in filtrate)/total radioactivity.
DFO Challenge Study
To demonstrate the stability of 89Zr-WS2/WOx (0.4) nanodots, DFO with the concentration of 1 mM was added into 250 µL of 89Zr-WS2/WOx (0.4)-PEG (~300 µCi) completely mouse serum solution (pH=7) at 37 °C under constant shaking (550 rpm) for 72 h. At each time point, 25 µL of mixture was taken out and resuspended in 100 µL of HEPES. A 300 kDa filter was used to separate potential 89Zr-DFO from 89Zr-labeled nanomaterials. The 89Zr-DFO and 89Zr-WS2/WOx (0.4) nanodots radio-activity was measured by using a gamma counter (PerkinElmer).
In vitro toxicity of WS2/WOx-PEG nanodots
4T1 murine breast cancer cells were cultured in the standard cell medium recommended by American type culture collection (ATCC) under 37 °C within 5% CO2 atmosphere. Cells seeded into 96-well plates at a density of 1*104 cells per well were incubated with different concentrations of WS2/WOx (0.4)-PEG nanodots for 24 h. Relative cell viabilities were determined by the standard methyl thiazolyl tetrazolium (MTT) assay.
Tumor model
All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. The 4T1 tumors were generated by subcutaneous injection of 1*106 cells in ~30µL serum-free RMPI-1640 medium onto the back of each female Balb/c mice. After the tumor volume grown to ~70 mm3, the mice were chosen for in vivo PET imaging.
In vivo PET imaging and biodistribution studies
For PET imaging, 4T1 tumor-bearing mice (3 mice per group) post i.v. injection of ~6.5 MBq of 89Zr-WS2/WOx (0.4)-PEG (dose = 1 mg/kg) were imaged using a microPET/microCT Inveon rodent model scanner (Siemens Medical Solutions USA, Inc.). Data acquisition, image reconstruction, and ROI analysis of the PET data were performed as described previously[29]. After the last PET scan at 48 h post-injection, biodistribution studies were carried out to confirm that the %ID/g values based on PET imaging would truly represent the radioactivity distribution in mice. For the control experiment, three Balb/C mice were i. v. injected with free 89Zr to investigate the imaging and biodistribution. For the investigation of long-term in vivo labeling stability, three ICR mice i. v. injected with 89Zr-WS2/WOx (0.4)-PEG nanodots were used to study the biodistribution at different time points. For in vivo lymph node mapping with PET, 30 µL of 89Zr-WS2/WOx (0.4)-PEG (~1.85 MBq) or free 89Zr was injected into the right footpad of mouse. The time points at 0.5, 1, 4, 8, and 24 h post injection were chosen for PET scans.
In vivo toxicity of WS2/WOx-PEG nanodots
Healthy Balb/c mice were i. v. injected with WS2/WOx (0.4)-PEG nanodots (200 µL, 40 mg/kg) and sacrificed at various time points (7 days and 30 days p.i., three mice per time point) after injection. For histological examination, major organs from the treated groups and the control group were fixed in 4% formalin and then conducted with paraffin embedded sections for H&E staining. The slices were examined and observed by a digital microscope (Leica QWin).
Blood analysis
Ten healthy Balb/c mice were i. v. injected with WS2/WOx (0.4)-PEG (a dose of 40 mg/kg). Other five mice were used as the un-treated control. Mice were sacrificed to collect the blood (0.8 mL) for blood biochemistry assay and complete blood panel test at 1 day and 14 days post injection of WS2/WOx (0.4)-PEG. The serum chemistry data and complete blood panel were measured in Shanghai Research Center for Biomodel Organism.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51525203, 51302180, 51572180), the National “973” Program of China (2012CB932601), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Post-doctoral science foundation of China (2013M531400, 2014T70542), the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520, 5T32GM08349), the National Science Foundation (DGE-1256259), and the American Cancer Society (125246-RSG-13-099-01-CCE). L. Cheng also acknowledges the Collaborative Innovation Center of Suzhou Nano Science and Technology (Nano-CIC) for the fellowship of “Young Scientists Overseas Exchanges and Cooperation Program”.
Footnotes
Supporting Information
Supporting Information is available online from the Wiley Online Library or from the author.
Contributor Information
Prof. Zhuang Liu, Email: zliu@suda.edu.cn.
Prof. Weibo Cai, Email: wcai@uwhealth.org.
References
- 1.a Weissleder R, Pittet MJ. Nature. 2008;452:580. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gambhir SS. Nat. Rev. Cancer. 2002;2:683. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]; c Weber WA, Grosu AL, Czernin J. Nat. Clin. Prac. Oncol. 2008;5:160. doi: 10.1038/ncponc1041. [DOI] [PubMed] [Google Scholar]; d Sun X, Cai W, Chen X. Acc. Chem. Res. 2015;48:286. doi: 10.1021/ar500362y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 3.a Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Sci. Transl. Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gao J, Gu H, Xu B. Acc. Chem. Res. 2009;42:1097. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]; c Thorek DLJ, Ulmert D, Diop N-FM, Lupu ME, Doran MG, Huang R, Abou DS, Larson SM, Grimm J. Nat. Commun. 2014;5 doi: 10.1038/ncomms4097. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Circulation. 2008;117:379. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Devaraj NK, Keliher EJ, Thurber GM, Nahrendorf M, Weissleder R. Bioconjugate Chem. 2009;20:397. doi: 10.1021/bc8004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. Nat. Nanotechnol. 2007;2:47. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 5.Cai W, Chen K, Li Z-B, Gambhir SS, Chen X. J Nucl. Med. 2007;48:1862. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao Y, Yang Y, Zhang Y, Nickles RJ, Cai W, Steeber DA, Gong S. Biomaterials. 2011;32:4151. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen F, Hong H, Zhang Y, Valdovinos HF, Shi S, Kwon GS, Theuer CP, Barnhart TE, Cai W. ACS Nano. 2013;7:9027. doi: 10.1021/nn403617j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. J Am. Chem. Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shaffer TM, Wall MA, Harmsen S, Longo VA, Drain CM, Kircher MF, Grimm J. Nano Lett. 2015;15:864. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Chen F, Ellison PA, Lewis CM, Hong H, Zhang Y, Shi S, Hernandez R, Meyerand ME, Barnhart TE, Cai W. Angew. Chem. Int. Ed. 2013;52:13319. doi: 10.1002/anie.201306306. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, Meyerand ME, Nickles RJ, Cai W. Adv. Mater. 2014;26:5119. doi: 10.1002/adma.201401372. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu T, Wang C, Gu X, Gong H, Cheng L, Shi X, Feng L, Sun B, Liu Z. Adv. Mater. 2014;26:3433. doi: 10.1002/adma.201305256. [DOI] [PubMed] [Google Scholar]; d Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, Cai W. ACS Nano. 2015;9:7950. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS. Nat. Nanotechnol. 2012;7:699. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]; b Duan X, Wang C, Pan A, Yu R, Duan X. Chem. Soc. Rev. 2015;44:8859. doi: 10.1039/c5cs00507h. [DOI] [PubMed] [Google Scholar]; c Chen Y, Tan C, Zhang H, Wang L. Chem. Soc. Rev. 2015;44:268. doi: 10.1039/c4cs00300d. [DOI] [PubMed] [Google Scholar]; d Yin W, Yan L, Yu J, Tian G, Zhou L, Zheng X, Zhang X, Yong Y, Li J, Gu Z, Zhao Y. ACS Nano. 2014;8:6922. doi: 10.1021/nn501647j. [DOI] [PubMed] [Google Scholar]
- 11.Zhu C, Zeng Z, Li H, Li F, Fan C, Zhang H. J Am. Chem. Soc. 2013;135:5998. doi: 10.1021/ja4019572. [DOI] [PubMed] [Google Scholar]
- 12.a Cheng L, Liu J, Gu X, Gong H, Shi X, Liu T, Wang C, Wang X, Liu G, Xing H, Bu W, Sun B, Liu Z. Adv. Mater. 2014;26:1886. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]; b Cheng L, Yuan C, Shen S, Yi X, Gong H, Yang K, Liu Z. ACS Nano. 2015;9:11090. doi: 10.1021/acsnano.5b04606. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Wang L, Li Y, Zhang F, Lin L, Niu S, Chenet D, Zhang X, Hao Y, Heinz TF, Hone J, Wang ZL. Nature. 2014;514:470. doi: 10.1038/nature13792. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C, Song X, Goel S, Barnhart TE, Cai W, Liu Z. ACS Nano. 2015;9:950. doi: 10.1021/nn506757x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a Xu S, Li D, Wu P. Adv. Funct. Mater. 2015;25:1127. [Google Scholar]; b Lin L, Xu Y, Zhang S, Ross IM, Ong ACM, Allwood DA. ACS Nano. 2013;7:8214. doi: 10.1021/nn403682r. [DOI] [PubMed] [Google Scholar]; c Wang Y, Ni Y. Anal. Chem. 2014;86:7463. doi: 10.1021/ac5012014. [DOI] [PubMed] [Google Scholar]; d Ha HD, Han DJ, Choi JS, Park M, Seo TS. Small. 2014;10:3814. doi: 10.1002/smll.201400988. [DOI] [PubMed] [Google Scholar]
- 16.Yong Y, Cheng X, Bao T, Zu M, Yan L, Yin W, Ge C, Wang D, Gu Z, Zhao Y. ACS Nano. 2015;9:12451. doi: 10.1021/acsnano.5b05825. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Xu Q, Li H, Wang Y, Yan B, Zhou Y, Chen J, Zhang J, Wang K. Angew. Chem. Int. Ed. 2015;54:15226. doi: 10.1002/anie.201508216. [DOI] [PubMed] [Google Scholar]
- 18.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. J Nucl. Med. 2010;51:1293. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epifani M, Comini E, Díaz R, Andreu T, Genç A, Arbiol J, Siciliano P, Faglia G, Morante JR. ACS Applied Mater. Interfaces. 2014;6:16808. doi: 10.1021/am504158r. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, He W, Gong H, Wang C, Chen Q, Cheng Z, Liu Z. Adv. Funct. Mater. 2013;23:5893. [Google Scholar]
- 21.Yella A, Tahir MN, Meuer S, Zentel R, Berger R, Panthöfer M, Tremel W. J Am. Chem. Soc. 2009;131:17566. doi: 10.1021/ja9007479. [DOI] [PubMed] [Google Scholar]
- 22.Mehlen P, Puisieux A. Nat. Rev. Cancer. 2006;6:449. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2004;22:93. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a Kobayashi H, Kawamoto S, Bernardo M, Brechbiel MW, Knopp MV, Choyke PL. J Control. Release. 2006;111:343. doi: 10.1016/j.jconrel.2005.12.019. [DOI] [PubMed] [Google Scholar]; b Kobayashi H, Kawamoto S, Sakai Y, Choyke PL, Star RA, Brechbiel MW, Sato N, Tagaya Y, Morris JC, Waldmann TA. J Natl. Cancer Institute. 2004;96:703. doi: 10.1093/jnci/djh124. [DOI] [PubMed] [Google Scholar]
- 25.a Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. New Eng. J. Med. 2003;348:2491. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]; b Cheng L, Yang K, Li Y, Chen J, Wang C, Shao M, Lee S-T, Liu Z. Angew. Chem. Int. Ed. 2011;50:7385. doi: 10.1002/anie.201101447. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, Yang X, Dobrucki LW, Chaudhury I, Yin Q, Yao C, Lezmi S, Helferich WG, Fan TM, Cheng J. Angew. Chem. Inter. Ed. 2012;51:12721. doi: 10.1002/anie.201205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a Kobayashi H, Hama Y, Koyama Y, Barrett T, Regino CAS, Urano Y, Choyke PL. Nano Lett. 2007;7:1711. doi: 10.1021/nl0707003. [DOI] [PubMed] [Google Scholar]; b Torabi M, Aquino SL, Harisinghani MG. J Nucl. Med. 2004;45:1509. [PubMed] [Google Scholar]
- 28.Cheng L, Huang W, Gong Q, Liu C, Liu Z, Li Y, Dai H. Angew. Chem. Int. Ed. 2014;53:7860. doi: 10.1002/anie.201402315. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Shen S, Shi S, Yi Y, Wang X, Song G, Yang K, Liu G, Barnhart TE, Cai W, Liu Z. Adv. Funct. Mater. 2016;26:2185. doi: 10.1002/adfm.201504810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.