Abstract
OSU-2S is a FTY720 (Fingolimod) derivative that lacks immunosuppressive properties but exhibits strong anti-tumor activity in several hematological and solid tumor models. We have recently shown OSU-2S to mediate potent cytotoxicity in human mantle cell lymphoma cell lines and primary cells. We report here the pre-clinical activity of OSU-2S in spontaneous B-cell lymphoma of dogs which shares many characteristics of human lymphoma. OSU-2S mediated apoptosis in canine B-cell lines and primary B-cell lymphoma cells obtained from spontaneous lymphoma bearing dogs. OSU-2S induced reactive oxygen species (ROS) in canine lymphoma cells and inhibition of ROS partially rescued OSU-2S mediated cell death. These studies provide a rational basis for the use of spontaneous lymphoma in pet dogs as a preclinical large animal model for the development of OSU-2S as small molecule for treating people and dogs with lymphoma.
Keywords: OSU-2S, FTY720, Canine lymphoma, B-cell lymphoma, small molecule
Short communication
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL) of dogs and has a similar incidence rate to that of humans.(1) Moreover, canine NHL imitate as low-high grade human NHL with 70–80 % of them with B-cell phenotype. The natural biology of the disease includes similar cellular signaling components, gene mutations and chemotherapeutic drug sensitivities to that of humans. As such, canine spontaneous lymphoma serves as a good model for the evaluation of anti-cancer compounds that are in clinical development for human malignancies.(2, 3) Unlike laboratory rodent models, the outbred nature of dogs more closely represents the human population genetically, and furthermore, pet dogs share common environmental exposures with people. Finally, with fewer regulatory obstacles, and less cost and time associated with clinical trials in veterinary patients, faster validation of investigational new drugs is possible.(4)
OSU-2S is a non-immunosuppressive FTY720 (Fingolimod) derivative small molecule which has been shown to have potent pre-clinical activity against human malignancies like chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), chronic myeloid leukemia (CML) and hepatocellular carcinoma (HCC).(5–8) Unlike FTY720, OSU-2S does not traffic T-cells in-vivo and has greater cytotoxic activity against various tumors. In hematological malignancies OSU-2S activates tumor suppressor protein phosphatase 2A (PP2A) and modulates a multitude of signaling components resulting in cell death.(6, 7) In HCC, OSU-2S activated the NADPH oxidase system leading to reactive oxygen species (ROS) induction and protein kinase C activation resulting in caspase mediated cell death.(5) In B-cell CLL, OSU-2S activated PP2A, SHP1 phosphorylation and reduction of TCL1 oncogene expression leading to cell death.(7) Further, in MCL OSU-2S increased CD74 expression and induced apoptosis of lymphoma cells as non- targeted naked drug and as tumor antigen ROR1 targeted nanoparticle formulation. In this communication, we report the anti-cancer activity of OSU-2S in B-cell lymphoma of dogs. (8)
Materials and Methods
Spontaneous lymphoma cells
Canine lymphoma samples were obtained from Veterinary Clinical Research Support Shared Resources (VCRSSR) of the Ohio State University Comprehensive Cancer Center (OSU CCC). Samples were obtained with owner signed informed consent under an approved Institutional Animal Care and Use Committee (IACUC) protocol. Fine needle aspirates were obtained from lymph nodes, or extra nodal masses, of dogs previously treated with various standard cytotoxic chemotherapy drugs (n=7) and from treatment naïve dogs (n=2) presenting with spontaneous lymphoma. B-cell lineage was determined using a diagnostic panel of monoclonal antibodies. The predominant cell population in all samples was CD21+ CD3−.
Cell Culture
Only freshly isolated cells were used for the experiment. Cells obtained from fine needle aspiration of lymph nodes or extra nodal masses were washed with sterile PBS, spun and resuspended in sterile PBS in a 50 ml conical tube. Ficoll (Ficoll: PBS 1:3) (Ficoll-Paque Plus; GE Healthcare, Piscataway, NJ) was under-layered in the tube and centrifuged at 1500 RPM for 30 minutes, before isolating mononuclear cells from the interface. The isolated cells were washed with RPMI 1640 media (Gibco, Life Technologies, Grand Island, NY), counted and resuspended at 0.5× 106 cells/ml in complete medium containing 10 % FBS (Sigma, St Louis, MO), 2 mM L-glutamine, penicillin (100 U/mL); streptomycin (100 µg/mL) (Gibco) and grown in multi-well cell culture plates at 37 °C with 5 % CO2 aeration.
Cell Staining
Cell viability was assessed by staining with annexin-V and propidium iodide (BD Bioscience, San Jose, CA, USA) or live dead stain (Invitrogen, Life Technologies) followed by flow cytometric analysis as previously described.(7) ROS positive cells were identified using flow cytometry after staining the cells with 10 µM dihydroethidium (Life Technologies) in the culture medium at room temperature for 30 minutes followed by washing with PBS. Flow cytometric data were analyzed using Kaluza software (Beckman-Coulter).
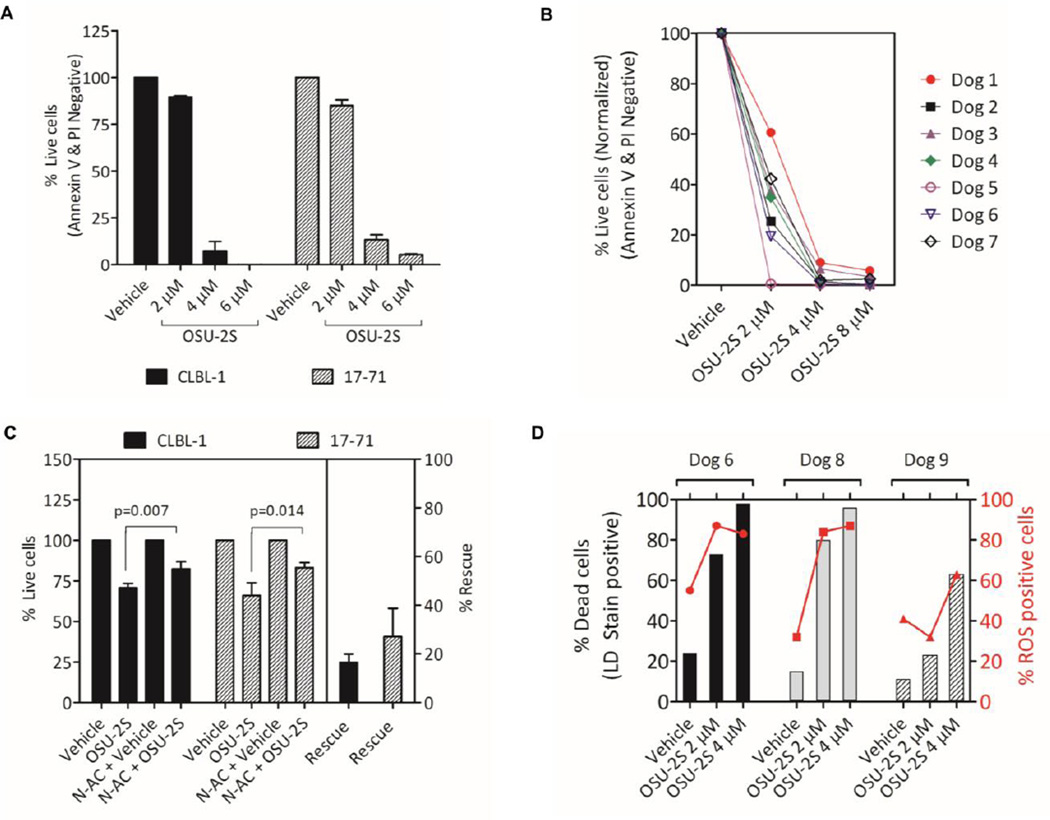
We first evaluated the cytotoxic activity of OSU-2S in canine B-cell lymphoma cell lines CLBL-1(9) and 17–71(10) and in primary canine patient samples. OSU-2S promoted cytotoxicity in these cells at concentration as low as 2 µM after 24 hours in culture (Figure 1A). Next we tested the effect of OSU-2S in canine B-cell lymphoma samples in ex-vivo cultures. OSU-2S induced apoptosis in canine lymphoma (N=7 dogs) as determined by flow cytometry based viability staining (Figure 1B). Since OSU-2S cytotoxicity was associated with ROS production in B-cell CLL, we sought to determine the role of ROS in canine B-cell lymphoma cytotoxicity. Pretreatment with scavenger N-acetyl cysteine (N-AC) partially prevented the OSU-2S cytotoxicity in the cell lines (Figure 1C). We then estimated the percentage of ROS positive cells in patient lymph node samples after ex-vivo OSU-2S treatment by staining cells using dihydroethidium (DHE). Interestingly, OSU-2S treatment resulted in ROS production in B-cell lymphoma samples; however, N-AC failed to reduce ROS or rescue cytotoxicity significantly (Figure 1D and not shown). Further characterization of OSU-2S cytotoxicity, target validation, and clinical activity in pet dogs with spontaneously occurring B-cell lymphoma will form a basis for accelerating the preclinical development of this compound and the initiation of first in human clinical trials.(11) Moreover, as very few small molecules have been developed/approved in veterinary oncology practice,(12) developing OSU-2S as a small molecule for treatment of veterinary patients would likewise be of benefit.
Figure 1. OSU-2S is active against canine B-cell lymphoma.
(A–B) Cytotoxicity of OSU-2S in canine B-cell lymphoma cell lines CLBL-1 and 17–71 and spontaneous canine B-cell lymphoma samples. Cell lines (N=3 independent experiments) (A) or mononuclear cells isolated from lymphoma mass (N=7 dogs) (B) by fine needle aspiration (FNA) and Ficoll centrifugation, were cultured with indicated concentration of OSU-2S and viability was determined after 24 hours by flow cytometer after annexin V and propidium iodide staining. Cytotoxicity of OSU-2S in CLBL-1 p=0.0124 at 2 µM and p<0.0001 at 4 µM; 17–71 p = 0.0015 at 2 µM and p<0.0001 at 4 µM. Bars represent mean + standard deviation. Cytotoxicity in primary lymphoma cells p = 0.0031 at 2 µM OSU-2S. (C) Rescue of cytotoxicity by N-AC in canine B-cell lymphoma cell lines. Cell lines were pre-treated with/without N-AC (10 mM) for 45 minutes before dosing with OSU-2S (3 µM) and viability was determined after 24 hours by flow cytometer by live dead stain. Bars represent mean + standard deviation, (N=3 independent experiments). (D) Cytotoxicity and ROS induction by OSU-2S in spontaneous B-cell lymphoma samples. Mononuclear cells isolated from lymphoma mass by FNA and Ficoll centrifugation were cultured with OSU-2S for 24 hours and ROS productionand viability were determined by flow cytometer after dihydroethidium and live dead staining. N=3 dogs. Cytotoxicity p=0.0012 and ROS production p=0.03 by OSU-2S. Analysis of variance (ANOVA) with repeated measures were used to analyze the data.
Acknowledgments
The authors are thankful to the VCRSSR of OSU CCC, Department of Veterinary Biosciences flow cytometry core for immunophenotyping lymphoma cells and grant support from Leukemia and Lymphoma Society (LLS 7004-11), National Cancer Institute (NIH-R01-CA197844-01) and Robert J Anthony Leukemia Fund.
Footnotes
Author contributions: R.M. designed and performed experiments, analyzed data, prepared the drafts of the paper. R.Y. synthesized OSU-2S. X.M. performed the statistical analysis. C.S.C., M.A.P., R.K. and J.C.B. provided necessary reagents, supported components of the research. W.C.K. and C.A.L. oversaw canine oncology patients, obtained clinical samples, and provided translation insight to the study. N.M. conceived the idea, supervised the study sought funding and approved the final version for submission.
Conflict of interest: A patent has been filed and awarded to OSU for the use of OSU-2S to treat hematologic malignancies. The authors do not have relevant conflict of interest.
References
- 1.Valli VE, Kass PH, San Myint M, Scott F. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Veterinary pathology. 2013;50(5):738–748. doi: 10.1177/0300985813478210. [DOI] [PubMed] [Google Scholar]
- 2.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer investigation. 2000;18(8):781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 3.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends in molecular medicine. 2011;17(7):380–388. doi: 10.1016/j.molmed.2011.02.004. PMCID: 3130881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito D, Frantz AM, Modiano JF. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications. Veterinary immunology and immunopathology. 2014;159(3–4):192–201. doi: 10.1016/j.vetimm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, et al. Antitumor effects of OSU-2S, a nonimmunosuppressive analogue of FTY720, in hepatocellular carcinoma. Hepatology. 2011;53(6):1943–19458. doi: 10.1002/hep.24293. PMCID: 3103615. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Neviani P, Harb JG, Oaks JJ, Santhanam R, Walker CJ, Ellis JJ, et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. The Journal of clinical investigation. 2013;123(10):4144–4157. doi: 10.1172/JCI68951. PMCID: 3784537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mani R, Mao Y, Frissora FW, Chiang CL, Wang J, Zhao Y, et al. Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia. Leukemia. 2015;29(2):346–355. doi: 10.1038/leu.2014.199. PMCID: 4272672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani R, Chiang CL, Frissora FW, Yan R, Mo X, Baskar S, et al. ROR1-targeted delivery of OSU-2S, a nonimmunosuppressive FTY720 derivative, exerts potent cytotoxicity in mantle-cell lymphoma in vitro and in vivo. Exp Hematol. 2015 doi: 10.1016/j.exphem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgen BC, Willenbrock S, Reimann-Berg N, Walter I, Fuchs-Baumgartinger A, Wagner S, et al. Authentication of primordial characteristics of the CLBL-1 cell line prove the integrity of a canine B-cell lymphoma in a murine in vivo model. PLoS One. 2012;7(6):e40078. doi: 10.1371/journal.pone.0040078. PMCID: 3386195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steplewski Z, Jeglum KA, Rosales C, Weintraub N. Canine lymphoma-associated antigens defined by murine monoclonal antibodies. Cancer immunology, immunotherapy : CII. 1987;24(3):197–201. doi: 10.1007/BF00205629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vail DM. Cancer clinical trials: development and implementation. The Veterinary clinics of North America Small animal practice. 2007;37(6):1033–1057. v. doi: 10.1016/j.cvsm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.London CA. Small molecule inhibitors in veterinary oncology practice. The Veterinary clinics of North America Small animal practice. 2014;44(5):893–908. doi: 10.1016/j.cvsm.2014.06.001. [DOI] [PubMed] [Google Scholar]