Abstract
Bcl-3 is an atypical member of the IκB family. It associates with p50/NF-κB1 and p52/NF-κB2 homodimers in nuclei where it modulates transcription in a context-dependent manner. A subset of B cell tumors exhibits recurrent translocations of Bcl-3, resulting in overexpression. Elevated expression without translocations is also observed in various B cell lymphomas and even some solid tumors. Here we investigated the role of Bcl-3 in AOM/DSS-induced colon tumors, a mouse model for colitis-associated colorectal cancers in humans. Contrary to expectations, Bcl-3 suppressed colorectal tumor formation: Bcl-3-deficient mice were relatively protected from DSS-induced epithelial damage and developed more polyps after AOM/DSS treatment, though polyp size was unaffected. DSS-challenged mutant mice exhibited increased recruitment of myeloid-derived suppressor cells (MDSCs), consistent with protection of the epithelium. Loss of Bcl-3 in intestinal epithelial cells was sufficient to increase tumorigenesis. The added tumor burden in mutant mice was dependent on TNFα, a tumorigenic, NF-κB-mediated signaling pathway that was dampened by Bcl-3. These findings reveal a tumor-suppressive role for Bcl-3 in this inflammation-associated cancer model. Bcl-3 thus functions as a tumor promoter or suppressor, depending on the cellular and environmental context.
Keywords: Bcl-3, NF-κB, colorectal cancer
INTRODUCTION
NF-κB transcription factors are homo- or heterodimers composed of members of the Rel-related protein family. These factors are activated by a vast array of stimuli, in particular stress- and pathogen-related signals as well as cytokines, and they regulate expression of numerous genes, most especially those involved in host defense and immune regulation, but also cellular proliferation and survival. The Rel-related family members RelA, RelB and c-Rel contain transcription activation domains, while the NF-κB1/p50 and NF-κB2/p52 family members do not; the latter two proteins can form homodimers that may associate with HDACs and may suppress expression of certain genes via competition with transactivating NF-κB dimers for DNA binding sites. Members of the IκB protein family regulate NF-κB activity. Classic members such as the prototypical IκBα inhibit transactivating NF-κB complexes primarily by retaining them in the cytoplasm, but also by preventing their DNA binding. Activating signals generally lead to proteolyic degradation of classical IκBs, thus liberating transactivating complex to migrate into the nucleus (background information reviewed in1,2). Bcl-3 is a member of the atypical subclass of IκB proteins, which also includes IκBζ and IκBNS. Unlike classic IκB proteins, Bcl-3 readily enters nuclei where it may associate with DNA-bound NF-κB1/p50 or NF-κB2/p52 homodimers to modulate transcription. Bcl-3 contains transactivating domains, and depending on gene and cellular context, Bcl-3 may promote or inhibit target gene expression. Bcl-3’s functions are regulated not only by its expression levels, but also by a multitude of post-translational modifications that may affect nuclear entry, stability and/or transcriptional activity (reviewd in3–5).
Bcl-3 is a bona fide proto-oncogene. Recurrent translocations into the immunoglobulin locus have been found in a number of B-cell chronic lymphocytic leukemias as well as a few lymphomas, resulting in high levels of Bcl-34. Abundant expression of Bcl-3 absent translocations has also been noted in a number of lymphomas, in particular Hodgkin’s lymphomas, and even some T cell lymphomas. Bcl-3 thus appears to contribute to the generation and/or progression of at least some hematologic malignancies. Elevated expression was also noted in some solid tumors, most especially breast cancer, but whether Bcl-3 actually contributed to the development of these cancers remains to be clearly established6. Nevertheless, some studies have implicated Bcl-3 in cellular proliferation and survival in cancer cell lines; Bcl-3 has been suggested to increase expression of cyclin D1 and Bcl-2, destabilize p53, stabilize c-Myc and protect against DNA-damage-induced cell death7–9. On the other hand, Bcl-3 was reported to have no role in either initiation or growth of primary mammary tumors in a mouse model, but instead promoted metastasis of these tumor cells10. Bcl-3 may thus have a distinct tumorigenic potential depending on cell type and contributions to solid tumor development remain to be investigated further.
Here we set out to explore the role of Bcl-3 in AOM/DSS-induced colon tumors, a mouse model of colitis-associated colorectal cancers. Colorectal cancer is one of the most commonly diagnosed cancers in the world, with frequent mutations found in APC, TP53, SMAD4, PIK3CA and KRAS genes. Inflammatory bowel disease (IBD) greatly increases the risk for such cancers11. A single dose of the carcinogen AOM, followed by repeated cycles of colitis-inducing DSS treatments can successfully induce colorectal tumorigenesis in mice within two months11,12. This model recapitulates many aspects of inflammation-associated colorectal carcinogenesis in human patients and thus provides an important tool to study this cancer. AOM/DSS-induced tumors have similar mutations as human colorectal cancer patients, including Tp53, Kras and Apc mutations, but the pathway to carcinogenesis likely differs when driven by inflammation in the colon11.
The largely inflammatory cell-derived cytokine TNFα has been reported to play a critical role in inflammation-associated cancers13,14. TNFα targets not only hematopoietic immune cells, but also intestinal epithelial cells; its actions are mediated at least in part via the IKK/NF-κB signaling pathway, a pathway essential for colitis-associated tumorigenesis, although some individual NF-κB factors may indeed have opposite functions15,16. In addition, NF-κB regulates the expression of the cytokine IL-6, which plays an important role in AOM/DSS-induced tumorigenesis by promoting proliferation and survival of intestinal epithelial cells, primarily via activation of Stat317.
Inflammation-associated tumorgenesis involves the interplay between immune and epithelial/stromal cells. In addition to the potential tumor-promoting functions of Bcl-3 in epithelial cells discussed above, prior work has shown that Bcl-3 can also modulate inflammatory responses via functions in both stromal and immune cells4,18,19. The role of Bcl-3 may therefore be complex and contrary to the notion that Bcl-3 acts as a tumor promoter, we demonstrate here that Bcl-3 instead suppressed colorectal tumor formation. Mice deficient in Bcl-3 were relatively protected from DSS-induced epithelial damage and developed more polyps after AOM/DSS treatment, while tumor growth was not impacted. DSS treatment led to increased numbers of myeloid suppressor cells (MDSCs) in the absence of Bcl-3, which may have afforded protection to the epithelial layer. The increased tumor burden also appeared to be dependent on TNFα signaling, a pathway dampened by Bcl-3. Finally loss of Bcl-3 in epithelial cells was sufficient for epithelial protection and for the increase in tumor polyps.
RESULTS
Bcl-3 suppresses AOM/DSS-induced colorectal tumorigenesis
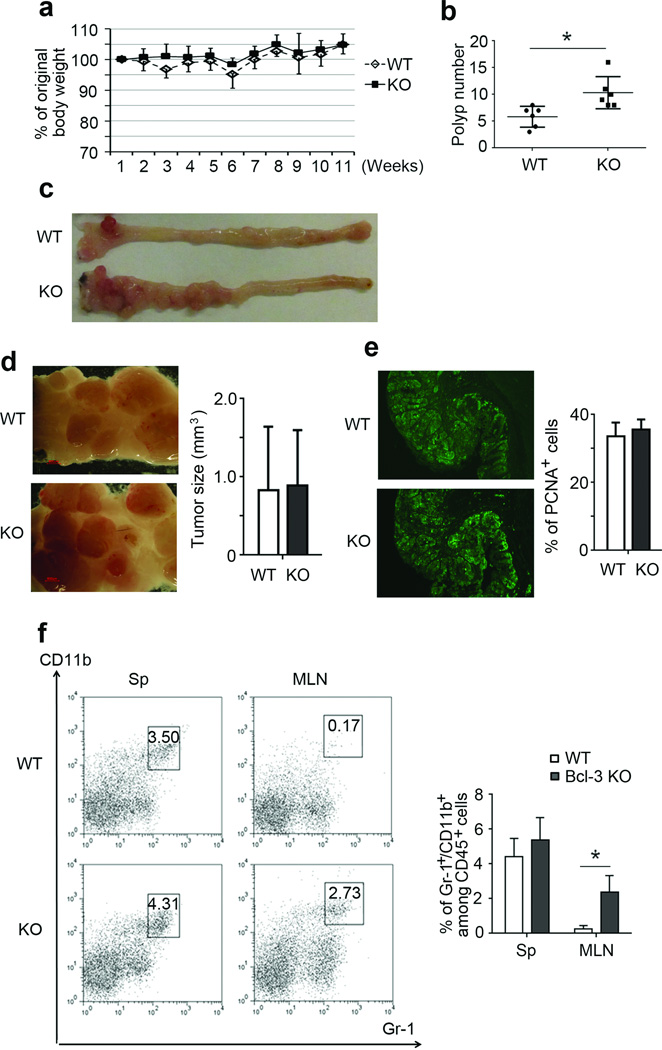
To study the role of Bcl-3 in inflammation-associated tumorigenesis, we utilized the AOM/DSS-induced colorectal mouse tumor model. Both wild type (WT) and Bcl-3 knockout (KO) mice were injected once with AOM and 5 days later subjected to three cycles of 2.5% DSS treatment (5 days with DSS, then 16 days without); mice were sacrificed 10 days after completion of the last cycle. Body weights were monitored weekly, and there was no statistically significant difference in weight loss between WT and KO mice (Figure 1a). AOM/DSS treatment induced tumorigenic polyps primarily in the distal part of the colon, which most closely resembles what is seen in patients with ulcerative colitis11,12. KO mice developed significantly more polyps than WT mice (Figure 1b and c). Polyp sizes were comparable (Figure 1d), suggesting that Bcl-3 primarily influenced tumor initiation rather than tumor growth. This conclusion was supported by histochemical analysis of polyp sections, which revealed a similar percentage of PCNA staining cells in WT and KO mice (Figure 1e).
Figure 1.
Loss of Bcl-3 promotes AOM/DSS-induced colorectal tumorigenesis. Weekly weights (a) and polyp numbers (b) of WT and (Bcl-3) KO mice after AOM/DSS treatment (n=6 mice per group; two additional experiments yielded similar results). (c), Representative examples of colons from WT and KO mice in (a,b) after AOM/DSS treatment. (d) Higher magnification of WT and KO tumors from a representative example as in (c) and average size of the tumors from WT and KO mice in (a,b). (e) PCNA staining of WT and KO tumor sections, representative of sections from n=5 mice per group from two independent experiments as in (a,b). (f) Representative example and summary of flow cytometric analysis of cells for markers as indicated, with cells isolated from spleen and mesenteric lymph node (MLN) from WT and KO mice after AOM/DSS treatment (n=6 mice per group with examples from two independent experiments as in (a,b)). Data represented as means ± SD. * p < 0.05.
Loss of Bcl-3 affected the immune response to AOM/DSS treatment. Spleens of KO mice were more enlarged than those of WT mice and they contained a higher proportion of activated T cells, especially of CD4+ effector/memory T cells (Supplementary Figure S1a and b). We additionally observed a notably higher proportion of myeloid-derived suppressor cells (MDSCs; Gr-1+ CD11b+) in mesenteric lymph nodes of treated KO compared to WT mice (Figure 1f).
Loss of Bcl-3 ameliorates epithelial cell death in DSS-induced colitis
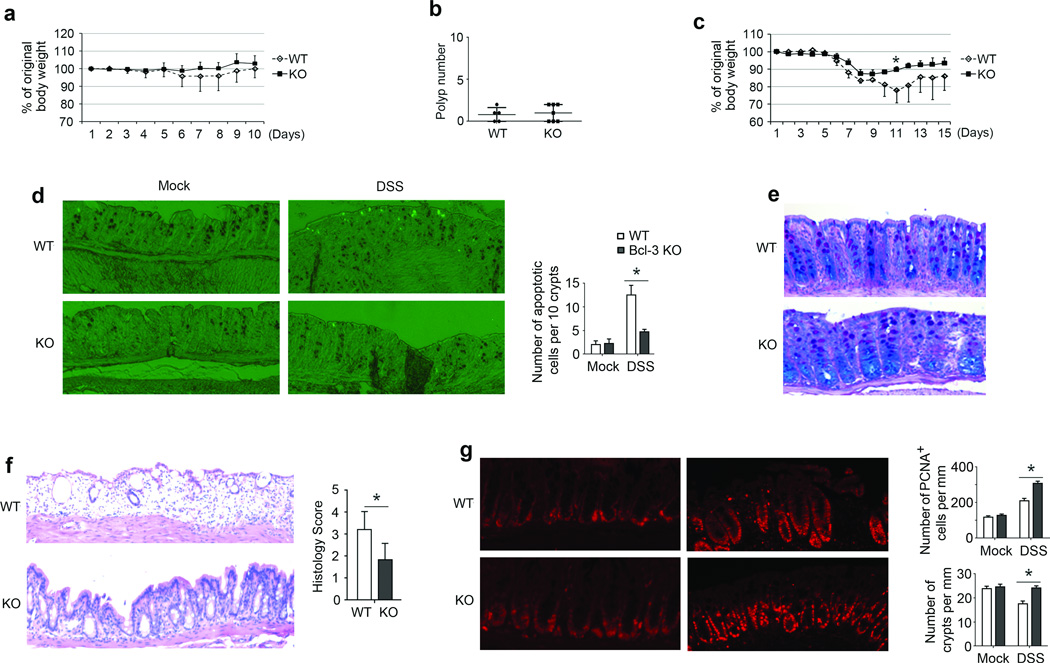
We investigated whether Bcl-3 played a role in responses to either AOM or DSS treatments alone. Injection with AOM alone failed to induce significant weight loss in KO or WT mice (Figure 2a), and also did not alter the appearance of the mucosal epithelium in H&E stained sections (Supplementary Figure S2a). AOM treatment did cause some apoptotic cell death in crypts by 12 h, but there was no significant difference between WT and KO animals (Supplementary Figure S2b). We injected mice with AOM once a week for 6 consecutive weeks. This regimen yielded only few polyps 5 months later, much less than what was observed upon AOM/DSS treatment, with no difference in numbers between KO and WT mice (Figure 2b).
Figure 2.
Loss of Bcl-3 protects against DSS-induced colitis. (a) Daily weights of WT and KO mice after a single AOM injection (n= 5 (WT) and 7 (KO) mice; an additional experiment yielded similar results). (b) Polyp numbers of WT and KO mice five months after six consecutive weekly AOM injections. (c) Daily weights of WT and KO mice treated with 3% DSS for 5 days (n= 5 (WT) and 6 (KO); two additional experiments yielded similar results). (d, e) Representative examples of tissue sections showing apoptotic cell death and summary graph (d) and PAS & Alcian blue staining (e) after 3 days of mock and/or DSS treatment of WT and KO mice. (f, g) H&E staining (with summary of histology scores) on day 6 of DSS treatment (f) and PCNA staining (g) on day 6 of mock (left panels) and DSS treatment (right panels) of KO and WT mice and quantitation for numbers of PCNA+ cells (top right graph) and numbers of crypts (bottom right graph). For statistical analyses in (d, f, g) we analyzed 5mm portions each of 3 sections per mouse from n=5 mice per group, taken from two independent experiments. Data represented as means ± SD. * p < 0.05.
Next we treated mice with 3% DSS for 5 days and monitored their body weight daily for 2 weeks. In agreement with a previous study20, WT mice lost more body weight and recovered more slowly than KO mice (Figure 2c). After 3 days of DSS, but not mock treatment we already observed more apoptotic epithelial cells in WT than KO mice (Figure 2d; quantitated in summary graph), although crypts were still largely intact at this time with no significant difference in mucus production between the two genotypes (Figure 2e). However, by day 6 (one day after 5 days of DSS) colons of WT mice exhibited severe epithelial damage, while the epithelium of KO mice was still largely intact, as assessed with H&E stained sections (Figure 2f). We also noted overall fewer proliferating cells in colons of WT relative to KO mice at this time of DSS treatment (but not after mock-treatment), as analyzed with PCNA staining; this was true even when examining WT sections with relatively better preserved architecture (Figure 2g, left panels; quantitation of total PCNA+ cells shown in top right graph). Rather than reflecting an inherently lower rate of proliferation in WT colonic epithelial cells, the lower numbers of such proliferating cells in WT mice may be due primarily to the increased loss of crypts in these mice (quantitated in bottom right graph in Figure 2g). Thus loss of Bcl-3 may not inherently promote proliferation in colonic epithelial cells in mice challenged with DSS. We furthermore analyzed primary WT and KO mouse colon epithelial cells isolated from unchallenged mice (no DSS) and found no significant differences in their growth in culture or their rate of cell death ex vivo (Supplementary Figures S2c and d). These data suggest that Bcl-3 did not intrinsically alter these parameters under homeostatic conditions, consistent with in vivo data.
Intestinal epithelial cell-specific loss of Bcl-3 promotes tumor formation
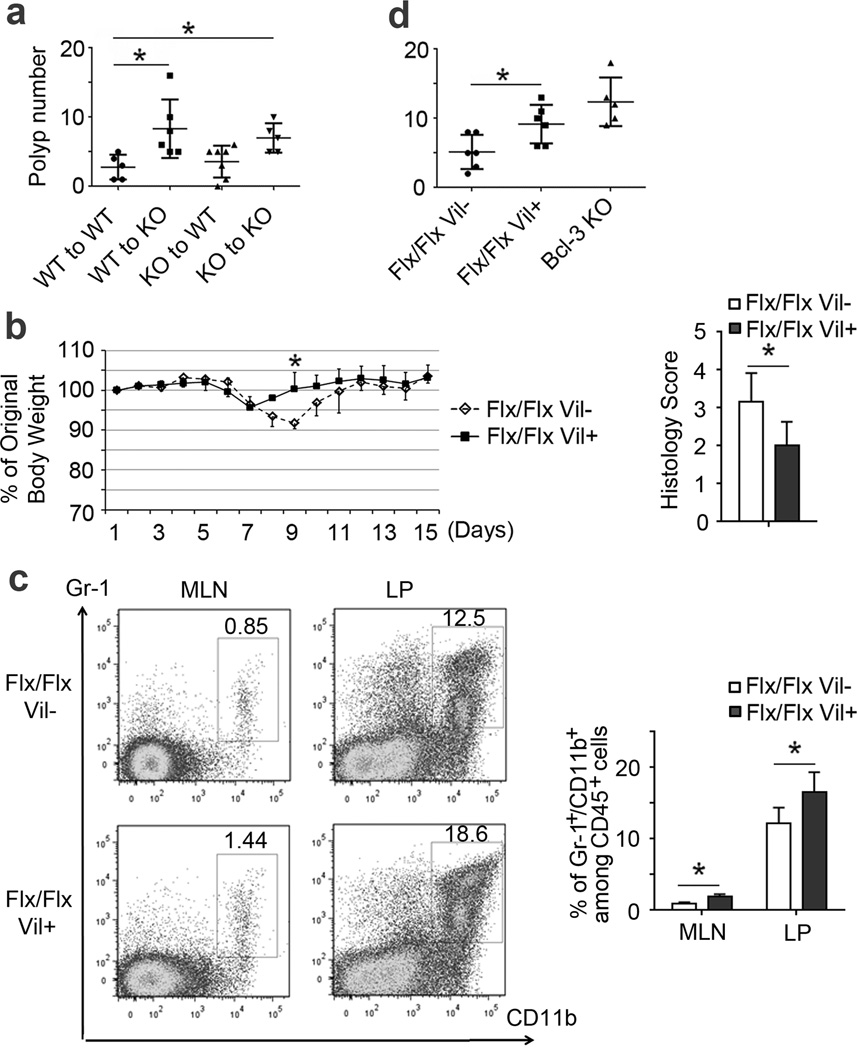
To determine in which cell type Bcl-3 acted to suppress inflammation-associated tumorigenesis in this model, we first performed reciprocal bone marrow transfer experiments and then treated recipient mice with AOM/DSS to induce colorectal tumors. We consistently observed more polyps in KO recipients compared to WT recipients, regardless of the type of bone marrow transferred (Figure 3a). This result indicated that radiation resistant cells (primarily stromal cells) were critical for the protective effect of Bcl-3.
Figure 3.
Intestinal epithelial-cell specific deficiency in Bcl-3 promotes tumor formation. (a) Polyp numbers of bone marrow chimeras with genotypes as indicated after AOM/DSS treatment (an additional experiment yielded similar results). (b) Daily weights and histology scores of WT (Flx/Flx) and intestinal epithelial cell-specific Bcl-3 KO mice (Flx/Flx, Vil+), treated with 3% DSS for five days (n= 6 (WT) and 5 (KO) mice; an additional experiment yielded similar results). Man-Whitney U tests were performed to determine significance in (a,b). (c) Representative example and summary of flow cytometric analysis of cells for markers as indicated, with cells from MLN and lamina propria (LP) of WT and intestinal epithelial cell-specific Bcl-3 KO mice on day six after DSS treatment (n=7 mice per group from two experiments). (d) Polyp numbers in individual mice with genotypes as indicated after AOM/DSS treatment (an additional experiment yielded similar results). Data represented as means ± SD. * p < 0.05.
To determine whether Bcl-3 might promote DSS-induced damage and suppress tumorigenesis via functions in colonic epithelial cells, we generated mice conditionally ablated of Bcl-3 in intestinal epithelial cells (Bcl-3flx/flx; Villin-Cre) (see Supplementary Figure S3a). Similar to mice globally lacking Bcl-3, those lacking Bcl-3 only in intestinal epithelial cells were largely protected from DSS-induced colitis when compared to Bcl-3-sufficient control mice, as judged by ameliorated weight loss and a better-preserved colonic epithelium (Figure 3b and Supplementary Figure S3b). Furthermore, DSS-treated mice lacking Bcl-3 in intestinal epithelial cells recruited more MDSCs into mesenteric lymph nodes and colon lamina propria than mice sufficient in Bcl-3 (Figure 3c). Finally, mice ablated of Bcl-3 in intestinal epithelial cells developed significantly more polyps compared with control mice after treatment with AOM and DSS, though possibly slightly fewer than mice with germline deletion (Figure 3d). Therefore, Bcl-3 functioned primarily in intestinal epithelial cells to suppress inflammation-associated tumorigenesis.
Bcl-3 suppresses TNFα responses
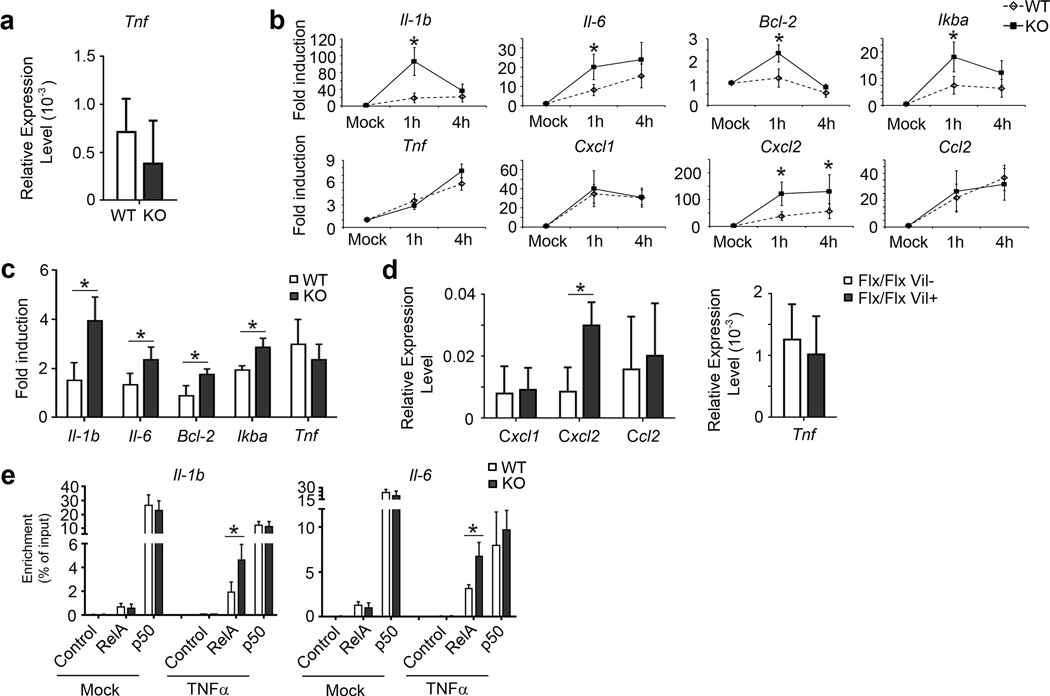
Anti-TNFα therapy has shown some efficacy in treatment of IBD patients21. Although it remains unsettled whether TNFα is critical for development of colitis in mouse models, possibly owing to differences in models and experimental details in various studies, this cytokine has been more clearly linked to tumor development in the AOM/DSS model13,22,23. TNFα mediates its functions in large part via activation of NF-κB, whose activity is also strongly associated with tumor formation. Given that Bcl-3 can dampen nuclear NF-κB activity, we hypothesized that Bcl-3 might suppress TNFα-mediated pro-tumorigenic activities. To this end we first examined TNFα mRNA levels after 3 days of DSS treatment and found no significant difference between WT and KO mice (Figure 4a). We then investigated whether Bcl-3 might modulate responses to TNFα. We generated primary MEFs from WT and KO animals, then treated these cells with 10 ng/mL TNFα for 1 h and 4 h and assessed mRNA levels for IL1β, IL-6, Bcl-2, IκBα and TNFα with RT-PCR assays. MEF cells lacking Bcl-3 (KO) exhibited higher expression levels for all these TNFα target genes than WT MEFs, except for TNFα itself (Figure 4b).. We additionally assessed mRNA levels of several chemokines that might play a role in recruiting MDSCs, and found higher TNFα-induced levels for CXCL2, but not CXCL1 or CCL2, in KO compared to WT MEFs (Figure 4b). We also isolated intestinal epithelial cells to examine their responses to TNFα in vitro. Responses were modest in these cells, but loss of Bcl-3 did result in higher TNFα-induced expression of IL1β, IL-6, Bcl-2 and IκBα, but not TNFα, consistent with the results obtained with MEFs (Figure 4c), although we could not discern a difference in what were very low expression levels of CXCL2. Nevertheless, we did detect significantly elevated expression of CXCL2 in colons of DSS-treated mice specifically lacking Bcl-3 in intestinal epithelial cells when compared with WT mice (Figure 4d, left graph), which supports the notion that this chemokine may have a role in increased recruitment of MDSCs. We found no difference in TNFα expression in the colons of these latter mice (Figure 4d, right graph), consistent with above findings. Together these data do suggest that Bcl-3 dampens several, though not all TNFα-induced responses.
Figure 4.
Bcl-3 deficiency leads to increased responses to TNFα. (a) RT-PCR analysis for TNFα expression in colons from WT and Bcl-3 KO mice after treatment with 3% DSS treatment for 3 days (n=5 mice per group; two additional experiments yielded similar results). (b) RT-PCR analysis of WT and KO mouse embryo fibroblasts (MEFs) for expression of genes as shown after TNFα treatment (10 ng/mL) for indicated times (n=6 mice per group from two experiments; an additional experiments yielded similar results). (c) RT-PCR analysis of intestinal epithelial cells from WT and KO mice for expression of genes as shown after TNFα treatment (10 ng/mL) for 4 h (n=9 mice per group from three experiments). (d) RT-PCR analysis for expression of genes as indicated in colons from mice treated with 3% DSS for 3 days (n=10 mice per group from three experiments). (e) ChIP analysis of indicated NF-κB subunits binding to IL1B and IL6 promoter in WT and Bcl3 KO MEF cells (n = 9 per group from three experiments). Data represented as means ± SD. * p < 0.05.
To gain insight into how Bcl-3 might inhibit responses to TNFα, we first investigated for potential effects on the activation of the classical IKK/NF-κB pathway in both MEFs and colon epithelial cells. The loss of Bcl-3 did not affect TNFα-induced degradation of IκBα in either cell type (Supplementary Figure S4a and b). Furthermore, there was no apparent difference between WT and KO MEFs in TNFα-induced nuclear translocation of RelA (Supplementary Figure S4c). However, ChIP analyses of the IL-1β and IL-6 promoters revealed higher levels of TNFα-induced association of RelA in KO as compared to WT MEFs (Figure 4e), consistent with the mRNA expression data above; this suggests Bcl-3 may delimit access/binding of RelA to these particular promoters (Figure 4e).
We furthermore investigated for potential effects of Bcl-3 on the alternative/non-canonical NF-κB signaling pathway. Upon treatment of primary mouse colon epithelial cells with Light in vitro we did not observe a difference in induced processing of p100/NF-κB2 to p52 between WT and KO cells (Supplementary Figure S4d). In addition, we detected similar levels of Light-induced nuclear translocation RelB/p52 complexes, as judged by co-immunoprecipitation (Supplementary Figure S4d, bottom panel). Finally, Bcl-3 did not affect p100 to p52 processing in MEFs upon treatment with TWEAK (Supplementary Figure S4e).
The suppressive effect of Bcl-3 on tumor formation is TNFα-dependent
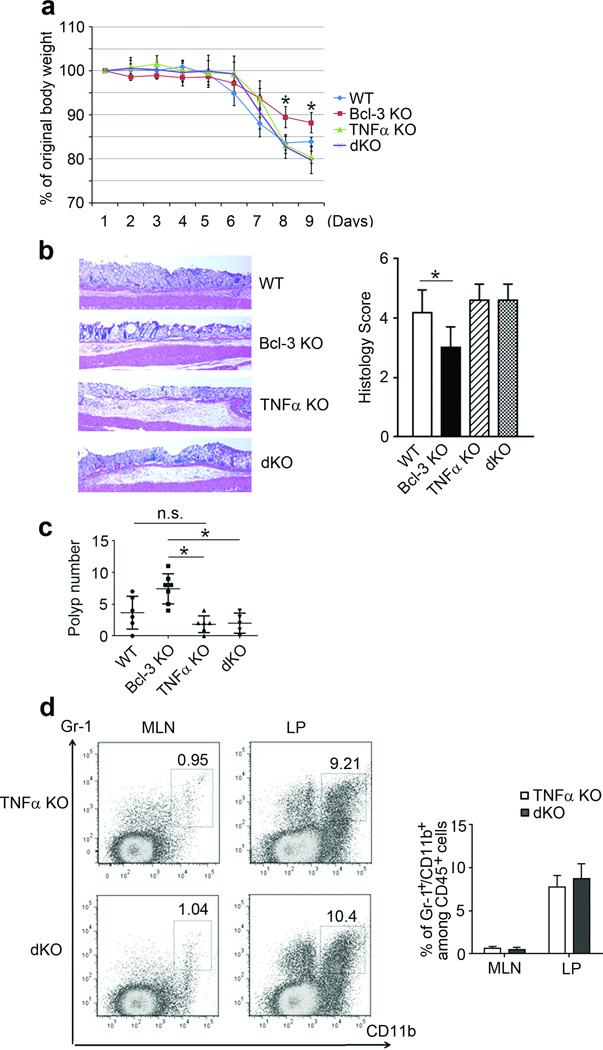
Since Bcl-3 negatively regulated TNFα responses in vitro, we asked whether the suppressive effect of Bcl-3 on tumor formation might be dependent on TNFα in vivo. To address this question we generated mice lacking both Bcl-3 and TNFα (double knockout; dKO) and initially subjected these mice, WT mice and mice lacking either Bcl-3 or TNFα to DSS for 5 days; we measured body weights for 9 days, at which point Bcl-3 KO mice had already lost less weight than WT mice. Absence of Bcl-3 in mice lacking TNFα (dKO) failed to reduce the weight loss experienced by TNFα KO mice, which was similar to the weight loss seen in WT mice (Figure 5a). Furthermore, dKO mice experienced epithelial damage to a degree similar to that in TNFα KO mice (and similar to that of WT mice), as revealed with IHC analysis (Figure 5b). These results indicated that loss of Bcl-3 on a TNFα knockout background no longer afforded these mice added protection against DSS-induced damage, while loss of Bcl-3 on an otherwise WT background did help to protect mice. We then challenged mice with AOM/DSS. Mice lacking TNFα had a tendency to develop slightly fewer polyps relative to WT mice, but this trend did not reach statistical significance with the numbers of animals analyzed. Importantly, loss of Bcl-3 on the TNFα KO background failed to increase the number of polyps, even though loss of Bcl-3 on a TNFα-sufficient background significantly increased polyp numbers relative to WT mice (Figure 5c). Consistent with this, dKO mice did not exhibit increased recruitment of MDSCs in mesenteric lymph nodes or lamina propria compared to TNFα KO mice, and there was no difference in expression of mRNA for CXCL2 (Figure 5d and Supplementary Figure S5). Thus the increased tumor burden seen in mice deficient in Bcl-3 no longer occurred in the absence of TNFα.
Figure 5.
The tumor-promoting effect associated with loss of Bcl-3 depends on TNFα signaling. (a) Daily weights of mice treated with 3% DSS for five consecutive days (n= 5 (WT), 6 (Bcl-3 KO), 5 (TNFα KO) and 7 (dKO) (an additional experiment yielded similar results). (b) Representative H&E stained sections and histology scores on day 9 of mice from (a). (c) Polyp numbers in mice with indicated genotypes after AOM/DSS treatment (an additional experiment yielded similar results). (d) Representative example and summary of flow cytometric analysis of cells for markers as indicated, with cells from MLN and LP of TNFα KO and Bcl-3/TNFα dKO mice on day six after treatment as in (a) (n=4 mice per group; an additional experiment yielded similar results). Data represented as means ± SD. * p < 0.05.
DISCUSSION
Here we investigated the role of Bcl-3 in an inflammation-associated colorectal carcinogenesis model. Bcl-3 has been ascribed oncogenic activities, based in large part on recurrent translocations of this gene in certain B cell leukemias, translocations that lead to overexpression of this protein, absent any mutations4. Although no translocations have been noted in solid tumors, some of these cancers exhibit elevated levels of Bcl-36. However, there is as yet no definitive evidence for a causative role of Bcl-3 in the genesis of these solid tumors. Nevertheless, some findings have linked Bcl-3 to cell survival and proliferation, which would be consistent with oncogenic activity6. It was therefore unexpected that Bcl-3 suppressed, rather than promoted colorectal tumor formation in the AOM/DSS model. We chose to investigate the role of Bcl-3 in this inflammation-associated carcinogenesis model, because prior work suggested that Bcl-3 functions in both immune cells and stromal/epithelial cells and thus may control the interplay between these cell populations at barrier sites18–20,24,25.
Our study demonstrates that Bcl-3 suppressed tumor initiation, limiting the number of polyps, rather than controlling tumor progression, as judged by the size of polyps and their proliferative status, which were similar in Bcl-3 sufficient and deficient mice. This suggests that Bcl-3 acted during the early phase in the AOM/DSS model to suppress tumorigenesis. Germline loss of Bcl-3 helped protect mice from excessive DSS-induced colitis, as also noted previously20. We now demonstrate here that loss of Bcl-3 specifically in colonic epithelial cells was sufficient to protect the epithelial cells from excessive DSS-induced cell death. The preservation of the epithelial layer in Bcl-3-deficient mice most likely underlies the increase in numbers of tumors, as presumably more of the AOM-mutated cells survived the DSS-induced stress, enlarging the pool of potential tumor-initiating stem cells.
The protection that loss of Bcl-3 afforded DSS-stressed epithelial cells may have been mediated, at least in part, by the significant increase in myeloid-derived suppressor cells (MDSCs) observed in mesenteric lymph nodes and the lamina propria of mutant mice. These cells usually accumulate at sites of strong inflammatory reactions/cell damage and are thought to help resolve inflammation and support tissue repair. As such, MDSCs are likely to provide an environment beneficial to survival of DSS-stressed and AOM-mutated epithelial cells, thereby promoting tumor development26,27. Increased accumulation of MDSCs correlated with increased expression of CXCL2 in the colon of DSS-challenged mice lacking Bcl-3 in intestinal epithelial cell; CXCL2 is a chemokine known to be involved in the recruitment of MDSCs28. However, the exact mechanisms by which Bcl-3 controlled recruitment of MDSCs remains to be determined.
Bcl-3 acted in colonic epithelial cells to suppress AOM/DSS-induced tumorigenesis. Loss of this regulator specifically in these cells was sufficient to increase polyp numbers to nearly the same degree as germline deficiency did. It is possible that other radioresistant mutant cells may have made additional, albeit limited contributions in mice with germline deficiency in Bcl-3. Nevertheless, the fact that loss of Bcl-3 in colonic epithelial cells was sufficient raised the possibility that Bcl-3 may function in these cells at least in part by modulating responses in the TNFα/NF-κB signaling pathway. TNFα signaling into colonic epithelial cells and the resulting activation of NF-κB has been reported as critical for AOM/DSS-induced tumor development13,22. While loss of Bcl-3 increased tumor burden relative to WT mice, loss of Bcl-3 in TNFα-deficient (dKO) mice failed to increase tumor burden relative to TNFα KO. Thus the tumorigenic effect resulting from loss of Bcl-3 appeared to be dependent on TNFα signaling. We furthermore found that Bcl-3-deficient fibroblasts and epithelial cells were relatively hyper-responsive to TNFα, exhibiting more pronounced induction of some NF-κB-dependent targets genes. Since Bcl-3 has previously been shown to inhibit certain NF-κB-regulated genes in epithelial/stromal cells18, Bcl-3 may blunt NF-κB activity on genes relevant in the present context. Indeed the roles of Bcl-3 revealed in our study are exactly opposite those of IKKβ or IKKγ/Nemo, primary mediators of classical NF-κB activation pathways. Signaling via IKKβ/Nemo in epithelial cells protected mice from DSS-induced colitis, while it promoted AOM/DSS-induced colorectal tumorigenesis15,29. Bcl-3 may thus suppress tumorigenic functions of classically activated NF-κB within epithelial cells, while also promoting DSS-induced colitis via these cells. Bcl-3 did not however modulate the classical IKK-dependent signaling pathway per se, as it did not impair TNFα-induced IκBα degradation or nuclear translocation of RelA, but instead delimited binding/access of RelA complexes to select regulatory gene regions, as shown here. Bcl-3 also did not modulate the non-canonical/alternative signaling pathway per se. It remains to be shown how exactly Bcl-3 functions to modulate actions of various NF-κB complexes in specific gene regulatory regions, including whether its functions are mediated locally via binding to p50/NF-κB1 and/or p52/NF-κB2 homodimers. Of note, different NF-κB factors appear to have distinct and even opposing roles in AOM/DSS-induced inflammation and tumorigenesis, based on studies with mice deficient in individual factors16; it is however unclear how these findings relate to our studies, given that an individual NF-κB factor can be part of physically and functionally distinct NF-κB dimers, and given that the study cited involved germline-deficient mice, in which functions of all cells are altered.
It is intriguing that IL-6 was among the TNFα-induced and NF-κB-dependent target genes whose expression was increased in Bcl-3-deficient epithelial cells and fibroblasts. This particular pro-inflammatory cytokine is considered crucial for colorectal tumor development via its ability to activate Stat3 in epithelial cells, which in turn is thought to promote proliferation and especially survival of these cells. Also, Stat3-deficiency in epithelial cells was reported to exacerbate DSS-induced colitis. Thus a TNFα/NF-κB/IL-6/Stat3 axis may help assure survival of DSS-stressed, AOM-mutated epithelial cells, preserving the epithelial barrier, but at the cost of promoting tumorigenesis17,30. Bcl-3 may normally dampen this tumorigenic pathway, and thus loss of Bcl-3 would enhance it.
Bcl-3 modulates NF-κB-regulated responses, and as such may help maintain homeostasis by properly balancing interactions between epithelial/stromal and immune cells. As shown here, loss of Bcl-3 ameliorated DSS-induced colitis damage, but at the same time promoted tumor development after AOM/DSS challenge. While colitic inflammation is required to induce tumors in this model, excessive inflammation may in fact suppress tumor initiation. Bcl-3 may act primarily in epithelial cells to dampen the tumor promoting effects of the TNFα/NF-κB pathway and to delimit the accumulation of MDSCs. Although Bcl-3 is an established tumor promoter in B cells, it appears to function as a tumor suppressor in epithelial cells in the AOM/DSS model. Bcl-3 is thus a prime example of a regulator whose contributions to tumor development are entirely dependent on context. The Bcl-3 pathway may provide new avenues to therapeutically control colitis-associated cancer development.
MATERIALS AND METHODS
Mice
Bcl-3 germline-deficient and conditionally-deficient mice have been described25,31,32. TNFα deficient (005540) and Villin-Cre (018963) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). 8 to 12 weeks old littermates were used for all experiments. Mice were housed in National Institute of Allergy and Infectious Diseases facilities, and all experiments were done with approval of the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee and in accordance with all relevant institutional guidelines.
Immunohistochemistry
Colon tissue samples were fixed with 10 ml of 10% formalin buffer and embedded in paraffin. Sections (5 µm) were cut and stained with hematoxylin and eosin (H&E). PAS and Alcian blue staining were performed to detect mucus production. The ApopTag kit (Millipore, Billerica, MA, USA) was used to detect apoptotic cells, following the manufacturer’s instructions. Histology of DSS treated colon was scored by a combination of inflammatory cell infiltration (score 0–3) and tissue damage (score 0–3) as previously described33. (Presence of occasional inflammatory cells in lamina propria scored as 0, increased numbers of inflammatory cells in lamina propria scored as 1, confluence of inflammatory cells extending into submucosa scored as 2, and transmural extension of the infiltrate scored as 3). For tissue damage, no mucosal damage was scored as 0, lymphoepithelial lesions scored as 1, surface mucosal erosion or focal ulceration scored as 2, and extensive mucosal damage and extension into deeper structures of the bowel wall scored as 3.
Flow cytometry
Spleen and lymph node samples were forced through 100 µm filters to prepare single-cell suspensions. Dead cells were removed by gradient centrifugation with lymphocyte M (Cedarlane, Burlington, NC, USA) and live cells were stained with surface antibodies for flow cytometric analysis. Data were collected in a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Antibodies used: Gr-1 (RB6-8C5, BD Biosciences, San Jose, CA, USA), CD11b (M1/70, eBioscience, San Diego, CA, USA), CD62L (MEL-14, eBioscience, San Diego, CA, USA), CD44 (IM-7, eBioscience, San Diego, CA, USA) and EpCAM (G8.8, eBioscience, San Diego, CA, USA).
AOM/DSS-induced colorectal tumor model and acute DSS treatment
8 to 12 week old mice were injected intraperitoneally with AOM (NCI, Frederick, Maryland, USA) at 12.5 mg/kg body weight. Five days after injection, 2.5% DSS (MW 36 – 50 kDa, MP Biomedicals, Santa Ana, CA, USA) was given in the drinking water for five days, followed by 16 days of regular water. This cycle was repeated twice more and mice were sacrificed ten days after the last complete cycle. The colon was washed with PBS and cut open longitudinally. Tumor polyps were measured under a dissecting microscope and their volume was calculated as 1/2 × length × (width)2. For acute DSS treatment 3% DSS was given in drinking water.
Intestinal epithelial cell isolation
To isolate intestinal epithelial cells, thoroughly washed intestine pieces were treated with 5 mM EDTA and 1 mM DTT in 1 × HBSS for 30 min at RT, then passed through 100 µm filters and centrifuged at 1600 rpm.
Real-time PCR
RNA was isolated using the RNeasy RNA isolation kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized with oligo(dT) using Superscript III (Life Technologies, Grand Island, NY, USA). Gene expression was quantified with the TaqMan real time PCR primers (Life Technologies). The results are then normalized against β-actin.
Chromatin immunoprecipitation
MEF cells were left untreated or treated with 10 ng/mL TNFα for 30 min (Peprotech, Rocky Hill, NJ, USA. ChIP analysis was performed using Magna ChIP A kit (Millipore, Billerica, MA, USA). ChIP grade antibodies indicated in Figures were from Santa Cruz Biotechnology (Dallas, TX, USA). Real-time PCR was performed with SYBR Green master mix (Life Technologies, Frederick, MD, USA) and previously reported promoter primers (Il1b 5’-CCCCTAAGAATTCCCATCAAGC-3’, 5’-GAGCTGTGAAATTTTCCCTTGG-3’and Il6 5’-CCCACCCTCCAACAAAGATT-3’, 5’-GCTCCAGAGCAGAATGAGCTA-3’)34.
Statistical analysis
All animals were randomized and analyzed in a blinded fashion for in vivo experiments. Each mouse in a given experiment was reported on, no mice were exluded. Group sizes were estimated based on the results of preliminary experiments. No statistical method was used to predetermine group size. There is an estimate of variation within each group, and the variance between groups was similar. Statistical significance was determined with the two-tailed unpaired Student’s t-test when comparing two groups that passed normality test, except when the Mann-Whitney U test was used, as indicated. One-way ANOVA test was used when comparing multiple groups. Normality test, Student’s t-test, Mann-Whitney U test and one-way ANOVA test were calculated in GraphPad Prism. All data are expressed as the mean ± SD. p values were considered to be statistically significant when less than 0.05.
Supplementary Material
Acknowledgments
We greatly appreciate the constructive inputs provided by all members of the Siebenlist laboratory. This research was supported by the Intramural Research Program of NIAID, NIH.
Footnotes
CONFLICTS OF INTEREST
Authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc).
REFERENCES
- 1.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 3.Hinz M, Arslan SC, Scheidereit C. It takes two to tango: IkappaBs, the multifunctional partners of NF-kappaB. Immunol Rev. 2012;246:59–76. doi: 10.1111/j.1600-065X.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S, Chen YH. Bcl-3, a multifaceted modulator of NF-kappaB-mediated gene transcription. Immunol Res. 2008;42:210–218. doi: 10.1007/s12026-008-8075-4. [DOI] [PubMed] [Google Scholar]
- 5.Schuster M, Annemann M, Plaza-Sirvent C, Schmitz I. Atypical IkappaB proteins - nuclear modulators of NF-kappaB signaling. Cell Commun Signal. 2013;11:23. doi: 10.1186/1478-811X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado V, Melendez-Zajgla J. Role of Bcl-3 in solid tumors. Mol Cancer. 2011;10:152. doi: 10.1186/1476-4598-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashatus D, Cogswell P, Baldwin AS. Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev. 2006;20:225–235. doi: 10.1101/gad.1352206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Jiang Y, Hou Y, Hu Y, Cao X, Tao Y, et al. The IkappaB family member Bcl-3 stabilizes c-Myc in colorectal cancer. J Mol Cell Biol. 2013;5:280–282. doi: 10.1093/jmcb/mjt020. [DOI] [PubMed] [Google Scholar]
- 10.Wakefield A, Soukupova J, Montagne A, Ranger J, French R, Muller WJ, et al. Bcl3 selectively promotes metastasis of ERBB2-driven mammary tumors. Cancer Res. 2013;73:745–755. doi: 10.1158/0008-5472.CAN-12-1321. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nature Medicine. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 15.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Burkitt MD, Hanedi AF, Duckworth CA, Williams JM, Tang JM, O'Reilly LA, et al. NF-kappaB1, NF-kappaB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. Journal of Pathology. 2015;236:326–336. doi: 10.1002/path.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassi I, Rikhi N, Claudio E, Wang H, Tang W, Ha HL, et al. The NF-kappaB regulator Bcl-3 modulates inflammation during contact hypersensitivity reactions in radioresistant cells. Eur J Immunol. 2015;45:1059–1068. doi: 10.1002/eji.201444994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W, Wang H, Claudio E, Tassi I, Ha HL, Saret S, et al. The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells. Immunity. 2014;41:555–566. doi: 10.1016/j.immuni.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Carroll C, Moloney G, Hurley G, Melgar S, Brint E, Nally K, et al. Bcl-3 deficiency protects against dextran-sodium sulphate-induced colitis in the mouse. Clin Exp Immunol. 2013;173:332–342. doi: 10.1111/cei.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne) 2014;1:3. doi: 10.3389/fmed.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onizawa M, Nagaishi T, Kanai T, Nagano K, Oshima S, Nemoto Y, et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G850–G859. doi: 10.1152/ajpgi.00071.2008. [DOI] [PubMed] [Google Scholar]
- 23.Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T, et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 24.Pene F, Paun A, Sonder SU, Rikhi N, Wang H, Claudio E, et al. The IkappaB family member Bcl-3 coordinates the pulmonary defense against Klebsiella pneumoniae infection. J Immunol. 2011;186:2412–2421. doi: 10.4049/jimmunol.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tassi I, Claudio E, Wang H, Tang W, Ha HL, Saret S, et al. The NF-kappaB regulator Bcl-3 governs dendritic cell antigen presentation functions in adaptive immunity. J Immunol. 2014;193:4303–4311. doi: 10.4049/jimmunol.1401505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manjili MH, Wang XY, Abrams S. Evolution of Our Understanding of Myeloid Regulatory Cells: From MDSCs to Mregs. Front Immunol. 2014;5:303. doi: 10.3389/fimmu.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasparakis M. Role of NF-kappaB in epithelial biology. Immunol Rev. 2012;246:346–358. doi: 10.1111/j.1600-065X.2012.01109.x. [DOI] [PubMed] [Google Scholar]
- 30.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Franzoso G, Carlson L, Scharton-Kersten T, Shores EW, Epstein S, Grinberg A, et al. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479–490. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 32.Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eigenbrod T, Bode KA, Dalpke AH. Early inhibition of IL-1beta expression by IFN-gamma is mediated by impaired binding of NF-kappaB to the IL-1beta promoter but is independent of nitric oxide. Journal of Immunology. 2013;190:6533–6541. doi: 10.4049/jimmunol.1300324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.