Abstract
Oncolytic viruses (OV), proteasome inhibitors, and NK cell immunotherapy have all been studied extensively as monotherapies but have never been evaluated in combination. Synergetic treatment of OV-infected glioblastomas (GBM) with a proteasome inhibitor induces necroptotic cell death to enhance NK cell immunotherapy, prolonging survival against human GBM.
Commentary
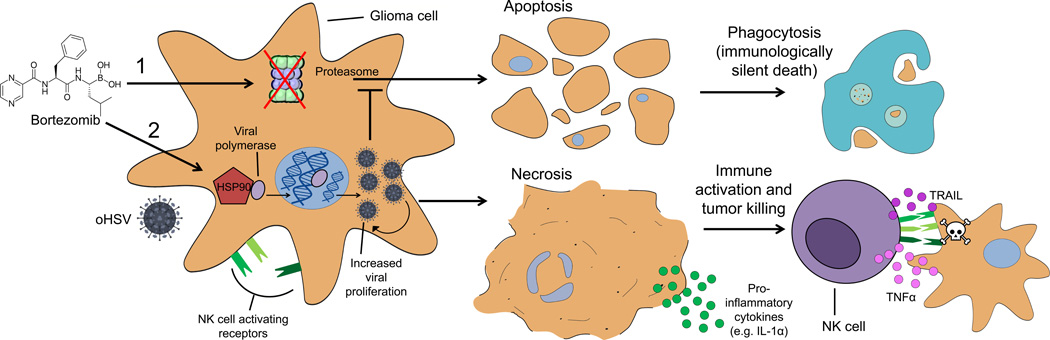
In this issue of Clinical Cancer Research, Yoo and colleagues report on a combination therapy that enhances the potency of an oncolytic virus (OV) to promote robust natural killer (NK) cell activity against glioblastoma (GBM) (1). OVs are an attractive therapeutic for brain tumors given their potential for selective replication in malignant cells while sparing normal brain. Oncolytic herpes simplex virus-1 (oHSV) has been under clinical investigation for GBM for more than a decade (2), and although its safety profile has been acceptable, its efficacy as a monotherapy has been disappointing. Bortezomib is a clinically available anticancer agent that functions by reversibly inhibiting the 26S proteolytic machinery, thereby interfering with protein turnover to promote tumor cell apoptosis (3). Interestingly, Bortezomib has recently been shown to synergize with oHSV therapy by increasing the expression of heat shock protein 90 (HSP90), which facilitates nuclear translocation of the oHSV viral polymerase to enhance viral replication (4). Herein, the authors show that combining oHSV therapy with Bortezomib prevents apoptosis and instead engenders inflammatory necrotic programmed cell death (i.e., necroptosis), which can be leveraged to enhance NK cell immunotherapy (Figure 1) after intracranial infusion to prolong survival against human GBM.
Figure 1. Bortezomib synergizes with oHSV to enhance NK cell activation and tumor killing.
(1) Bortezomib as a single agent: Bortezomib inhibits the proteasome to induce apoptotic cell death. Apoptotic bodies are subsequently engulfed by phagocytes, escaping immune recognition. (2) Bortezomib in combination with oHSV: Dual therapy leads to the upregulation of NK cell activating receptors and enhanced oHSV proliferation in infected glioma cells. Bortezomib increases the expression of HSP90, which facilitates nuclear translocation of oHSV viral polymerase, leading to enhanced viral proliferation and tumor necrosis (oHSV inhibits apoptotic cell death). Necrotic cells release pro-inflammatory factors (e.g., IL-1α) to enhance NK cell activation and the production of effector cytokines (e.g., TNFα and TRAIL), resulting in immune-mediated tumor killing.
OVs are an attractive biological therapy because of their ability to induce direct tumor cell lysis and simultaneously elicit innate and adaptive immune responses, which has only recently become appreciated in this age of cancer immunotherapy. Although the reasons for their failure remain controversial, the disappointing results of OVs as monotherapies may be explained by the need to strike a delicate balance between anti-viral suppression and immune-mediated tumor cell killing (5). Therefore, it is unsurprising that this approach requires a rethinking in order to favorably manipulate tumor microenvironments (TMEs) to promote a coordinated immune response. Alternatively, given improvements in technology and the escalation of cell transfer therapies, co-administering OVs with an autologous immune cell product has also become an attractive option.
Immunogenic cell death is believed to be a primary pathway of activating the immune system to recognize cancer, and the depth and breadth at which this occurs may explain the limitations of current therapies. The rationale is clear; non-specific therapies (e.g. traditional chemoradiation) are either 1) too toxic, 2) insufficiently penetrant and spread, or 3) prone to selecting for the outgrowth of tumor resistant clones. Despite being a promising alternative, targeted immunotherapies, on the other hand, suffer from a dearth of tumor-specific antigens that are homogeneously expressed, with the rare exception of lineage-derived cancers that share a common marker. Furthermore, immunological tumor killing is often dampened by various forms of tumor-mediated immunosuppression prevalent across many cancer types, particularly glioma (6). Key obstacles for the immunotherapy of solid tumors therefore include the need to adequately promote an immunogenic TME, the identification of tumor-specific antigens, and the need to address tumor heterogeneity, underscored by the clinical recurrence of antigen-negative tumor cells following targeted therapy (7).
Necroptosis is a critical cell death pathway involved in an array of physiological and pathological processes, governed by the receptor interacting protein kinases (RIPKs). In contrast to apoptosis which confers an “immunologically silent” cell death, necroptosis has gained attention for its potential role in instigating secondary immune responses, as its execution involves the production of pro-inflammatory cytokines and a concomitant spillover of intracellular contents following membrane disintegration (8). The study by Yoo and colleagues is encouraging because it demonstrates that it is possible to supersede chemotherapy-induced apoptosis with virally-induced necroptosis (i.e., an immunogenic cell death) and leverage these events for therapeutic gain.
Following combined treatment with Bortezomib and oHSV, tumor cells secrete higher levels of IL-1α and upregulate CD58, CD112, CD155. Given the putative role of this cytokine and these cell surface molecules in NK cell activation, the authors rationally evaluated the addition of NK cell immunotherapy as an adjuvant to this regimen, with success. There have been relatively few clinical trials evaluating NK cell therapy, however, given the historical difficulty of expanding these cells ex vivo. The study by Yoo and colleagues further demonstrates a robust strategy for activating antitumor NK cells, warranting continued investigations for improving the technical feasibility of this approach.
NK cell immunotherapy comes with great promise, as NK cells do not require the presence of target antigens for tumor cell recognition and killing, allowing for their pervasive activity in heterogeneous tumors or in settings where tumor-specific antigens have not yet been identified as potential targets. This versatility can be taken one step further; a recent study demonstrated the antitumor efficacy of human NK cells expressing a chimeric antigen receptor (CARs) with cross-reactivity for the epidermal growth factor receptor and its type III tumor-specific mutation, which are both expressed by GBM (9). Importantly, CAR-armed NK cells (CAR-NK cells) were also shown to synergize with oHSV, in line with the evidence presented by Yoo et al. It would be interesting to determine whether CAR-NK cells stand to benefit from activation signals induced by necroptosis, as CAR activation alone may obviate the need for this signaling. Notably, Alvarez-Breckenridge and colleagues have also shown that the acute responsiveness of NK cells to oHSV can hinder virotherapy by clearing infection before it sufficiently spreads (10), leading us to question whether the survival benefits observed by Yoo and colleagues can be improved by further delaying NK cell transfer after oHSV infection.
As alluded to earlier, understanding whether secondary antigen-specific immune responses are elicited in this context remains a top priority given the induction of necroptotic cell death when Bortezomib and oHSV are used in combination. This is, however, complicated by the need to study such mechanisms in a syngeneic and immunocompetent mouse model, where available strains are either impervious to oHSV infection or the viral replication rate in mouse glioma cells is incomparable to the physiological levels found in clinical specimens. Nonetheless, determining whether this combination treatment can favorably augment immune cell recruitment and intratumoral immune cell interactions in the central nervous system will be critical.
Acknowledgments
Financial support: This work was supported by funding from the National Institutes of Health: 5R01-NS085412-04 (J.H. Sampson), 5R01-CA177476-04 (J.H. Sampson) 5R01-NS086943-03 (J.H. Sampson), 4P01-CA154291-05 (D.D. Bigner and J.H. Sampson), 5U01-NS090284-02 (J.H. Sampson), 5R25-NS065731-08 (J.H. Sampson), 5P50-CA190991-02 (J.H. Sampson), and T32-CA009111 (C.M. Suryadevara). Additional support was provided by the Pediatric Brain Tumor Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Yoo JY, Jaime-Ramirez AC, Bolyard C, Dai H, Nallanagulagari T, Wojton J, et al. Bortezomib treatment sensitizes oncolytic HSV-1 treated tumors to NK cell immunotherapy. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ning J, Wakimoto H. Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy. Front Microbiol. 2014;5:303. doi: 10.3389/fmicb.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 4.Yoo JY, Hurwitz BS, Bolyard C, Yu JG, Zhang J, Selvendiran K, et al. Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clin Cancer Res. 2014;20:3787–3798. doi: 10.1158/1078-0432.CCR-14-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Breckenridge CA, Choi BD, Suryadevara CM, Chiocca EA. Potentiating oncolytic viral therapy through an understanding of the initial immune responses to oncolytic viral infection. Curr Opin Virol. 2015;13:25–32. doi: 10.1016/j.coviro.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 9.Chen XL, Han JF, Chu JH, Meisen W, Zhang JY, Chiocca EA, et al. Modulation of Natural Killer Cell Activity in the Setting of Oncolytic Virotherapy and with a Chimeric Antigen Receptor. Blood. 2015;126 [Google Scholar]
- 10.Alvarez-Breckenridge CA, Yu J, Price R, Wojton J, Pradarelli J, Mao H, et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med. 2012;18:1827–1834. doi: 10.1038/nm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]